Intron retention via alternative splicing affects the thermotolerance regulation of ZmHsf17
Abstract
Heat shock transcription factor (Hsf) plays a pivotal role in promoting rapid heat-induced transcriptional reprogramming in plants. The thermotolerance regulatory function of Hsfs is influenced by their own alternative splicing. In this study, we found that ZmHsf17-II, an intron retention isoform of subclass A2 gene ZmHsf17 of maize (Zea mays), accumulated in large amounts as a result of severe or sustained heat stress. It was confirmed by expression and purification that ZmHsf17-II encodes a small truncated peptide with 115 amino acids. ZmHsf17-II was found to be located in the nucleus and have no transcriptional activity. Overexpressing ZmHsf17-I in Arabidopsis could enhance plants' thermotolerance, while overexpressing ZmHsf17-II does not. Based on the results of molecular docking, Y2H and split LUC experiments, we found that ZmHsf17-II could bind to DBD region of ZmHsf17-I through the hydrogen bond interaction between the truncated DBD of ZmHsf17-II and three amino acid residues (Arg105, Thr109 and Lys142) of ZmHsf17-I DBD region. Further experiments showed that ZmHsf17-I could bind to its own promoter and exhibited transcriptional activation activity, while ZmHsf17-I interaction with ZmHsf17-II, transcriptional activation activity was interfered. Those findings indicate that ZmHsf17 can negatively regulate its own transcription by producing more intron retention isoforms via alternative splicing under heat stress.
1 INTRODUCTION
Maize (Zea Mays) stands as one of the pivotal cereal crops cultivated extensively across the globe. Its primary applications span the realms of food production, livestock feed, and industrial processing (Parmar et al., 2017). However, in recent years, global warming has brought about a surge in both the frequency and duration of extreme high temperatures, significantly impacting the yield and quality of major crops, maize included (Hoegh-Guldberg et al., 2019). Typically, maize thrives within the optimal daytime temperature range of 28–32°C. Any deviation from this range results in a reduction in maize yield, with a decrease of 7.4% for every 1°C increase (Sánchez et al., 2014; Zhao et al., 2017). Importantly, during the critical stages of pollination and grain filling, ambient temperatures exceeding 35°C can cause detrimental effects. These elevated temperatures slow down pollen growth, inhibit the fertilization process in maize, lead to the formation of abnormal ovaries, result in kernel abortion and a daily yield reduction of 101 kg per hectare (Li and Howell, 2021; Naveed et al., 2014). At the cellular and molecular levels, heat stress (HS) compromises the integrity of mitochondrial and chloroplast membranes, disrupts the structure and stability of functional proteins, triggers an excessive accumulation of reactive oxygen species (ROS) within cells, and ultimately induces apoptosis (El-Sappah et al., 2022). Consequently, it becomes imperative to unravel how plants perceive and respond to high temperatures, with the aim of safeguarding global maize production.
In the realm of plants, the phenomenon of alternative splicing (AS) in precursor mRNA (pre-mRNA) is a common occurrence. Clark et al. (2019) conducted a comprehensive analysis and discovered that AS had a prevalence of approximately 65% throughout the tomato genome, which is akin to what is observed in Arabidopsis thaliana. This conclusion was reached by amalgamating data from mRNA, EST, and RNA-sequence reads from 27 different published projects. Previous research endeavors have shed light on the prevalence of AS in intron-containing genes in rice (Oryza sativa) and maize. More specifically, it has been found that over 40% of such genes in rice undergo AS (Lu et al., 2010), and this figure rises to over 50% in maize (Lu et al., 2013). The selection of distinct splicing sites within a single type of pre-mRNA is orchestrated by a ribonucleic protein complex known as the spliceosome. This intricate molecular machinery gives rise to various AS events, including exon skipping, intron retention, alternative 5′ or 3′ splicing, mutually exclusive exon usage and alternative first or last exon utilization (Lam et al., 2022). This process significantly augments the complexity of genetic output and is widely regarded as a pivotal post-transcriptional regulatory mechanism. It plays a crucial role in a diverse array of biological processes, encompassing the regulation of metabolic pathways, intracellular signal transduction, control of flowering time and circadian rhythms, as well as responses to both biotic and abiotic stresses (John et al., 2021; Lam et al., 2022; Petrillo et al., 2020). Recent investigations have highlighted the role of AS in pre-mRNA as a “molecular thermometer” that enables plants to adapt to temperature variations, including extreme heat (John et al., 2021). For instance, a study conducted in environmental conditions unveiled that elevated daily temperatures heightened the occurrence of major AS modes in maize, particularly retained introns and skipped exons (Li et al., 2021). While high-throughput sequencing techniques have detected AS events induced by HS, the precise regulatory mechanisms governing plant responses to high temperatures through AS remain largely elusive. Prior research has indicated that some transcript isoforms carrying in-frame premature termination codons (PTCs) are likely subjected to degradation through the nonsense-mediated decay (NMD) pathway (Kerényi et al., 2008; Kervestin and Jacobson, 2012). AS coupled with NMD represents a widely conserved eukaryotic mechanism for post-transcriptional regulation, which influences the transcription levels of full-length mRNAs (Sugio et al., 2009; Ge and Porse, 2014). Notably, it has been reported that approximately 11 to 18% of splice variants are linked to NMD in Arabidopsis (Kalyna et al., 2012). Recent studies have unveiled that AS of certain transcription factors is associated with peptide interference (PEPi) by generating small interfering peptides (siPEPs) (Seo et al., 2012; Seo et al., 2013; John et al., 2021). These siPEPs have the capacity to bind to transcription factors, forming homo- or heterodimers, and consequently attenuate the DNA-binding ability of full-length peptides (Seo et al., 2011).
High temperature triggers the heat shock response (HSR), during which heat shock transcription factor (Hsf) activates a cascade of genes encoding heat shock protein (Hsp) (Li and Howell, 2021). In contrast to yeast and mammals, plants boast a remarkable abundance of Hsf members, forming a complex, plant-specific superfamily (Guo et al., 2016). Arabidopsis has 21 members, tomato possesses 24, maize contains 31, and wheat exhibits a staggering 82 Hsf members (Duan et al., 2019; Guo et al., 2008; Guo et al., 2016; Lin et al., 2011; Nover et al., 2001; Scharf et al., 2012; Zhang et al., 2020a). Serving as pivotal regulatory components in response to HS, plant Hsfs exhibit conserved modular structures. Their N-terminal domains bear a characteristic DNA binding domain (DBD) featuring a central helix-turn-helix motif, while the oligomerization domain (OD) possesses a bipartite heptad pattern of hydrophobic amino acid residues (HR-A/B region) (Guo et al., 2016). Plant Hsfs have been categorized into three classes (A, B and C) based on the sequence length between their two domains and the number of amino acid residues inserted into HR-A/B (Scharf et al., 2012). Among these classes, HsfA members typically possess C-terminal aromatic, hydrophobic, and acidic amino acid residues (AHA motifs), which are essential for their transcriptional activity. In contrast, HsfB and HsfC lack AHA motifs and often do not function as transcriptional activators (Li and Howell, 2021). Hsf genes expanded in plants, and most Hsfs can respond to HS. In maize seedlings, 22 genes showed expression activity in maize leaves under HS (Lin et al., 2011). In anthesis stage or post-anthesis stage of maize, 24 Hsf genes respond to HS (Zhang et al., 2020a). In an analysis of gene expression profiles under various environmental stresses, Nishizawa et al. (2006) identified HsfA2 as the most highly up-regulated class A gene in response to HS. While the regulatory functions of individual plant Hsf in response to HS are well understood, there is limited experimental evidence suggesting that plant Hsfs regulate their own gene expression through AS to mitigate the adverse effects of high temperatures.
Our previous work showed that the alternatively spliced forms of ZmHsf04 and ZmHsf17 increased after heat shock (42°C) (Zhang et al., 2020a). According to the analysis of HEATSTER (https://applbio.biologie.uni-frankfurt.de/hsf/heatster/home.php) that is a relatively authoritative platform for identification, classification and characterization of Hsfs (Scharf et al., 2012), ZmHsf17 (Zm00001d018941, ZmHsfA2b) from maize Hsf family were identified and classified as one member of subclass A2. In this study, we found that ZmHsf17 generates two isoforms (ZmHsf17-I and ZmHsf17-II) by AS of pre-mRNA. ZmHsf17-II accumulated at elevated temperatures and may be translated into a truncated protein localized in the nucleus but had no transcriptional activity. Furthermore, it was found that ZmHsf17-II interacted with ZmHsf17-I to effectively suppress the transactivation function of ZmHsf17-I. In summary, the results suggest that intron retention isoforms of ZmHsf17 play a negative regulatory role in regulating the transcription level of ZmHsf17 itself.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
In this study, the maize (Zea mays) inbred line H21 was employed. Seeds underwent surface sterilization using 0.1% HgCl2, followed by thorough rinsing and a 12-hour soak in distilled water. Subsequently, they were placed in an incubator set at 28°C. After germination, the seedlings were transplanted in pots filled with Hoagland nutrient solution and cultivated in a controlled-environment greenhouse. The greenhouse maintained conditions of 16 hours of daylight and 8 hours of night (with a light intensity of 500 μmol m−2 s−1), temperatures of 28°C during the day and 20°C at night, and relative humidity ranging from 60% to 80%. After two weeks, the second leaf of each seedling was fully expanded and shoots and roots of about ten seedlings were sampled and immediately immersed in liquid nitrogen for subsequent gene expression analysis. Meanwhile, other plants continued to grow in the greenhouse. Functional leaves, ears, pollen, and immature embryos from two-month-old plants at different growth stages were harvested for gene expression analysis.
Arabidopsis thaliana [(L. Heynh.), ecotype; Col-0] and athsfa2 mutant (SALK_ 008978) were used for genetic transformation of ZmHsf17 in this study (Charng et al., 2007). The athsfa2 mutant cannot generate all three AS informs (AtHsfA2-I, AtHsfA2-II and AtHsfA2-III) due to the T-DNA insertion in upstream of the splice site in the AtHsfA2 gene. The seeds of Arabidopsis were surface-sterilized in 75% alcohol for 30 seconds and followed by a 10-minute treatment with 10% sodium hypochlorite. The sterile seeds were sown on 0.5× Murashige and Skoog (MS) medium, which included 1% (w/v) sucrose and 0.8% (w/v) gelrite (San-Ei Gen FFI Inc., 1× MS salt and vitamin, pH 5.8) in a plastic Petri plate. After a 3-day incubation at 4°C in the dark, the seeds were transferred to a growth chamber with the following conditions: a 16/8 hour light/dark cycle with temperatures of 22°C/18°C, a photosynthetic photon flux density (PPFD) of 400 μmol m−2 s−1, and a relative humidity of 50%.
2.2 Stress treatments
For HS tests, two-week-old uniformly-grown maize seedlings with two fully expanded leaves were subjected to the following treatments: 37°C, 42°C, and 45°C in an incubator. The second leaves were sampled at different times after initiation of the treatment (0, 10, 20, 30, 60, 90, 120 and 240 min) and stored in liquid nitrogen for expression analysis. Seedlings grown at 28°C were used as the control.
2.3 RNA extraction, semi-quantitative PCR and quantitative real-time PCR
Total RNA was extracted using Plant RNA Kit R6827 (Omega) according to the manufacturer's protocol. The RNA quantity was determined on a NanoDrop ND-2000 spectrophotometer (Thermo Scientific). Each RNA sample was treated with DNase I for 30 minutes at 37°C to remove residual DNA contamination, and cDNA was synthesized using PrimeScript RT reagent Kit with gDNA Eraser (Takara). Primers designed for semi-quantitative RT-PCR and quantitative RT-PCR are listed in Table S1. The maize β-ACTIN gene was used as reference. Quantitative real-time PCR was performed in a 7500 Real-Time PCR System using the TB Green Premix EX Taq II (Takara) following the manufacturer's protocol (Applied Biosystems). The data were analyzed with GraphPad Prism 8, and each dataset was repeated at least three times to calculate the standard deviation.
2.4 Cloning of the complete coding sequence of ZmHsf17
The coding regions of ZmHsf17-I and ZmHsf17-II were amplified using high-fidelity DNA Polymerase PrimeSTAR (TaKaRa) with the specific full-length cDNA primers ZmHsf17-FR (Table S1). The reaction mixture consisted of 5× PrimeSTAR GC buffer, 10 μL; dNTP mixture (2.5 mmol L−1), 4 μL; template cDNA, 100 ng; 10 μmol L−1 forward primer, 1 μL; 10 μmol L−1 reverse primer, 1 μL; PrimeSTAR HS DNA polymerase 0.5 μL; ddH2O, up to 50 μL. The reaction program was as follows: 98°C 10 s, 55°C 5 s, 72°C 2 min, 30 cycles. After ligation into T-vector (pEasy-Blunt Simple Cloning Kit, TransGen Biotech), the products were sequenced (Sangon biotech). The sequences of ZmHsf17-I and ZmHsf17-II were submitted to NCBI, and they have been assigned the accession numbers MK736813 and OR354318, respectively.
2.5 Protein prokaryotic induction and purification
The coding sequences of ZmHsf17-I and ZmHsf17-II were respectively inserted into the purification vector pET30a. The recombinant plasmids were transformed into the BL21 strain of E. coli cells for the prokaryotic expression of His-tagged fusion proteins into the BL21 fusion protein according to the manufacturer's instructions. The BL21 positive monoclonal colonies were cultured in LB liquid medium with shaking until the OD600 value of bacterial fluid reached 0.6–0.8 at 37°C, then 3 mL of bacterial solution was transferred into 200 mL LB medium to expand cultivation, and IPTG was added at a final concentration of 3 mmol L−1 for inducing protein expression. Cultivating the bacterial fluid at 16°C for 16 hours was optimal inducing conditions based on preliminary experiments. The expanded bacterial solution was centrifuged at 2500 g at 4°C. Bacterial precipitation was resuspended in MCAC buffer solution (Metal chelate affinity chromatography; 20 mmol L−1 Tris HCl, 0.5 mol L−1 NaCl, 10% glycerol, 10 mmol L−1 β- mercaptoethanol). Triton X-100 (10%) and PMSF (0.1 mol L−1) were added to the resuspension in the ratio of 1/100. After ultrasonic crushing, the solution was centrifuged at 10000 g at 4°C. The supernatant was filtered through a 0.45 μm membrane. The fusion protein tagged with 6 × His was purified using Ni2+ affinity chromatography column (Sangon biotech). The affinity column was washed with ddH2O and MCAC buffer solution. The supernatant containing the target protein was added to the balanced column at a flow rate of 10–15 mL h−1. The column was sequentially washed with 5 mL of the following MCAC buffer solution containing a segmented elution manner: 20, 50, 80,100, 200 and 300 mmol L−1 imidazole. The gradient eluent was collected and traces of denatured proteins were detected with the SDS-PAGE method. The concentration of purified proteins was measured with Bradford Protein Assay Kit (Beyotime).
2.6 Subcellular localization of ZmHsf17-II
The coding regions of ZmHsf17-II and ZmHsf17-I tagged with GFP were cloned to pCAMBIA1300 using the ClonExpress II one-step cloning kit (Vazyme). Primers for constructing vectors are listed in Table S1. The reconstructed plasmid and empty plasmid were transformed into Agrobacterium strain GV3101, and injected into the leaves of 4- to 6-week-old N. benthamiana with a needleless 1 mL syringe. Before imaging, the nucleus was stained with 4′, 6-diamidino-2-phenylindole (DAPI, 1 mg mL−1) dye. Fluorescence observations of GFP and DAPI in the transformed N. benthamiana were performed with a confocal laser scanning microscope (META510, Zeiss). The GFP showed a strong emission at around 507 nm when excited at 488 nm, and DAPI emission was at 461 nm when excited at 405 nm. The experiment was repeated three times with similar results.
2.7 Detecting protein expression of ZmHsf17-II and ZmHsf17-I in transgenetic Arabidopsis
The above Agrobacterium GV3101 carrying the ZmHsf17-II-GFP or ZmHsf17-I-GFP were used to transform wild Arabidopsis by vacuum dipping method (Clough and Bent, 1998). The MS medium containing 25 μg ml−1 hygromycin was used to screen the progeny plants until the homozygous seeds were harvested. The transgenic lines of T3 homozygous were selected to detect the expression of ZmHsf17-II-GFP and ZmHsf17-I-GFP fusion proteins. The leaves of two-week-old transgenic Arabidopsis were sampled, rapidly frozen in liquid nitrogen, and homogenized. Total protein extraction was performed using RIPA lysis buffer (Sangon biotech) containing 1 mmol L−1 PMSF. Protein detection was carried out using GFP monoclonal antibodies (Sigma-Aldrich) by Western blot.
2.8 Transactivation analysis in yeast
Transcriptional activation activity assays were performed using the yeast strain AH109 and pGBKT7 vector (Clontech). The coding sequences of ZmHsf17-II and ZmHsf17-I were fused to GAL4 BD (pGBKT7) and then introduced into yeast strain AH109 by lithium acetate-polyethylene glycol-mediated transformation procedure (Gietz and Woods, 2002). Primers for constructing vectors are listed in Table S1. The transformed yeast clones were serially diluted (1:10) and spotted on the yeast synthetic triple-dropout SD medium (-WAH, SD/−Trp/−Ade/-His) contains 5-bromo-4-chloro-3-indolyl-α-D-galactopyranoside acid (X-α-Gal) for growth at 30°C. The transcriptional activation activity was evaluated according to its growth status and the activity of α-galactosidase.
2.9 Generation of transgenic Arabidopsis plants and assays of basal and acquired thermotolerance
The coding regions of ZmHsf17-II and ZmHsf17-I were respectively cloned into pCAMBIA1300 vector using ClonExpress II one-step cloning kit (Vazyme). Primers for constructing vectors are listed in Table S1. Agrobacterium GV3101 carrying one of the above-mentionned vector were used to transform wild Arabidopsis and deletion athsfa2 mutants by vacuum dipping method. The MS medium containing 25 μg ml−1 hygromycin was used to screen the progeny plants until the homozygous seeds were harvested. The transgenic lines of T3 homozygous were selected for thermotolerance assay. The expression levels of the ZmHsf17-II and ZmHsf17-I were detected through semi-quantitative PCR and quantitative PCR experiments. In semi-quantitative PCR, primers were designed and verified to exclusively amplify ZmHsf17. In quantitative experiments, another pair of primers was designed and ensured to simultaneously amplify ZmHsf17 and AtHsfA2. All the primers used are listed in Table S1.
The sterilized seeds of WT, athsfa2, and Arabidopsis transgenic lines were all placed in 1/2 MS solid plates containing 0.8% agar according to the above method. Five-day-old Arabidopsis seedlings were subjected to heat shock. The heat shock experiments were carried out in the incubator based on previous research (Li et al., 2019). The basal and acquired thermotolerance assay conditions were done according to Charng et al. (2007). In WT and overexpressing line group, the basal thermotolerance treatment protocol involves exposing the sample to a heat shock of 45°C in an incubator for 50 minutes, followed by a recovery period at 22°C in a growth chamber with above optimal conditions for 8 days. The acquired thermotolerance treatment protocol includes a heat shock at 37°C for 60 minutes, followed by a recovery period at 22°C for 2 days, then another heat shock at 46°C for 60 minutes, and a final recovery period at 22°C for 8 days. In WT, athsfa2, and complementary lines group, the thermotolerance treatment protocol is 44°C HS for 70 minutes and 22°C for 8 days. Arabidopsis seedlings were photographed and the chlorophyll contents were determined subsequently. Acetone extraction method was used to detect the chlorophyll contents, and maximum absorption peaks of acetone solutions of chlorophyll a/b in the visible light range are located at 663/645 nm, respectively. At least three independent biological replicates were employed, and significant differences were determined using t-test and one-way ANOVA in Graphpad Prism 8.0.
2.10 Yeast two-hybrid (Y2H) assay
For the Y2H assay, the coding sequences of ZmHsf17-II and ZmHsf17-I were cloned into pGBKT7 and pGADT7 vector (Clontech) to generate the BD-ZmHsf17-II and AD-ZmHsf17-I constructs, respectively. The cryptic exon and DBD domain of ZmHsf17-II were truncated successively by replacing the downstream primers. The truncated sequences of ZmHsf17-II were cloned into pGBKT7 vector, and the fusion vectors were used to verify the domain of protein interaction. Besides, the ZmHsf17-I containing base mutation sites (Arg105, Thr109 and Lys142) was amplified by overlap PCR. The sequences containing mutation sites were cloned into pGADT7 vectors for Y2H to verify the protein interaction sites. All the primers in Y2H assay are listed in Table S1, and the truncated or mutated coding sequences are shown in Table S2. The BD-ZmHsf17-II and AD-ZmHsf17-I constructs were co-transformed into yeast strain AH109 by the lithium acetate method and grown on the yeast synthetic double-dropout and quadruple-dropout SD medium (-LW, SD/−Leu/−Trp and -LWAH, SD/−Leu/−Trp/−Ade/-His) for 3 days at 30°C following the manufacturer's protocol. The interaction was evaluated according to their growth status and the activity of α-galactosidase.
2.11 Molecular docking by Autodock Vina
The fragmented small peptide ZmHsf17-II was used as the ligand, and the receptor protein for molecular docking was ZmHsf17-I (Uniprot accession number: AFB6SVD5F1). The protonation state of all the compounds was set at pH 7.4, and the compounds were expanded to 3D structures using Open Babel (O'Boyle et al., 2011). AutoDock Tools (ADT3) were applied to prepare and parameterize the receptor protein and ligands. The docking grid documents were generated by AutoGrid of sitemap, and AutoDock Vina (1.2.0) was used for docking simulation (Eberhardt et al., 2021). The optimal pose was selected to analyze interaction. Finally, the protein-ligand interaction figure was generated by PyMOL.
2.12 Split firefly luciferase complementation (SFLC) assay
The coding sequences of ZmHsf17-I and ZmHsf17-II were individually cloned into the vectors pCAMBIA1300-nLUC and pCAMBIA1300-cLUC to generate ZmHsf17-II-nLUC, cLUC-ZmHsf17-II and cLUC-ZmHsf17-I (see primers in Table S1). The indicated recombinant constructs were transformed into Agrobacterium strain GV3101 and injected into the leaves of 4- to 6-week-old N. benthamiana with a needleless 1 mL syringe. After two days, the N. benthamiana leaves were infiltrated with 1 mmol L−1 D-Fluorescein and visualized after 5 min using the Tannon 5200 plant imaging system (Tannon).
2.13 EMSA
A pair of 40 bp oligonucleotide probes containing typical heat shock element (HSE) (5’-nGAAnnTTCn-3′) sequences were synthesized (Sangon biotech). The HSE regions from ZmHsf17 were used to synthesize the biotin-labeled probes, and the concentrations of unlabeled competitive probes were 10 times, 100 times and 1000 times that of the biotin-labeled probes. The mutant probes were designed according to the ways of G → A and C → A in nGAAnnTTCn. The probes and mutation probe sequences are listed in Table S1. Biotin-labeled dUTP was added to the 3′ end of single-stranded DNA probes using Terminal Deoxynucleotidyl Transferase (TdT) with Probe Biotin Labeling Kit (Beyotime). Annealed double-stranded probes were used for EMSA assays according to the EMSA/Gel-Shift Kit instructions (Beyotime). Three higher concentrations of unlabeled probes were used as competitors.
2.14 Dual luciferase reporter assay
To confirm the transcriptional activation of ZmHsf17-II and ZmHsf17-I, dual luciferase reporter assay was performed by referring to previous research (Hellens et al., 2005). The −1 kb promoter segment of ZmHsf17 was cloned into the pGreenII 0800-LUC to generate Hsf17pro: LUC. For effector plasmid, the ORFs of ZmHsf17-II and ZmHsf17-I were inserted into the pCAMBIA1300 vector to generate 35S: ZmHsf17-II and 35S: ZmHsf17-I. Primers for constructing vectors are listed in Table S1. The pCAMBIA1300 vector without ZmHsf17 was set as blank control. The constructed plasmids were transformed into Agrobacterium strains GV3101 using the freeze–thaw method (Weigel and Glazebrook, 2006). The strains were infiltrated into the lower epidermis of N. benthamiana leaves for transient expression according to the method mentioned above. After two days, the N. benthamiana leaves were divided into two groups for visualization and enzyme activity assay of luciferase. The N. benthamiana leaves were infiltrated with 1 mmol L−1 D-Fluorescein and visualized after 5 minutes using the Tannon 5200 Imaging System. Another group of N. benthamiana leaves was sampled and ground in liquid nitrogen. After 13,000 g centrifugation, the supernatant liquid was used to determine the activity of firefly luciferase (LUC) and Renilla reniformis luciferase (REN) according to the instruction of Dual-Luciferase Reporter Assay System (Promega). The luminescence values were measured using the Tecan Infinite M200 PRO microplate reader and the relative luciferase (LUC/REN) was calculated and plotted. Data are means ± SD of three independent sample replicates. Significant differences were determined using t-test and one-way ANOVA in Graphpad Prism 8.0.
3 RESULTS
3.1 ZmHsf17-II encodes a small truncated protein
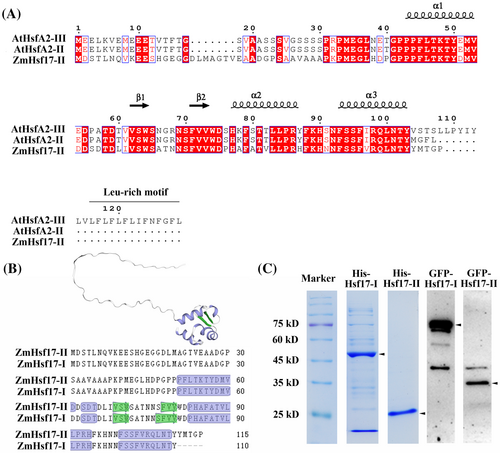
In our previous study, a new splice variant of the ZmHsf17 gene was identified through the analysis of nanopore third-generation full-length transcriptome sequencing data, and it was named ZmHsf17-II (Zhang et al., 2020a). The retained intron regions contain premature translation termination codon (PTC) and ZmHsf17-II was hypothesized to form a small truncated protein with 115 amino acids. To confirm the above speculation, we obtained the coding sequence of ZmHsf17-II from the leaves of maize seedlings after HS treatment and analyzed the corresponding amino acid sequence. Multiple sequence alignment analysis revealed that ZmHsf17-II has no C-terminal Leu-rich motif and shares a higher similarity with AtHsfA2-II (Figure 1A). By comparing the DBD structure of ZmHsf17-I and ZmHsf17-II, it was found that the C-terminal truncated DBD of ZmHsf17-II contained helixes α1, α2, and α3, and strands β1 and β2, but lacked strands β3 and β4, which were replaced by a short peptide of five amino acids (YMTGP) (Figure 1A,B). The protein structure of ZmHsf17-II was predicted by using the SWISS-MODEL server, and the homology-modelling template was ZmHsf17 (B6SVD5.1.A), modeled by AlphaFold v2.0 (Figure 1B). The predicted small peptide of ZmHsf17-II still retained the basic helix-turn-helix structure (Figure 1B). To confirm the presence of the truncated protein, ZmHsf17-II was tagged with His or GFP labels and subjected to heterologous expression in E. coli or Arabidopsis. By prokaryotic protein induction and His-tag affinity purification, the 25-kDa His-ZmHsf17-II was detected (Figure 1C). The ZmHsf17-II protein, tagged with GFP at its N-terminus, was detected through Western blot analysis (Figure 1C). These results suggested that ZmHsf17-II can be well translated into a small truncated protein in E. coli and Arabidopsis.
3.2 Elevated temperature promotes the accumulation of ZmHsf17-II
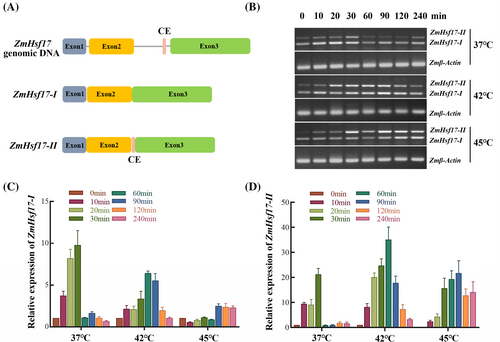
To validate the induction of ZmHsf17-II under various temperature conditions, we assessed the transcript levels of ZmHsf17-II in maize seedlings subjected to heat shock treatments at 37°C, 42°C, and 45°C, respectively. Specific primers were designed to amplify ZmHsf17-I and ZmHsf17-II based on the sequences of different ZmHsf17 transcripts (Figure 2A). Semi-quantitative reverse transcription PCR (semi-qRT-PCR) results demonstrated a substantial increase in ZmHsf17-II transcripts with prolonged treatment duration; with severe heat showing a particularly pronounced effect on ZmHsf17-II accumulation (Figure 2B). As anticipated, quantitative reverse transcription PCR (qRT-PCR) further confirmed that heat shock induced a temperature-dependent elevation in the levels of ZmHsf17-II transcripts (Figure 2C,D). It is worth noting that under 45°C high-temperature stress, the expression levels of ZmHsf17-I transcripts increase only minimally (Figure 2C). This forms a striking contrast with the high expression levels of ZmHsf17-II transcripts under prolonged extreme high-temperature treatment.
3.3 ZmHsf17-II is localized in the nucleus but lacks transcriptional activation activity
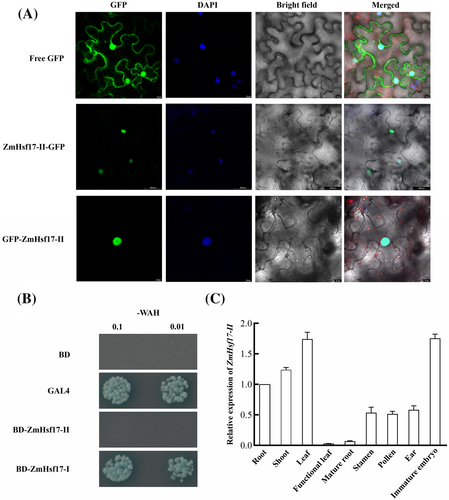
Considering that ZmHsf17-II encodes a small truncated ZmHsf17 that retains only a portion of DBD and lacks functional domains such as activation domain and nuclear localization signal (NLS), we investigated into the localization and transcriptional activity of ZmHsf17-II proteins. The ZmHsf17-II proteins were tagged with GFP in N-terminal (35S::GFP-ZmHsf17-II) and C-terminal (35S::ZmHsf17-II-GFP). Transient expression of fusion proteins in N. benthamiana leaf epidermal cells indicated a conspicuous fluorescence concentration in the nucleus, aligning perfectly with the fluorescence pattern of the nuclear-specific dye DAPI (Figure 3A). In contrast, the free GFP protein was evenly distributed throughout the entire cell. This result indicates that ZmHsf17-II exhibits nuclear localization, even in the absence of NLS. Following that, we examined the transcriptional activity of ZmHsf17-II in yeast. The yeast harboring pGBKT7-GAL4 or pGBKT7-ZmHsf17-I grew well on the yeast medium (SD/-WAH) and could catalyze 5-bromo-4-chloro-3-indolyl-α-D-galactopyranoside acid (X-α-Gal), while the yeast harboring pGBKT7-ZmHsf17-II and negative controls carrying pGBKT7 barely grew at all (Figure 3B), which indicated ZmHsf17-II had no transcriptional activation activity. Furthermore, we assessed the relative expression levels of ZmHsf17-II in different tissues. ZmHsf17-II transcripts exhibited relatively higher expression levels in young leaves and immature embryos (Figure 3C).
3.4 Heterologous expression of ZmHsf17 different spliced isoforms in Arabidopsis
To validate the thermotolerance function of two ZmHsf17 isoforms, we generated overexpressing lines and complementary lines of both ZmHsf17-I and ZmHsf17-II using wild-type Arabidopsis and athsfa2 mutant. Chlorophyll content in Arabidopsis seedlings was measured as a physiological indicator. The transcription levels of both ZmHsf17-I and ZmHsf17-II were assessed in overexpression and complementary lines, and two ZmHsf17 transcripts were overexpressed to varying degrees (Figure S1). At optimal temperature (22°C), all the transgenic plants and wild type plants exhibited healthy growth (Figure 4A,D) and showed no significant differences in chlorophyll content (Figure 4C,F). After basal thermotolerance treatment at 45°C for 50 minutes followed by a recovery period at 22°C for 8 days, the overexpressed lines of ZmHsf17-I (18_9, 24_21 and 29_18) grew better than the wild-type plants and displayed higher chlorophyll contents (Figure 4B,C). However, the truncated isoform ZmHsf17-II overexpressing lines (7_8, 20_9 and 25_21) did not exhibit increased thermotolerance and the chlorophyll contents were similar to those of the wild type (Figure 4E,F). Similarly, in acquired thermotolerance assays, ZmHsf17-I enhanced the thermotolerance of transgenic Arabidopsis plants, but ZmHsf17-II did not (Figure S2). As a result of the complete mortality observed in athsfa2 mutant plants subjected to 45°C treatment for 50 minutes, we have redefined the heat shock conditions for the basal thermotolerance experiment. After treatment at 44°C for 70 minutes, the ZmHsf17-I/athsfa2 complementary lines (2_5, 5_2 and 10_20) restored the reduced thermotolerance of the athsfa2 mutant seedlings (Figure 4H). The chlorophyll contents of these complementary lines were significantly higher than those of the mutant plants, with line 5_2 even surpassing the wild type (Figure 4I). In contrast, the ZmHsf17-II/athsfa2 complementary lines (5_10, 14_3 and 18_14) failed to enhance the growth status of athsfa2 mutant seedlings (Figure 4K), and the chlorophyll contents in these lines were not significantly different from those of the athsfa2 mutant seedlings (Figure 4L).
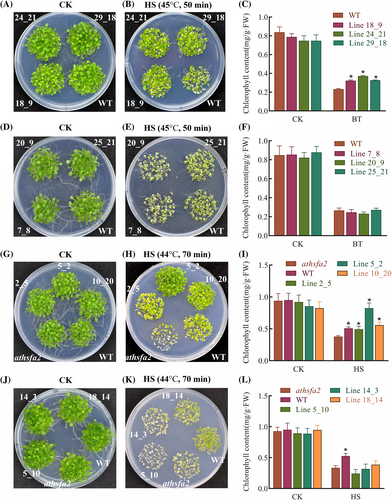
The thermotolerance phenotype of ZmHsf17-I or ZmHsf17-II transgenetic Arabidopsis. (A-C) Phenotype of WT and three ZmHsf17-I overexpressing lines (18_9, 24_21 and 29_18) under (A) normal condition or (B) basal thermotolerance treatment (45°C for 50 minutes and 22°C for 8 days) . (C) The chlorophyll contents of WT and three ZmHsf17-I overexpressing seedlings under normal and HS conditions.
(D-F) Phenotype of WT and three ZmHsf17-II overexpressing lines (7_8, 20_9 and 25_21) under (D) normal conditions or (E) after basal thermotolerance treatment. (F) The chlorophyll contents of WT and three ZmHsf17-II overexpressing seedlings under normal or HS conditions.
(G-I) Phenotype of WT, athsfa2 mutant and three ZmHsf17-I complementary lines (2_5, 5_2 and 10_20) under (G) normal condition or (H) HS treatment (44°C for 70 minutes and 22°C for 8 days). (I) The chlorophyll contents of WT, athsfa2 mutant and three ZmHsf17-I complementary lines under normal and HS conditions. (J-L) Phenotype of WT, athsfa2 mutant and three ZmHsf17-II complementary lines (5_10, 14_3 and 18_14) under (J) normal condition or (K) HS treatment. (L) The chlorophyll contents of WT, athsfa2 mutant and three ZmHsf17-II complementary lines under normal and HS conditions. Data are means ± SDs of three independent sample replicates. The significant differences were determined using t-test (N = 3), and one asterisk (*) shows a significant difference (P < 0.05).
3.5 ZmHsf17-II interacts with ZmHsf17-I
To investigate the interaction between ZmHsf17-II and ZmHsf17-I, we utilized both Y2H and SFLC assays. Y2H assay revealed that ZmHsf17-II can indeed interact with ZmHsf17-I (Figure 5A). Interestingly, we also observed an interaction between ZmHsf17-II and itself, suggesting that ZmHsf17-II might form homodimers (Figure 5A). Furthermore, SFLC assays showed that strong fluorescence signals were observed in N. benthamiana leaves transiently co-expressing N-terminal part of luciferase protein fused with ZmHsf17-II (ZmHsf17-II -nLUC) and the C-terminal part with ZmHsf17-I or ZmHsf17-II (cLUC-ZmHsf17-I or cLUC-ZmHsf17-II), but not in leaves co-expressing ZmHsf17-II-nLUC and cLUC, cLUC-ZmHsf17-I and nLUC, or nLUC and cLUC (Figure 5B,C).
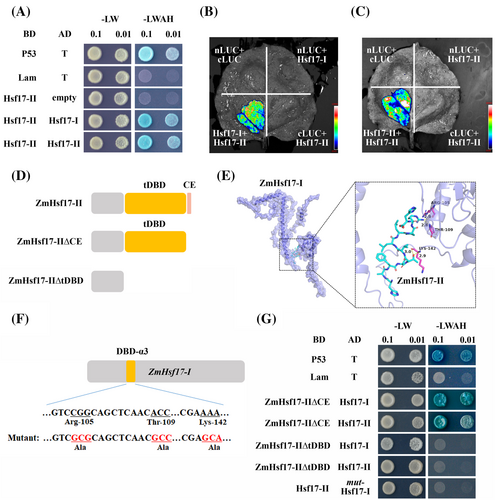
To investigate the interaction region between ZmHsf17-II and ZmHsf17-I further, we employed segment truncated ZmHsf17-II in a macromolecular docking assay. The protein-ligand interaction analysis revealed that the DBD region of ZmHsf17-II interacts with the DBD-α3 region of ZmHsf17-I (Figure 5E). Specifically, three amino acid residues Arg105, Thr109 and Lys142 of ZmHsf17-I were identified to form hydrogen bonds with truncated ZmHsf17-II ligand (Figure 5E). These interactions contributed to a binding energy of the protein-ligand complex, which predicted −6.1 kcal mol−1, indicative of a strong binding affinity. Furthermore, we introduced mutations in ZmHsf17-I, where these three Arg/Thr/Lys residues were replaced with Ala, and also created corresponding yeast transformation vectors with segment-truncated ZmHsf17-II for Y2H assays (Figure 5D,F). The results demonstrated that the truncated DBD (tDBD) of ZmHsf17-II is essential for its interaction with ZmHsf17-I in yeast cells, whereas the short peptide of five amino acids (YMTGP) was not sufficient for interaction. Additionally, the mutated ZmHsf17-I could not bind to ZmHsf17-II (Figure 5G). These findings indicate that ZmHsf17-II can interact with ZmHsf17-I, and that the Arg/Thr/Lys residues of ZmHsf17-I are specifically recognized by ZmHsf17-II.
3.6 ZmHsf17-II suppressed the transcriptional activation activity of ZmHsf17-I
In general, some transcription factors can regulate their expression by binding to their own promoters. The DBD-α3 is crucial for Hsf to bind to the HSE located in its promoter. The above-mentioned protein interaction showed the DBD-α3 region was also a target of ZmHsf17-II. To investigate whether ZmHsf17-I could regulate its own transcription and elucidate the biological function of ZmHsf17-II in the self-regulation of ZmHsf17-I expression, we conducted an EMSA assay. After analyzing the fragment of ZmHsf17 promoter, a typical cis-acting HSE was identified (Figure 6A). Using a probe containing this HSE, we observed a binding of ZmHsf17-I to its own promoter (Figure 6B). Furthermore, the addition of ZmHsf17-II or an excess of unlabeled probes decreased this binding (Figure 6B). Notably, we found that ZmHsf17-II alone could not bind to the promoter of ZmHsf17 (Figure S3).
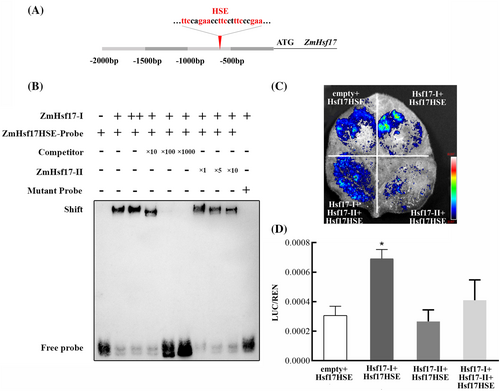
Subsequently, we conducted a dual-luciferase reporter assay in N. benthamiana leaf cells to determine whether ZmHsf17-II affected the transcriptional activation activity of ZmHsf17-I. In this assay, the firefly luciferase (LUC) gene driven by the -1 kb ZmHsf17 promoter served as the reporter, while the individual coding sequence of ZmHsf17-I and ZmHsf17-II driven by the 35S promoter acted as effectors. As shown in Figure 6C, the expression of ZmHsf17-I alone could activate its own transcription, as evidenced by the higher relative LUC/REN ratio compared to the control (Figure 6D). However, overexpression of ZmHsf17-II alone did not significantly affect LUC activity compared to the control and even reduced LUC activity when co-expressed with ZmHsf17-I (Figure 6C,D). In summary, these findings suggest that ZmHsf17-II negatively regulates ZmHsf17-I by interacting with it and directly suppressing its transcriptional activation activity.
4 DISCUSSION
Transcription factors regulate the development, cellular differentiation and adversities resistance in eukaryotes by directly influencing the transcription of downstream genes. Beyond the transcriptional regulation, post-transcriptional regulation, including pre-mRNA alternative splicing (AS), plays a critical role in plant response to abiotic stress (Mastrangelo et al., 2012; Ling et al., 2021). AS in plants generates additional splice variants and reprograms the transcriptome, precisely and rapidly (Laloum et al., 2018; Filichkin et al., 2018). Transcriptome sequencing analysis of immature kernels from 368 maize inbred lines revealed that 50% of protein-coding genes undergo AS (Chen et al., 2018). Differential usage of alternative splice sites under stress conditions generates diverse transcript isoforms (Mandadi et al., 2023). In our previous study, different spliced isoforms of ZmHsf17 were identified under different temperature HS conditions in maize by the nanopore third-generation transcriptome sequencing (Zhang et al., 2020a). In this study, we conducted heterologous expression of different splicing variants fused with a GFP tag in N-terminal in Arabidopsis, and observed the detectable presence of both the full-length form GFP-ZmHsf17-I and truncated form GFP-ZmHsf17-II (Figure 1C). Based on sequence alignment, we also found that the distance between the last PTC and exon was relatively short in ZmHsf17-II isoforms, about 57-nt. The distance between the PTC signal and the last exon junction affects the NMD efficiency: the closer the distance, the lower the efficiency within a range of 100-nt (Lindeboom et al., 2016). In recent years, a growing body of studies have found that not all the PTC signal can deliver the transcripts to NMD pathway (Reddy et al., 2013; Seo et al., 2013; Chen et al., 2018; John et al., 2021). Two AS transcripts from radish (RsMYB1-IR1/2) were predicted to encode truncated proteins due to the generation of PTC signal, and proved to increase the anthocyanin contents as a completely spliced transcript (Kim et al., 2021). Therefore, we predicted that ZmHsf17-II isoform was translated to one truncated protein and associated with thermotolerance in maize.
The HsfA2 proteins in plants, characterized by their DNA-binding domains and activation domains, have been demonstrated as the central components responsible for regulating downstream genes associated with thermotolerance, such as Heat Shock Proteins (Hsps) (Gu et al., 2019; Liu et al., 2022; Kappel et al., 2023). The HsfA2 from Arabidopsis, rice, tomato, cabbage, poplar and wheat were reported to undergo AS regulation (Ling et al., 2021). Responses to high temperature vary among splice variants of HsfA2 genes in different species. OsHsfA2d-II had only a slight response to 42°C HS, but instead had a main response to long-day light stress (Cheng et al., 2015). AtHsfA2-II was the main splicing isoform at moderate HS condition (Sugio et al., 2009); however, the transcription level of ZmHsf17-II increased with the prolongation of treatment time at severe HS (45°C). For maize, elevated 42°C HS treatment led to significant growth arrest and temperature extremes (45°C) will cause irreversible damage to the cell membrane system (Sánchez et al., 2014). With the prolongation of HS treatment time at 45°C, the expression levels of ZmHsf17-II isoform were higher (Figure 2B,C). Although the ZmHsf17-II did not contain nuclear localization signal, subcellular localization experiment found that ZmHsf17-II was localized to the nucleus (Figure 3A). The subcellular localization of OsHsfA2d-II was in both nucleus and cytoplasm (Cheng et al., 2015), and the AtHsfA2-III and GFP fusion protein was observed mainly in the nucleus (Liu et al., 2013). The consistent nuclear localization of splicing variants suggests their roles as transcription factors; however, ZmHsf17-II showed no transactivation activity in yeast (Figure 3B). We speculate that ZmHsf17-II does not affect gene expression through transcriptional regulation like typical transcription factors, but rather through protein–protein interactions that interfere with receptor function. Of course, before that, we need to compare the extent of thermotolerance improvement in Arabidopsis after heterologous expression of different splice isoforms of ZmHsf17.
The full-length variants of HsfA2 members (including ZmHsf01, ZmHsf04 and ZmHsf05) enhanced the thermotolerance of transgenetic Arabidopsis plants (Jiang et al., 2017; Li et al., 2019; Zhang et al., 2020b). ZmHsf17-I, as the full-length variant from another A2 subclass member of Hsfs, has also conservative DNA-binding domains and activation domains. So, we inferred ZmHsf17-I should have thermotolerance function. Indeed, Arabidopsis plants overexpressing ZmHsf17-I demonstrated significantly enhanced thermotolerance compared to their wild-type counterparts (Figure 4B). However, heterologous overexpression of ZmHsf17-II in wild type Arabidopsis could not enhance the thermotolerance, and not rescue the heat-sensitive phenotypes of the athsfa2 mutant (Figure 4E,K). It appears that the overexpression of ZmHsf17-II alone may not enhance the heat tolerance of plants. Therefore, we consider whether ZmHsf17-II interacts with ZmHsf17-I protein. AS of TFs is often associated with PEPi to limit excessive transcriptional activation by forming a negative feedback loop (Seo et al., 2013; Gil et al., 2017). LlHsfA3B undergoes AS to form a siPEP LlHsfA3B-III as the repressor for controlling the activation of LlHSFA3A (Wu et al., 2019). Y2H and SFLC results indicated that ZmHsf17-II can interact with ZmHsf17-I as well as itself (Figure 5A-C). As a truncated peptide lacked OD region, how ZmHsf17-II interacts with ZmHsf17-I appears confusing. The OD region is generally believed to be required for the interaction between HsfAs (Chan-Schaminet et al., 2009; Wang et al., 2018). While in Lily, the LlHsfA3B-III lacking OD region could still interact with other LlHsfA3 proteins (Wu et al., 2019). Molecular docking simulations can predict the structure of protein complexes and protein interaction sites. Here, we further identified the hydrogen bond interaction between the α2-helical coiled coil of ZmHsf17-II and three amino acid residues of ZmHsf17-I (Figure 5E). Highly conserved Arg105 and Thr109 of these three amino acid residues both located in the α3-helical coiled-coil (often referred to as “the recognition helix”). The Arg105 residue is considered to be the key amino acid binding to the guanine in “GAA” of HSEs (Jaeger et al., 2016). The Lys142 located on the wing domain of the DBD region may be helpful to the formation of DBD dimerization (Feng et al., 2021). Through segmenting ZmHsf17-II and mutating specific amino acid sites in ZmHsf17-I, we employed Y2H assay to validate this novel interaction pattern (Figure 5G). We speculate that this interaction occurring in the critical DBD region may potentially impact the normal function of transcription factors. Previous studies in PEPi mechanism have found that siPEPs inhibit the DNA binding activity by forming nonfunctional heterodimers with functional TFs (Yun et al., 2008; Chen et al., 2018).
Can ZmHsf17-II also perform a function similar to siPEPs? Based on the conclusion that the alternative splicing variant of AtHsfA2 can activate its own expression in Arabidopsis (Liu et al., 2013), we cloned the promoter of ZmHsf17. EMSA and dual luciferase reporter assays indicated that ZmHsf17-I can bind to the HSE elements in its own promoter and activate its expression (Figure 6). Subsequently, due to the addition of ZmHsf17-II, both the DNA binding ability and transcription activation capability of ZmHsf17-I exhibited a certain degree of decline (Figure 6). When severe HS was experienced, increased accumulation of ZmHsf17-II variants mitigated the potential adverse effects caused by ZmHsf17-I over-activation. Due to energy and resource limitations, plants have evolved a complex regulatory mechanism to coordinate growth and resistance under diverse stress conditions (Huot et al., 2014; Karasov et al., 2017). Unrestricted energy transfer in plants for enhancing resistance may lead to growth stagnation and death (Major et al., 2022). Excessive HSR is not always advantageous to survive upon prolonged high-temperatures (Wang et al., 2023). The plant response to a variety of heat regimes encompasses diverse mechanisms operating across a wide range of temperatures, timescales and cellular components (Hayes et al., 2021). At post-translational modification level, a dynamic ON/OFF switch controlling HSR was found in wheat undergoing prolonged heat exposure by suppressing the SUMOylation of TaHsfA1 (Wang et al., 2023). Triggering the continuous release of functional Hsf may not always be beneficial for the survival of plants under HS. For Arabidopsis at lethal temperatures, the immediate protection or controlled breakdown of cellular structures, instead of heat priming, was the dominant response process (Hayes et al., 2021).
5 CONCLUSION
Severe HS caused irreversible damages to plant growth, photosynthesis and membrane system (Sánchez et al., 2014). How plants appropriately control the tradeoff between growth and heat resistance in a variety of thermal regimes to avoid improper reallocation of resources remains obscure. Our study found that intron retention isoform ZmHsf17-II was accumulated in large amounts during severe HS. Protein interaction between intron retention variants ZmHsf17-II and full-length functional variants ZmHsf17-I interfered with the transcription activation activity of ZmHsf17-I. We deem that the alternative splicing of ZmHsf17 itself acts as a negative regulation of its thermotolerance function.
AUTHOR CONTRIBUTIONS
Xiulin Guo and Guoliang Li: conceptualization; Huaning Zhang, Ran Liu, Xiangzhao Meng, Zhenyu Ma and Ran Li: Methodology; Wenying Zhang, Shuonan Duan and Zihui Liu: formal analysis; Huaning Zhang and Xiangzhao Meng: Writing – Original Draft Preparation; Xiulin Guo and Guoliang Li: Writing – Review & Editing.
FUNDING INFORMATION
This work is supported by the Hebei Natural Science Foundation (C2021301038), the Basic Research Funds of Hebei Academy of Agriculture and Forestry Sciences (2021110301) and the HAAFS Agriculture Science and Technology Innovation Project (2023KJCXZX-HZS-3).
Open Research
DATA AVAILABILITY STATEMENT
The data, encompassing gene sequences, recombinant plasmids and transgenic lines supporting the findings of this study, are available from the corresponding author upon reasonable request.




