HLS1 promotes apical hook formation by regulating YUCCA8 and GH3.17 expression differently in the inner and outer side of the hook in cotton
Abstract
HOOKLESS1 (HLS1) plays an indispensable role in apical hook development by promoting asymmetric auxin distribution in the hook. However, the precise mechanisms by which HLS1 regulates the differential gene expression in the inner and outer sides of the apical hook to promote this asymmetric auxin distribution remains unknown. The inner and outer sides of the cotton apical hook were separated for gene sequencing to search for differently expressed auxin-related genes. Then, the GhYUCCA8 and GhGH3.17 genes, which are highly expressed in the inner and outer side, respectively, as well as GhHLS1, were silenced by the SSA-VIGS method to study their interaction and function on auxin asymmetric distribution and hook formation. The hook formation was inhibited in the VIGS-GhHLS1, VIGS-GhYUCCA8 and VIGS-GhGH3.17 seedlings. Silencing GhHLS1 decreased the expression of GhYUCCA8 on the inner side but increased the expression of GhGH3.17 on the outer side of the apical hook, which weakened the auxin asymmetric distribution in the hook. Our results indicated that the high expression of GhHLS1 in the inner side caused high expression of GhYUCCA8 and GhGh3.17 in the inner and outer side of the apical hook, respectively, which caused the gradient of free auxin between the inner and outer side and ultimately promoted apical hook formation.
1 INTRODUCTION
For most spermatophytes, seeds that germinate in the soil need to break through the soil after germination under darkness. To manage to break the surface, dicotyledonous plants form an apical hook in the dark, which provides mechanical protection for the cotyledons and apical meristem as the seedlings penetrate the soil (McNellis and Deng, 1995; Von Arnim and Deng, 1996; Leivar et al., 2008). The mechanical pressure from the topsoil induces ethylene biosynthesis in the seedling, which increases radical expansion and exaggerates apical hook formation by regulating EIN3, ERF1, PIF3, and HLS1 in dicotyledonous plants (Zhong et al., 2014; Shen et al., 2016; Shi et al., 2016).
The apical hook is formed through asymmetric cell division and cell growth at opposite sides of the apical portion of the hypocotyls. The growth and division rates of the cells on the concave (inner) side are lower than those on the convex (outer) side (Raz and Ecker, 1999; Raz and Koornneef, 2001). Plant hormones play important roles in promoting apical hook formation. Auxin (IAA), especially the asymmetrical auxin gradient in the apical hook, plays an important role for the different cell division and cell growth between the inner and outer sides of the apical hook (Zadnikova et al., 2010; Gallego-Bartolomé et al., 2011; Mazzella et al., 2014; Du et al., 2022). During hook formation and maintenance, inner cells within the hook region accumulate more auxin, thereby inhibiting the growth of the inner side through the auxin-TMK1 signalling pathway (Zadnikova et al., 2010; Gallego-Bartolomé et al., 2011; Cao et al., 2019). Seedlings exhibit a hookless phenotype when the asymmetrical accumulation of auxin is abolished by applying naphthylphthalamic acid to block auxin transport (Lehman et al., 1996; Zadnikova et al., 2010). The asymmetric auxin distribution in hook tissues is regulated by multiple levels of auxin-related events, including biosynthesis, transport and signalling at the apical hook (Li et al., 2006; Vandenbussche et al., 2010; Wu et al., 2010; Zadnikova et al., 2010; Béziat & Kleine-Vehn, 2018). The asymmetrical expression and localization of auxin carriers, such as PIN-FORMED auxin efflux carriers PIN1, PIN3, PIN4 and PIN7 (Zadnikova et al., 2010), the AUXIN-RESISTANT1/LIKE-AUX3 (AUX1/LAX3) auxin import carriers and the ATP-binding cassette (ABC-type) subfamily B number 19 (ABCB19) auxin efflux transporter play important role on the auxin gradient between inner and outer sides of apical hook. Auxin synthesis is also required for hook development, as hook development is inhibited in the mutants of some IAA biosynthetic genes (yucca, sur1/sur2, and taa1/tar2) (Boerjan et al., 1995; Zhao et al., 2001; Stepanova et al., 2008; Mazzella et al., 2014).
The HOOKLESS 1 (HLS1) gene encodes an N-acetyltransferase, which plays a key role in apical hook formation (Lehman et al., 1996; Shen et al., 2016). The hls1 mutant fails to form the apical hook due to defects in asymmetric auxin distribution and differential expression of auxin-dependent genes (Li et al., 2004). Ethylene plays a vital role in apical hook formation, directly increasing the HLS1 expression through EIN3/EIL1 and promoting apical formation (Li et al., 2004; An et al., 2012). Interestingly, gibberellins (GAs) and jasmonate (JA) also regulate apical hook development through EIN3/EIL1 (An et al., 2012; Song et al., 2014; Zhang et al., 2014, 2018a). In addition, GAs may directly enhance apical hook formation by promoting cell elongation and cell division at the outer side of the hook (Vriezen et al., 2004). PIF3 and EIN3 are dominant transcription factors which can independently bind to the promoter of HLS1 to activate HLS1 expression and promote apical hook formation cooperatively (Shi et al., 2018; Zhang et al., 2018a). COP1 is an E3 ubiquitin ligase and represses light signalling predominantly through the proteasome degradation system (Shi et al., 2016; Hoecker, 2017). Mutation in COP1 decreases the expression of the HLS1 gene and causes severe defects in the ability to penetrate the soil since the apical hook opens before it emerges from the soil (Shi et al., 2016). Although HLS1 plays a key role in apical hook development by regulating asymmetric auxin distribution in the inner and outer sides of the apical hook (Li et al., 2004), the direct evidence for the N-acetyltransferase of HLS1 on auxin distribution and signalling remains to be explored so far (Wang and Guo., 2019).
Cotton is a dicotyledonous plant. The apical hook forms after seed germination in the field to minimize the damage to the shoot apical meristem during growing out of the soil (Kong et al., 2018; Zhou et al., 2022). Because of the large size of cotton seeds, the apical hook of cotton seedlings is large enough to easily separate the inner and outer sides of the apical hook, making it possible to accurately determine the physiological and molecular events on each side. In the present study, the apical hook of cotton seedlings from sand culture was harvested, and its inner and outer sides were evenly separated along the longitudinal axis. The molecular mechanisms of the apical formation shown as differences in cell growth and cell division between the inner and outer sides were investigated through transcriptomic and genetic methods. We hypothesized that HLS1 promotes asymmetric auxin distribution for apical hook formation by regulating auxin-related genes differently expressed on the inner and outer sides of the apical hook. Therefore, this study focused on (1) the expression pattern of differently expressed hormone and transcription factor-related genes between the inner and outer sides; (2) the function of differently expressed auxin-related genes GhYUCCA8 and GhGH3.17 between the inner and outer sides of apical hook on apical hook formation; (3) the mechanism that HLS1 promotes asymmetric IAA distribution and apical hook formation by regulating GhYUCCA8 and GhGH3.17 genes differently expressed in the inner and outer sides.
2 MATERIALS AND METHODS
2.1 Plant materials, treatment, growth conditions, sampling and anatomical analysis
The commercial cotton (Gossypium hirsutum L.) cultivar K638 was used in this experiment. Acid-delinted seeds were sown at ~1 and ~ 3 cm depths in plastic boxes (20 × 15 × 10 cm) containing sterilized wet sand and allowed to germinate in a growth chamber under darkness at 28 ± 2°C. As for 1-amino cyclopropane-1-carboxylic acid (ACC, ethylene precursor) and 1-methylcyclopropene (1-MCP, functional inhibitor of ethylene) treatment, acid-delinted seeds were sown at 1 cm depths in the plastic boxes containing sterilized wet sand. The sterilized wet sand, containing 40 μM ACC and 10 μM 1-MCP, was used as ACC /1-MCP treatment and sand containing only water was used as control. The seedlings were harvested 3 days after planting (DAP), and the inner and outer sides of the apical hook were separated equally along the longitudinal axis using a blade (Figure S1). They were frozen in liquid nitrogen and stored at −80°C. For each treatment, three biological replicates were taken; the samples of each biological replicate were pooled from 50 randomly selected seedlings.
To observe the anatomical structure of the apical hook, the complete apical hook was harvested and fixed in FAA (formalin: alcohol: glacial acetic acid was 90:5:5, V). The fixed material was then dehydrated using an ethanol series, cleared in xylene, embedded in paraffin wax and cut into 4–6-μm-thick sections using a rotary microtome (RM2016, Leica). The sections were stained with safranine and fast green and fixed in neutral balata. They were subsequently observed and photographed with a microscope (Nikon Eclipse E100, Nikon digital sight DS-U3). The Image J program (http://rsbweb.nih.gov/ij/) was used for image analysis. The scale was appropriately adjusted based on the image dimensions, the scale was set at 50 μm, and the settings were saved for future reference. Subsequently, the line drawing tool was selected to meticulously delineate a closed shape encompassing the cell outline. The “measure” function was employed to calculate and record the cell area, repeating the measurement process 4–6 times for accuracy.
2.2 RNA extraction, DGE sequencing and analysis
Total RNA was extracted using the TRIzol reagent (Invitrogen), and mRNA was isolated from total RNA using Dynabeads Oligo (dT) (Invitrogen Dynal), following the manufacturer's instructions. The inner or outer sides of the apical hook from 50 representative individual plants were mixed as one biological replicate for total RNA extraction. For RNA-Seq, 8 μg of total RNA was used. Tag libraries were prepared using the Illumina Gene Expression Sample Prep Kit, following the manufacturer's protocol (Luan et al., 2011). The libraries were then sequenced using an Illumina HiSeq™ 2500 with 50-bp single-end (SE) reads each. The genome of G. hirsutum (http://ftp.ncbi.nih.gov/repository/unigene/Gossypium_hirsutum/Ghi.Seq.uniq.gz) was used as a reference sequence to align and identify the sequencing reads. To map the reads to the reference, the alignments and the candidate gene identification procedure were conducted using the mapping and assembly with qualities software package (Li et al., 2008). Clean tags mapping to reference sequences from multiple genes were filtered out, and the remaining clean tags were designated as unambiguous clean tags. For gene expression analysis, the number of unambiguous clean tags for each gene was calculated and then normalized to TPM (number of transcripts per million clean tags) (Morrissy et al., 2009).
2.3 Identification of differentially expressed genes and functional analysis
To identify differentially expressed genes (DEGs) across the inner and outer sides of the apical hook, pairwise comparisons among the inner and outer sides of the apical hook samples were performed using a rigorous algorithm method based on a previous method by Audic et al. (1997). The DEGs were obtained after filtering using a threshold FDR of ≤0.001 and an absolute value of log2 Ratio ≥2. GO enrichment analysis was performed for functional categorization of DEGs using agriGO software, and the p-values were corrected by applying the FDR correction to control falsely rejected hypotheses during GO analysis (Du et al., 2010). The pathway analysis was conducted using KEGG (www.genome.jp/kegg/). The genes related to hormones and transcription factors were selected manually.
2.4 Quantitative real-time (qRT)-PCR analysis
The gene expression level was determined using qRT-PCR. The inner or outer sides of the apical hook from 50 representative individual seedlings were mixed as one biological replicate to extract RNA. Then, 2 μg of RNA was reverse transcribed into cDNA with a Super Script III RTS First-Strand cDNA Synthesis Kit (Invitrogen). The primers used for real-time PCR are listed in Table S1. 20 μL of each sample was run in triplicate on a Bio-red IQ2 Sequence Detection System with Applied Biosystems software using 0.1 μL of first-strand cDNA and SYBR Green PCR Master Mix (Applied Biosystems, Cat. No.: 4385617). Thermal cycling was performed at an initial denaturation step of 95°C for 3 min, followed by 40 cycles (95°C for 10 s, 60°C for 10 s, and 72°C for 10 s). Baseline data were collected between cycles 3 and 15. All amplification plots were analyzed with a 0.2 fluorescence signal threshold to obtain CT values. The CT values of all genes were normalized to the CT value of the β-actin to compare data from different PCR runs and cDNA samples. The PCR efficiency (E) was estimated from the data obtained from the exponential phase of each individual amplification plot with the equation E = 10−1/slope − 1 (Ramakers et al., 2003). The expression level of each gene of interest (GOI) is presented as (1 + E)−△CT, where △CT = CTGOI − CTREF.
2.5 IAA and IAA-glu extraction, purification, and quantification
The quantification of endogenous IAA and IAA-Glu was performed according to the method previously reported by Xin et al. (2020) with some modifications. The ground powder of 200 mg fresh plant tissues was extracted overnight in methanol containing [2H2]-IAA as internal standards. The crude extracts were further purified by the Oasis MCX SPE cartridge, which was first activated and equilibrated with methanol, water and 0.05% formic acid (FA). The samples were loaded into the cartridge and sequentially washed with 5% FA and water. Finally, IAA and IAA-Glu were eluted with methanol and analyzed on a liquid chromatography–tandem mass spectrometry system comprising an ACQUITY UPLC (Waters) and Qtrap 6500 system (AB SCIEX) equipped with electrospray ionization (ESI) source. The separation of IAA and IAA-Glu was achieved on a Waters ACQUITY UPLC BEH C18 column (100 × 2.1 mm i.d., 1.7 μm) with mobile solvents of 0.1% FA in water (solvent A) and acetonitrile (solvent B) at a programmed gradient from 95:5 (v/v) A:B to 5:95 (v/v) A:B within 5 min. The multiple reaction monitoring (MRM) transitions for IAA, IAA-Glu and [2H2]-IAA were as follows: IAA 176.2 > 130.1, IAA-Glu 305.1 > 130.1, [2H2]-IAA 178.2 > 132.0. The analysis was performed on three biological replicates. Three biological replicates were analyzed for each treatment, and each sample was quantified three times.
2.6 Seed soak agroinoculation-virus-induced gene silencing (SSA-VIGS) procedure
The pTRV-VIGS vectors were constructed as described previously (Gao et al. 2011). Briefly, cDNA fragments of GhHLS1, GhYUCCA8 and GhGH3.17 were amplified from K638 by PCR with gene-specific primers. The resulting products were digested with restriction enzymes (EcoRI and KpnI) and cloned into the pTRV2 vector to produce the recombinant vectors pTRV::GhYUCCA8, pTRV::GhGH3.17, and pTRV::GhHLS1, respectively. The GFP gene was also cloned into the pTRV2 vector to produce the recombinant vector pTRV::GFP, which was used as the control vector. These recombinant vectors and the empty vector (pTRV::00) were then introduced into the Agrobacterium strain GV3101 and resuspended in a solution consisting of 10 mM 3-(N-morpholino) ethane sulfonic acid, 10 mM MgCl2, 200 μM acetosyringone (AS), and 5% (w/v) sucrose. The densities of the solutions were set as OD values of 2.0 at 600 nm, as reported previously (Kim et al., 2016; Zhang et al., 2018b). Then, Agrobacterium cultures containing pTRV1 and pTRV2 or their derivatives were mixed in a 1:1 ratio.
The vectors containing target genes were transformed into cotton seeds according to the method described by Zhang et al. (2018b). The seeds were surface-sterilized with 3% hydrogen peroxide solution for 60 min and then washed with sterilized distilled water six times. After that, the seeds were immersed in sterilized distilled water for 12 h at 28°C, and the seed coats were completely removed. The naked seeds were dipped into Agrobacterium suspensions for 4 h with 200 μM AS at 26°C. The inoculated seeds were sown at ~2 cm depth in plastic boxes (20 × 15 × 10 cm) containing sterilized wet sand. The boxes were placed in an incubator under 22°C and continuous darkness. The seedlings were harvested three days after planting.
2.7 Statistical analysis
Data were statistically analyzed using the analysis of variance, and treatment means were compared using Duncan's Multiple Range at p = 0.05.
3 RESULTS
3.1 Difference in cell growth and IAA content between the inner and outer sides of the apical hook
The cell size of the inner side was smaller than that of the outer side of the apical hook, both in 1 and 3 cm sand-covered seedlings (Figure 1A-C). The apical hook was enhanced by increasing depths of sand covering at 3 DAP (Figure S1). The inner side cell length of the apical hook in the 1 cm sand covered seedlings was larger than that of the 3 cm sand covered seedlings, while the cell size of the outer side did not differ between 1 and 3 cm depths of sand covering (Figure 1C). The IAA content in the inner side was higher than that in the outer side of the apical hook under both 1 cm and 3 cm sand covering treatment (Figure 1D). The asymmetrical gradient of the IAA between outer and inner side of the apical hook was enhanced as the sowing depth increased (Figure 1E).
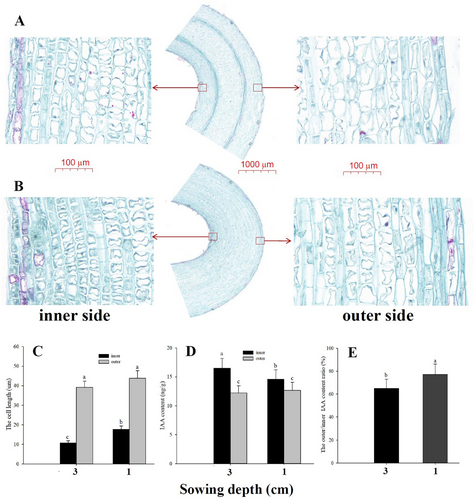
3.2 Transcriptome analysis and identification of differentially expressed genes between inner and outer sides of the apical hook
Transcriptome analysis was performed on the inner and outer sides of the apical hook under 1 and 3 cm sand covering treatment, Clean tags ranging from 45.4 to 56.1 million per library (SRA submission number: SRP068502) were obtained. The gene sequences of G. hirsutum genome were used as reference to align and identify the sequencing reads. This allowed for the mapping of approximately 92% of the distinct clean tags that passed our filters, representing more than 42.2 million reads per library, with about 85% mapped unique reference genes (Table S2).
The differentially expressed genes with an average change of more than 2-fold between the two treatments were finally selected. Accordingly, 474 and 989 differentially expressed genes (DEGs) in the inner and outer side of the apical hook were identified in the 1 and 3 cm sand-covered seedlings, respectively (Figure 2A). Among them, 315 DEGs appeared simultaneously in both sowing depth treatments. There were 103 genes up-regulated and 212 genes down-regulated on the outer side compared to the inner side of the apical hook (Figure 2A). Therefore, these DEGs were selected for in-depth analysis.
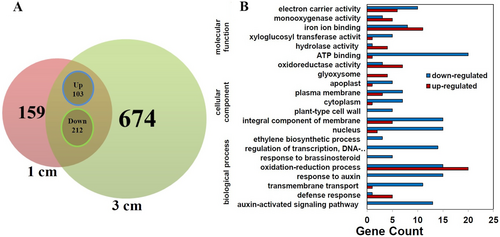
3.3 Hormone-related genes and Transcription factor genes differentially expressed in the inner and outer sides of the apical hook
Gene ontology (GO) analysis was performed by mapping each DEG into the records of the GO database (http://www.geneontology.org/). The GO annotation of the 315 DEGs showed that 22 groups, such as electron carrier activity, monooxygenase activity, ethylene biosynthetic process, regulation of transcription, response to brassinosteroid, oxidation–reduction process, response to auxin and auxin-activated signalling pathway, etc. were identified (Figure 2B). Interestingly, these genes related to ethylene biosynthetic process, regulation of transcription, response to brassinosteroid, response to auxin and auxin-activated signalling pathway were all down-regulated in the outer side compared to those in the inner side of apical hook (Figure 2B).
To understand the functions of the DEGs, we mapped all these genes to the terms in the KEGG database and compared them with the whole transcriptome background to search for significantly enriched genes. The KEGG analysis showed that 14 pathways were enriched. Among them, the plant hormone signal transduction pathway was the most significantly enriched pathway, with 28 genes down-regulated in the outer side compared the inner side of the apical hook (Table S3).
Hormone-related pathways were enriched in the GO and KEGG data suggesting that hormones may play an important role in regulating apical hook formation. Among the 315 DEGs, there were 51 hormone-related genes, which include 46 auxin, 3 ethylene and 2 JA-related genes (Table 1). The 3 ethylene related genes were ethylene biosynthesis genes 1-aminocyclopropane-1-carboxylate synthases (ACS), and their expression in the inner side was higher than that in the outer side of the apical hook (Table 1). On the contrary, the expression of the 2 JA-related genes were lower in the inner side than in the outer side of the apical hook (Table 1). Auxin-related genes were the most enriched hormone-related DEGs. Compared to the inner side of the apical hook, there were 6 up-regulated and 40 down-regulated auxin-related genes in the outer side. Most of the auxin-related genes were auxin signalling genes such as SAUR and AUX/IAA genes, except one IAA biosynthesis gene, YUCCA8 and one IAA-amido synthetase gene, GH3.17.
| Hormone | Gene ID | log2(inner vs outer) of 1 cm treatment | log2(inner vs outer) of 3 cm treatment | Gene annotation |
|---|---|---|---|---|
| Auxin | evm.TU.Gh_A04G0617 | 1.02 | 1.05 | SAUR family protein [Theobroma cacao] |
| evm.TU.Gh_A06G1664 | 1.27 | 1.90 | Auxin-responsive IAA26 -like protein [Gossypium arboreum] | |
| evm.TU.Gh_A11G2575 | 1.74 | 2.90 | Indole-3-acetic acid-amido synthetase GH3.17 [Theobroma cacao] | |
| evm.TU.Gh_D04G1076 | 1.12 | 1.25 | SAUR family protein [Theobroma cacao] | |
| evm.TU.Gh_A13G2013 | 1.67 | 3.38 | Auxin response factor 18 [Gossypium arboreum] | |
| evm.TU.Gh_D07G0892 | 2.58 | 1.68 | Auxin-induced in root cultures 12 -like protein [Gossypium arboreum] | |
| evm.TU.Gh_A03G1771 | −2.13 | −2.54 | Auxin-induced SAUR protein [Theobroma cacao] | |
| evm.TU.Gh_A03G1775 | −1.61 | −1.31 | Auxin-induced SAUR protein [Theobroma cacao] | |
| evm.TU.Gh_A03G1776 | −3.42 | −2.41 | Auxin-induced SAUR protein [Theobroma cacao] | |
| evm.TU.Gh_A03G1779 | −2.26 | −2.49 | Auxin-induced SAUR protein [Theobroma cacao] | |
| evm.TU.Gh_A03G1780 | −1.51 | −1.84 | Auxin-induced SAUR protein [Theobroma cacao] | |
| evm.TU.Gh_A03G1782 | −2.70 | −3.06 | SAUR-like auxin-responsive protein family, putative [Theobroma cacao] | |
| evm.TU.Gh_A03G1838 | −2.35 | −2.29 | SAUR-like auxin-responsive protein family, putative [Theobroma cacao] | |
| evm.TU.Gh_A03G1840 | −1.02 | −1.08 | SAUR-like auxin-responsive protein family, putative [Theobroma cacao] | |
| evm.TU.Gh_A05G1068 | −2.60 | −3.18 | Indole-3-acetic acid inducible 19 [Theobroma cacao] | |
| evm.TU.Gh_A07G2154 | −1.28 | −1.77 | Auxin-induced 22D [Gossypium arboreum] | |
| evm.TU.Gh_A08G2485 | −2.60 | −2.07 | auxin-responsive protein [Gossypium hirsutum] | |
| evm.TU.Gh_A02G0340 | −1.88 | −2.08 | Gbiaa-Re [Gossypium barbadense] | |
| evm.TU.Gh_D02G0405 | −1.55 | −1.29 | Gbiaa-Re [Gossypium barbadense] | |
| evm.TU.Gh_A09G1530 | −1.68 | −1.99 | Auxin transporter-like protein 4 [Gossypium arboreum] | |
| evm.TU.Gh_A09G1945 | −1.77 | −2.53 | Auxin-induced 22D [Gossypium arboreum] | |
| evm.TU.Gh_A10G1020 | −1.28 | −1.04 | AUX/IAA transcriptional regulator family protein [Theobroma cacao] | |
| evm.TU.Gh_A11G1627 | −2.94 | −4.33 | SAUR family protein, partial [Theobroma cacao] | |
| evm.TU.Gh_A13G1674 | −1.63 | −1.31 | SAUR family protein [Theobroma cacao] | |
| evm.TU.Gh_A13G2186 | −2.25 | −2.16 | SAUR-like auxin-responsive protein family [Theobroma cacao] | |
| evm.TU.Gh_D02G2200 | −2.04 | −2.037 | Auxin-induced SAUR protein [Theobroma cacao] | |
| evm.TU.Gh_D02G2203 | −2.50 | −1.95 | Auxin-induced SAUR protein [Theobroma cacao] | |
| evm.TU.Gh_D02G2204 | −1.79 | −1.32 | Auxin-induced SAUR protein [Theobroma cacao] | |
| evm.TU.Gh_D02G2209 | −1.75 | −1.56 | Auxin-induced SAUR protein [Theobroma cacao] | |
| evm.TU.Gh_D02G2214 | −1.37 | −1.68 | SAUR-like auxin-responsive protein family, putative [Theobroma cacao] | |
| evm.TU.Gh_D02G2215 | −2.35 | −1.69 | SAUR-like auxin-responsive protein family, putative [Theobroma cacao] | |
| evm.TU.Gh_D05G0568 | −2.20 | −2.30 | Auxin-responsive IAA29 -like protein [Gossypium arboreum] | |
| evm.TU.Gh_D05G1217 | −3.11 | −3.22 | Indole-3-acetic acid inducible 19 [Theobroma cacao] | |
| evm.TU.Gh_D06G0009 | −1.43 | −1.30 | Auxin-induced 15A [Gossypium arboreum] | |
| evm.TU.Gh_D07G1540 | −2.13 | −2.17 | Flavin-containing monooxygenase YUCCA8 -like protein [Gossypium arboreum] | |
| evm.TU.Gh_D07G2124 | −1.25 | −1.47 | Auxin-induced 22D [Gossypium arboreum] | |
| evm.TU.Gh_D08G0665 | −2.12 | −2.52 | Auxin-induced 22B [Gossypium arboreum] | |
| evm.TU.Gh_D09G1548 | −2.21 | −2.53 | Auxin transporter-like protein 2 [Gossypium arboreum] | |
| evm.TU.Gh_D09G2152 | −4.17 | −3.10 | Auxin-induced 22D [Gossypium arboreum] | |
| evm.TU.Gh_D10G1512 | −1.37 | −1.74 | AUX/IAA transcriptional regulator family protein [Theobroma cacao] | |
| evm.TU.Gh_D12G0291 | −4.21 | −2.53 | SAUR family protein [Theobroma cacao] | |
| evm.TU.Gh_D13G0965 | −1.89 | −1.63 | SAUR-like auxin-responsive protein family [Theobroma cacao] | |
| evm.TU.Gh_D13G2038 | −1.16 | −1.11 | SAUR family protein [Theobroma cacao] | |
| evm.TU.Gh_D02G0405 | −1.55 | −1.30 | Gbiaa-Re [Gossypium barbadense] | |
| evm.TU.Gh_A05G0429 | −1.82 | −2.00 | Auxin-responsive IAA29 -like protein [Gossypium arboreum] | |
| Ethylene | evm.TU.Gh_A11G0453 | −5.72 | −4.42 | 1-aminocyclopropane-1-carboxylate synthase [Theobroma cacao] |
| evm.TU.Gh_D11G0525 | −6.18 | −4.37 | 1-aminocyclopropane-1-carboxylate synthase [Theobroma cacao] | |
| evm.TU.Gh_D11G2060 | −5.01 | −3.74 | 1-aminocyclopropane-1-carboxylate synthase [Theobroma cacao] | |
| JA | evm.TU.Gh_D12G0393 | 1.34 | Inf | Fatty acid hydroperoxide lyase 2 [Theobroma cacao] |
| evm.TU.Gh_D06G0087 | 2.35 | 2.14 | chloroplast allene oxide synthase [Gossypium hirsutum] |
Transcription factors (TFs) are important in promoting apical hook formation. There were 9 differently expressed TF genes between the inner and outer sides of the apical hook. The expression of the ERF TF in the inner side was lower than that in the outer side of the apical hook, but the expression of the Heat stress, MYB and GATA TFs in the inner side was higher than that in the outer side of the apical hook (Table S4). There were 4 differently expressed bHLH TFs, 2 were expressed higher on the inner side of the apical hook (Table S4).
3.4 YUCCA8 and GH3.17 regulated apical hook formation
The expression of YUCCA8 was higher on the inner than on the outer side of the apical hook, but GH3.17 had an opposite expression pattern (Table 1, Figure 3A, B). The GhYUCCA8 and GhGH3.17 genes were silenced in the cotton seedlings by the SSA-VIGS method. The expression of GhYUCCA8 in the inner and outer side of the apical hook of the VIGS-YUCCA8 seedlings decreased by 73and 53% at 3 DAP (Figure 3A). The expression of GhGH3.17 in the inner and outer side of the apical hook of the VIGS-GH3.17 seedlings was decreased by 51 and 77% (Figure 3B). The apical hook formation was inhibited by silencing the GhYUCCA8 and GhGH3.17 genes and therefore the apical hook angle of the VIGS-YUCCA8 and VIGS-GH3.17 seedlings were higher than that of the VIGS-GFP control (Figure 3C, D and Figure S2). VIGS-YUCCA8 and VIGS-GH3.17 seedlings has an increased cell size of the inner side of the apical hook by 73 and 69% compared to the VIGS-GFP control, respectively (Figure 3E, F).
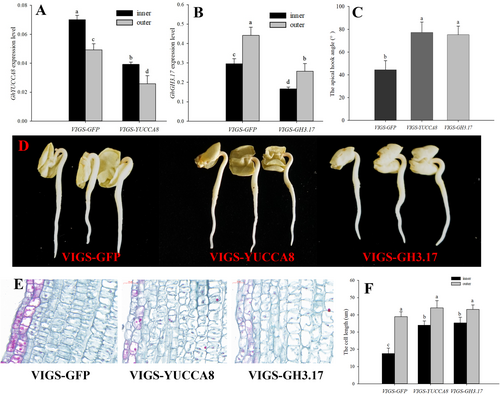
The IAA content in the inner side of the apical hook of VIGS-YUCCA8 seedlings decreased by 30% but only by 8% on the the outer side, compared to the VIGS-GFP control (Figure 4A). Therefore, the asymmetrical gradient of IAA between inner and outer side of the apical hook was decreased by silencing VIGS-YUCCA8 gene (Figure 4B). The IAA-glu content in the outer side was higher than that in the inner side of the apical hook in the VIGS-GFP seedlings (Figure 4C). Silencing GH3.17 decreased the IAA-glu content in the apical hook, and the IAA-glu content decreased by 21% on the outer side, and by 7% on the inner side, compared to the VIGS-GFP control (Figure 4C). The silencing GH3.17 increased the IAA content in the inner and outer side of the apical hook by 7 and 35%, therefore silencing GH3.17 decreased the asymmetrical gradient of the IAA between inner and outer side of the apical hook (Figure 4A,B).
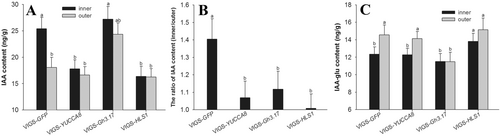
3.5 HLS1 regulate apical hook formation by YUCCA8 and GH3.17 genes
HLS1 plays key roles in apical hook formation, and the hls1 Arabidopsis mutant is defect in the asymmetric auxin distribution and fails to form the apical hook. In this study, the GhHLS1 was silenced by the SSA-VIGS method to study its function on asymmetric auxin distribution and apical hook formation. The expression of GhHLS1 in the inner and outer sides of the apical hook of the VIGS-HLS1 seedlings decreased by 74 and 58%, respectively, compared control at 3 DAP (Figure 5A). The apical hook formation of the VIGS-HLS1 seedlings was inhibited (Figure 5B) and the cell length of the inner side of the apical hook in the VIGS-HLS1 silenced seedlings was increased by 93% compared to control (Figure 5C, D and Figure S2). The IAA content in the inner side of the apical hook of the VIGS-HLS1 seedlings decreased by 36% and on the outer side by only 9%, compared to the VIGS-GFP control (Figure 4A). The IAA-glu content increased by 12% on in the inner side while the content on the outer side was increased by 4%, compared to the control (Figure 4C). Therefore, the asymmetrical gradient of IAA in the apical hook of VIGS-HLS1 silenced seedling was decreased compared to the VIGS-GFP control (Figure 4B).
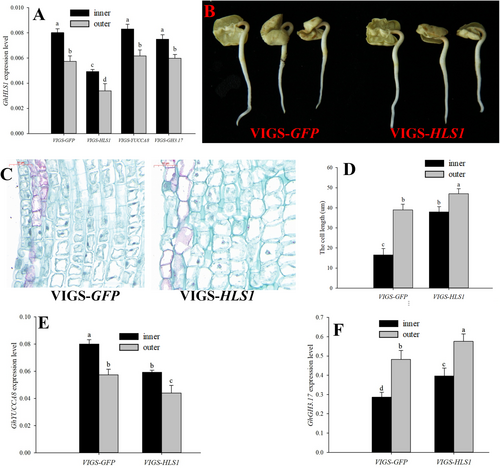
The expression of the GhYUCCA8 and GhGH3.17 genes in the VIGS-HLS1 silenced seedlings was determined to study if GhHLS1 regulated GhYUCCA8 and GhGH3.17 expression during apical hook formation. Compared to the VIGS-GFP control, VIGS-HLS1 silenced seedlings decreased the expression of GhYUCCA8 in the inner and outer sides of the apical hook but increased the expression of GhGH3.17 in both sides of the apical hook (Figure 5E, F). On the contrary, VIGS-YUCCA8 and VIGS-GH3.17 silenced seedlings had no changes in the expression of GhHLS1 in the inner and outer sides of the apical hook (Figure 5A).
3.6 Ethylene regulated YUCCA8 and GH3.17 by HLS1
The GhHLS1 expression in the inner side of the apical hook of the VIGS-GFP seedlings was higher than in the outer side (Figure 6A). Exogenous application of ACC increased the expression of the GhHLS1 and GhYUCCA8 genes in the inner and outer side of the apical hook of the VIGS-GFP seedlings, but the expression of GhGH3.17 in the inner and outer side of the apical hook was decreased by ACC treatment (Figure 6A-C). On the contrary, 1-MCP decreased the expression of GhHLS1 and GhYUCCA8 genes but increased the expression of GhGH3.17 on both sides of the apical hook (Figure 6A-C). The IAA content in the inner and outer side of the ACC-treated apical hooks was increased, but the IAA-glu content in that of the ACC-treated seedlings were decreased (Figure 6D, E). On the contrary, 1-MCP decreased the IAA content but increased the IAA-glu content in the inner and outer sides of the apical hook (Figure 6D, E).
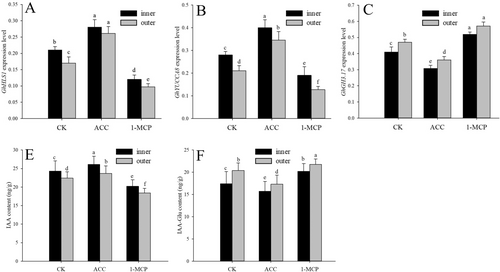
In this study, we modulated the expression level of GhHLS1 by exogenously applying ACC and its inhibitor 1-MCP. These treatments successfully modulated the intracellular ethylene content in seedling cells, subsequently impacting the expression of GH3.17 and YUCCA8. The expression of GhHLS1, GhYUCCA8 and GhGH3.17 genes in the apical hook of the VIGS-HLS1 silenced seedlings was not affected by ACC and 1-MCP treatments (Figure S3). The expression of GhHLS1 in the apical hook of the VIGS-YUCCA8 and VIGS-GH3.17 silenced seedlings were increased by ACC treatment but decreased by 1-MCP treatment (Figure S3a). ACC treatment increased the expression of GhYUCCA8 in the apical hook of the VIGS-GH3.17 silenced seedlings, but 1-MCP had an opposite effect (Figure S3b). ACC treatment decreased the expression of GhGH3.17 in the apical hook of the VIGS-YUCCA8 silenced seedlings, but 1-MCP increased the expression of GhGH3.17 (Figure S3c).
4 DISCUSSION
The apical hook of dicotyledonous seedlings protects the apical meristematic tissues and cotyledons from damage and promotes the successful exit of seedlings from the darkness in the soil. Apical hook development is well characterised as a differential growth process on the concave (inner) and convex (outer) sides of the apical hook in Arabidopsis (Raz and Koornneef, 2001). The differential growth is driven by an asymmetrical distribution of auxin with higher accumulation in the inner than in the outer side of the apical hook. The auxin levels above the optimum inhibit cell growth on the inner side of the hook (Zadnikova et al., 2010; Gallego-Bartolomé et al., 2011; Mazzella et al., 2014). In this study, the cells on the outer side grew faster than the ones on the inner side during the formation of the apical hook of cotton. The inner side accumulated much more IAA than the outer side, which might contribute to the small cell size of the inner side of the apical hook (Figure 1). The result suggested that the asymmetrical distribution of IAA in the apical hook could play an important role in the formation of the apical hook in cotton.
Apical hook development is regulated by diverse internal and external factors, which makes it a good model for dissecting the interplay of external and internal factors in controlling differential cell growth (Béziat and Kleine-Vehn, 2018; Zádnáková et al., 2016). As for the external factors, mechanical pressure promotes, but light inhibits apical hook formation (Liscum et al., 1993; Shi et al., 2016). Plant hormones play a key role in regulating apical hook formation. Ethylene and GA play positive roles, while JA plays a negative role on apical hook formation (Mazzella et al., 2014; Zhang et al., 2018a; Wang and Guo, 2019). These hormones contribute to establishing or diminishing the proper asymmetric distribution of auxin within the apical region of the hypocotyl, leading to the formation or opening of the apical hook (Abbas et al., 2013; Zhang et al., 2018a). Exogenous application of ethylene induces dark-grown seedlings to form a dramatically exaggerated hook curvature (Ecker, 1995; Raz and Ecker, 1999). The ethylene overproducer mutant eto2, which overexpression of the ethylene biosynthesis gene aminocyclopropane-1-carboxylate synthase (ACS5), shows an exaggerated hook phenotype (Vogel et al., 1998). Ethylene regulates both auxin biosynthesis and polar auxin transport to promote apical formation by enhancing the auxin gradients at the hook region (Béziat and Kleine-Vehn, 2018; Wang and Guo, 2019). In this study, there were 315 DEGs which simultaneously up- or down-regulated in the inner side of the apical hook of both 1 and 3 cm sand covered seedlings (Figure 2). Interestingly, 51 hormone-related genes were found in the 315 DEGs, suggested that hormone may play important roles on apical hook formation in cotton. The 51 hormone-related genes included 46 auxin, three ethylene and two JA-related genes, which indicated that auxin might play important roles on the apical hook formation of cotton (Table 1). The three ethylene-related genes were all 1-aminocyclopropane −1-carboxylate synthases (ACS) genes and these were higher on the inner than on the outer side of the apical hook. There was one differently expressed JA biosynthesis gene, chloroplast allene oxide synthase (AOS), and the expression of the AOS gene in the inner side was lower than that on the outer side of the apical hook.
Auxin is an important growth integrator in plants, determining growth in a concentration- and cell-type-dependent manner through direct modulation of the expression of many genes (Lavy and Estelle, 2016). The expression of three primary auxin-responsive gene families, namely Aux/IAA (auxin/indole 3-acetic acid), SAUR (small auxin-up RNA) and GH3 (Gretchen Hagen3) is rapidly induced in response to auxin (Rogg et al., 2001; Timpte et al., 1995; Staswick et al., 2005; Wang and Guo, 2019). Recently, Cao et al. (2019) found that high levels of auxin on the inner side of the apical hook inhibited cell growth by promoting C-terminal cleavage of transmembrane kinase 1 (TMK1) and therefore stabilised the noncanonical transcriptional repressors IAA32 and IAA34. Wang et al. (2020) found that many SAUR genes expressed in the apical hook could regulate apical hook formation in Arabidopsis. The SAUR12, SAUR16, and SAUR50 displayed stronger expression in the inner side of the apical hook and overexpression of SAUR50 suppressed the altered hook curvature and resulted in open cotyledons in the dark (Wang et al., 2020). On the contrary, SAUR57 displayed stronger expression in the outer side of the apical hook and SAUR57 could enhance apical hook formation by inhibiting PP2C-D1 activity in convex cells of the hook (Wang et al., 2020). In this study, the 51 hormone-related DEGs contained 46 auxin-related genes indicating that auxin has an important role in apical hook formation. There were 22 SAUR genes and 15 Aux/IAA genes that were differentially expressed on the inner and outer sides of the apical hook and most of them were highly expressed in the inner side, which may be an attribute to the high IAA content in the inner side of the apical hook. These differentially expressed SAUR Aux/IAA genes might have an important role in apical hook formation. The candidate genes that may regulate the apical hook formation need to be further studied in the future.
The flavin monooxigenases (YUCCA) is an IAA biosynthesis gene and mutation in YUCCA impairs hook development (Zhao et al., 2001). On the contrary, the dominant yuc1-D mutant, which contains elevated levels of auxin also has a hookless phenotype (Zhao et al., 2001; Vandenbusche et al., 2010). GH3.17 is a member of the eight predicted IAA-amido synthetases in Arabidopsis that conjugate the carboxyl group of IAA to amino acids, forming inactive amide-linked auxin for storage or degradation (Staswick et al., 2005; Zheng et al., 2016). The loss of function in GH3.17 mutant vas2 has high free IAA content at the expense of IAA-Glu in the hypocotyls epidermis, which promotes hypocotyl elongation in light conditions (Zheng et al., 2016). A previous study shows that the auxin gradient is probably not the only result of asymmetric auxin synthesis driven by YUCCA, although auxin synthesis is required for hook development because the expression of YUCCA is symmetric (Vandenbussche et al., 2010). In this study, we found that the GhYUCCA8 gene expressed more on the inner side, but the GhGH3.17 gene expressed more in the outer side. Silencing YUCCA8 or GH3.17 genes increased the cell size of the inner side and inhibited apical hook formation. The IAA content in the inner and outer sides all decreased in the VIGS-YUCCA8 silenced seedlings, but the IAA content in the outer side decreased less. Therefore, the IAA gradient between the inner and the outer sides in VIGS-YUCCA8 silenced seedlings was decreased, which may be the reason for the inhibited apical hook formation (Figure 4). In VIGS-GH3.17 silenced seedlings, the IAA-glu content on the inner and outer sides decreased, but the IAA content on the apical hook increased and the IAA content increased more strongly on the outer side than on the inner side, which also decreased the IAA gradient between the inner and outer sides of the apical hook (Figure 4). Therefore, the inhibited development of the apical hook in VIGS-GH3.17 silenced seedlings may also be attributed to the reduced IAA gradient between the inner and outer side. These results suggested that the differently expressed GhYUCCA8 and GhGH3.17 genes had an important function on the apical hook formation of cotton. During the formation of the apical hook, the GhYUCCA8 gene was highly expressed on the inner side to synthesise IAA, while the GhGH3.17 gene was highly expressed on the outer side to decrease the IAA content by synthesising IAA-glu. Therefore, the formation of the auxin gradient was largely attributed to the differential expression of GhYUCCA8 and GhGH3.17 on different sides of the apical hook, which promoted the apical hook formation.
HOOKLESS1 (HLS1) is a central regulator of hook development, acting as a hub integrating various external and internal signals (Lehman et al., 1996; Shen et al., 2016; Lyu et al., 2019). The HLS1 protein is present as an oligomer in the nucleus of dark-grown seedlings and oligomerization is required for HLS1 activation because the mutated HLS1 protein that abolishes self-association exists as non-functional monomers (Lyu et al., 2019). HLS1 is required to establish the asymmetric auxin gradient between the inner and outer sides of the apical hook and to enhance auxin-dependent gene expression on the inner side of the apical hook (Li et al., 2004). The asymmetric auxin distribution in the apical hook is defected and the apical hook disappeared in the hls1 mutant (Li et al., 2004). But the regulatory components that participate in the convergence to an asymmetric auxin response and that act downstream of HLS1 have not been identified so far (Wang and Guo, 2019). In this study, we found that silencing the GhHLS1 gene inhibited the apical hook formation of the VIGS-HLS1 silenced seedlings by increasing the cell growth of the inner side of the apical hook. The IAA content on the inner side of the apical hook decreased, and therefore the auxin gradient between the inner and outer sides of the apical hook was weakened in VIGS-HLS1 silenced seedlings. Increasing GhHLS1 expression by ethylene treatment improved the auxin gradient in the apical hook and promoted apical hook formation, but the ethylene biosynthesis inhibitor 1-MCP decreased the auxin gradient in the apical hook and inhibited cotton apical hook formation. These results suggests that GhHLS1 plays a key role formation of the auxin gradient and the apical hook.
The expression of GhYUCCA8 in VIGS-HLS1 silenced seedlings and 1-MCP treated seedlings decreased, but increased in ethylene-treated seedlings. The expression of the GhHLS1 gene did not change in the VIGS-YUCCA8 seedlings compared to the control seedlings. These results indicated that GhYUCCA8 worked downstream of GhHLS1 and GhHLS1 could up-regulate GhYUCCA8 during apical hook formation. The expression of GhGH3.17 in the VIGS-HLS1 silenced seedlings, and the 1-MCP treated seedling increased, but that in the ethylene treated seedlings decreased. The expression of the GhHLS1 gene was not changed in the VIGS-GH3.17 seedlings compared to the control seedlings. These results indicated that GhHLS1 worked upstream of GhGH3.17 and GhHLS1 could down-regulated GhGH3.17 during apical hook formation.
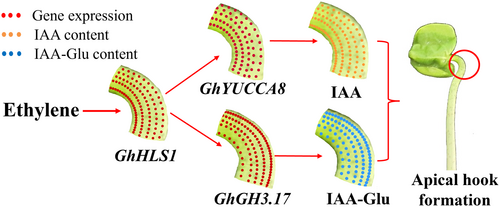
During the development of the apical hook, the expression level of GhHLS1 on the inner side was higher than on the outer side of the hook. GhHLS1 up-regulated GhYUCCA8 but down-regulated the GhGH3.17 gene, therefore, was GhYUCCA8 expressed more highly on the inner side and the GhGH3.17 expressed more highly on the outer side of the apical hook. Therefore, the IAA on the inner side was biosynthesised through GhYUCCA8, but the IAA on the outer side was changed to IAA-glu through GhGH3.17, which finally caused the auxin gradient between the inner and outer side of the apical hook and promoted the formation of the apical hook.
In summary, GhYUCCA8 and GhGh3.17 were significantly expressed on the inner and outer sides of the apical hook, respectively. They were regulated by GhHLS1, which was highly expressed on the inner side. The differential expression of GhYUCCA8 and GhGh3.17 on different sides of the apical hook resulted in the formation of a free auxin gradient between the inner and outer sides, which ultimately promoted the apical hook formation in cotton (Figure 7). This study provides new insights into the function and interaction of HLS1, YUCCA8 and GH3.17 genes in auxin asymmetric distribution and hook formation. Monoseeding is a crucial practice in cotton production that helps reduce seed use and labor inputs while maintaining cotton yield stability (Zhou et al., 2023). Additionally, it has an impact on GhHLS1 expression (Li et al., 2021; Zhou et al., 2022). Thus, this study also offers valuable insights into how monoseeding enhances apical hook development and promotes successful seedling emergence in cotton.
AUTHOR CONTRIBUTIONS
HD supervised the whole study. XK designed the research. XK, JZ and XL performed most of the experiments, CL and JC determined the IAA and IAA-glu contents. XK, JZ, XL and HZ performed the statistical analysis. XK and JZ wrote the paper with the input from all other authors. JZ and HD completed the revision.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (No. 31971857), the National Key Technologies Research and Development Program of China (No. 2018YFD0100306), the special found for Taishan Scholar (No. Tsqn201812120), and the earmarked fund for China Agricultural Research System (No. CARS-15-15).
CONFLICT OF INTEREST STATEMENT
All authors disclosed no relevant relationships.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, upon reasonable request.




