Unraveling the Molecular Functions of Multifaced Plant-Vacuolar Processing Enzymes
Abstract
In the plant development cycle, different types of proteases play essential functions. Plant vacuolar processing enzymes (VPEs) are a group of cysteine proteases localized in the cell vacuoles that process and regulate several larger proprotein precursors. VPEs activate the proteolytic cleavage of proproteins precursors within aspartic acid and asparagine residues to trigger the tightly controlled programmed cell death process induced by different stages of plant development and environmental cues, impacting myriad plant physiological processes. In plants, VPEs are categorized into embryo, vegetative, and seed coat-types, in which embryo and seed coat-type VPEs are mainly involved in processing the seed storage protein precursors to provide nutrients for early growth, while the vegetative type induces programmed cell death in a varied range of vegetative tissues. Recently, several plant VPEs exhibited transpeptidase activity, which is essential for the cyclization mechanism during the production of cyclotides. In this review, we explore and understand the complex functions of plant VPEs, which can lead to the production of stress-tolerant and higher-yield crops, and provide the recent advances in the application of VPEs in molecular pharming, which highlights the significant implications of VPEs in protein science and agriculture.
1 INTRODUCTION
Vacuolar processing enzymes (VPEs) are classified as the members of the cysteine proteases present in the plant vacuole (Rawlings et al., 2014; Dall and Brandstetter, 2016). In plants, VPEs are responsible for the maturation of the seed storage proteins and initiation of programmed cell death (PCD) by cleaving a peptide bond at the substrate C-terminal aspartate (Asp) and asparagine (Asp) residue of several vacuolar proteins (Hara-Nishimura et al., 1991; Becker et al., 1995; Hiraiwa et al., 1999). Recently, further analysis of their activity revealed their involvement in the cyclisation of peptides precursors (Jackson et al., 2018; Poon et al., 2018). VPEs are folded as inactive precursors called zymogens in the endoplasmic reticulum (ER). These zymogens contain a C-terminal pro-domain, which acts as an auto-inhibitory domain inhibiting the substrate accessioning the VPE active site (Hara-Nishimura et al., 1993). It has been determined that during stress response, the ER bodies fuse and release inactive VPEs into the vacuole, where the acidic environment of the vacuole initiates the proteolytic maturation to provide VPEs active site (Chen et al., 1998b; Hayashi et al., 2001; Kuroyanagi et al., 2002; Yang et al., 2017; Zauner et al., 2018). Active VPEs are considered to be the executioner of PCD by inducing the proteolysis of the various vacuolar proteins, which eventually leads to cell death in plants (Hayashi et al., 2001; Hatsugai et al., 2015). In Nicotiana benthamiana, the RNAi silencing of the VPE reduces the PCD process induced by a viral infection, which demonstrates the importance of the VPEs in triggering the different types of PCD process (Hatsugai et al., 2015). Plant VPEs are primarily expressed in seeds and vegetative tissues (Gruis et al., 2002; Ariizumi et al., 2011; Radchuk et al., 2011; Kumar et al., 2015). In Arabidopsis thaliana, four types of VPEs were identified, alpha VPE (αVPE/AtVPE1), Beta VPE (βVPE/AtVPE2), Gamma VPE (γVPE/AtVPE3), and Delta VPE (δVPE/AtVPE4), which were further categorized into embryo-type (βVPE), seed coat-type (δVPE) and vegetative-type (αVPE, γVPE) according to their expression patterns (Rojo et al., 2003; Yamada et al., 2005; Hatsugai et al., 2015). The vegetative-type αVPE and γVPE are shown to participate in the hypersensitive response signal transduction pathway (Kinoshita et al., 1999). Additionally, during lateral root formation and leaf senescence, the upregulated expression of the vegetative-type VPEs exhibits their essential role in the maturation of vacuolar proteases in vegetative organs to mediate PCD response induced by different developmental stages and environmental cues (Kinoshita et al., 1999; Hatsugai et al., 2004). On the contrary, the embryo and seed coat-type VPEs (βVPE and δVPE) are involved in processing the seed storage proteins, in which the δVPE has been reported to trigger PCD during seed coat formation (Nakaune et al., 2005), and it is the only one among all VPEs to localized outside cells. Mutation in βVPE resulted in reduced VPE activity in the seed storage vacuole (Shimada et al., 2003). In contrast, the activity was utterly lost in the quadruple mutant (Kuroyanagi et al., 2005), which indicates the dominant function of the embryo and seed coat-type VPEs in processing most of the seed storage protein activity, and the vegetative type VPEs could complement their activity (Gruis et al., 2002; Wang et al., 2009; Kumamaru et al., 2010; Radchuk et al., 2011).
Despite the significant roles of the VPEs in peptide cleavage of the various proproteins, few VPEs isolated from the plant species have also been reported to exhibit peptide ligase activity (Saska et al., 2007; Jackson et al., 2018; Chen et al., 2021). Plants employ VPE peptide ligase activity to promote post-transcriptional modification, which leads to the production of cyclotides (Bernath-Levin et al., 2015; Jackson et al., 2018). These cyclic peptides exhibit a range of plant bioactivities (Colgrave et al., 2008; Plan et al., 2008). For instance, a cystine-knotted macrocyclic peptide kalata B1 was shown to be cyclized by a VPE in Oldenlandia affins. Kalata B1 showed a higher tolerance response towards chemical denaturation and thermal stress and exhibits insecticidal activity towards Helicoverpa punctigera (Jennings et al., 2001; Jennings et al., 2004). Recently, many studies have shown the essential role of VPEs in mediating the activity of the vacuolar proteins. However, detailed research on the molecular mechanism of VPEs in plant development and in response to environmental stresses still needs to be conducted. This review aims to discuss the various functions of plant VPEs throughout different stages of plant development. Additionally, we also summarize their role in recent developments in enzyme biology.
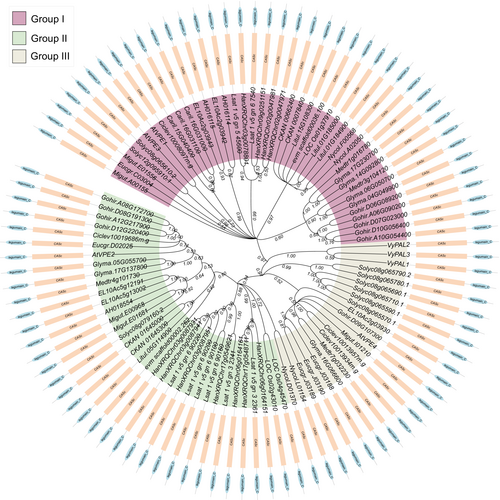
2 EVOLUTION AND STRUCTURAL FEATURES OF THE PLANT VPEs
The study by Hara-Nishimura et al. (1991) initially reported the function of VPE involved in the maturation of the seed storage proteins, which was later confirmed by several studies displaying the critical part of VPEs in the activation of the vacuolar proteins and the regulation of several tissues' programmed cell death (Hiraiwa et al., 1993; Mitsuhashi 2001; Shimada et al., 2003). Plant VPEs are also known as peptide asparaginyl ligase, asparaginyl endopeptidase (AEP), or legumins as they are identified in the activation of the CATHEPSINS B, L, and H lysosomal hydrolytic enzymes by performing a YVADase and caspase-1-like activity (Shirahama-Noda et al., 2003). VPEs originate from seeded plants, in which the ancestral protease in the plant cell might have played a vital part in the processing and maturation of the cytoplasmic proteins, which then have been recruited to the vacuole during the duplication events, resulting in the formation of the VPEs. Through this recruitment process, the plant utilizes VPEs as a storage compartment for proteases. This allowed the plant to target the proteases to a specific tissue for biological functions like programmed cell death. Homology searches in the plant genomes indicated that the VPEs evolve from the prokaryotes' caspase-conserved domain, which plays an essential role in the animal PCD process (Cohen 1997; Coll et al., 2011); nevertheless, the absence of aspartic acid specificity in plant caspases causes them to cleave substrate after arginine (Arg) and lysine (Lys) residues (Tsiatsiani et al., 2011). The evolution of the VPEs from mosses and ferns led them to be widely distributed in land plants (Hatsugai et al., 2015) (Figure 1). The VPE genes in the angiosperms are classified into three di-phyletic clades (embryo-type, seed coat-type, and vegetative-type), whereas the gymnosperms VPEs formed only a single monophyletic clade, indicating that the formation of the embryo, seed coat-type and vegetative-type VPEs occurred only in the angiosperms after several rounds of duplication events (Poncet et al., 2015). Additionally, β-type and γ-type VPEs exist in the monocots, basal eudicots, and basal angiosperm, while the δ-type VPE are only found in the super rosids and superasterids (Figure 1). However, most of the higher plants retain all types of VPEs; for instance, the genome of A. thaliana contains four kinds of VPEs; these VPEs are separated into three groups: group I (vegetative-type), group II (embryo-type), and group III (seed coat-type) (Figure 1). The amino acid sequences of VPE orthologs shared a highly conserved cysteine-dependent and pro-legumain-C domain (Figure 1). Nevertheless, these orthologs were identified to have dissimilar expression patterns in several tissues (Hara-Nishimura et al., 1995; Hara-Nishimura et al., 1998a; Kinoshita et al., 1999; Ariizumi et al., 2011; Cilliers et al., 2018), suggesting the functional diversification events might have occurred in the VPEs since their duplication. The first reported VPE subfamily is the seed type, also known as embryo-type, due to their higher expression level in the maturing seed (Hara-Nishimura et al., 1993; Kinoshita et al., 1995b). Different studies have suggested that embryo-type VPEs direct the activation of the antibiotic peptides and the maturation of the seed storage proteins in various plant seeds (Hara-Nishimura et al., 1991; Hara-Nishimura et al., 1998a; Kinoshita et al., 1999; Yamada et al., 1999; Shimada et al., 2003). Furthermore, in A. thaliana βvpe mutant, the loss of function mutation in the vegetative-type VPEs promotes the abnormal effect of the seed maturation proteins, indicating the possible function of the αVPE and γVPE in the processing of the seed storage proteins (Gruis et al., 2002; Shimada et al., 2003). The orthologous of the δVPE in N. benthamiana and Lycopersicon esculentum was expressed explicitly in the maturing seeds, siliquas, roots, leaves, and fruits (Lemaire-Chamley et al., 1999; Zakharov and Muntz 2004). The different expression levels and functional diversification of VPEs in plants might display their different roles in the various cellular processes.
2.1 Structural features of the plant VPEs
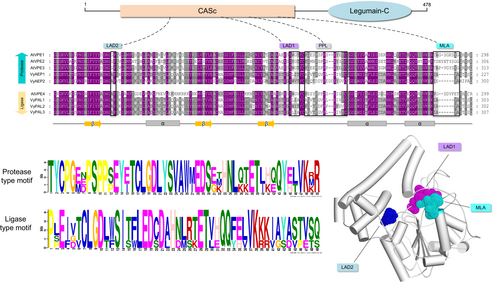
VPEs are vacuole-associated proteins in the plant cell, which play a necessary part in the different biological pathways, such as in the production of cyclic peptides by post-transcriptional modification of RECEPTOR-INTERACTING PROTEINS (RiPs) and for the activation of the environmental- or developmental-induced PCD to protect the plant from pests, bacteria and various ecological stress responses (Hara-Nishimura et al., 1991; Li et al., 2012; Tan et al., 2014; Dall and Brandstetter 2016; Yamada et al., 2020). Due to their essential function in plant development and protein engineering, there has been an expansion of studies in understanding their molecular structure and features to enhance their enzymatic activity (Dall and Brandstetter, 2013; Nguyen et al., 2016b; Zauner et al., 2018; Hemu et al., 2019b).
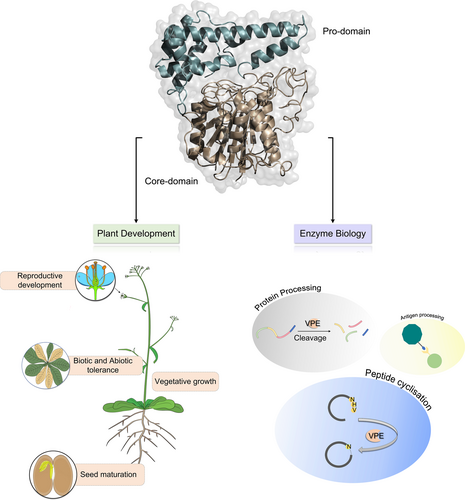
In plants, VPEs are produced as large inactive precursor molecules, which are then self-catalytically converted into mature form in the vacuole (Hara-Nishimura et al., 1993). VPEs are members of the C13 protease superfamily, which comprises the glycosyl-phosphatidylinositol-anchor transamidase (GPI), responsible for mediating the peptide ligation or caspase activity. The VPEs subfamily contains a two-signature conserved catalytic core domain, the N-terminal cystine-dependent (CASc) domain, which belongs to the Peptidase C13 protease superfamily, and the C-terminal pre-pro-legumain-C domain (Figure 2). The autocatalytic deletion of the C-terminal pro-legumain domain results in the active pro-legumain, which suggests the auto-inhibitory role of the C-terminal pre-pro legumain domain (Muntz and Shutov 2002; Zauner et al., 2018). It has been shown that removing the C-terminal propeptide is required for producing active VPE. Consistent with this, the recombinant production of the C-terminal pre-pro legumain domain reduces VPE enzymatic activity, in which the Cys83, His180, and Cys222 residues in the catalytic dyad were involved in the autocatalytic activation of VPE (Kuroyanagi et al., 2002). Studies on the castor bean shown that the precursors of VPEs are translocated to the specialized vesicles and protein storage vacuoles where they can self-catalyzed into the mature form (Hara-Nishimura et al., 1993; Hara-Nishimura et al., 1998b). Despite having lower sequence identity, enzymatic activity, and structural properties, VPEs are comparable to mammalian caspase-1(Chen et al., 1998a). The VPEs orthologs from mammalian have a rigorous substrate specificity towards aspartic acid and are involved in PCD, antigen processing, and mediating T cell activation (Manoury et al., 1998; Sepulveda et al., 2009). The first isolated VPE from castor beans displayed a substrate specificity towards Asp and Asn residues. Yet, the exploration of substrate specificity by VPEs identified additional VPEs from O. affinis, Clitoria ternatea (C. ternatea), and several other plant species (Chen et al., 1998a; Bernath-Levin et al., 2015; Haywood et al., 2018). For instance, a Papaver rhoeas VPE (PrVPE1) cleaved the substrate-specific site DEVD, and a NtTPE8 isolated from the N. benthamiana seeds displayed the cleavage activity towards the FVR site of the cathepsin H (Bosch et al., 2010; Wang et al., 2019). In contrast, the loss of function mutation in the A. thaliana VPEs displayed no caspase-like activity (Kuroyanagi et al., 2005; Cilliers et al., 2018). Additionally, studies performed on the A. thaliana VPE revealed a unique two-chain autoactivation mechanism, which adopts a monomeric state at acidic pH 4.0, allowing for autocatalysis of the prolegumain domain, hence revealing the active site (Dall and Brandstetter 2013; Harris et al., 2015; Zauner et al., 2018). Moreover, the N-terminal CASc domain of the VPEs is suggested to be cystine-dependent as it utilizes a cysteine residue as a nucleophile to form an acyl-enzyme intermediate by attacking the Asn residue (Nguyen et al., 2014; Harris et al., 2015; Haywood et al., 2018). Different isoforms of the VPEs isolated from the different organisms displayed protease and ligase activity (Jackson et al., 2018; Hemu et al., 2019b; James et al., 2019), which were reported to be governed by the pH condition, amino acid residues, and the substrate peptides (Jackson et al., 2018; Zauner et al., 2018; Harris et al., 2019). It has been shown that the N-terminal CASs domain of the VPEs contains a gatekeeper residue known as ligase-activity determinant (LAD1 and LAD2) (Figure 2), which influences the substrate accessibility to the active site of the VPEs (Yang et al., 2017; Hemu et al., 2019b). However, the mutation on the LAD1 site of the O. affinis VPEs ortholog suppresses its ligase activity, whereas adding the smaller residue to the same site increases the cyclization activity (Yang et al., 2017). This modulated form of the OaAEP1 has been utilized in several studies to perform various protein labeling reactions (Rehm et al., 2019; Rehm et al., 2021). Additionally, in ligase VPEs, the marker of ligase activity (MLA) was identified: a five amino acid deletion located within the ligase-related VPEs (Figure 2). The addition of the five amino acid residues in the MLA site restrained the peptide cyclization activity; in contrast, the ligase VPEs from C. ternatea did not contain the MLA site in their sequence, suggesting that the MLA region is not an essential part of the VPEs activity (Jackson et al., 2018) (Figure 2). Furthermore, in the LAD2 site, the replacement of the Tyr with Gly was reported to shift the protease activity of the Viola canadensis AEP (VcAEP) to ligase activity (Hemu et al., 2019b; Nonis et al., 2021), implying the importance of these critical determinants in dictating the VPEs activity. However, the mechanistic determination of the proproline region (PPL) in the functional preference of VPEs has yet to be elucidated (Figure 2).
3 BIOLOGICAL FUNCTION OF VACUOLAR PROCESSING ENZYME IN REGULATING PLANT PHYSIOLOGY
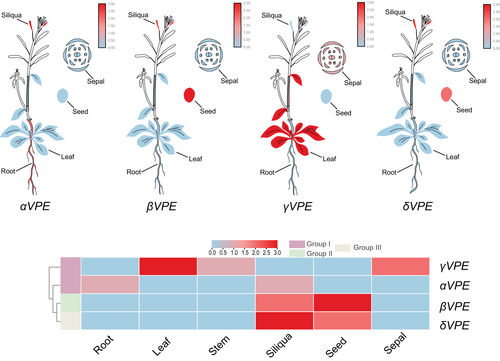
The plant vacuole holds several larger vacuolar proprotein precursors involved in degrading the cellular proteins to direct plant morphology. These larger precursors convert to their active form by cleavage of the protease inhibitor, which is catalyzed by the VPEs. VPEs were first reported to function as a propeptide in processing seed storage proteins, but recently, many studies have stated their role in different plant developmental processes and during environmental stress response, which is confirmed by their diverse expression pattern in various plant tissues (Hara-Nishimura et al., 1995; Nakaune et al., 2005; Zheng et al., 2017; Cheng et al., 2019; Teper-Bamnolker et al., 2019; Ferreira et al., 2020; Li et al., 2020; Guo et al., 2022) (Figure 3). The different members of the VPEs not only play essential functions in the seed maturation or plant PCD (Table 1), but the application of these proteins in the production of biopharmaceuticals, protein-labeling, and semi-synthesis of the small peptides highlights their significant importance in enzyme biology (Chau et al., 2019; Thompson and Muir 2020) (Table 2).
| Gene Symbol | Molecular Function | Tissue Expression | Mutant Phenotype | References |
|---|---|---|---|---|
| αVPE | Trigger immune response by BRASSINOSTEROID INSENSITIVE 1; Induce cell death in response to pathogens; Act as a virulence factor in modulating aldose; Dismantling of the tonoplast; Perform protein cyclization. | Hypocotyl, flower, inflorescence meristem, carpel, cauline leaf, rosette leaf, stem carpel | OE: Contributes to the modification of plant extracellular aldoses and activates defense signal cascade. Directed the expression in senescent tissues; LOF: Mutants remain green after drought treatment and show decreased sucrose content. | (Kinoshita et al., 1999; Mylne et al., 2012; Have et al., 2017; Xu et al., 2021; Tang et al., 2023) |
| βVPE | Processing of vacuolar seed proteins into mature form; Involved in Pollen development and teptal cell, also involved in post-translational proteolysis of seed protein | Seed specific | Direct the expression of the β-glucuronidase reporter gene in seeds and the root tip of transgenic Arabidopsis plants; delayed vacuolar degradation and reduced pollen fertility. | (Kinoshita et al., 1999; Lu et al., 2015; Cheng et al., 2020) |
| γVPE | Functions in the lytic vacuoles: Involved in Heat shock-induced programmed cell death and in xylem fiber cell PCD during stem development. | Senescent tissues | LOF: increased toxin-induced cell death response; Directed the expression in senescent tissues; VPE deficiency abolished the PCD process induced by TMV infection, which increases susceptibly of viral infection. | (Kinoshita et al., 1999; Yamada et al., 2005; Mylne et al., 2012) |
| δVPE | It might regulate the activity of other proteases with caspases-like functions and mediate cell death during seed coat formation. | Expressed in developing pericarp, Inner integument | LOF: Delayed PCD; Abnormal seed coat formation | (Nakaune et al., 2005; Mylne et al., 2012; Tran et al., 2014; Shimada et al., 2021) |
| OE: (Overexpression); LOF: (Loss of Function) | ||||
| VPEs | Specie | Recognition Site | Activity | Usage | References |
|---|---|---|---|---|---|
| OaAEP | Oldenlandia affinis | GL-Xn-NGL | Highest | Cyclotide biosynthesis, including kB1, kB2, R1, GFP, Rhau23. Involved in the cyclization of an intrinsically disordered protein (MSP2). Utilized in segmental isotopic labeling and nanobody labeling | (Harris et al., 2015; Mikula et al., 2017; Mikula et al., 2018; Harris et al., 2019; Rehm et al., 2019; Ngo et al., 2020) |
| VyPAL2 | Viola yedoensis | GI-Xn-NSL | Highest | Display the highest sequence homology with the OaAEP1 and butelase-1. Formed peptide bonds through transpeptidation. Utilized in site-specific labeling and protein macrocyclization | (Jackson et al., 2018; Hemu et al., 2019b; Tang and Luk 2021) |
| Butelase-1 | Clitoria ternatea | GI-Xn-NHV | Moderate | Cyclotide biosynthesis, including kB1, Thanatin, SFTI, AS-48, Somatropin, IL-1Ra, Apelin, Galanin, and Salusin. Structural investigation of p53 binding domain. Utilized in the N-terminal labeling of the GFP and ubiquitin. | (Nguyen et al., 2014; Nguyen et al., 2015b; Hemu et al., 2016; Nguyen et al., 2016a; Pi et al., 2019) |
| MCoAEP2 | Momordica cochinchinensis | GG-Xn-NAL | Moderate | Utilized in the cyclization of cyclic trypsin inhibitor (MCoSST-01) | (Du et al., 2020) |
| HeAEP3 | Hybanthus enneaspermus | Not reported | Moderate | Cyclotide biosynthesis | (Jackson et al., 2018; Hemu et al., 2019b) |
3.1 VPEs in the maturation of seed storage proteins
During the seed maturation stage, different types of seed storage proteins, such as albumins and globulins, accumulate in the protein storage vacuoles, which are utilized as a source of amino acids for seed germination and seedling growth (Figure 4). VPEs control the maturation and activation of these seed storage proteins through proteolytic cleavage of the peptide bond (Hara-Nishimura et al., 1998a; Yamada et al., 2005). For instance, a 100-kDa PV100 component, composed of three domains and a signal peptide, is cleaved by VPE on an Asn-Gln site. The cleavage releases a 50-kDa vicilin-like protein, Arg/Glu-rich peptidases, and a Cys-rich peptide (Hara-Nishimura et al., 1991; Yamada et al., 1999) (Figure 4). Previous studies have shown that the Arg/Glu-rich and Cys-rich peptides act as cytotoxic peptides and trypsin inhibitors, respectively (Naisbitt et al., 1988; Yamada et al., 1999). Additionally, in Leguminosae seeds, VPE was also reported to process the proteolytic cleavage of the ABRIN-A, which is a toxic protein involved in suppressing the synthesis of the proteins by inhibiting the B-chain to bind with its receptor and through the inactivation of 60S ribosomal subunits (Funatsu et al., 1988) (Figure 4).
3.1.1 Functional aspects of embryo-type and seed coat-type VPEs
The seed-type VPEs, in which βVPE is mainly expressed in the seeds, is reported to be responsible for the activation of the antibiotic peptides and the proteolytic cleavage of the seed storage proteins in several plants (Figure 3) (Hara-Nishimura et al., 1998a; Yamada et al., 2005). For instance, the overexpression of the Vitis vinifera VPE (VvβVPE) in A. thaliana enhanced seed germination and ovule formation (Gong et al., 2018), while the downregulation of N. benthamiana βVPE (NtTPE8) caused seed abortion (Wang et al., 2019). A mutation in the A. thaliana and rice βVPE abolished the VPE proteolytic cleavage activity, which resulted in the incomplete accumulation of seed storage protein (Shimada et al., 2003). Contrarily, the triple VPE mutant (αvpe, βvpe, γvpe) didn't properly process the mature storage protein at the proper cleavage site, resulting in a large amount of storage protein precursors (Gruis et al., 2004), whereas the peptide sequence analysis of the double mutant lacking αvpe and γvpe had a normal process and accumulated no protein precursors in their seeds (Shimada et al., 2003); additionally, the expression of the βVPE was also detected in the mature pollen grain and flower bud, which shows the vital role of the βVPE in processing seed storage proteins and also provide a nutritional role for tapetal degradation and pollen development (Kinoshita et al., 1995a; Noguchi 2006; Wang et al., 2019; Cheng et al., 2020). It has also been shown that the βVPE co-operates with aspartic proteases to process 2S albumin seed storage protein precursors, in which the VPE active site Asn-Pro processes the 2S albumin into two subunits and the aspartic-protease holding Phe-Asp site trims these subunits (Yamada et al., 2005). Furthermore, one more member of the VPEs family (δVPE) was also reported to function in a seed development stage, and its expression was detected in the seed and siliquas (Figure 3). Seed coat-type δVPE is mainly expressed in the integuments of the seed coat during the primary stages of seed development to abate the cell layer (Nakaune et al., 2005). Unlike other VPEs, the C-terminal region of the δVPE lacks the vacuolar sorting signals, which direct them to the apoplast to mediate PCD of specific layers during seed coat formation (Ariizumi et al., 2011). The loss of function mutation of the A. thaliana δVPE gene hinders the degradation of the seed coat cell layers (Nakaune et al., 2005), forming the thick seed coat during the seed maturation stage. The enzyme activity of the embryo-type and seed coat-type VPEs in processing and compartmentalization of the seed storage proteins have been reported in several plants; for instance, the VPE from pumpkin and castor bean processed different seed storage proteins in in-vitro conditions (Hara-Nishimura et al., 1993; Yamada et al., 1999) (Figure 4), while the mutation in the rice VPE (OsVPE1) resulted in reduced processing activity of seed storage protein and modulation in the structure morphology of the storage proteins vacuoles (Wang et al., 2009; Kumamaru et al., 2010). In compliance with this, barley VPE degraded maternal tissues, including the nucleus and pericarp of barley seeds (Julian et al., 2013; Tran et al., 2014; Radchuk et al., 2018), indicating the vital role of βVPE and δVPE during seed maturation.
3.2 VPEs in directing Plant PCD under different developmental stages
Programmed cell death (PCD) plays a fundamental role in vegetative and reproductive development in plants, including seed maturation, pollen maturation, xylem formation, and embryogenesis (Maizel, 2015; Olvera-Carrillo et al., 2015). PCD triggers the expression of lipases, nucleases, and proteases, which remove the cells at the margins and create clear boundaries between different tissue types to ensure the normal plant development processes at the cellular level (Lam, 2004; Van Hautegem et al., 2015; Valandro et al., 2020). In eukaryotes, PCD is completed by the caspases, which are the cysteine-containing aspartate-specific proteases that cleave cellular proteins after the aspartic acid (ASP) residues (Earnshaw et al., 1999). However, the cleaving position might be different among caspase family members. Regulating the expression of the caspases is frequently seen as a fundamental signal in many pathways to control PCD during stressful conditions (Baehrecke, 2002; Lam, 2005). In plants, the caspase-like proteinase activity was first predicted in tobacco plants (del Pozo and Lam, 1998). Despite numerous previous attempts to locate proteinases with caspase activities (Jones, 2001; Woltering et al., 2002), Hatsugai et al. (2004) initially identified the role of VPE in performing a caspase-1-like activity directing PCD during different stages of plant development.
3.2.1 VPEs in regulating PCD during pollen and embryonic development
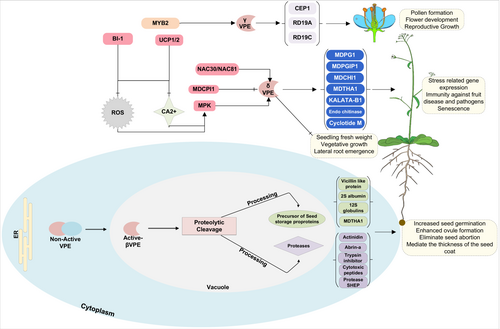
On top of the PCD process mentioned above, VPEs also regulate PCD during pollen development (Hara-Nishimura, 2013), xylem growth (Han et al., 2012), breaking of apical bud dominance (Teper-Bamnolker et al., 2012), as well as during the development and senescence of petal, leaf, and root nodules (van Doorn and Woltering, 2008; Cilliers et al., 2018). In flower plants, the formation and development of the pollen and microspores are carried out by the degradation of the tapetum, which provides essential elements, such as enzymes, lipids, nutrients, and polysaccharides, for the formation of pollen (Parish and Li, 2010). In contrast, abnormal tapetum degradation results in male sterility and disrupts pollen formation (Hanamata et al., 2020). Different types of proteases associated with plant PCD trigger tapetum degradation. For example, the A. thaliana ANTHER-SPECIFIC CYSTEINE PROTEASE 51 (CP51) and PAPAIN-LIKE CYSTEINE PROTEASE (CEP1) are involved in mediating the tapetum stability and pollen exine formation (Yang et al., 2014; Zhang et al., 2014; Guo et al., 2022). Moreover, the Brassica napus cystine proteas (BnaC.CP20.1) was identified to direct pollen wall formation and tapetal degradation (Song et al., 2016). Recently, a study by Cheng et al. (2020) reported that the βVPE directly induces the processing of cysteine proteases, including RESPONSIVE TO DEHYDRATION 19A (RD19A), RESPONSIVE TO DEHYDRATION 19C (RD19C), and CEP1, to mediate pollen formation (Figure 4). Conversely, the maturation of these proteins in βvpe mutant was completely suppressed, resulting in reduced pollen fertility due to retarded tapetal PCD (Cheng et al., 2020). Furthermore, a promotor activity analysis of the βVPE indicated they have a high activity during maturating embryos and pollen development. The transcriptional factor MYB2 interacted with the βVPE promoter to activate its expression (Guo et al., 2022); in compliance with this, the expression of the βVPE in cultivated tobacco was also identified in the pollen and late embryonic development stages (Zakharov and Muntz, 2004), indicating the potential function of the VPEs during pollen development and maturating embryos (Figure 4).
3.2.2 Functional aspects of VPEs during vegetative-organ development
Vegetative-type VPEs (αVPE and γVPE) are mainly expressed in the vegetative tissues to initiate PCD during various plant developmental processes (Vorster et al., 2019) (Figure 3). For instance, during stem development, a higher expression level of the γVPE was observed in the cambium to process the protease CEP1 into its mature form (Cheng et al., 2019). Furthermore, a higher mRNA expression of the VPE gene was also reported in some fruit crops, such as Lycopersicum esculentum and Citrus sinensis, where their expression gradually increased during the fruit maturation stage (Alonso and Granell, 1995; Lemaire-Chamley et al., 1999; Ariizumi et al., 2011). Intriguingly, the higher expression level of the vegetative-type VPEs was also observed in the roots and leaves of the A. thaliana (Hara-Nishimura et al., 1998a; Kinoshita et al., 1999), which is in line with our expression analysis (Figure 3). γVPE deficiency in leaves reduces the stomatal opening in the A. thaliana γvpe mutant, suggesting a role of γVPE in mediating the cellular components regulating the stomatal functioning (Albertini et al., 2014).In contrast, expression of seed coat-type VPE, specifically δVPE, was observed in the maturing seeds (Zakharov and Muntz, 2004). In A. thaliana δvpe mutant, the reduced expression of δVPE shows the delayed vacuolization of the embryo during seed maturation stage, indicating the function of seed coat-type VPE in governing the embryo morphology (Endo et al., 2012). In plants, the xylem is an essential tissue for water transport and provides mechanical support in the stem. During xylem development, different xylem cells, such as tracheary elements, parenchymal cells, and xylary fibers, undergo PCD (Fukuda 1996, 2004). Previously, it has been reported that CEP1, which is a papain-like cysteine protease, participates in PCD during xylem fiber development in cell wall thickening (Han et al., 2019). Additionally, CEP1 plays a role during the PCD in regulating pollen and tapetal development (Zhang et al., 2014). The mutation in the γvpe delays the PCD process, resulting in incomplete degradation of the cellular components, reduced pollen fertility, and thicker cell wall formation (Cheng et al., 2019), indicating the possible role of γVPE in activating CEP1 maturation. Furthermore, γVPE also promoted the degradation of various glycosyl hydrolases proteins, including FRUCTOSIDASE4 (FRUCT4), α-mannosidases, α-galactosidases, and β-glycosidase, which are essential for regulating root elongation by mobilizing sugar molecules (Rojo et al., 2003; Sergeeva et al., 2006; Nagele et al., 2010; Ariizumi et al., 2011), suggesting the possible function of the vegetative-type VPEs in regulating sugar accumulation during plant development. Additionally, during root velamen radicum development in the Cymbidium tracyanum, the five VPEs were upregulated and played an essential role in cell autolysis or cell death, which indicates the involvement of the VPEs in velamen radicum development (Li et al., 2020).
Furthermore, in Aponogeton madagascariensis, the higher activity of vegetative-type VPE induces ethylene-related PCD during leaf morphogenesis stage (Rantong and Gunawardena, 2018); however, the inhibitor of VPE activity (Ac-ESEN-CHO) reduced the progression of ethylene-related PCD in the lace plant leaf, indicating the possible involvement of the vegetative type VPEs in ethylene-induced PCD during leaf development (Hatsugai et al., 2009; Li et al., 2012; Lord et al., 2013). In addition, the VPE interaction with the actin filaments was also reported to activate the PCD in aleurone layers (Zheng et al., 2017) (Figure 4). Disrupting the structure of actin filaments induces PCD by promoting vacuole fusion (Liu and Palevitz, 1992; Kost and Chua, 2002). In contrast, the inhibition of rice VPE blocked the depolymerization of actin filaments, which ultimately delayed the fusion of vacuoles, indicating the relation of vegetative type VPEs in mediating the actin filaments related PCD in aleurone layers.(Uemura et al., 2002; Zheng et al., 2017; Zhang et al., 2020). Moreover, abscisic acid (ABA) accumulates in the integument layers to delay aleurone vacuolation and induce seed dormancy (Bethke et al., 1999; Bethke et al., 2002; Bethke et al., 2007), whereas gibberellins (GA) promoted vacuolation to mobilize the reserve enzymes and compounds to incite aleurone PCD (Bethke et al., 2002; Zhang et al., 2020). Interestingly, the seed coat-type VPE (δVPE) has been identified to control PCD in the inner integument layers during seed development, suggesting that δVPE might be involved in a GA response in the inner integument cells during seed development. However, more evidence is needed to thoroughly examine the molecular mechanism of VPEs in regulating ABA and GA response in the aleurone layers.
3.3 VPEs in Mediating Plant Defense Mechanism
3.3.1 VPEs in directing PCD during biotic stresses
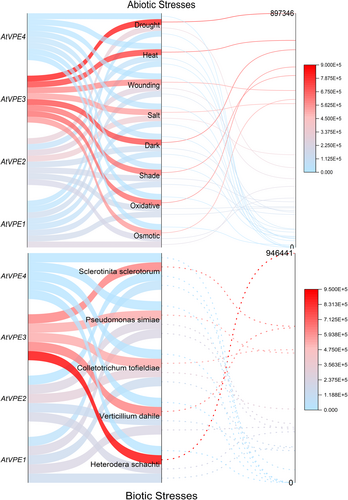
Plants defend themselves from pathogen infections by utilizing various molecular processes to ultimately stop the infection from spreading to healthier tissues (Lord and Gunawardena 2012). For instance, increased salinity and pathogen infection activate cell death of affected tissues, limiting the spread of stress; thus allowing the plants to conserve resources and allocate them to the healthier tissues (del Pozo and Lam, 1998; Lin et al., 2006; Kosova et al., 2018). During stress responses, VPEs activate the proteolytic cascade by releasing proteases into the cytoplasm, leading to the PCD of the infected tissue (Li et al., 2012). Originally, transient VPE-deficient tobacco unraveled the essential role of VPE in virus-induced hypertensive response (Hatsugai et al., 2004). The hypertensive response provides plant immunity to several pathogens and is amongst the known triggers of the VPE transcription. Nevertheless, some pathogens induce plant cell death by releasing toxins for their infection. However, the VPE-silenced A. thaliana mutant (four-VPEs null mutant) completely abolished the fungal toxin-induced cell death, which suggests the key role of the VPEs in mycotoxin-induced cell death (Kuroyanagi et al., 2005). Furthermore, δvpe loss-of-function mutant in A. thaliana exhibited necrosis, curly leaf, and sensitive response to the aphid Myzus persicae infection (Alpuerto et al., 2017). Recently many reports have also shown the involvement of the VPEs in plant immunity against different pathogens, including Botryosphaeria dothiea (Dong et al., 2022), Phaeoisariopsis personata (Kumar et al., 2015), Phytophthora parasitica (Gao et al., 2023), Hyaloperonospora arabidopsidis (Misas-Villamil et al., 2013), and Heterodera filipjevi (Labudda et al., 2020). In compliance with this observation, βVPE and γVPE expression increased mildly sets in A. thaliana treated with Heterodera schachtii, Verticillium dahile, and Colletotrichum tofieldiae in RNA seq-data (Figure 5). The importance of VPEs in mediating plant PCD during biotic stresses has been documented in many studies. These studies reported the upregulated expression of the VPEs induced by aphid infestation, wounding, bacterial pathogens, and fungi (Julian et al., 2013; Christoff and Margis 2014). For instance, the VPEs were reported to process the proteolytic cleavage of the vacuolar proteins, such as chitinase, lectins, and pathogenesis-related proteins, which are involved in the plant defense against biotic stresses (Neuhaus et al., 1991; Ponstein et al., 1994; Rojo et al., 2003; Poon et al., 2018) (Figure 4).
Regulation of stomatal aperture plays an essential part in plant innate immunity (Pandey et al., 2007; Melotto et al., 2008). The opening and closing of the stomata can be induced by the elicitors, PAMPs, and different pathogenic bacteria (Melotto et al., 2006; Zeng and He, 2010). In N. benthamiana, VPE deficiency suppresses the elicitors-induced stomatal closure via inhibiting the accumulation of the NO in guard cells, suggesting the significant role of VPE-dependent stomatal activity in plant innate immunity (Zhang et al., 2009; Zhang et al., 2010). Additionally, recent studies have also reported the involvement of the VPEs in autophagy. Autophagy plays an important part in all stages of the plant life cycle; however, this process increases during biotic stresses to degrade infected cells (Hashimi et al., 2021; Yang et al., 2021). The initiation of the autophagy process is regulated by the autophagy-related genes (Atg), which are involved in targeting the negative regulator of the autophagy response TOR signaling pathway (Fu et al., 2020). RNAi silencing of the Atg4 resulted in a reduced VPE response and cell death rate. Consistent with this, Atg8IL and VPE are colocalized in the outer membrane of the autophagosome, implying that the VPE activity might be dependent upon the autophagy regulators, which translocate them to the vacuole through the autophagy pathway where it can induce stress-promoted cell death (Teper-Bamnolker et al., 2019; Li et al., 2020); however, there is still limited information available to describe the function of VPEs in the autophagy process.
3.3.2 VPEs in mediating abiotic stress responses
VPEs also regulate PCD during different abiotic conditions, such as salinity, cold, drought, waterlogging, and cadmium treatment. For example, vegetative-type VPE and seed-coat type VPE showed higher transcriptional response to salinity and drought treatment than cold and cadmium stress (Song et al., 2020; Zhu et al., 2022) (Figure 5). For instance, γVPE-silenced rice induced plant tolerance to salt stress by delaying the collapse of vacuolar membrane during salt-induced PCD (Lu et al., 2016). Additionally, in Medicago sativa, the expression level of γVPE in root meristem increased under salinity stress. Nevertheless, the expression decreased during in vitro experiments where melatonin, a growth regulator inhibiting the abiotic stress-induced PCD, was supplemented in a highly saline medium (Jalili et al., 2022). Consequently, it can be assumed that melatonin might affect vegetative-type VPE activity during abiotic stresses.
Intriguingly, during heat shock stress, the expression of the A. thaliana γVPE was regulated by MPK6 (Li et al., 2012). In contrast, the MPK6-deficient mutant exhibited reduced γVPE enzyme activity during heat shock stress, whereas MPK6 overexpression enhanced γVPE enzyme activity, which resulted in a significant decrease in seed fresh weight, suggesting the possible role of the MPK signaling in regulating γVPE expression during heat shock stress (Li et al., 2012) (Figure 4). Furthermore, in soybean, the NAC transcriptional factors (NAC30/NAC81) induced VPEs expression (Figure 4). This NAC-VPE signaling induced PCD in response to leaf senescence and osmotic stress (Mendes et al., 2013). Furthermore, it was also shown that senescence-induced A. thaliana γVPE activity [i.e, proteolytic cleavage of the CARBOXYPEPTIDASE Y (CPY), and vacuolar invertase (AtFruct4)] in aging tissues, which leads to the deterioration of several metabolic processes in plants (Figure 4). Drought stress impacts various metabolic and physiological pathways in plants. Several studies have reported the importance of the stomata and ABA in controlling the water content during drought stress (Fan et al., 2004; Nadeau, 2009). An increased transcriptional level of the vegetative type VPE (γVPE) in guard cells enhanced drought tolerance by regulating the stomatal opening during abscisic acid (ABA) treatment in many species, indicating the possible relation between VPEs and ABA in governing stomatal opening during drought stress (Zhang et al., 2010; Bauer et al., 2013; Albertini et al., 2014; Tan et al., 2017).
Results from the above studies examined the higher expression level of the vegetative-type VPE (γVPE) in a range of vegetative and reproductive tissues, such as seeds, siliquas, leaves, roots, cotyledons, and sepal, which have undergone PCD induced by biotic and abiotic stresses In compliance with this, we have also predicted the different transcriptional levels of the AtVPEs during abiotic and biotic stresses using the A. thaliana RNA seq-data sets (Figure 5): γVPE expression increased less upon both biotic and abiotic stress conditions than other VPEs. Therefore, it suggests the putative role of the γVPE in PCD induced by different biotic and abiotic stresses or by different developmental stages.
4 INVESTIGATING THE INTRAMOLECULAR ROLE OF VPEs
4.1 Functional Aspects of VPEs in peptide cyclization
Cyclic peptides are short (50 amino acid-long), simple structure molecules involved in a broad range of bioactivates, including antimicrobial agents and drug development (de Veer et al., 2019). In the last decade, several approaches have been used to produce cyclic peptides from different organisms with lower yields (Arnison et al., 2013; Craik and Malik, 2013). In recent years, the introduction of enzymes into the production of cyclic peptides has significantly improved the yield and synthesis rate (Pawlas et al., 2019). Different types of plant species, such as Viola odorata, C. ternatea, Canavalia ensiformis, and O. affins, produced several types of cyclic peptides involved in antibiotic activities (Sheldon et al., 1996; Mylne et al., 2011; Goransson et al., 2012; Nguyen et al., 2014). Production of these cyclic peptides requires a ligation step, where a peptide ligase enzyme forms a peptide bond in the precursor proteins. It has been shown that the VPEs isolated from several plant species produced a cyclic peptide by joining an amino-terminal and carboxyl group (Arnison et al., 2013; James et al., 2018) (Table 2). Hence, the wide variety of VPEs serves as a valuable tool in peptide cyclization.
The ligation activity of the VPEs was first discovered in the processing of the C-terminal Asp/Asn of kalata B1 precursors (Jennings et al., 2001). A. thaliana and N. benthamiana, which lack the cyclic peptides, accumulated higher levels of the kalata B1 peptides; however, the amount of the kalata B1 peptide significantly reduced when the activity of the VPEs was transiently suppressed (Saska et al., 2007; Gillon et al., 2008), suggesting the involvement of the VPEs in the biosynthetic process of the kalata B1 peptide. VPEs process three plant cyclic peptides: PawS-derived, cyclic knottins, and kalata-type (Conlan et al., 2012). These cyclic peptides participate in various types of bioactivities in plants and show higher stability than their counterparts (Colgrave et al., 2008). For instance, a kalata B1 isolated from a native African plant (O.affins) exhibits higher insecticidal activity against H. punctigera and exhibits higher chemical denaturation and thermal tolerance (Jennings et al., 2001; Colgrave and Craik, 2004; Jennings et al., 2004). Several types of VPEs have recently been identified, and their role in the production of cyclic peptides confirmed their macro-cyclizing ability. For instance, the βVPE of the jack bean was shown to be required for the peptide ligation and post-translational modification of the concanavalin A (Abe et al., 1993), while the γVPE from C. ternatea and O. affins exhibits ligation activity to produce cyclotide peptide (Nguyen et al., 2014; Harris et al., 2015; Yang et al., 2017). Additionally, the VPE from gac fruit and sunflower was reported to process the precursors of the PawS1 to produce trypsin inhibitors MCoTI-II and SFTII, respectively, which indicates the dual function of the VPEs in ligation and peptidase reactions (Mylne et al., 2011; Bernath-Levin et al., 2015; Franke et al., 2017; Jackson et al., 2019; Swedberg et al., 2019; Du et al., 2020) (Table 2). Furthermore, it has also been shown that during the VPE peptide cyclization reaction, the ligation and cleavage reaction occurred simultaneously at the C-terminal processing sites of the VPE precursor proteins (Carrington et al., 1985; Bowles et al., 1986), which suggests that the VPEs process cyclic peptides via transpeptidation reactions. Notably, the peptide ligation activity is common in both groups of eudicots VPEs (Zauner et al., 2018). However, the peptide ligation activity of the VPEs differ widely; some plant VPEs exhibit strong ligation activity, whereas others show weak ligation activity, which indicates that the evolutionary events of the cyclic peptides might have optimized the ligation activity of the different VPEs (Jackson et al., 2018; Hemu et al., 2019b; James et al., 2019).
4.2 Other functional aspects of VPEs in enzyme biology
The VPEs' ability to process backbone cyclization and peptide ligation via a short substrate recognition sequence shows the significant importance in the various applications of protein science, such as protein labeling, post-translational modification, semi-synthesis of small peptides, and production of biopharmaceuticals. Recently, different types of VPEs derived from various plant species have been engineered and employed in the cyclization of various peptides and proteins, including anti-malarial-peptide-R1 (Harris et al., 2019), bacteriocins (Hemu et al., 2016), conotoxins, neuropeptides (Hemu et al., 2016), sunflower trypsin inhibitor, AS-48 (Nguyen et al., 2016a; Hemu et al., 2019a), histatin-3 (Nguyen et al., 2014), MCoTI-II J (Du et al., 2020), somatropin (Cao et al., 2015), p53 binding domain (Pi et al., 2019), and GFP (Nguyen et al., 2015b; Yang et al., 2017). The plant-derived VPE (Butelase-1) generated anti-microbial cyclic peptide θ-defensin and conotoxin MrIA with a yield of up to 95% in 1 minute under pH 6.0 (Nguyen et al., 2016a) (Table 2). Furthermore, butelase-1 cyclized the large protein p53-binding domain, which is involved in anti-cancer therapeutics, for X-ray crystallography and NMR structural studies; the cyclization significantly improved the ligand binding ability and protein conformational stability of the p53-binding domain (Pi et al., 2019). Additionally, a 25 kDa protein MEROZOITE SURFACE PROTEIN 2 (MSP2) from Plasmodium falciparum was also cyclized by the VPE for the development of the malarial vaccine (Harris et al., 2019) (Table 2). Moreover, a recent study explored the effect of VPEs from C. ternatea and Viola. yedoensis in enzyme immobilization (Hemu et al., 2019a). This study reported that the VPEs offered repeated use for cyclization, protein labeling and offered great stability in the storage of polypeptides, which enables the usage of VPEs at low concentrations in performing peptide cyclization for large-scale and industrial applications (Hemu et al., 2019a) (Table 2). This highly catalytic activity of VPEs enables fast reactions and minimizes the demand for the enzyme-to-substrate ratio. However, there are other protease derivatives, including omniligases and sortases, that allow constant peptide ligation with a broad range of substrates that only carry C-terminal ester, which limits their application only to a range of non-recombinant proteins. Moreover, VPEs ligases also allow the labelling of proteins with a wide variety of markers under mild reaction conditions. This VPEs-labeling enables the modification of several protein substrates, such as ubiquitin, DARPin, omPA, GFP, nano-bodies and maltose binding proteins with a varied range of synthetic labels, such as drug molecules, polyethylene glycol, and fluorophores (Nguyen et al., 2015a) to enable these isotopically labeled proteins for NMR studies and examining the protein dynamics (Cao et al., 2015; Luk et al., 2015; Bi et al., 2017; Mikula et al., 2017; Mikula et al., 2018; Rehm et al., 2019; Ciragan et al., 2020). However, labeling of peptides utilizing VPEs has the drawback of being reversible, necessitating the supply of an excess nucleophilic peptide, which serves as a substrate for re-ligation. An additional drawback associated with VPEs is the meager recombinant protein efficiency. To overcome these limitations, genetic engineering, functional characterization, and bioinformatics can be used to recognize the structural features of different VPEs that favor ligation over hydrolase activity, which ultimately broadens the VPEs substrate specificity and enhances their effectiveness.
5 FUTURE OUTLOOK
VPEs function as endopeptidases contributing to the processing of several vacuolar proteins, such as defensive proteins, proteolytic enzymes, and seed storage proteins, by cleaving a substrate peptide bond with specificity for Asp and Asn residues. This cleavage allows VPEs to mediate various vacuolar roles, including wounding response, seed maturation, and plant signal transduction. Additionally, the participation of VPEs in different types of PCD has been reported. PCD is an integral part of plant physiology and of the plant response to internal and external stimuli. VPEs, along with other proteases, trigger PCD by allowing the outflow of the vacuolar hydrolases to the cytoplasm, which ultimately causes cell death. However, the molecular targets of VPEs in triggering the PCD in different stages of plant development are still obscure. In this regard, proteomic studies on VPE-deficient mutants could uncover the VPE targets in isolated vacuoles. Furthermore, modifying the plant VPEs expression might provide a strategy to produce stress tolerance crops or increase seed maturation and protein content. In higher plants, three types of VPEs have been identified: embryo-type (βVPE), vegetative-type (αVPE, and γVPE), and seed coat-type (δVPE). These VPE types are categorized based on their transcriptional expression in the seeds and vegetative tissues (Kinoshita et al., 1995b; Santos-Silva et al., 2012). However, the roles of these types were reported to be ubiquitous in different organs (Julian et al., 2013), and no substantial difference has been identified in their motif's distribution. Thus, much remains to be investigated, specifically in examining each VPE-type function and the transcriptional factors influencing the transcriptional activity of the VPEs during plant development and stress responses. Furthermore, some reports have reported the involvement of the VPEs in salicylic acid, methyl jasmonate, and ABA-induced plant development. However, there is still a lack of understanding of VPEs in controlling phytohormonal signal transduction pathways in response to multiple biotic and abiotic stresses, which is an attractive goal in future research.
VPE accumulates as an inactive enzyme in ER but can be self-activated in acidic conditions. Additionally, VPEs utilize a short recognition sequence of protein precursors to convert them into their active forms. These features allow the VPEs to be adopted as a biocatalyst in developing various polypeptides and used in multiple applications. Although there has been some recent progress on plant VPEs, there is still a need for detailed research about their role and function in plant defense signaling, enzyme biology, and in identifying and characterizing the VPE gene family across various plant species. Unraveling the regulatory network through future studies will undoubtedly advance the innovative applications of VPEs in protein science and allow the elucidation of a full range of VPE activities in plant response to biotic and abiotic stresses. Although the research on plant VPEs is limited to date, we have provided an overview of the recent processes of VPEs during plant development and in enzyme biology (Figure 6).
AUTHOR CONTRIBUTIONS
R.S. conceived and conceptualized the data. J.U., M.Z., Y.W, R.S., and Z.K.M gathered the data. R.S. wrote the original draft of the manuscript. R.S. generated the figures and table. R.S., J.U., Z.K.M analyzed the transcriptomic data. X-LT revised and supervised the manuscript. All the authors reviewed, revised, and agreed to the final draft of the manuscript.
ACKNOWLEDGMENTS
This work was supported by the Jiangsu Agriculture Science and Technology Innovation Fund (CX (21) 2009), and Key R&D in the Jiangsu Province (BE2023342; BE2022340).
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




