Rhizobium rhizogenes rolC gene promotes leaf senescence and enhances osmotic stress resistance in Arabidopsis: the positive role of abscisic acid
Abstract
The root oncogenic loci (rol) C oncogene has been identified as a key player during the plant-Rhizobium rhizogenes interaction and is considered to confer resistance to plant abiotic stresses, yet the underlying mechanisms remain largely elusive. In this study, Arabidopsis plants constitutively overexpressing rolC were produced. Biometric analysis showed that rolC induced diverse phenotypic modifications, including dwarfism, increased number of stem branches (1.3 times more than WT), weak root growth, early flowering (1.6 days earlier than WT) and premature leaf senescence. In addition, senescence stimulus (exogenous ethylene: 10 or 100 μL L−1) and 10% (w/v) polyethylene glycol 6000 (PEG) treatments demonstrated that rolC mediated leaf abscisic acid (ABA) enhancement in Arabidopsis, and this might be involved in rolC-induced premature leaf senescence and resistance enhancement to osmotic stress. It is concluded that rolC-induced premature leaf senescence may be involved in an abiotic stress escape mechanism in Arabidopsis, which is closely related to the increase of endogenous ABA levels. These findings provide new insights into the role of rolC in plant-bacterial interaction and uncover the potential of biotechnological application associated with R. rhizogenes/rol genes in plant drought defense.
1 INTRODUCTION
Rhizobium rhizogenes, previously Agrobacterium rhizogenes (Young et al. 2001), is a gram-negative pathogenic bacterium living in the soil. It was first identified in 1930 (Riker et al.), formally named in 1942 (Conn) and subsequently studied with great interest for its role in inducing the ‘hairy root’ syndrome. When R. rhizogenes infects plants, it transfers and integrates part of its root-inducing (Ri) plasmid into the plant genome, which results in an abundant root proliferation, termed hairy roots, at the site of infection. The Ri-plasmid of agropine-type strains is divided into two regions designated as the left T-DNA (TL-DNA) and right T-DNA (TR-DNA). These regions harbor specific genes that are referred to as root oncogenic loci (rol) genes (White et al. 1985) as well as other less characterized open reading frames (ORFs). The rolC is located on TL-DNA and considered to be a key functional gene of R. rhizogenes with respect to plant hairy root syndrome induction (Rangslang et al. 2018, Paolis et al. 2019).
As a natural breeding strategy (Intrieri et al. 2001, Matveeva et al. 2012, Kyndt et al. 2015), R. rhizogenes-mediated transformation could be ideally divided into two main aspects for both commercial and research purposes. First, the R. rhizogenes transformation, an important bio-sustainable alternative to chemical growth retardants, has been widely used for the generation of compact ornamental plants since rol genes trigger plant dwarfism with varying degrees (Christensen et al. 2008, Zhu et al. 2015, Hegelund et al. 2018). Secondly, the interest has also been focused on plant metabolic engineering since R. rhizogenes and single rol genes are powerful inducers of bioactive molecules, such as secondary metabolites and recombinant proteins (Bulgakov 2008, Shkryl et al. 2008, Bulgakov et al. 2013, Martínez et al. 2020). However, increased attention has been recently paid to the potential use of R. rhizogenes strains or single rol genes to confer stress resilience to plants, e.g., drought resistance (Arshad et al. 2014, Bulgakov et al. 2018, Veremeichik et al. 2022).
The rolC gene, one of the key denominators of R. rhizogenes, has been considered to have a great potential in endowing plants stress resistance (Bulgakov et al. 2012, Shkryl et al. 2022). Enhanced stress tolerance was revealed in rolC-expressing cell cultures, as shown by decreased oxidative damage, increased salinity tolerance, etc. (Victor P. Bulgakov 2008, Kiselev K V et al. 2010). Besides, the increased number of secondary metabolites (e.g., phytoalexin and anthraquinones) produced in rolC transgenic tissues of several plant species (Bulgakov et al. 2004, Bulgakov 2008, Matveeva et al. 2015) could contribute to resistance to pathogen attack. In addition, plants transformed solely with rolC gene display a series of morphological abnormalities, including reduced height, increased number of branches, and better rooting ability, etc. (Favero et al. 2021). The morphological alterations and abiotic stress resistance induced by rolC indicate its mechanism of action interferes with common factors that modulate plant development and stress responses. Among them, phytohormones play essential roles in both aspects (Christensen et al. 2009, Lütken et al. 2012, Pieterse et al. 2012) since endogenous levels or ratios of different hormones [e.g., abscisic acid (ABA), auxins (IAA), cytokinins (CTK) and gibberellins (GA)] are closely linked to both plant morphogenesis and environmental adaptation (Bettini et al. 2010, Vanstraelen et al. 2012).
It is well known that ABA is an important regulator of growth and development, affecting diverse processes, specially leaf senescence (Xue-Xuan et al. 2010, Zhao et al. 2016). ABA could induce plant oxidative damage, thereby triggering plant senescence (Li et al. 2020, Asad et al. 2021). Besides, ABA is also a stress hormone that induces quick responses, such as stomatal closure, and long-term responses, such as growth inhibition, osmotic regulation, senescence and abscission (Zhao et al. 2017). Leaf senescence is known to be a highly regulated process in the plant life cycle and a typical programmed cell death (PCD) (Ren et al. 2021), during which cells encode/control their own death to regulate the growth and development of organisms (De Pinto et al. 2012). More importantly, the senescence and abscission of older leaves and subsequent re-allocation of nutrients are known to increase plant survival under abiotic stresses, including drought and low temperatures, etc. (Zhao et al. 2016). Thereby, senescence is one of the multiple defense strategies employed by plants to cope with stress. Leaf senescence and plant responses to abiotic stress are closely related to each other, and ABA is hypothesized to be a key conjunction mediator between these processes. Recent studies revealed that rolC is able to trigger changes in endogenous ABA levels in plants (Bettini et al. 2010) and provide plant tissues with enhanced stress resistance (Bulgakov et al. 2012). This provides a research basis for further exploring rolC-mediated defense strategies to abiotic stresses.
This study aimed to investigate the rolC-mediated response mechanism to abiotic stress. Therefore, R. rhizogenes rolC-overexpressing Arabidopsis plants (rolC-OX) were generated. Moreover, ethylene exposure and polyethylene glycol (PEG) 6000 treatment, which result in senescence stimuli (Ueda et al. 2015) and osmotic stress (Li et al. 2022), respectively, were applied to the rolC-OX. We hypothesized that rolC exerts an important role in mediating leaf senescence and osmotic stress resistance by modulating endogenous ABA levels in plants.
2 MATERIALS AND METHODS
2.1 Plant Materials and Growth Conditions
Wild-type (WT) Arabidopsis thaliana Columbia-0 ecotype (Col-0, stock code N1092) and rolC-OX Arabidopsis seeds (see 2.2) were germinated on standard ½ MS media. The seedlings were then transplanted to pots filled with soil (peat: vermiculite: perlite 1:1:1) and grown in a growth chamber at a photosynthetic active radiation of 120 μmol m−2 s−1 and 22°C day/20°C night temperature as well as a 16-h photoperiod.
For the PEG treatments, Arabidopsis seedlings at the four-leaf stage were transplanted to a hydroponic system and grown in containers filled with nutrient solution (Li et al. 2022). The nutrient solution was changed every five days and was continuously aerated using an air compressor. The pH of the nutrition solution was monitored daily and adjusted to 6.0 with 0.1 M HCl or 0.1 M NaOH. The growth conditions of the chamber were the same as described above for pot-grown plants. For the ethylene treatment experiment, plants in pots were grown in transparent airtight 128 L containers containing 10 μL L−1 or 100 μL L−1 ethylene (Jurdak et al. 2021). For both concentrations, treated and control plants were maintained under the same assay conditions described for the pot-grown plants. Additionally, pot-grown Arabidopsis plants at four growth stages (i.e., 35, 45, 55 and 65 day (d)) were sampled for further tests: ABA, chlorophyll quantification and RNA extraction, etc. (methods as below). These four growth stages correspond to flowering initiation (35 d), flowering termination (45 d), silique ripening (55 d), and complete senescence and seed harvesting (65 d), according to Boyes et al. (2001).
2.2 Vector Construction and Transformation of Arabidopsis
The rolC sequence was amplified from the total DNA of R. rhizogenes A4 strain (ATCC43057) (Favero et al. 2021) on a MyCycler (Biorad) using TaKaRa LA Taq polymerase (Mountain View). The amplification product was BP-cloned into the entry vector pDONR221 and subsequently recombined with the destination vector pK2GW7 to generate 35S:rolC fusion constructs according to Favero et al. (2021). The recombinant plasmids containing rolC clones were transferred into electrocompetent Agrobacterium tumefaciens C58C1 (pGV3850) by electroporation, then rolC gene was transformed to Arabidopsis plants by the floral dip method (Chen et al. 2022). Among the second generation (F2) of rolC-OX plants, 12 independent rolC-OX lines were generated for preliminary characterization (data not shown). From these lines, rolC presence and phenotypic characteristics of three lines (rolC1-OX, rolC2-OX and rolC5-OX) showing a common and stable phenotype are presented in Figure S1. Furthermore, the rolC5-OX line, which had representative morphological features, was selected for seed collection. Plants of the corresponding F3 generation were used in the PEG, ethylene and life cycle experiment. The primers used for rolC cloning are listed in Table S1.
2.3 RNA Extraction, cDNA Synthesis, and Quantitative Real-time PCR Reactions
Leaf RNA Extraction was conducted using the RNeasy Plant Mini Kit (Qiagen). Total RNA (500 ng) was treated with DNase I Amplification Grade (Sigma Aldrich), and cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad). The quantitative real-time (qRT)-PCR was performed using a SYBR FAST qPCR Kit (Bio-Rad) on a CFX Connect Real-Time System (Bio-Rad) following the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines (Wang et al. 2023). Before qRT-PCR reactions, four reference genes (ACTIN8, GAPDH, 18sRNA and EXPRS) were used for normalizing the transcript level of each tested gene, and it turned out that the expression of ACTIN8 was the most stable in different leaf samples and it was, therefore, selected as the reference gene. The comparative CT (2−ΔΔCT) method (Livak et al. 2001) was used to calculate relative gene expression levels. Each sampling point was performed on two technical replicates and four biological replicates. Specific primers used for qRT-PCR are listed in Table S1.
2.4 PEG 6000 Treatments
After five weeks of growth in the hydroponic system, rolC-OX and WT plants were treated with 10% (w/v) PEG for 0 and 24 h (directly in the hydroponic medium; expressed as P0 and P24) to mimic short-term osmotic stress. Stressed plants were then transferred back to PEG-free nutrient solution for 24 h as short-term recovery (expressed as R24). According to the equation developed by Michel and Kaufmann (1973), the osmotic stress level in the hydroponic solution was −0.16 MPa for 10% PEG solution. Four plants were sampled at each time point, and the experiment was repeated two times displaced in time.
2.5 Senescence Treatment
For the senescence treatment, five-week-old potted plants were incubated in transparent airtight containers containing 10 μL L−1 or 100 μL L−1 ethylene (Jurdak et al. 2021) for 0, 24, 48 and 72 h. Four plants were sampled at each time points and the experiment was repeated two times displaced in time.
2.6 Biometric Analysis
Five-week-old rolC-OX and Col-0 Arabidopsis plants were used for root structure characterization. Fresh roots of four replicates were sampled and then photo-scanned by root scanner (Epson Expression 12000XL). The images were subsequently analyzed by the software WinRHIZO Pro (Version 2009c, 32-bit) to determine root length (cm), root diameter (mm), root surface area (cm2) and the number of root tips. Root branching intensity (tips cm−1) was calculated as the number of root tips divided by the corresponding root length. The number of days until first flowering of each plant was recorded continuously, then the days to first flower were counted as the average value (n = 30). The total number of branches (≥3 cm) was recorded after senescence completion (nine weeks) (n = 30). Experiments for each biometric parameter measurement were conducted two times displaced in time.
2.7 Chlorophyll Contents, Leaf ABA Concentrations and Total Antioxidant Capacity Measurement
Chlorophyll contents of upper fully unfolded leaves were measured as SPAD value by using a Chlorophyll Meter (SPAD-502DL Plus). For ABA measurement, leaf samples were ground into powder in liquid nitrogen and transferred (27–33 mg) to a 1.5 mL Eppendorf tube. The ABA was extracted with 1.0 mL milli-Q water on a shaker at 4°C overnight. The extracts were centrifuged at 14,000 g and 0.7 mL supernatants were collected for leaf ABA analysis. Leaf ABA concentration [ng g−1 fresh weight (FW)] was assayed by ELISA (Enzyme Linked Immuno Sorbent Assay) following the protocol described by Asch (2000). Total antioxidant capacity [nmol μL−1 fresh weight (FW)] was assessed by the Total Antioxidant Capacity Assay kit MAK187 (Sigma) with minor modifications according to Fraser et al. (2017). The concentration of antioxidant in samples was calculated as Trolox Equivalents.
2.8 Leaf Carbon and Nitrogen Concentrations
The concentration of leaf carbon (C) and leaf nitrogen (N) was determined using leaves collected from 55 d and 65 d of plant samples grown under normal growth conditions. Dry samples were thoroughly ground into powder, and 3–4 mg of the powder was employed for C, N analysis using a CHNS/O Elemental Analyzer (Flash 2000, Thermo Fisher Scientific).
2.9 Statistical Analyses
Statistical analyses were carried out using Duncan Test and two-way ANOVA in SPSS 21. Differences between treatments were considered as significant when P ≤ 0.05. Heatmaps were made by Origin 2020 and presented based on qRT-PCR data computed by 2−ΔΔCt.
3 RESULTS
3.1 Biometric traits of aerial parts and roots of rolC Transgenic Arabidopsis
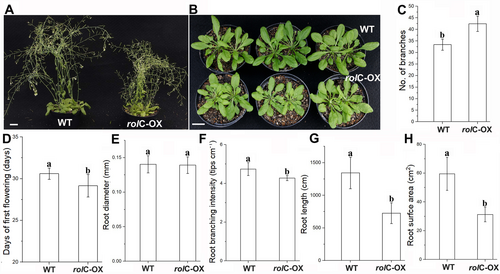
The rolC-OX exhibited a pronounced dwarf phenotype with more compact rosettes than WT (Figure 1A, B). Moreover, more stem branches and early flowering were observed in rolC-OX plants (Figure 1C, D). Specifically, the number of branches of rolC-OX (42.4 ± 3.3) was about 1.3 times greater than that of WT plants (33.3 ± 2.4), while the time to first flower of rolC-OX (29.2 ± 1.5 d) was 1.6 days earlier than that of WT (30.8 ± 0.7 d). In addition, the root morphology of rolC-OX was determined (Figure 1E-H). There was no significant difference in the average root diameter between rolC-OX and WT plants; both were 0.14 ± 0.01 mm. However, the root branching intensity, root length and root surface area were significantly decreased in rolC-OX (4.2 ± 0.1 cm−1, 725 ± 158 cm, 31.3 ± 5.1 cm−2) compared to WT plants (4.7 ± 0.3 cm−1, 1343 ± 242 cm, 59.5 ± 11.5 cm−2).
3.2 Premature Leaf Senescence in rolC-Overexpressing Arabidopsis Under Normal Growth Conditions
Leaf phenotype of both rolC-OX and WT plants was monitored throughout the growth cycle under normal growth conditions. Six weeks after sowing, notable differences appeared in the leaves of both lines (Figure 2A). The rolC-OX started to show signs of leaf yellowing at around 48 d (marked with red circle), a feature that became more severe thereafter. In contrast, the WT leaves only showed signs of senescence at around 55 d (marked with red circle) and remained greener than that of rolC-OX even at 65 d.
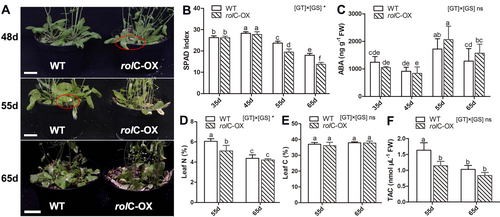
To reveal the reasons behind the leaf phenotypic differences, parameters related to leaf senescence and oxidative stress (Wang et al. 2013, Zhang et al. 2022) were measured at different growth stages (Figure 2), including chlorophyll contents, ABA concentrations, total antioxidant capacity (TAC) and leaf C and N concentrations. Besides, the expression of genes involved in chlorophyll degradation (Figure S2) and reactive oxygen species (ROS) generation and scavenging (Figure S3) was also analyzed. At 55 d, SPAD values for chlorophyll contents in both rolC-OX (19.5 ± 1.5) and WT (23.7 ± 0.9) plants were significantly lower than at 45 d, this reduction being more pronounced in rolC-OX lines (Figure 2B). Moreover, the ABA levels significantly increased at 55 and 65 d in both rolC-OX and WT plants than that at 45 d, and the largest value (2064.6 ± 483.1 ng g−1) occurred in rolC-OX at 55 d (Figure 2C). In addition, the leaf C and N concentrations and TAC were measured in both lines during late growth stages (55 and 65d; Figure 2D-F). The leaf N concentration at 55 d was significantly lower in rolC-OX (5.1 ± 0.5%) than in WT (6.1 ± 0.3%). Consistently, TAC was 1.2 ± 0.1 nmol μL−1 in rolC-OX at 55d, being 1.3-fold lower than that of WT (1.6 ± 0.2 nmol μL−1). On the other hand, the leaf C concentrations of both lines exhibited no significant differences at 55 and 65 d, being approximately 37% in both time points (Figure 2E).
Regarding transcript level analysis, expression levels of NOL, PAO and PPH, which are crucial genes involved in chlorophyll catabolism, were dramatically upregulated at 55 d in rolC-OX. However, this up-regulation was delayed in the WT, appearing only at 65 d (Figure S2). In both rolC-OX and WT plants, the respiratory burst oxidase homologues F (RBOHF; a calcium-dependent NADPH oxidase that generates superoxide) and the antioxidant system genes [i.e., Catalase 1 (Cat1), ascorbate peroxidase 1(Apx1), ascorbate peroxidase 2 (Apx2), Cu/Zn superoxide dismutase 1 (CSD1) and Cu/Zn superoxide dismutase 2 (CSD2)] were generally upregulated at 65 d. Notably, a clear up-regulation of RBOHF (2.1-fold) and Cat1 (6.5-fold) expression in rolC-OX compared to WT plants was observed at 65 d (Figure S3).
3.3 Accelerated Leaf Senescence and ABA Accumulation in rolC-Overexpressing Arabidopsis Under Enhanced Senescence Stimulus
The rolC-OX leaves exposed to both ethylene concentrations (10 μL L−1 or 100 μL L−1) started to show yellowing symptoms after 48 h of treatment (Figure 3A). This phenotype was exacerbated at 72 h, with most of the leaves turning yellow compared to the WT.
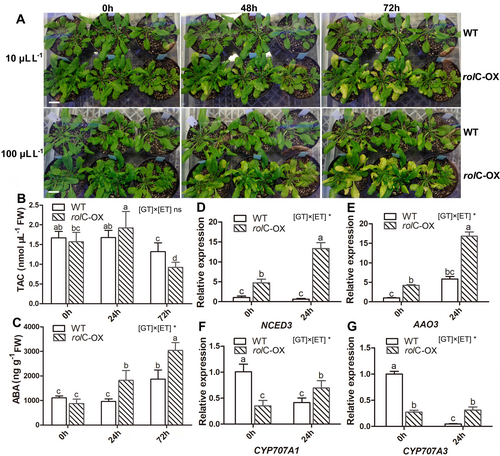
With a 100 μL L−1 concentration of ethylene treatment, TAC was 0.8-fold lower in WT at 72 h than at previous time points (0 h and 24 h), whereas the TAC of rolC-OX notably increased at 24 h (1.9 ± 0.2 nmol μL−1) and dramatically decreased at 72 h (0.9 ± 0.1 nmol μL−1) (Figure 3B). Besides, a significant difference in leaf ABA concentrations between the two genotypes were found at both 24 h and 72 h, of which ABA accumulated in rolC-OX lines were 1.9-fold and 1.6-fold higher than those in WT, respectively (Figure 3C).
In addition, the expression of target genes involved in ABA metabolism was also investigated in leaves treated with 100 μL L−1 ethylene (Figure 3D-G). A down-regulated expression of CYP707A1 and CYP707A3, which are involved in ABA catabolism, was observed in WT at 24 h compared to 0 h. However, the expression levels of CYP707A1 as well as those of NCED3 and AAO3, which are involved in ABA biosynthesis, were dramatically upregulated in the rolC-OX after 24 h of treatment.
3.4 Expression Analysis of Genes Involved in Phytohormone Pathways in rolC Overexpressed Arabidopsis Under Normal Growth Conditions
Given the close correlation between phytohormones, leaf senescence and dwarfing phenotypes, key genes implicated in the biosynthesis, catabolism and signaling transduction pathways of phytohormones, including ABA, ethylene, CTK, GA, salicylic acid (SA) and IAA, were selected for qRT-PCR analysis under normal growth conditions. The location of these genes in the corresponding hormone metabolic pathways (metabolite diagrams) is shown in Figure S4-S9.
Regarding the ABA pathway (Figure 4A), the expression levels of the ABA-responsive genes AREB2 and RD29B were significantly upregulated by 2–6 folds at 45, 55 and 65 d in rolC-OX compared to WT plants, indicating a possible ABA signaling activation in rolC-OX lines. Moreover, the NCED3 gene, which is involved in ABA biosynthesis, was highly expressed at the tested time points in rolC-OX lines; besides, AAO3, another gene involved in ABA biosynthesis, showed 3.5-fold higher expression in rolC-OX plants at day 65 in comparison to WT plants. In addition, the expression of both CYP707A1 and CYP707A3, which are involved in ABA catabolism, was generally down-regulated by 1.5–15 folds during the life cycle of rolC-OX plants.
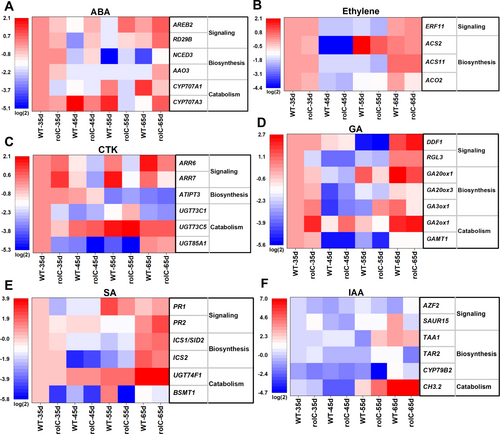
For the ethylene pathway, gene expression differences were only detected during the biosynthesis, where ACS2 was expressed 2-fold lower in rolC-OX than in WT at 55 d, and ACO2 was expressed 2.5-fold lower in rolC-OX than in WT at 65d (Figure 4B). Overall, the expression of genes involved in CTK biosynthesis and catabolism showed no significant differences between rolC-OX and WT plants (Figure 4C). At 35 d, the CTK signaling response factors ARR6 and ARR7 were highly expressed in rolC-OX in relation to WT plants; in contrast, at 45, 55 and 65 d, the expression of ARR6 and ARR7 was significantly decreased in rolC-OX plants compared to the WT. As shown in Figure 4D, the expression of RGL3, a key gene associated with GA signaling activation, was down-regulated 5-fold in rolC-OX leaves at 35 d compared to the WT. On the contrary, a negative regulator of GA signaling DDF1 was expressed 1.5-fold higher in rolC-OX than in WT at 65 d. However, the expression of genes related to GA biosynthesis and catabolism pathways fluctuated irregularly to varying degrees due to the introduction of rolC.
As shown in Figure 4E, the genes implicated in the SA biosynthetic pathway showed no big differences in expression between rolC-OX and WT plants. However, the expression of genes involved in SA signaling was significantly perturbed, with PR1 significantly down-regulated at 55 d and PR2 significantly upregulated at 65 d in rolC-OX compared to WT plants. Notably, the expression of BSMT1, a gene involved in SA catabolism, was significantly lower in the whole life cycle of rolC-OX than in WT, especially at 55 d, at which BSMT1 was 157-fold lower in rolC-OX lines compared to that of WT. Although the changes in expression of genes implicated in IAA metabolism had no obvious pattern, this pathway was clearly disturbed to varying degrees (Figure 4F). This was especially evident at 55 and 65 d, in which distinctive expression profiles of the genes implicated in IAA signaling transduction and biosynthesis was observed between rolC-OX and WT plants.
3.5 Improved Stress Resistance and Enhanced ABA Accumulation in rolC Transgenic Arabidopsis Under Polyethylene Glycol 6000 Treatment
The rolC-OX plants showed no obvious osmotic stress symptoms after 24 h of PEG treatment (P24), whereas the WT plants exhibited a serious wilting phenotype (Figure 5A). Regarding the TAC, there was no significant difference before and after PEG stress in rolC-OX plants, with a mean of 3.0 nmol μL−1 during the whole treatment. However, TAC of WT was significantly increased after 24 h of PEG recovery (R24), being 4.0 ± 0.5 nmol μL−1 (Figure 5B). In addition, the ABA concentrations were higher by 2.1 and 1.4-fold, respectively, in rolC-OX and WT plants at P24 compared to those before stress (P0). Subsequently, it increased to 2152 ± 264 ng g−1 in the WT plants but was reduced to 1352 ± 147 ng g−1 in rolC-OX plants at R24 (Figure 5C). During the period of PEG treatment, the expression of all tested ABA pathway genes in both WT and rolC-OX plants was significantly upregulated after 24 h of treatment. However, this up-regulation was much lower in the overexpressing line than in the WT. Moreover, a significant general down-regulation of these genes was observed after 24 h of recovery (R24) in the WT, whereas a stable expression level could be noted from P24 to R24 in rolC-OX plants (Figure 5D-I).
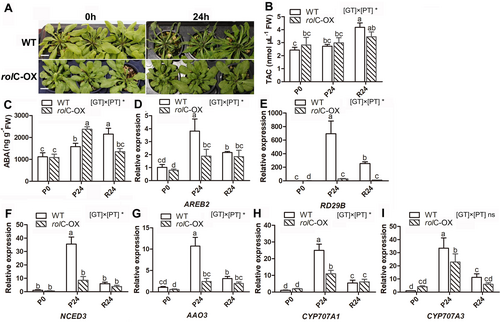
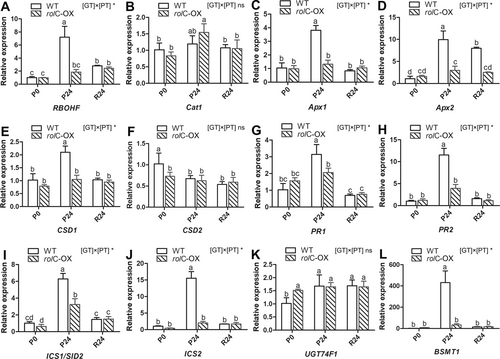
As crucial factors linked to plant defense and oxidative stress response, expression levels of ROS generation and scavenging-related genes (Mittler et al. 2011), as well as of genes implicated in SA metabolism (Jogawat et al. 2021), were determined under osmotic stress. As shown in Figure 6A-F, the expression of both RBOHF, a main producer of ROS, and antioxidant system genes responsible for ROS scavenging were generally upregulated in the WT upon 24 h of treatment, at which only Cat1 was expressed significantly higher in the rolC-OX than that at P0. On the other hand, in the SA pathway (Figure 6G-L), a significantly upregulated expression of SA pathway genes was observed in WT plants subjected to PEG stress. However, only the ICS1/SID2 was expressed higher (3.8-fold) at P24 than at P0 in rolC-OX (Figure 6I). Remarkably, BSMT1, a gene involved in the methylation of active SA, was expressed markedly lower (by 13-fold) in rolC-OX lines at P24 compared to WT (Figure 6L).
4 DISCUSSION
4.1 The rolC gene—a key plant phenotype modifier from Rhizobium rhizogenes
In this study, the constitutive overexpression of rolC conferred a markedly different phenotype in Arabidopsis plants, including dwarfism, increased number of stem branches and early flowering (Figure 1), which are consistent with those observed in other species transformed with rolC, e.g., carnation and tomato, etc. (Amir Zuker et al. 2001, Bettini et al. 2010). Besides, weak root growth was noticed in rolC-OX plants in comparison with the WT, as exemplified by the lower root branching intensity, root length and root surface area (Figure 1F-H). This indicates that rolC overexpression in Arabidopsis inhibited root growth, which contradicts previous observations that rolC enhances rooting ability in fruit trees (Kaneyoshi et al. 1999, Koshita et al. 2002) and carnations (Zuker et al. 2001). As previously described, we also observed that rolC triggered varying degrees of perturbations on hormonal pathways in Arabidopsis plants at different growth stages (Figure 4). These perturbations, in turn, could have contributed to the phenotypic alterations observed in rolC-OX plants, since plant morphogenesis is closely related to the alterations of phytohormones (Vanstraelen et al. 2012, Zdarska et al. 2015).
Among the phenotypic alterations conferred by rolC, dwarfing was the most striking. Our expression data, however, did not support the idea that such dwarfism could be linked to alterations in transcript levels of GA- and IAA-related genes (Figure 4D, F), although IAA and GA are important determinants of plant size (Vanstraelen et al. 2012, Ke et al. 2021). This suggests that the reduced growth of rolC-OX plants is likely a result of crosstalk between multiple hormones. The flowering time of plants is highly regulated by GA (Cho et al. 2017). Here, however, we did not find a direct link between the expression level of GA-related genes and the early flowering phenotype in rolC-OX plants. On the other hand, evidence suggests that ABA might be an endogenous component that can affect floral initiation and promote flowering in plants (Du et al. 2018). Thus, based on our results, it is plausible that the detected up-regulation in transcript levels of ABA-related genes under normal growth conditions (Figure 4A) may contribute to the early flowering of rolC-OX plants.
The dwarfing and well-branched phenotypes observed in the present study indicate reduced apical dominance of rolC-OX plants (Figure 1A-C), an effect that was also seen in carnation and tobacco plants overexpressing rolC (Eva Casanovaa 2003). It has been previously speculated that over-expression of rolC may lead to increased CTK levels (Favero et al. 2021), a feature that might contribute to the phenotypes observed here. In general, high ratios of CTK-to-auxins promote plant shoot differentiation (Naseem et al. 2012); however, here, the gene expression data did not support the idea based on the CTK transcript levels. Instead, it revealed that genes implicated in CTK signaling were suppressed in the rolC-OX (Figure 4C). Intriguingly, although the introduction of the rolC gene suppressed root growth in Arabidopsis (Figure 1E-H), this phenotype was not observed in carnation and some fruit trees (Mauro et al. 2017) in which rolC overexpression enhanced root growth. Therefore, it seems that the effects of the rol genes on root phenotype could be species-dependent.
Overall, the diversity of phenotypes shown by rolC-OX plants indicates that rolC, one of the crucial oncogenes of R. rhizogenes, is a key factor modulating plant phenotypes. Whether rolC-induced hormonal perturbations of transcript levels contribute to this process requires further studies.
4.2 Is rolC-mediated ABA enhancement involved in inducing leaf premature senescence in Arabidopsis?
Leaf yellowing due to chlorophyll degradation and leaf nitrogen re-translocation are the most obvious symptoms of leaf senescence (Ahmad et al. 2021, Roni et al. 2022). In this study, we report the early leaf yellowing in rolC-OX plants under normal growth conditions (Figure 2A). This, combined with an early decline in leaf nitrogen and chlorophyll contents as well as early activation of expression of chlorophyll degradation genes in the rolC-OX (Figures 2B, D and S2), collectively provide a clear indication that the overexpression of rolC promotes leaf senescence in Arabidopsis. In general, earlier senescent leaves should be accompanied by increased ABA levels (Asad et al. 2021). Here, although the increase was not significant in ABA metabolism levels, the transcript levels were, as expected, significantly activated in rolC-OX plants in comparison with the WT (Figure 4A). In line with this, a dramatic decrease of TAC and a sharp up-regulation of superoxide generation-related RBOHF and antioxidant system gene Cat1 in senescent leaves of rolC-OX (Figure S3A, B) indicate a more pronounced oxidative stress response due to its premature leaf senescence. Furthermore, under enhanced senescence stimuli from ethylene exposure (Ueda et al. 2015), a more severe leaf senescence was triggered in rolC-OX lines as shown by highly yellowing symptoms and a notable decrease of TAC (particularly at 72 h) compared with WT (Figure 3A,B). This was confirmed by a nearly doubled leaf ABA level under ethylene treatment, along with a strong up-regulation of genes implicated in the biosynthesis and catabolism of ABA in rolC-OX leaves compared to WT (Figure 3C-G). These results imply that rolC plays an important role in modulating endogenous ABA levels in Arabidopsis under senescence-stimulated conditions.
Additionally, the results of this study suggest that the function of rolC in promoting ABA levels could be regulated by a positive feedback of senescence stimuli, i.e. senescence-dependent manner, for the following reasons: (1) the yellowing phenotype of leaves of rolC-OX appeared at late growth (48d) instead of early growth (Figure 2A); (2) qRT-PCR analysis showed that rolC triggered greater perturbations in various hormonal pathways during late developmental stages (Figure 4), suggesting that rolC tends to function during plant senescence; (3) compared with the WT, enhanced senescence stimulus (ethylene exposure) triggered a greater accumulation of ABA in the rolC-OX (Figure 3C), and both natural senescence and ethylene exposure conditions strongly upregulated the transcript levels of ABA-related genes in the rolC-OX (Figures 4A and 3D-G). Excessive accumulation of endogenous ABA is known as the main cause of plant senescence by triggering oxidative damage (Xue-Xuan et al. 2010). Besides, in vitro studies have shown that an inhibitor of ABA biosynthesis effectively retarded leaf senescence, while an ABA catabolism inhibitor obviously accelerated leaf senescence by enhancing the endogenous ABA concentration in senescent leaves (Asad et al. 2021). Taken together, we suggest that rolC-mediated ABA enhancement could be involved in inducing leaf premature senescence.
4.3 Does rolC-mediated ABA enhancement contribute to osmotic stress resistance in Arabidopsis?
In the PEG experiment, the data indicated that TAC and expression of genes implicated in ROS generation and scavenging were not induced by PEG-induced osmotic stress in rolC-OX plants (Figure 6A-F). This implies that rolC-OX plants would have been less affected by oxidative stress as exemplified by their better leaf performance under PEG treatment compared with the WT (Figure 5A). These, therefore, suggest that the introduction of rolC gene improved osmotic stress resistance in Arabidopsis. ABA is a key signaling molecule in the response to plant environmental adaptation (Asad et al. 2019). In the rolC-OX, the expression of genes involved in ABA signaling transduction and biosynthesis was less affected by osmotic stress than in the WT (Figure 5D-G), which would imply a lower ABA level in this plant; oppositely, the leaves of rolC-OX plants treated with PEG retained higher ABA concentration (2376 ± 113 ng g−1) compared to the WT (1580 ± 152 ng g−1), suggesting that rolC has a specific role in upregulating endogenous ABA levels under osmotic stress. Notably, although the expression of genes involved in ABA signaling transduction and biosynthesis was strongly lower in rolC-OX plants than in WT, the expression of ABA catabolic genes was also significantly suppressed in rolC-OX (Figure 5H, I). This indicates that, although accumulating lower levels of ABA after PEG treatment, the rate of ABA degradation seems to be even lower in rolC-OX leaves than in the WT plants during the same period. ABA accumulated under stress (stress ABA) is known to possess a catabolic rate that is more than 11 times higher than the catabolic rate of non-stress ABA (Ren et al. 2007). Thus, under stressed conditions, ABA catabolism has a greater effect than ABA biosynthesis on ABA accumulation, resulting in higher ABA levels in the rolC-OX leaves than in the WT. In this context, RD29B, a key signaling activator gene of ABA pathway, was shown to be significantly down-regulated (24-fold) in PEG-treated rolC-OX plants compared to the WT (Figure 5E). RD29B is known to respond to water deprivation and may be an important target gene involved in the rolC-induced osmotic stress response. Accordingly, these results suggest that rolC gene upregulates endogenous ABA levels in Arabidopsis leaf, primarily through modulating the ABA catabolic pathway under osmotic stress.
ABA, as a stress hormone, plays a crucial role in regulating plants' response to drought and other abiotic stresses (Cao et al. 2013). Increased ABA, occurring in response to low water potential, is able to induce short-term responses, such as stomatal closure, and long-term responses, such as osmotic regulation and the enhancement of root hydraulic conductivity (Sharipova et al. 2016, Zhao et al. 2017, Fang et al. 2019). Besides, there is also evidence that ABA increases the influx of ions across membranes in the root and the synthesis and accumulation of osmotically active solutes (Alves et al. 2004, Verslues et al. 2006). Given the improved osmotic stress resistance accompanied by a simultaneous enhanced accumulation of ABA in the rolC-OX, we postulate that rolC-mediated up-regulation of endogenous ABA levels contributes to rolC-induced higher osmotic stress resistance in Arabidopsis. In addition to ABA, we provided transcript level evidence that the SA, a key plant defense-associated phytohormone (Jogawat et al. 2021), seems to be involved in the rolC-induced osmotic stress responses since a strongly suppressed expression of BSMT1 was detected under PEG treatment (Figure 6L). However, further data on hormone levels are needed to prove it.
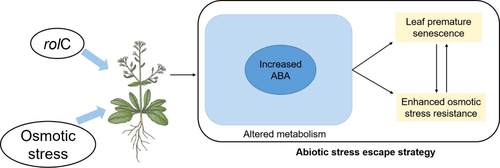
4.4 rolC-Induced Leaf Senescence May be Involved in Abiotic Stress Escape Mechanism of Arabidopsis
It is well known that abiotic stress escape (ASE) is a classical adaptive mechanism involving rapid plant development to enable the completion of the full life cycle prior to a forthcoming stress event, e.g., drought. (Shavrukov et al. 2017). In this ASE mechanism, the plant undergoes a series of changes in response to the shortening of the growth cycle, i.e. the call for plant pre-senescence accompanied with a high metabolic rate (Kooyers 2015). The increase in metabolic rate contributes greatly to enhancing plant stress resistance (Piasecka et al. 2015, Chen et al. 2022). Thus, the induction of leaf pre-senescence could be a smart strategy triggering the enhancement of plant stress resistance (Zhao et al. 2017, Du et al. 2018). In this study, rolC-OX plants showed a shortened growth cycle, exemplified by premature leaf senescence and early flowering (Figures 1D and 2A), the latter being considered a typical manifestation of the ASE mechanism (Shavrukov et al. 2017). More importantly, rolC-OX plants also exhibited enhanced resistance to osmotic stress during early growth (Figure 5A). In view of these features, we speculate that rolC gene could be involved in this ASE strategy, which enables rolC not only to activate metabolic processes and promote the accumulation of defense-related secondary metabolites in plant tissues, such as phytoalexin (Chandra 2012, Matveeva et al. 2015), but also to enhance stress resistance in plants (Veremeichik et al. 2022) and rolC-expressing cell cultures (Victor P. Bulgakov 2008, Shkryl et al. 2022). As one of the metabolites altered by the call for premature senescence, ABA has been shown to be regulated by the rolC gene in this study (Figures 3-5), thus it might serve as a key conjunction mediator between early senescence and resistance enhancement in rolC-OX plants. Interestingly, ABA was reported to promote plants' ASE mechanism by stimulating early flowering (Du et al. 2018). Taken together, the rolC-mediated regulation of endogenous ABA levels and the early flowering phenotype of rolC-OX plants give strong support to the ASE strategy in rolC-OX plants.
From the perspective of evolution, Mauro et al. (2017) proposed that R. rhizogenes acts on plant roots to evolve a species-specific ecological niche over time, i.e. achieve mutual benefit and symbiosis between R. rhizogenes and plants. Specifically, when R. rhizogenes infects plants, hairy roots are formed at the site of infection; importantly, hairy roots can synthesize opines, which can be utilized by R. rhizogenes as a source of energy and food (Nilsson et al. 1997, Vladimirov et al. 2015). Therefore, the benefits for R. rhizogenes under this mutually beneficial model are clear. However, what would be the benefits to plants?
R. rhizogenes infects plants as a pathogen, which inevitably triggers plant defense mechanisms, although R. rhizogenes meanwhile have developed strategies to suppress plant defense reactions (Shkryl et al. 2010, Mauro et al. 2017). This defense mechanism includes the activation of secondary metabolisms, such as anthraquinones, and activation of defense-related proteins, etc. (Bulgakov et al. 2013). Based on the present and previous studies on the view that rol genes of R. rhizogenes are able to enhance stress resistance in plants (Bulgakov et al. 2013, Veremeichik et al. 2022), we propose that the attack of plants by R. rhizogenes does not render them lethal, rather, it could subsequently induce resistant mechanisms improving plants' ability to survive in adverse environments. Therefore, the enhanced resistance to abiotic stress could be the benefit that plants derive from their symbiotic pattern with R. rhizogenes. Under this mutually beneficial model, the activation and execution of the types of ASE mechanisms are believed to be a selection for long-term interaction between R. rhizogenes and plants under certain environments. Here, as a key functional gene of the R. rhizogenes, rolC can (at least partially) perpetuate the activation of this ASE mechanism. Taken together, we propose a hypothetical model of the rolC-induced ASE mechanism in Arabidopsis in response to osmotic stress, in which increased ABA plays a key regulatory role in mediating the responses (Figure 7).
AUTHOR CONTRIBUTIONS
XC, HL, FL, BF and IGM conceptualized the present idea. XC, HL, FL and BF planned the experiments and contributed to the interpretation of results. XC performed the experimental work and data processing, and RN and JH assisted with experiment execution and data analysis. XC wrote the original manuscript and organized the figures and tables. HL, FL, BF and IGM provided critical feedback to the original manuscript editing. All authors contributed to the article and approved the submitted version.
ACKNOWLEDGEMENTS
Kehao Liang, Lene Korsholm Jørgensen, Mia Jin Hasselmose Hansen, Katrine Sikker Hansen and Katrine Kristensen are acknowledged for experimental assistance.
FUNDING INFORMATION
The Chinese Scholarship Council provided scholarships to XC (PhD grant no. 202106850009) and JH (PhD grant no. 201906760024). HL, IGM and BF were supported by a SPRINT grant (2019/08846–2) provided by The São Paulo Research Foundation (FAPESP). The Brazilian Federal Agency for Support and Evaluation of Graduate Education (Capes) provided a scholarship to BF (PRINT/Capes grant no. 88887.469034/2019–00). IGM is a Brazilian National Council for Scientific and Technological Development (CNPq) productivity research fellow (CNPQ: 301043/2018–3).
Open Research
DATA AVAILABILITY STATEMENT
All data supporting the findings of this study are available within the paper and within its supplementary data published online.




