Insights into the molecular mechanism of Trichoderma stimulating plant growth and immunity against phytopathogens
Abstract
Trichoderma species have received significant interest as beneficial fungi for boosting plant growth and immunity against phytopathogens. By establishing a mutualistic relationship with plants, Trichoderma causes a series of intricate signaling events that eventually promote plant growth and improve disease resistance. The mechanisms contain the indirect or direct involvement of Trichoderma in enhancing plant growth by modulating phytohormones signaling pathways, improving uptake and accumulation of nutrients, and increasing soil bioavailability of nutrients. They contribute to plant resistance by stimulating systemic acquired resistance through salicylic acid, jasmonic acid, and ethylene signaling. A cascade of signal transduction processes initiated by the interaction of Trichoderma and plants regulate the expression of defense-related genes, resulting in the synthesis of defense hormones and pathogenesis-related proteins (PRPs), which collectively improve plant resistance. Additionally, advancements in omics technologies has led to the identification of key pathways, their regulating genes, and molecular interactions in the plant defense and growth promotion responses induced by Trichoderma. Deciphering the molecular mechanism behind Trichoderma's induction of plant defense and immunity is essential for harnessing the full plant beneficial potential of Trichoderma. This review article sheds light on the molecular mechanisms that underlie the positive effects of Trichoderma-induced plant immunity and growth and opens new opportunities for developing environmentally friendly and innovative approaches to improve plant immunity and growth.
1 INTRODUCTION
There has been a growing interest in understanding the intricate interactions between plants and beneficial microorganisms, particularly in the context of their promotion of plant growth and immunity against phytopathogens (Lyu et al., 2019; Konappa et al., 2020; Saia et al., 2020; Zhang et al., 2023). Among these beneficial microorganisms, Trichoderma is of interest for its strong host-friendliness and functionality and its presence as a non-toxic opportunistic plant symbiont in almost all soil types and rhizosphere ecosystems (Leylaie and Zafari, 2018; Sood et al. 2020). Research on Trichoderma has expanded beyond its traditional agricultural applications to encompass a broader range of industrial uses (Zhang et al., 2022a; Zhang et al., 2023). Currently, more than 375 species of Trichoderma have been identified and their molecular and morphological characteristics have been documented. The functions of many of these Trichoderma strains have been explored, all of which have been shown to possess a property known as “rhizosphere capacity,” the ability to colonize and grow in association with plant roots (Nascimento et al., 2022). This Trichoderma-plant association is categorized as mutualistic because fungi confer advantages to the plants, mainly related to resistance induction and growth promotion and received shelter and carbon source (Macías-Rodríguez et al., 2018).
Numerous studies have shown that Trichoderma stimulates plant growth through multiple mechanisms, including the production of plant growth-promoting substances, as well as the enhancement of nutrient uptake and stress tolerance in plants. Trichoderma-induced plant growth promotion is also frequently linked to the modulation of phytohormone signaling pathways, which control several facets of plant growth and development. Besides the growth promotion effect, the Trichoderma-triggered plant immunity against plant pathogens is characterized by the priming of plant defenses, which enhances the plant's capacity to initiate an effective and rapid defense signaling against pathogen attack. The regulation of genes, including those that support ROS generation, antimicrobial compounds and pathogenesis-related proteins' production and defense responses, forms the molecular basis of Trichoderma-triggered plant immunity.
Recent developments in molecular tools have given useful understandings of the complex molecular interplay between plants and Trichoderma. These approaches have made it easier to identify the main signaling pathways: the genes and proteins that control the Trichoderma-induced plant growth stimulation and immunity. Deciphering the molecular mechanism behind Trichoderma's induction of plant defense and immunity is essential for harnessing the full plant beneficial potential of Trichoderma. This review provides a comprehensive overview of the understanding of molecular mechanisms involved in Trichoderma-mediated plant immunity and growth promotion. The potential roles of the Trichoderma elicitors in these processes are discussed, and the key players and interconnected signaling networks are highlighted. By deciphering the molecular complexities of the Trichoderma-plant relationship, we can pave the path for developing novel biotechnological approaches to increase plant resistance and productivity.
2 COLONIZATION OF PLANT ROOTS BY TRICHODERMA
2.1 Mutual sensing of plant root and Trichoderma
Trichoderma root colonization is a prerequisite of the diverse interaction between plants and Trichoderma (Kredics et al., 2018). Several species of Trichoderma are known to colonize the root surface, inducing drastic changes in plant metabolism (Contreras et al., 2016). Chemical signals that facilitate the recognition process are released both by the plant and Trichoderma. For instance, tomato plants under pathogen attack secrete reactive oxygen species ROS and oxylipins, which have the ability to act as specific chemo-attractants to Trichoderma (Lombardi et al., 2018) (Figure 1A). Wheat plants were also reported to produce a variety of phenolics, including carbohydrates, lipids, terpenoids, and amino acids, in T. atroviride presence, which promotes the Trichoderma growth toward the plant (Lucini et al., 2019). Likewise, differences were observed in the types of carbohydrates released by the host plants under pre and post-colonization of roots by the Trichoderma. An increase in the release of carbohydrates was observed in plants before colonization, indicating that these chemicals may act as chemo-attractants, guiding the fungi toward the roots (Macías-Rodríguez et al. 2018).
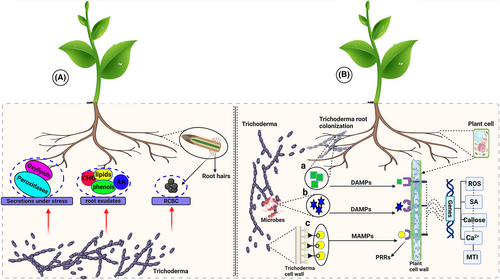
Trichoderma also produces cysteine-rich hydrophobins that facilitate recognition and root colonization (Tyśkiewicz et al., 2022). Hydrophobins, along with CAZymes, play key roles in Trichoderma-roots interaction (Samolski et al., 2012). Hydrophobin gene qid74 from T. harzianum mediates the fungal root colonization in tomato plants (Samolski et al., 2012). On the contrary, when the TasHyd1 hydrophobin gene was deleted from T. asperellum, it caused an altering hyphae wettability and drastic disablement in root adherence (Viterbo and Chet, 2010). The interconnection between Trichoderma-intracellular signaling pathways and host-induced signals might be the G-protein located in the cell membrane of the host plant that results in an increase in the mycoparasitism-related processes and is crucial in recognition of Trichoderma by the plant (Omann and Zeilinger 2010; Zhong et al., 2019). Besides, Trichoderma secondary metabolite clusters, which are essential for communication with a plant for efficient root colonization, were also discovered. These plant recognition clusters mediate early chemical communication between plants and fungus and are required for colonization (Schalamun et al., 2023).
Previously it was believed that Trichoderma colonizes mainly the elongation zone and root hair, but current findings reveal their ability to colonize root cap border cells (RBCs) as well (Jaroszuk-Ściseł et al., 2019). The RBCs play a vital role during the interaction between the host plants and microflora in the rhizosphere (Hawes et al., 2016). These cells are delivered into the rhizosphere from the external boundary of the cap cells during the process of root elongation. It is believed that the cells and the affiliated root efflux are composed of a rich source of nutrients and metabolites for rhizosphere microflora (Jaroszuk-Ściseł et al., 2009). Furthermore, RBCs determine the structure and composition of the rhizosphere community, playing a role as attractants for microbes and assisting the interaction with the plants, thereby facilitating root colonization (Jaroszuk-Ściseł et al., 2009; Jaroszuk and Kurek, 2008). Penetration of roots is assisted by cellulose-binding expansins released by the microbes that disrupt the integrity of plant cell walls through channel formation, which facilitates the entry (Sood et al., 2020; Kumar and Khurana, 2021; Ramírez-Valdespino et al., 2019). Proteins, like swollenins and cerato-platanins, having enhanced affinity for cellulose, are also released by Trichoderma, which are vital for the efficient colonization of plant roots (Brotman et al., 2010; Sánchez-Cruz et al., 2021). Efficient root colonization with vigorous plant growth-enhancing impact was observed in pepper (Capsicum annuum L.) roots when T. atroviride showed enhanced expression of the Taswo1 swollenin gene (Sánchez-Cruz et al., 2021). Moreover, there is a strong correlation between Trichoderma root colonization and the release of proteolytic, cellulolytic, xylanolytic, and pectinolytic plant cell wall degrading enzymes (PCWDEs), facilitating the root colonization of epidermis and cortex areas (Sood et al., 2020; Contreras et al., 2016; Chen et al., 2020). During the process of colonization, Trichoderma may alter its transcriptional profile either to save the energy needed for the enhanced transcription of proteins required for root colonization or to avoid stimulation of plant defenses. This was observed during the interaction between T. viren and maize roots, where T. virens repressed the transcription of genes across a broad spectrum of activity upon recognition of plant roots. During root colonization, T. virens increases the expression of genes necessary to assist the entry of T. virens into the maize root and established there (Malinich et al., 2019).
2.2 Plant response to Trichoderma colonization
The pattern-recognition receptors (PRPs) are activated by damage or microbe-associated molecular pattern (DAMP or MAMP) before the colonization of plant roots by Trichoderma sp. (Jacoby et al., 2017; Sood et al., 2020; Mendoza et al., 2018; Ramírez-Valdespino et al., 2019; Alfiky and Weisskopf, 2021). The structural elements of the Trichoderma cell membrane and cell wall (sterols, chitin, and β-glucans) behave like MAMPs (Alfiky and Weisskopf, 2021). The host cell wall oligomers released as a result of the hydrolytic action of Trichoderma on plant surface or the derivatives due to biocontrol activity of other microbes (antagonism) might act as DAMPs, which on recognition by PRRs, trigger MAMP-stimulated immunity (MTI) and hence activating plant resistance (Woo et al., 2023). A number of biochemical and physical responses are stimulated, limiting the fungal invasion to only a few cortical cell layers of host roots. These physical or biochemical responses include strengthening and modification of the host cell wall, production of antimicrobial secondary metabolites (SMs), and reactive oxygen species. Deposition of callose, oxidative burst, ROS, and Ca2+ signaling are triggered quickly after the attack; as at that stage, the plant could not comprehend it as a friendly assault. Trichoderma arrival is also responded with SA level enhancement (a vital plant hormone controlling root colonization) to restrict Trichoderma in the apoplastic area of the cortex and epidermis (Carrero-Carrón et al., 2018; Alonso-Ramírez et a., 2014) (Figure 1B).
2.3 Management of plant immunity against root colonization
In order to maintain a long-term mutualistic alliance, Trichoderma has developed several mechanisms to suppress plant immunity. During the host root colonization, Trichoderma strains exploit their ability to compete in the rhizosphere, as they can effectively tolerate increased levels of ROS produced as an initial defense response by the host plant (Morán et al., 2010; Montero et al., 2011; Liu et al., 2016). Trichoderma gets host tolerance through the suppression of plant defense responses by the activity of different effectors (Alfiky and Weisskopf, 2021). Significant insight into global proteome and transcriptome changes mediated by Trichoderma has revealed sophisticated interactive mechanisms that help root colonization of host plants by Trichoderma (Martínez-Medina et al., 2019). Components of the Trichoderma secretome were reported to induce susceptibility in host plants by suppressing initial plant resistance, which helps the Trichoderma to penetrate the root surface (Lamdan et al., 2015). In maize plants, 36% less proteins in secretome was observed, including important families of peroxidases and glycosyl hydrolases that participate in plant defense against the invasion of microbes during the root colonization process by T. virens. In response, T. virens releases a plethora of SMs and proteins, facilitating root colonization synergistically in a synchronized way. This includes the release of cell wall degradation enzymes to ease entry into the host roots, antioxidant production to neutralize defense-stimulated oxidative burst (comprising of hydrogen peroxide, hydroxyl radical and superoxide, and several SMs as well as effector-like proteins) that intervene with plant hormones homeostasis that plays vital roles in plant defense (Lamdan et al., 2015). Similarly, drastic changes have been reported in the transcriptome of Arabidopsis thaliana affiliated with transient suppression of the plant immune reactions and repression of defense genes in the roots during root colonization by T. asperelloides (Brotman et al., 2013). Furthermore, high resistance has been observed in Trichoderma sp. against phytoalexins, phenols, aglycones, terpenoids, and flavonoids released by plants during infection caused by foreign micro-organisms. It facilitates the swift colonization of this fungi and its alteration of the root system of host plants (Błaszczyk et al., 2014; Tyśkiewicz et al., 2022). The compounds like maleic acid, citric acid, and oxalic acid released by Trichoderma may also assist root colonization (Zhang et al., 2014).
3 MECHANISM OF TRICHODERMA-INDUCED PLANT GROWTH
3.1 Trichoderma modulates phytohormones signaling pathways for plant growth
Phytoregulators and phytohormones produced by microbes are among the direct mechanisms that kick in the quick and durable soil colonization with microbes promoting the growth and development of host plant (Tamayo and Osorio et al., 2017; Zhou et al., 2018). The mechanism of plant growth stimulation by Trichoderma invasion is through the release of compounds having a direct impact on several plant hormone-mediated signaling pathways (Garnica-Vergara et al., 2016) (Figure 2A). A number of Trichoderma spp. have been characterized with the potential to release phytohormones (auxin, gibberellin, and ethylene) and phytoregulators that are vital for plant growth by controlling the biosynthesis pathway of phytohormones in host plants (Jaroszuk-Ściseł et al., 2019; Alfiky and Weisskopf, 2021; Ozimek et al., 2018). Indole-3-Acetic Acid (IAA), an auxin phytohormone, is vital for the majority of the procedures required for the appropriate development and growth of plants (Fu et al., 2015; Nieto-Jacobo et al., 2017). At reduced concentrations, IAA promotes root length, whereas high IAA concentration is vital for the proper production of adventitious and lateral roots (Jaroszuk-Ściseł et al., 2019). Inoculation of Trichoderma spp. capable of releasing IAA (T. virens and T. atroviride) increased the total biomass and number of lateral roots of A. thaliana seedlings. In a study investigating the roles of the phytohormone IAA released by Trichoderma on Arabidopsis growth and development, Contreras et al. (2009) concluded that Arabidopsis plants mutated in genes regulating auxins transport or important in signaling pathways (AXR1, EIR1, BIG, and AUX1) were non-responsive to the favorable impacts of T. virens in contexts of fungi- or IAA-triggered root branching and shoot growth. The understanding of IAA and related auxin-like compounds by changes induced in the root tips in auxin-sensitive marker DR5:GUS has been related to increases in lateral root production and cell proliferation. Moreover, the exogenic employment of IAA itself or/and its precursor (indole-3-acetaldehyde) to Arabidopsis plants has resulted in enhancing a concentration-dependent accumulation of foliar biomass and promoting plant growth. A number of other crops, including tomato (Bader et al., 2020), sorghum (Saber et al., 2017), bean (Hoyos et al., 2009), wheat (Illescas et al., 2021), and pepper (Sánchez-Montesinos et al., 2020) revealed growth improvement in plants as a result of IAA action procured from the Trichoderma species. Environmental factors, including nutrient status and pH, are believed to be responsible for regulating the IAA production in Trichoderma strains (Nieto-Jacobo et al., 2017).
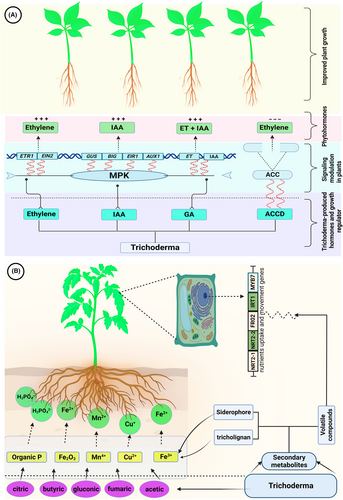
Another phytohormone, ethylene (ET), is important in controlling several different physiological activities in plants, partially through complicated and sophisticated interactions with different phytohormones (Dubois et al., 2018). It has been proclaimed that T. atroviride enhances growth in plants via ET emission in a reaction that encompasses a unique molecular mechanism conglomerating a network of signals governed by ETHYLENE INSENSITIVE 2 (EIN2), a modulator of ET response. Release of fungal ET is involved in this mechanism, which stimulates the production of root hairs and impacts the formation of lateral roots through modulation of the ET receptors ETR1 and EIN2 via MITOGEN-ACTIVATED PROTEIN KINASE 6 (MPK6) regulation. It is interesting to note that T. atroviride and its IAA metabolite have been found to promote MPK6 efficiency, which in turn regulates both ET and auxin signaling pathways through an information exchange mechanism (Contreras et al., 2015). Although phytohormones play a crucial role in regulating plant growth, their excess can harm plants and Trichoderma assists the plants in maintaining the phytohormones' levels. For example, an increased amount of ET can restrict root elongation, eventually resulting in the death of plants (Glick, 2014; Nascimento et al., 2014). Trichoderma spp. have the capacity to release the ACC-deaminase enzyme (ACCD), an enzyme that cutdown the ethylene precursor (ACC) into ammonia and α-ketobutyrate, which assists in adjusting the level of plant ET; hence promoting plant development and growth (Todorovic and Glick, 2008). Several species of Trichoderma are known to have the potential to release ACCD, which enhances plant growth (Jaroszuk-Ściseł et al., 2019; Saravanakumar et al., 2018a; Zhang et al., 2020). Rauf et al. (2021) have reported increased growth in wheat due to the beneficial influence of ACCD released by T. asperellum. Furthermore, ACCD produced by T. harzianum beneficially impacted maize seedling growth and germination in a greenhouse environment (Zhang et al., 2020). The ACCD enzyme encoding gene (Tas-acdS) was characterized in Trichoderma genomes. Gene silencing in T. asperellum resulted in a reduced capacity to promote root growth in canola crop (Viterbo et al., 2010). By changing the plant hormonal homeostasis, Trichoderma modifies the physiology and enhances the fitness of the host plant (Martínez et al., 2014).
Gibberellins (GAs) are major phytohormones whose deficiency has a negative impact on proper seed germination, shoot and root growth in plants, expansion of leaf, and development of flowers (Ozimek et al., 2018). Several research findings reported the potential of several species of Trichoderma to produce GAs (Jaroszuk-Ściseł et al., 2019; You et al., 2016; Kamaloy et al., 2018). It has been reported that inoculation of T. koningiopsis isolates in tomato resulted in significant growth improvement due to the release and activity of GA (You et al., 2016). Furthermore, T. asperellum releasing GA enhanced the amount of these hormones in cucumber seedling leaves (Zhao et al., 2015). The deposition of GA synthesized by T. harzianum along with IAA was reported to improve plant growth (Kamalov et al., 2018). Moreover, the release of GA by the strains of Trichoderma was favorably linked with the production of IAA phytohormone and ACCD in wheat seedlings (Jaroszuk-Ściseł et al., 2019). Few Trichoderma spp. are known to produce a mixture of growth hormones (gibberellin, ABA, IAA) that stimulate the growth of seedlings and roots (Zhao et al., 2015; Jaroszuk-Ściseł et al., 2019). Other secretions from different Trichoderma spp., like histone deacetylase (HAD), were also reported to improve plant growth (Estrada et al., 2019).
3.2 Improved uptake and accumulation of nutrients in plants
Trichoderma-plant interactions revealed that one of the aspects of triggering plant growth is the result of improved uptake of biogenic elements. Association of Trichoderma in rhizospheres are reported with an effective absorption of nutrients, like metallic Zn, MnO2, and rock phosphate, especially Ca3(PO4)2, after their early solubilization (Li et al., 2015; Singh et al., 2018a, b). The T. harzianum inoculated tomato plants have been reported to enhance Mg2+, Ca2+, P, and K+ concentrations. The enhanced concentrations of these nutrients are beneficial for various shoot parameters, including diameter, height, dry and fresh weights (Azarmi et al., 2011). Li et al. (2018) reported that pre-inoculated tomato plants with T. asperellum resulted in improved nutrient (K, P, Zn, K, and Mg) absorption. In another study, K+/Na+ ratio was improved in cowpea by applying T. longibrachiatum (Liu et al., 2023). Moreover, in tobacco plants, an increase in the production of NO, levels of total nitrogen, and piling up of cytosolic Ca2+ have been found in response to the addition of T. asperellum. Trichoderma improves the ability of nutrient absorption and its transport by modulating their translocation genes in the host plant. T. asperellum activates various genes in tobacco plants that translate nitrate transporters (NRTs), like NRT 2.1 and NRT 2.2. All these alterations were reported to be linked with Trichoderma-mediated growth enhancements, thus revealing that T. asperellum improves the utilization efficiency of nitrogen in tobacco plants (Singh et al., 2018a). Furthermore, the growth of roots and shoots in reaction to Trichoderma inoculation led to improved uptake of Na, Cu, Zn, and other micronutrients (Li et al., 2015).
3.3 Mechanism of increasing bioavailability of nutrients to plants
Trichoderma has a vital role in increasing the growth in plants through improved solubility of nutrients present in the rhizosphere (Cu2+, Fe3+, ZnO, Mn4+, phosphate); hence augmenting their absorption by plants (mainly phosphorus, potassium, nitrogen, and other microelements) and boosting plant growth and yield (Contreras et al., 2016; Jaroszuk-Ściseł et al., 2019; Sood et al., 2020) (Figure 2B). Among the different nutrients required by plants, phosphorus (P) is present in the soil in a form having the least bioavailability to the host plants (Menezes et al., 2018). It was demonstrated that the experimental application of Trichoderma in soil improved the solubilization of inorganic phosphate as a result of exogenous phytase activity (Saravanakumar et al., 2013; Yin et al., 2021) and soil acidification due to citric, butyric, acetic, and fumaric acids synthesis (Scervino et al., 2010). Improved growth in different plants (Borges et al., 2015; Paul and Rakshit, 2021) has been correlated with the phosphate solubilization potential of Trichoderma. Some strains of Trichoderma strains have the ability to synthesize gluconic acid and coumaric acid and can increase the acidity of the environment to improve solubilization of micronutrients, for example, Mn4+, Fe3+, and Mg2+, making them available for utilization by the plant (Shoresh et al., 2010; Li et al., 2018) (Figure 2B).
Iron deficiency, because of its insoluble form for plant uptake, is also a major constraint for proper plant growth. T. asperelloides application increased iron availability by up to 30% in the rhizosphere via siderophore production. Siderophores are high-affinity iron-chelating compounds secreted by microbes to solubilize Fe3+. T. asperellum treated roots of cucumber not only enhanced siderophore contents and Fe2+ in soil but also improved the activity of Fe3+ chelate reductase that helps in Fe3+ oxidation (Harman et al., 2004). Moreover, Colla et al. (2015) have shown the production of two types of siderophores (catechol and hydroxamate) by the T. atroviride that cause Fe3+ reduction. These findings reveal that the application of Trichoderma in soil facilitates Fe uptake of host plants by reducing Fe3+ to Fe2+, which ultimately enhances its solubilization and absorption. Besides siderophores, Trichoderma also secretes other metabolites that help nutrient availability and improve their uptake from the soil by plants. A polyketide, tricholignan-A, secreted by T. harzianum, acts as a redox-active ortho-hydroquinone having the ability to reduce Fe3+ (Vinale et al., 2014). Similarly, the synthesis of tricholignan A assists the growth of plants under Fe deficiency conditions (Chen et al., 2019). Trichoderma was also found to enhance the expression of nutrient uptake marker genes, assisting the plants in coping with low nutrient uptake. For example, VOCs synthesized by T. harzianum and T. asperellum induce plants' iron absorption by stimulating the expression of iron transport and regulation genes IRT1 and FRO2. They also express MYB72, encoding for a factor important for the transcription of genes having the double role of regulating iron uptake and controlling defense responses (Martínez-Medina et al., 2017b).
4 ACTIVATION OF PLANT IMMUNE RESPONSE BY TRICHODERMA AND PHYTOPATHOGENS
4.1 The basic plant immunity triggered by Trichoderma and phytopathogens
Pattern-recognition receptors (PRRs; present on plant cell walls) identify the structural components of the pathogen, at the time of infection, as pathogen-associated molecular patterns (PAMPs). This identification activates the host immunity, which is called pathogen-triggered immunity (PTI). The PTI is amplified and transmitted to an array of mitogen-activated protein kinases that change the exogenous signal into endogenous reactions, reprogramming the transcription that results in fortification of the plant cell wall, enhances concentrations of endogenous calcium, synthesis of reactive oxygen species, production of anti-microbial SMs and PRPs, and increase of phytohormones related to defense (such as ET, JA, and SA). Pathogens deploy relevant effector proteins in the cell cytoplasm of the host to overcome and repress PTI. The nucleotide-binding site leusine-rich repeat protein (NLR) of the cytoplasmic receptors then identifies cytoplasmic effector placed by pathogens. This perception activates effector-triggered immunity (ETI), which is quicker and stronger than PTI. It is characterized by cell death and early oxidative burst due to hypersensitive reactions restricting the attack of pathogens (Figure 3).
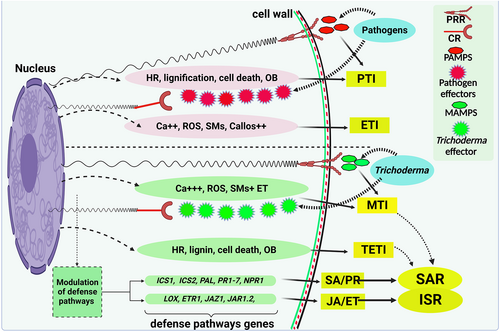
The plant immune response induced by Trichoderma is much quicker and more intense compared to ETI and PTI. Trichoderma-triggered immunity (TTI) is initiated when the plant's PRRs recognize Trichoderma-originated DAMPs and MAMPs, which stimulate MAMP-triggered immunity (MTI), a type of immunity that is stronger than PTI or ETI, hence providing resistance to the host plant (Woo et al., 2023). Trichoderma also releases a cascade of effectors and metabolites like LysM protein Tal6 (Romero-Contreras et al., 2019), xylanase EIX (Ramírez-Valdespino et al., 2019), the peptaibol alamethicin, cerato-platanin EPL1, and the terpenes trichodiene (Malmierca et al., 2015) and harzianum A (Ramírez-Valdespino et al., 2019) in the apoplastic area of the cortex and epidermis. Those effectors are identified by host plant NLR receptors, which ultimately triggers Trichoderma effector-triggered immunity (TETI) (Carrero-Carrón et al., 2018; Alonso-Ramírez et al., 2014). Nonetheless, it is not yet known how the NLR receptors of plants correlate with the Trichoderma-generated effectors. NLR receptors in tomato plants are over-expressed in the leaves proteomes where roots of the host plants are invaded with Rhizoctonia solani and T. atroviride (Marra et al., 2006), and NLR receptors were also expressed by harzianic acid produced by T. harzianum (Manganiello et al., 2018). NLR receptors are overexpressed in the maize leaf proteome when host roots are inoculated with T. afroharzianum. Trichoderma effectors are not thought to act as ligands for NLR, the subsequent processes and signal transduction triggered by Trichoderma interaction, as well as their effects on host plants, are subjects of active and in-depth research (Morán-Diez et al., 2021; Ramírez-Valdespino et al., 2019).
The host plant induces a “transcriptional memory” when the Trichoderma-triggered signals progressively disappear. In this case, the cells previously stimulated with certain stimuli exhibit an intensified expression of the genes when re-stimulated subsequently (Liu and Avramova, 2016). The ability of defense induction is heritable, prompting subsequent memory levels named ‘heritable priming’, which can be transferred to the offspring. The level of expression of defense response is much stronger in the next-generation plants compared to the seedlings of non-induced plants. The beneficent impacts of Trichoderma on host plants are regulated by molecular networks, which shape the initial as well as long-term systemic reactions, crafting the metabolic exchanges between defense response and plant (Morán-Diez et al., 2021). During the nematode-tomato-Trichoderma interrelationship, the host resistance to root-knot nematodes and growth enhancement is inherited by the tomato seedlings without jeopardizing the defense status in the offspring of tomato plant while responding to the invasion of nematode (Medeiros et al., 2017).
The resistance developed in response to localized attacks systematically extends to the organs that are not damaged. The vasculature serves as a main route for diffusible signals that help in communication between roots and shoots, thereby assisting immunity in distant plant parts (Van Bel and Gaupels, 2004). For example, the xylem path was reported to transport signals from roots to shoots for triggering systemic resistance (Constantino et al., 2013). The mobile signaling molecules α-ketol of octadecadienoic acid (KODA) and 12-oxo-phytodienoic acid (12-OPDA) are implicated in Trichoderma-triggered ISR against maize anthracnose disease (Wang et al., 2019).
4.2 Trichoderma-triggered Induced Systemic Resistance and Systemic Acquired Resistance
Recent evidence from several research findings reveals that Trichoderma-induced defenses (TETI and MTI) against different pathogens are regulated by ET, SA, and JA (Woo et al., 2023). The increased concentrations and accumulation of Trichoderma-induced elicitors are affiliated with the induction of the immune response in the host plant, which comprises induced systemic resistance (ISR) and systemic acquired resistance (SAR) (Tyśkiewicz et al., 2022; Kumar and Ashraf, 2017). ISR is a vital mechanism in which Trichoderma residing in the rhizosphere stimulates the defense response in host plants against hemibiotrophic and biotrophic pathogens (Tyśkiewicz et al., 2022; Kumar and Ashraf, 2017; Nawrocka and Małolepsza, 2013). It is characterized by the release of ET and JA, which may cause rapid aging, chlorosis, and ultimately death of invaded tissue (Wang et al., 2021; Ding and Ding, 2020; Yang et al., 2015; Kumar and Khurana, 2021; Monfil and Casas, 2014). It has been reported that roots of cucumber plants inhabited with T. asperellum had significantly higher amounts of ET and JA within 24 h (Shoresh et al., 2005), quite in agreement with the idea that Trichoderma triggers ISR response (Pieterse et al., 2014). The ISR signals, JA, and ET are also important for generating several defense molecules like peroxidases, polyphenol oxidase, β1-3glucanase, catalase, β1-4-glucanase, chitinases and N-acetylglucosaminidases (Peng et al., 2021; Vargas et al., 2009). Trichoderma may also directly enhance the production of these defense molecules (Zhan et al., 2023). The first MAMP signal (xylanase) detected from Trichoderma was also reported for ET-modulation activity, triggering defense reactions in tobacco and tomato (Rotblat et al., 2002).
An alteration in the gene expressions, controlled by JA and ET, has been reported in many plant species invaded with Trichoderma. For instance, lipoxygenases (LOX) oxidize the polyunsaturated fatty acids, and the oxidation product is then transformed into JA precursor, i.e., 12-oxo-phytodienoic acid (12-OPDA), which is then transformed to JA (Feussner and Wasternack, 2002; Andreou et al., 2009; Borrego and Kolomiets, 2016). The lox mutants are unable to synthesize JA, which weakens maize immunity (Christensen et al., 2013; Gao et al., 2008). It is reported that T. asperellum regulates the expression of LOX and ETHYLENE RESPONSE 1 (ETR1) in cucumber (Shoresh et al., 2005). Trichoderma also assists the plants by countering pathogen-suppressed host defense response through JA regulation. For example, T. harizanum uplifted the JA-regulated defenses involving the expression of MULTICYSTATIN and PROTEINASE INHIBITOR II (PI-II) and hence resisted the downregulation of the JA-induced defense response activated by the nematodes, thereby inversely affecting the fecundity and galling by M. incognita (Martínez-Medina et al. 2017a). Analogous research findings have been shared by Leonetti et al. (2017), reporting T. harzianum-induced ET and SA synthesis in M. incognita-infected tomato plants.
The synthesis of SA and pathogenesis-related proteins (PR) is associated with the SAR response and restricts the establishment of pathogens within the plant by stimulating programmed cell death (Kumar and Khurana, 2021; Monfil and Casas-Flores, 2014; Yang et al., 2015; Ding and Ding, 2020; Wang et al., 2021). SA is synthesized in plants using the isochorismate synthase (ICS) route, beginning with chorismate (Lefevere et al., 2020). ICS genes (ICS1, ICS2) affiliated with pathogenesis-associated proteins, like PR-1-7, are among the important genes involved in the SA pathway in many plant species interacting with Trichoderma when attacked by different pathogens (Ali et al. 2018). SA activates a number of PR genes, the proteins of which have antimicrobial properties (Boamah et al., 2023). The NONEXPRESSOR OF PR GENES1 (NPR1) protein is involved in the regulation of defense genes by SA, such as PR-1. (Uquillas et al., 2004). T. hamatum stimulates the expression of PR-1 and PR-7 in tomato plants infected with TMV (Abdelkhalek et al., 2022). Martínez-Medina et al. (2017a) demonstrated T. harzianum-regulated ISR immunity in tomato plants against Meloidogyne incognita, which is achieved through SA-induced defense mechanisms employing the expression of PR-P6 and PR-1a to restrict the infection of host plant roots by M. incognita. In tomato plant roots where the root-knot nematode completes its life cycle, Trichoderma helps to reprogram immune response through JA and SA-induced defense mechanisms, depending on the infective stage of nematode (Medeiros et al., 2017; Martínez-Medina et al., 2017a).
The defense enzyme PHENYLALANINE AMMONIA-LYASE (PAL) is necessary for the biosynthesis of phenylpropane unit, which is a component of lignin and thus contributes to cell wall lignifications and resistance to pathogen invasion (Chen et al., 2020). Upon biotic stress, the plants increase lignification and use it as an adaptive mechanism to limit pathogen entry (Sarma et al., 2015; Chen et al., 2020). Trichodermainduced plant immunity by enhancing PAL activities in plants is evident from several studies. A substantial increase in the level of PAL activities is correlated with improved disease resistance in rice and tomato (Singh et al., 2016; Wang et al., 2021). Through transcriptional analyses, Wang et al. (2023) found that T. asperellum improved C. canephora resistance to anthracnose by up-regulating the expression of phenylpropane biosynthesis pathway genes (including PAL). Moreover, Zhang et al. (2022b) reported that T. asperellum is involved in the regulation of the watermelon phenylpropane synthesis pathway against Fusarium oxysporum. The defense mechanism of T. longibrachiatum in cucumber plants against Botrytis cinerea was reported to be due to the phenylalanine pathway synthesis of SA because of the enhanced PAL expression both at the gene and protein level (Yuan et al., 2019).
4.3 Role of different Trichoderma elicitors as regulators of plant immunity
4.3.1 Trichoderma enzymes
The first recognized MAMP of Trichoderma origin was an enzyme xylanase associated with T. viride that acts as an elicitor for defense activation in tobacco and tomato plants (Rotblat et al., 2002). On account of its ET-producing character, it has also been named ET-inducing xylanase (Eix). The LeEix1 and LeEix2 (pattern recognition receptors) of Eix in tomato crops have exogenous leucine-rich repeat (LRR) domains located in the plasma membrane (Ron and Avni, 2004; Hermosa et al., 2013). The xylanase-regulated systemic resistance activated through the ET synthesis and extended by ISR in the leaves is accomplished through S-Adenosyl methionine synthesis from 1-aminocyclopropane-1-carboxylic acid (ACC), which is an ET precursor (Mathur et al., 2023). The pectinases (such as EPGs and endopolygalacturonases) help in securing entry into the cell/tissues of the host plant during the pathogen-plant interaction. The systemic resistance in host plants is stimulated by the elicitor action of EPGs associated with the generation of oligogalacturonides oligomers, which activate defense mechanisms (Federici et al., 2006). The elicitor action of the polygalacturonase is confirmed by using polygalacturonases inhibition protein (PGIP), which restricts the pectinase activity and consequently blocks the production of oligomers that may not further behave as elicitor (D'Ovidio et al., 2004). The two isomorphs of polygalacturonase, tvpg1 and tvpg2, are required for the induction of polygalacturonase-inhibitor gene Lepgip1 in the interaction of tomato with T. virens (Sarrocco et al., 2017). The immune system is strongly hindered by the pre-treatment of tomato plants with T. virens tvpg2 mutant against the invasion of B. cinerea, hence depicting tvpg2 gene role in triggering ISR in tomato plants (Sarrocco et al., 2017). Phosphopantetheinyl transferases (PPTases) belong to a superfamily of enzymes required for the production of numerous compounds in eukaryotes and prokaryotes. In biosynthetic pathways, PPTases also stimulate carrier proteins. Biosynthesis of siderophore (Seidle et al., 2006; Ollinger et al., 2006), surfactin activating ISR in bean and tomato plants (Ongena et al., 2007), and antibiotics are induced by PPTases. An enhanced expression of pLox2:uidA and pPr1a:uidA markers was recorded in A. thaliana treated with wild-type T. virens having PPTase, which correlated with increased camalexin, SA, and JA accumulation, as well as resistance against B. cinerea (Velazquez-Robledo et al., 2011). Trichoderma-provoked cellulases are also known to stimulate defense reactions. Cellulase from T. harzianum enhanced the defense of maize plants against Fusarium graminearum by activating ET and JA (Saravanakumar et al., 2018b).
4.3.2 Trichoderma proteins and peptides
Cerato-platanins (CPs) are low molecular weight cysteine-rich proteins. Trichoderma CPs, such as ELICITING PLANT RESPONSE-LIKE (Epl1), play a role in the development of local and systemic plant resistance (Gao et al., 2020). Upon B. cinerea and tomato interaction, Epl1 from T. harzianum increased the defense priming at the initial phase of interaction, stimulated the defense mechanism through SA signaling in the host, and modified the pathogen virulence (Gomes et al., 2017). Generally, JA/ET and SA signaling interact antagonistically in plants. However, the synergistic action of JA/ET and SA in plants has been reported because of the Epl1 activity, suggesting that JA/ET and SA may interact synergistically (Cheng et al., 2018; Sharma et al., 2022). The simultaneous and additive induction of both ISR and SAR in Nicotiana benthamiana enhanced immunity against the Tomato mosaic virus (Cheng et al., 2018). T. formosa in tobacco plants released an Epl1-analogous polypeptide, which identically activates systemic resistance (Cheng et al., 2018). T. virens has also been reported to release non-ribosomal peptides of 11, 14, and 18 residues, out two synthetic 18-residue-amino acid peptaibols isomorphs (TvBI and TvBII), which stimulate systemic resistance in cucumber against phytobacteria P. syringae (Viterbo et al., 2007). Regulation of elicitors of Trichoderma is achieved in several distinctive ways depending on the type of fungal species. EPL1/SM1 of T. atroviride and T. virens activates defense mechanisms in host plants. However, SM1 of T. harzianum downregulated the defense mechanisms in host plants, permitting only the colonization of roots (Lopes et al., 2022). These findings reveal that type and species elicitor are key factors to look upon while stimulating TISR in host plants; hence, it is important to look deeply into the elicitors and activation pathways of host plants in defense mechanisms.
Sm1 (a CP protein), representing a secretary proteins group, behaves like an elicitor. T. virens Sm 1 is able to induce defense mechanisms both in monocot and dicot plants (Buensanteai et al., 2010). It is a non-enzymatic proteinaceous elicitor that exists either in monomer or dimer form (Djonovic et al., 2006; Vargas et al., 2008). It has biological importance in rice and cotton, triggering SAR as an effector (Djonovic et al., 2006); on the other hand, in corn, it plays an important role in ISR, revealing signaling through ET / JA / volatile substances (Djonovic et al., 2007). The colonization of corn plant roots with Sm1-deficient T. virens strain resulted in reduced resistance against C. graminicola (Nawrocka and Małolepsza, 2013). The Sm1-Tvi elicitor of T. virens is a strong activator of defense mechanisms in rice, maize, and cotton (Buensanteai et al., 2010; Freitas et al., 2014; Salas-Marina et al., 2015). Hydrophobins, which are low molecular weight cysteine-rich secretory proteins, can behave like elicitors for immunity induction in plants (Linder et al., 2005; Bayry et al., 2012). Its interaction in plant roots is through UBL/Ubiquilin 1-like protein receptor modulating Trichoderma-induced ISR (Yu et al., 2020). Systemic resistance against B. cinerea invasion in tomato plants is induced by HYTLO1, encoding hydrophobin of Trichoderma (Ruocco et al., 2015). Hyd1 protein from Trichoderma interacts with Ubiquilin1-like protein (UBL) in maize and this interaction stimulates the resistance development, through ET/JA signaling pathways, against C. lunata in maize plants (Yu et al., 2020).
4.3.3 Volatiles of Trichoderma as elicitor of plant immunity
The volatile substances produced by Trichoderma behave like MAMPs, improving defense reactions and shielding the plants against pathogen attacks (Nawrocka et al., 2018; Estrada-Rivera et al., 2019; Zhang et al., 2022). 6PP, a metabolite having dual roles in plant growth and defense, is one of the most prevalent VOCs released by Trichoderma (Kottb et al., 2015; Garnica-Vergara et al., 2016). The application of 6-PP to maize seedlings caused enhanced activities of defense enzymes such as B-1,3-glucanase, peroxidase, and polyphenol oxidase, which linked with the control of blight disease (El-Hasan and Buchenauer 2009). Kottb et al. (2015) reported the upregulation of defense-related genes in A. thaliana by applying 6-PP of T. harzianum, which correlated with the reduction in symptoms caused by B. cinerea and Alternaria brassicicola. Another VOC trichothecenes from T. arundinaceum caused the upregulation of SA-responsive genes in tomato against B. cinerea infection (Malmierca et al., 2015). T. virens released a mixture of VOCs that express the JA-responsive marker LOX2, along with the accumulation of free H2O2 and JA (Contreras-Cornejo et al., 2014). It has also been shown that VOCs produced by T. harzianum and T. asperellum stimulate JA-associated defense mechanisms and induce the expression of the genes PLANT DEFENSIN 1.2 (PDF1.2) and VEGETATIVE STORAGE PROTEIN 2 (VPS2) in shoots of Arabidopsis, improving the resistance against B. cinerea invasion (Martínez-Medina et al., 2017b). In another study, the induction of enhanced activity of defense enzymes in tomato plants by Trichoderma metabolites was linked to their tolerance to bacterial wilt disease (Khan et al., 2023).
5 TRICHODERMA-INDUCED BALANCING OF PLANT GROWTH AND IMMUNITY
The interaction between Trichoderma and plant is multi-dependent and highly dynamic because both members of this relationship synthesize metabolites that work collectively and because their biosynthesis is dependent on various abiotic and biotic factors and is varied over time. This interaction shapes the specific homeostasis and hormone regulation system. Trichoderma-produced phytohormones and the enzyme that controls plant ET levels are primarily responsible for this regulation (Jaroszuk-Sciseł et al., 2019). The Trichoderma-induced balancing of plant growth and immunity can be derived from the cross-communication between SA, JA, ET, and IAA and the response pathways of other growth hormones (GA and ABA) (Hermosa et al., 2012). Jasmonate ZIM-domain (JAZ) proteins regulate JA signaling; they function as JA receptors and, at reduced levels of JA, act as transcriptional repressors of gene expression related to JA response. Elevation in JA level resulted in the degradation of JAZ proteins, which quickly triggered a slew of JA responses (Kazan and Manners, 2012). The MYC2, a multipurpose transcription factor, is released after JAZ degradation to stimulate the expression of JAZ-induced genes. JAZ proteins suppress MYC2, which causes the upregulation of JA-responsive genes but downregulates JA/ET-responsive genes. MYC2 participates in the suppression of early MTI reactions (Hermosa et al., 2013).
The defense is suppressed and growth is promoted when under favorable conditions. ACCD activity limits the ACC availability required for ET synthesis, which in turn downregulates plant defense and induces growth through GA signaling. GA promotes the growth by stimulating the breakdown of a set of plant growth repressor proteins (DELLAs), while the defense is obstructed by suppressing the transcriptional activity of MYC2 through its binding to JAZ proteins, which downregulates the JA-defense (Navarro et al., 2008). The ET and IAA biosynthesis in plant roots is regulated in a reciprocal manner. Trichoderma-produced IAA, as an exogenous auxin, helps plants grow, develop lateral roots, and increase ET production. The latter, in turn, activates enhanced ABA production, which is frequently linked to plant growth as well as the expression of defense genes by activating JA biosynthesis (Pieterse et al., 2009) (Figure 4).
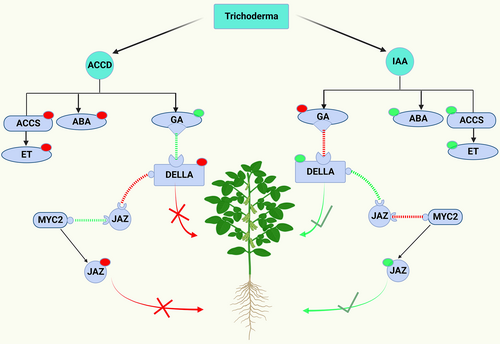
The defense is stimulated, and the growth is suppressed when the plant is challenged by Trichoderma, and the transcriptional activity of MYC2 is made possible by the JA-induced JAZ protein degradation. This quickly helps to activate JA signaling, which further suppresses growth by reducing DELLAs' breakdown and shifting the balance towards a defense mechanism (Kazan and Manners, 2012). Due to the competition between MYC2 and DELLAs for JAZ binding, the JA-GA signaling communication is anticipated to establish equilibrium within a brief colonization stage, which will be identified by reduced expression levels of defensive genes (Kazan and Manners, 2013). In addition to the involvement of Trichoderma to host defense and growth by maintaining the appropriate level of IAA and ET, the antagonistically mutual crosstalk interactions between DELLA-JAZ balance and GA-JA signaling, both of which are controlled by MYC2, have been suggested as switching features that help the plants to resolve the conflict of whether to prioritize investing in growth or defense (Hermosa et al., 2012; Brotman et al., 2012).
Mitogen-Activated Protein Kinases (MAPKs) are enzymes involved in cellular signaling and they also play a role in balancing growth and defense in Trichoderma-plant interaction. Trichoderma inoculation in Arabidopsis enhances MPK6 expression, increasing proton ATPase activity for growth promotion (Guo et al., 2019). The MPK3/6 cascade stimulates phytoalexin biosynthesis, leading to ET signaling-dependent plant immunity (Meng et al., 2013). It also regulates the WRKY transcription factors involved in defense gene expression (Birkenbihl et al., 2012) and induces the balance between SA- and JA-linked responses in bean plants during interaction with T. velutinum (Mayo et al., 2016). This suggests that MPK3/6-induced signal transduction is not only required to activate defense responses but also establishes a molecular node that fine-tunes the phytohormone signiling that gives rise to a balance between defence and growth. Histone alterations at WRKY40 and MYB77 promoters in Arabidopsis, post-Trichoderma interaction, influence long-term balance in growth and defense (Roosjen et al., 2018). This multifaceted process indicates the plant approaches in dealing with growth-defense trade-offs upon Trichoderma interactions. The Trichoderma-colonized roots of Arabidopsis plants showed an undulating expression pattern in different MYB genes; the highest expression was shown by MYB15 and MYB51 at 24 h. At this time point, the growth-regulating MYB77 gene exhibited its lowest expression level, restricting lateral root growth while augmenting defenses, which would validate the Trichoderma capability to balance the costs of plant defense and growth (Hermosa et al., 2013).
The corresponding actions of methylation and demethylation on H3K4me3 at the WRKY40 promoter are possibly responsible for the extended downregulation of this transcription factor, thus influencing both SA and ABA responses. This regulation assists in balancing defense and plant growth. In the interaction between Trichoderma and Arabidopsis, the MYB77, induced by auxin, showed downregulation 24 hours post-inoculation (Brotman et al., 2013), which therefore restricts the development of lateral roots. After a pathogen attack, the constant MYC2-dependent gene activation aligns with the continuing deacetylations noticed at the MYB77 promoters (Roosjen et al., 2018). This response supports the plant to control auxin-related responses, moving the balance to defense as required.
6 CONCLUSIONS
In recent years, several key publications on the mutualistic interaction of Trichoderma and plants have unveiled the variety of action mechanisms involved in this relationship. These findings have increased our understanding of this intricate association, which probably suggests the discoveries of several new elicitors and effectors molecules that activate and regulate plant response, producing beneficial effects by the fungus, such as the induction of plant growth as well as protective effects against biotic challenges. Several plant receptors playing a specific recognition role for Trichoderma effectors were also discovered. Despite the presence of significant information related to this beneficial interaction, there are several aspects remaining largely unknown, such as the molecules and their biosynthesis factors that contribute to Trichoderma-plant and plant-Trichoderma recognition. Furthermore, the discovery of bidirectional cross-kingdom communication facilitated by sRNAs between plants and fungi raises the query of whether the plant or the Trichoderma employs sRNAs to regulate the expression of its partner's genes in order to develop a mutualistic association.
Trichoderma's induction of growth and defense trade-offs in plants can be revealed by elucidating the elicitors/effectors interactions with plant hormonal pathways, most notably SA, JA, and ET, for which key genes have been discovered. These genes stand out as prospective targets for biotechnological applications that involve the molecular regulation of cellular responses and root fungal colonization. Generally, whether the Trichoderma fungi naturally exist in the root microbiome of the plant or is applied in the field, they can act as important probiotics to enhance plant adaptability and defense against a variety of biotic stressors. Significant progress has been accomplished in recent years to discover the signaling of core hormonal pathways (SA, JA, ET) triggering plant immunity. Understanding in detail the process that results in the induction of plant immunity could be achieved by searching more on the factors that participate downstream and upstream of the other signaling cascades and how the activated signal pathways interact during signal integration. Although there are still many unsolved questions, our understanding of this plant-beneficial interaction of Trichoderma is growing and will aid in the development of more useful and novel ways to utilize Trichoderma for boosting plant growth and development and immunity.
AUTHOR CONTRIBUTIONS
Raja Asad Ali Khan performed the initial literature search, designed and wrote the initial draft of the paper. Saba Najeeb edited the final version of the paper and prepared figures. Jie Chen, Riu Wang, and Jiang Zhang designed and edited the final version of the paper. Jumei Hou prepared figures. Tong Liu performed overall supervision, edited the final version, and funding.
ACKNOWLEDGEMENTS
We gratefully recognize the financial support from National Natural Science Foundation of China (32060609) and Scientific research project of Collaborative Innovation Center of Hainan University (XTCX2022NYB12).
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




