Insight into the nodal cells transcriptome of the streptophyte green alga Chara braunii S276
Abstract
Charophyceae are the most complex streptophyte algae, possessing tissue-like structures, rhizoids and a cellulose-pectin-based cell wall akin to embryophytes. Together with the Zygnematophyceae and the Coleochaetophycae, the Charophyceae form a grade in which the Zygnematophyceae share a last common ancestor with land plants. The availability of genomic data, its short life cycle, and the ease of non-sterile cultivation in the laboratory have made the species Chara braunii an emerging model system for streptophyte terrestrialization and early land plant evolution. In this study, tissue containing nodal cells was prepared under the stereomicroscope, and an RNA-seq dataset was generated and compared to transcriptome data from whole plantlets. In both samples, transcript coverage was high for genes encoding ribosomal proteins and a homolog of the putative PAX3- and PAX7-binding protein 1. Gene ontology was used to classify the putative functions of the differently expressed genes. In the nodal cell sample, main upregulated molecular functions were related to protein, nucleic acid, ATP- and DNA binding. Looking at specific genes, several signaling-related genes and genes encoding sugar-metabolizing enzymes were found to be expressed at a higher level in the nodal cell sample, while photosynthesis-and chloroplast-related genes were expressed at a comparatively lower level. We detected the transcription of 21 different genes encoding DUF4360-containing cysteine-rich proteins. The data contribute to the growing understanding of Charophyceae developmental biology by providing a first insight into the transcriptome composition of Chara nodal cells.
1 INTRODUCTION
Originally considered as a model organism for plant electrophysiology (reviewed in Beilby, 2016) and studies of plant polarized growth and gravity sensing (Braun & Limbach, 2006), the Charophyceae alga Chara braunii Gmel. 1826 (Gmelin, 1826) has recently gained considerable interest as a model organism to study early land plant evolution. Charophyceae, along with the Zygnematophyceae and the Coleochaetophyceae, form the ZCC grade, from which the common ancestor of land plants evolved (de Vries & Archibald, 2018; Hess et al., 2022; Wickett et al., 2014; Wodniok et al., 2011). While Zygnematophyceae are the sister group to, and share a last common ancestor with, land plants (Cheng et al., 2019; Ruhfel et al., 2014; Wickett et al., 2014), the earlier branching Charophyceae form the only class that includes streptophyte algae with tissue-like structures and functional rhizoids (Bonnot et al., 2019), traits that were probably already possessed by the common ancestor of land plants, at least in a rudimentary fashion (Fürst-Jansen et al., 2020). Moreover, Charophyceae of the ZCC grade possess a huge cellulose-pectin-based cell wall with many polymers similar to land plants as well as plasmodesmata (Mikkelsen et al., 2014; Sørensen et al., 2011; Umen, 2014), which are features relevant for the successful colonization of the terrestrial habitat. Charophyceae have indeed been considered as the sister lineage to land plants early on (Pringsheim, 1863).
The model organism Chara braunii produces both a high number of oospores within a few months and has a short annual life cycle compared to other Charophyceae species. Reproduction can occur generatively by means of oospores, but can also proceed vegetatively by fragmentation of thalli (Casanova, 2015; Holzhausen et al., 2022). The sequenced draft genome (Nishiyama et al., 2018) revealed evolutionarily significant, plant-like features as well as a striking secondary complexity. Furthermore, standardized protocols for cultivation and generative germination have been established, enabling a more detailed insight through transcriptomic studies (Holzhausen et al., 2022).
Roughly 500 million years ago, an algal concestor of Zygnematophyceae and embryophytes colonized land and gave rise to land plants (Martin & Allen, 2018). Generally, a comprehensive understanding of early molecular innovations towards the dynamic terrestrial environment is still limited (Zhang et al., 2022). The extant Charales, such as Chara braunii, bear striking similarities to the morphology of land plants, yet also display important differences in cell biology (reviewed in Domozych & Bagdan, 2022). Therefore, analysis of the Chara braunii developmental program can shed light on the evolutionary adaptions associated with these morphological features.
The monoecious macroalgae grows from a terminal apical cell and consists of a central stem with branchlets radiating from axial nodes at regular intervals. Nodal and elongated, multinucleate internodal cells are derived from the apical cell in alternating fashion, separating each whorl by an internodal cell. The nodes contain at their center a pair of small cells, called central cells, and an additional 6 to 20 cells surrounding this pair. These nodal cells derive from a nodal initial cell (Kuczewski, 1906; Schubert et al., 2016) and can develop into lateral branches (Nishiyama et al., 2018), suggesting a stem cell-like character for the initial cells. Along its branchlet nodes, the resulting Chara braunii bears both male (antheridia) and female (oogonia) gametangia (Moody, 2020; Nishiyama et al., 2018).
Characeae present certain evolutionary novelties of streptophyte algae, leading to three-dimensional growth: apical cell growth, tip growth and division plane rotation (involving the phragmoplast and cell plates). The nodal cells, found at morphologically key positions between the long internodal cells and branchlets along central cells, can still undergo cell divisions. In contrast to internodal cells, this enables them to generate distinct nodal discs and the founder cells for branches (reviewed by Buschmann, 2020). Compared to oogonia and antheridia, this de novo formation of apical cells also enables asexual propagation (Nishiyama et al., 2018).
Due to their prevailing ability to divide and differentiate, we hypothesized that the transcriptional profiles of nodal cells might differ from vegetative cells in Chara braunii and, therefore, might provide insight into cell-type specific gene expression.
Here, we performed a transcriptome analysis of a Chara braunii sample enriched for nodal cells compared to total plantlet material, providing the first insight into the gene expression in these cells. Whole transcriptome sequencing of Chara braunii central and nodal cells revealed the relative enrichment of several signaling-related mRNAs and of mRNAs encoding sugar-metabolizing enzymes, while photosynthesis-and chloroplast-related genes were expressed at a comparatively lower level.
2 MATERIALS AND METHODS
2.1 Culture of Chara braunii, growth conditions and dissection of central and nodal cells
Cultures of the non-axenic freshwater strain Chara braunii S276 (KU-MACC) were vegetatively propagated. Chara braunii S276 was originally isolated from Lake Kasumigaura (Ibaraki, Japan), then maintained at Kobe University (Kawai et al., 2022; Sato et al., 2014) and consecutively propagated at the Universities of Marburg and Freiburg. Plantlets were grown using the protocol of Holzhausen et al. (2022) in double autoclaved culture vessels containing sieved compost (Gardol Pure Nature, Bauhaus), lime (Gardol Garten- & Rasenkalk, Bauhaus), quartz sand (0.4–0.8 mm in diameter, Carl Roth GmbH) and distilled water, sealed with surgical tape (Micropore, 3 M). Algae were kept under long-day conditions at 22°C in a 16 h light: 8 h dark cycle with white light lateral illumination (30–70 μmol photons m−2 s−1, Lumilux L36W/840, OSRAM).
Algae were cut along their central thallus below and above each node, removing internodal and lateral cells (including branchlets), at the University of Marburg using razor blades and a stereomicroscope (Leica DM6000 CS). Harvested nodes from multiple algae were pooled, collected in 1.5 ml Eppendorf tubes, frozen in liquid nitrogen and stored at −80°C until further analysis.
2.2 Preparation of total RNA and northern hybridizations
Total RNA was extracted from complete thalli utilizing a modified acid guanidinium thiocyanate-phenol-chloroform protocol (Chomczynski & Sacchi, 1987, 2006) using PGTX (Pinto et al., 2009), but omitting Triton X-100.
At least 100 mg FW total Chara braunii samples were ground into a homogenate using mortar and pestle under liquid nitrogen. Samples were suspended in 1 ml Z6 buffer (8 M guanidiniumhydrochloride, 20 mM MES, 20 mM EDTA, 50 mM 2-mercaptoethanol, pH 7), mortar rinsed with 500 μl Z6 buffer, and transferred to a screw-cap tube. 3 ml PGTX was added and samples were incubated for 30 min at room temperature while vortexing every 5 min. Two chloroform extractions were performed consecutively: each adding 3 ml chloroform/isoamylalcohol (24:1), incubating samples for 10 min at room temperature under occasional vortexing, centrifugation for 3 min (3.273 g, at room temperature) and transferring the aqueous layer to a fresh tube. After chloroform extractions, RNA was precipitated using one volume 2-propanol overnight at −20°C. RNA was collected by centrifugation for 30 min (13,000 g, 4°C), and washed two times using 70% ethanol and air-dried for 10 min. RNA was resuspended in 100 μl RNase-free H2O and stored at −80°C until further analysis.
Nodal RNA was extracted similarly. Pooled nodal cells were suspended in 250 μl Z6 buffer in ice-cooled 2 ml reaction tubes. Cell disruption was performed using a mixer mill (MM 400, Retsch; 5 cycles of 90 s with varying frequencies: 30 s 8/s, 30 s 16/s, 30 s 25/s, and 2 min cooled on ice) using one glass bead (2.85–3.45 mm diameter, Carl Roth GmbH). The supernatant was transferred into a new reaction tube, together with 125 μl of Z6 buffer used for rinsing the glass beads. 750 μl PGTX was added and samples incubated for 30 min at room temperature while vortexing every 5 min. Addition of 750 μl chloroform/isoamylalcohol (24:1) was followed by incubation for 10 min at room temperature under occasional vortexing. Afterwards, sample tubes were centrifuged for 3 min (room temperature, 3273 g). Following another chloroform extraction, the upper aqueous phase was precipitated with one volume 2-propanol overnight at −20°C. RNA was collected by centrifugation for 30 min (13,000 g, 4°C) and air-dried for 10 min. RNA was resuspended in 20 μl RNase-free H2O and stored at −80°C until further analysis.
RNA concentration and purity were measured using a NanoDrop ND-1000 spectrophotometer according to manufacturer instructions (PEQLAB Biotechnologie GmbH). Residual DNA was removed using the Turbo DNase-free™ Kit (Thermo Fisher Scientific) following manufacturer instructions. RNA was recovered using RNA Clean & Concentrator kits (Zymo Research). RNA integrity was controlled on a 5200 Fragment Analyzer System, according to manufacturer instructions (Agilent).
Eight μg total RNA was separated by 1.4% denaturing agarose gel electrophoresis and transferred by capillary transfer on Hybond N+ nylon membranes (Cytiva Europe GmbH) overnight. Northern hybridization was performed with radioactively labeled probe for chlorophyll a/b binding protein (cab) mRNA (primers for template; see Table 1 for sequences), generated using [α-32P]-UTP and the Maxiscript T7 in vitro transcription kit (Thermo Fisher Scientific). Blotted RNA was crosslinked to the membrane via 240 mJ using a UV-Stratalinker (Stratagene). Hybridizations were performed in Northern buffer (50% deionized formamide, 7% SDS, 250 mM NaCl and 120 mM Na2HPO4/NaH2PO4 pH 7.2) overnight at 62°C. The membranes were washed at 57°C in buffer 1 (2× SSC (3 M NaCl, 0.3 M sodium citrate, pH 7.0), 1% SDS), buffer 2 (1× SSC, 0.5% SDS) and buffer 3 (0.1× SSC, 0.1% SDS) for 10, 5, and 2 min. Hybridization signals were detected via Typhoon FLA 9500 imaging system (GE Healthcare) using phosphorimaging and quantified using Quantity One software (Bio-Read Laboratories Inc.).
| ID | Sequence | Description |
|---|---|---|
| CBr-CABconsensus-F | TAATACGACTCACTATAGGGTAGGCCCAGGCGTTGTT | Northern Blot |
| T7-CBr-CABconsensus-R | GACCGCCCCAAGTACC | Northern Blot |
| TruSeq_Sense_nodal | AATGATACGGCGACCACCGAGATCTACAC-CAAGTGGG-ACACTCTTTCCCTACACGACGCTCTTCCGATCT | TruSeq_Sense_primer i5 Barcode |
| TruSeq_Sense_control | AATGATACGGCGACCACCGAGATCTACAC-AGATTTGG-ACACTCTTTCCCTACACGACGCTCTTCCGATCT | TruSeq_Sense_primer i5 Barcode |
| TruSeq_Antisense_nodal | CAAGCAGAAGACGGCATACGAGAT-TCCGCGAA-GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT | TruSeq_Antisense_primer i7 Index |
| TruSeq_Antisense_control | CAAGCAGAAGACGGCATACGAGAT-TCCGCGAA-GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT | TruSeq_Antisense_primer i7 Index |
- Note: An added T7 promoter sequence is highlighted in boldface letters.
Templates were amplified from Chara genomic DNA using OneTaq Quick-Load 2× Master Mix with Standard Buffer. To facilitate the use of PCR products as templates for in vitro transcription, the T7 RNA polymerase promoter (TAATACGACTCACTATAGGG) was included in the 5′ sections of the corresponding reverse primers (Table 1).
2.3 Preparation of total DNA from Chara braunii
Genomic DNA was extracted from at least 100 mg FW total Chara braunii samples ground into a homogenate using mortar and pestle under liquid nitrogen. Samples were suspended in 1 ml SET buffer (1 mM EDTA, 25% sucrose, 50 mM Tris) and lysed overnight at 50°C in a screw-cap tube after adding 1/4 volume 0.5 M EDTA, 1/10 volume 20% SDS and 100 μg/ml proteinase K (Scholz et al., 2019).
After the addition of 2 volumes of ROTI Aqua-Phenol (DNA; Carl Roth GmbH) and 2 volumes of chloroform/isoamylalcohol (24:1), samples were incubated for 5 min at room temperature while vortexing intermediately. Samples were centrifuged for 5 min (3273 g, room temperature), the aqueous layer was transferred to a fresh tube and the chloroform extraction step was repeated. Afterwards, DNA was precipitated using one volume 2-propanol overnight at −20°C, collected by centrifugation for 30 min (13,000 g, 4°C), and washed two times using 70% ethanol and air dried for 10 min. DNA was resuspended in 100 μl RNase-free H2O and stored at −20°C until further analysis.
2.4 cDNA sequencing
cDNA libraries were constructed and sequenced as a service provided by Vertis Biotechnologie AG (Germany). Poly(A)+ RNAs were fragmented using ultrasound (one pulse of 30 s at 4°C), after which an oligonucleotide adapter was ligated to the 3′ termini. First-strand cDNA synthesis was performed using M-MLV reverse transcriptase and the 3′ adapter as primer. First-strand cDNA was purified and the 5’ Illumina TruSeq sequencing adapter ligated to the 3′ end of the antisense cDNA. The resulting cDNA was PCR-amplified using a high fidelity DNA polymerase. The cDNA was purified using an Agencourt AMPure XP kit (Beckman Coulter Genomics).
For Illumina NextSeq sequencing, samples were pooled in approximately equimolar amounts, purified using the Agencourt AMPure XP kit (Beckman Coulter Genomics) and analyzed by capillary electrophoresis. Primers used for PCR amplification were designed for TruSeq sequencing according to manufacturer instructions. The cDNA pool was single-read sequenced on an Illumina NextSeq 500 system using 75 bp read length. Single replicates were sequenced for the pooled nodal cells and the control cell samples, respectively.
2.5 Bioinformatic analyses
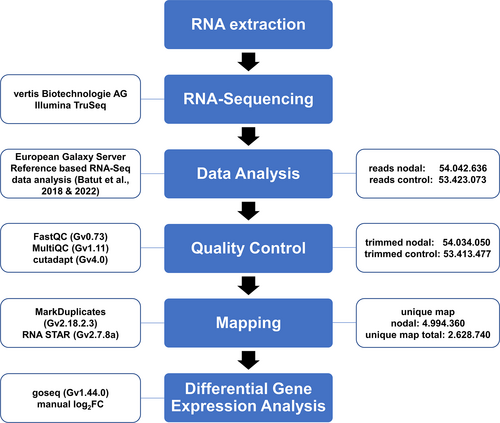
Data analysis was performed on the European instance galaxy server (The Galaxy Community, 2022) following the available guidelines for reference-based RNA-seq analysis (Batut et al., 2018, 2022). The quality of raw reads was assessed using FastQC (Andrews, 2010) and MultiQC (Ewels et al., 2016). Trimming of adapter and barcode contamination was performed using cutadapt (Martin, 2011). Mapping and counting of reads against the published Chara genome (Nishiyama et al., 2018) was performed via RNA STAR (Dobin et al., 2013). Differential gene expression analysis was performed via log2FC calculation following the given guidelines. Differentially expressed genes were determined with a cutoff as log2FC ≥ |1|. Gene ontology (GO) analysis of RNA-seq data was performed using goseq (Galaxy version 1.44.0; Young et al., 2010).
2.6 Phylogenetic analysis
A set of amino acid sequences (Table S1) was selected based on position within the green lineage and sequence similarity to Chara braunii putative amylase homologous genes of interest, g31563 and g41182, and putative MurE-like PAP11, g32224. The sequences were aligned with M-coffee (Di Tommaso et al., 2011; Notredame et al., 2000) and further analyzed using the BEAST 2 software package version 2.7.3 (Bouckaert et al., 2019). Calculations were performed using standard BEAUTi settings utilizing the tree prior yule model, substitution model Blosum62 and MCMC chain length of 1e6, with logged parameters every 1e4 steps. Tracer v1.7.2 software (Rambaut et al., 2018) was used to validate the effective sample sizes (ESS > 200). TreeAnnotator was used to build maximum clade credibility trees using burnIn of 50% and a posterior probability limit of 0.5 for median node heights. FigTree v1.4.4 software (Rambaut, 2018) was used to visualize the generated dendrograms.
3 RESULTS
3.1 The most abundantly transcribed genes in Chara nodal cells
The nodal cells of Chara braunii are embedded in the zone between two adjacent large internodal cells from where the branchlets are emanating (Figure 1). By cutting the algae below and above each node, only the nodal cells from the central thallus were collected. Separated nodes were kept in liquid nitrogen until RNA extraction. RNA was extracted from 28 pooled nodes, yielding 0.68 μg of total RNA after DNase treatment. In parallel, RNA was extracted from fresh total algal material. We decided to use complete above-ground thalli, including nodal cells, as control. The inclusion of nodal cells in the control allowed, after cDNA sequencing, the direct calculation of log2 fold change (log2FC) factors for the differentially expressed genes. Accordingly, positive log2FC factors indicated mRNAs enriched in nodal cells. Judged by the visible sharp rRNA bands and the test hybridization for cab mRNA, the prepared RNA samples were of high quality (Figure S1). After polyA-mRNA enrichment, cDNA libraries were generated for the two different Chara braunii RNA sample types and sequenced, yielding a total of 54.042.636 raw single read counts for enriched nodal cells and 53.423.073 for total cells. From these, 10.991.291 (20.6%) reads for total and 13.763.747 (25.5%) for the enriched sample remained unmapped. After trimming and mapping steps, 4.994.360 nodal (9.4%) and 2.628.740 total (4.9%) reads were uniquely mapped to the Chara genome (Figure 2). Mapped reads matched 11.319 putative genes for the nodal sample and 15.627 genes for total sample. The 15 most highly expressed genes for each tissue sample are given in Tables 2 and 3, while the full list of genes is given in Table S2.

| Gene ID | Description | Control counts | Nodal counts |
|---|---|---|---|
| g49772 | n/a | 820,153 | 1875 |
| g50349 | Chlorophyll a-b binding protein | 76,820 | 7262 |
| g49773 | n/a | 74,655 | 178 |
| g48962 | n/a | 56,585 | 128 |
| g20157 | Chlorophyll a-b binding protein 151, chloroplast | 53,471 | 1408 |
| g18875 | PSII 5 kDa PsbT protein, chloroplast | 50,667 | 5196 |
| g20098 | 60S ribosomal L6-like protein | 39,190 | 38,394 |
| g20154 | Major chlorophyll binding protein | 37,336 | 2194 |
| g20151 | Chlorophyll a-b binding protein 151, chloroplast | 36,381 | 740 |
| g50044 | 60S ribosomal protein L35a-3-like | 33,211 | 20,144 |
| g29937 | 60S ribosomal protein L10 | 29,890 | 21,826 |
| g21828 | 40S ribosomal protein S19-3 | 29,726 | 19,115 |
| g32357 | 40S ribosomal protein S8 | 29,627 | 18,184 |
| g19118 | Chlorophyll a-b binding protein 151, chloroplast | 26,204 | 1230 |
| g46838 | PAX3- and PAX7-binding protein 1 | 25,206 | 30,172 |
- Note: The 15 genes for which the highest read counts in the control sample were detected in descending order. The gene ID in the first column is followed by the annotation and the normalized read counts in the control and in the nodal samples.
| Gene ID | Description | Nodal counts | Control counts |
|---|---|---|---|
| g20098 | 60S ribosomal L6-like protein | 38,394 | 39,190 |
| g46838 | PAX3- and PAX7-binding protein 1 | 30,172 | 25,206 |
| g29937 | 60S ribosomal protein L10 | 21,826 | 29,890 |
| g10845 | n/a | 20,269 | 19,117 |
| g50044 | 60S ribosomal protein L35a-3-like | 20,144 | 33,211 |
| g21828 | 40S ribosomal protein S19-3 | 19,115 | 29,726 |
| g17012 | ribosomal protein L3, conserved site | 18,638 | 20,362 |
| g32357 | 40S ribosomal protein S8 | 18,184 | 29,627 |
| g41408 | 60S ribosomal protein L5 | 13,381 | 23,292 |
| g26356 | thioredoxin H-type | 13,127 | 6048 |
| g12280 | n/a | 13,114 | 9764 |
| g38728 | n/a | 13,063 | 22,161 |
| g23412 | 60S ribosomal protein L17-2-like | 12,797 | 25,171 |
| g8281 | 40S ribosomal protein S21-2 | 11,504 | 10,917 |
| g36718 | n/a | 10,474 | 5103 |
- Note: The 15 genes for which the highest read counts in the nodal sample were detected, in descending order. The gene ID in the first column is followed by the annotation and the normalized read counts in the nodal and in the control samples.
In the control sample, the 15 most expressed genes were mostly associated with photosynthesis and protein synthesis, such as chlorophyll a-b binding proteins (5/15 instances), the photosystem (PS) II 5 kDa protein PsbT, or the ribosomal proteins RpL6-like, RpL35a-3-like, RpL10, RpS19-3, and RpS8 (Table 2). Moreover, mRNA for g46838, a gene encoding a GC-rich sequence DNA-binding factor-like protein with similarity to the PAX3- and PAX7-binding proteins, was found enriched in both samples (Tables 2 and 3). Last, three of these highly expressed mRNAs encode unknown proteins.
In the sample enriched for nodal cells, the 15 most strongly expressed genes differed from the control by lacking all genes related to photosynthesis. However, even 9/15 mRNAs were encoding ribosomal proteins, indicating that the drive for protein synthesis was high in this tissue (Table 3). g26356, encoding a thioredoxin-encoding mRNA, and g46838, a protein with similarity to the PAX3- and PAX7-binding proteins, were also found enriched. Another four enriched mRNAs encode unknown proteins.
When looking at the full list of genes expressed, 117 different mRNAs for proteins with domains of unknown function (DUF) were identified in both samples. Among them, we found 21 instances of genes encoding DUF4360-containing cysteine-rich proteins. While DUF4360-containing proteins exist in bacteria and eukaryotes (Paysan-Lafosse et al., 2023), it is striking that these Chara braunii DUF4360-containing proteins are more similar to homologs from fungi (mainly Ascomycota) than to any other. Although potential instances of horizontal gene transfer events between algae and fungi or soil bacteria were reported (Donoghue & Paps, 2020), we cannot unambigiously exclude the possibility of fungal contamination given the non-axenic nature of the Chara cultures. However, the genes encoding DUF4360-containing proteins are part of the Chara braunii genome annotation. Therefore, if these reads resulted from a contaminating fungus, the same contamination had to be present already in the cultures that were taken for the initial genome analysis. Most of the mRNAs for DUF4360-containing proteins were more abundant in the Chara total control sample (e.g., genes g9217, g68682, g2717, g2719, g58375, g11068, g36724, g68700, g9208, g68689, g11072, and g76151; for details see Table S1).
3.2 Functional assignments to differentially expressed genes
To obtain an overview of genes differentially expressed between the two samples, we set cutoffs of log2FC ≥ |1| and reads per kb (RPK) ≥10, leaving a set of 3145 differentially regulated genes (2762 up, 383 down in the sample enriched for nodal cells compared to control). The full list of genes can be found in Table S2.
Gene ontology (GO) assignments were used to classify the putative functions of the differently expressed Chara braunii genes (Figure 3). We sorted the GO assignments according to biological process, cellular components and molecular function, and into the up- or down-regulated categories. Most prevalent among the biological processes upregulated in the nodal sample were the GO terms “DNA integration” (225 genes), “protein phosphorylation” (121 genes), “proteolysis” (119 genes), “metabolic process” (63 genes) and “regulation of transcription, DNA-templated” (50 genes). Prominent cellular component GO terms were “integral component of membrane” (103 genes), “nucleus” (91 genes), “membrane” (78 genes), “intracellular anatomical structure” (59 genes) and “cytoplasm” (39 genes). Among the upregulated GO terms for molecular function, “protein binding” (459 genes), “nucleic acid binding” (517 genes), “ATP binding” (287 genes), “zinc ion binding” (138 genes), and “DNA binding” (138 genes) were highly enriched.
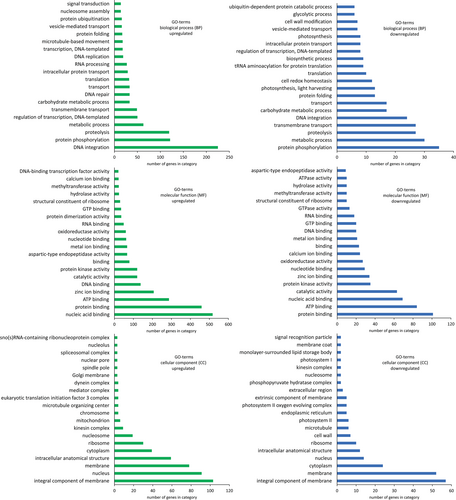
In contrast, the biological process GO terms “protein phosphorylation” (35 genes), “metabolic process” (30 genes), “proteolysis” (27 genes), “transmembrane transport” (27 genes) and “DNA integration” (24 genes) were downregulated. Among downregulated cellular component GO terms, “integral component of membrane” (57 genes), “membrane” (52 genes), “cytoplasm” (24 genes), “nucleus” (14 genes), and “intracellular anatomical structure” (12 genes) were most prevalent. Prominent GO terms for downregulated molecular functions were “protein binding” (101 genes), “ATP binding” (84 genes), “nucleic acid binding” (69 genes), “catalytic activity” (63 genes), and “protein kinase activity” (35 genes). Interestingly, 30 genes associated with the GO term “ribosome” were upregulated and 10 genes downregulated. Within this subset, two paralogous genes encoding 40S ribosomal S21-2 proteins were inversely regulated (g8281, log2FC 2.06 and g28482, log2FC −1.59), similarly two genes for the 60S ribosomal L22-2 protein were inversely regulated (g50176, log2FC 1.40 and g41227, log2FC −1.20). Besides their crucial function in translation, ribosomal proteins can have extraribosomal functions. One intriguing example of this is provided by RPL18aB, which is involved in sexual reproduction and male gametophyte development in Arabidopsis (Xiong et al., 2021; Yan et al., 2016). A putative 60S ribosomal protein L18-2 was found downregulated within the ribosomal subset (g50118, log2FC −3.06). A full list of GO terms can be found in Table S3.
Because there were many genes with unclear annotation or similarity to retrotransposons, we focused on genes with an available functional annotation (Table 4). Among these genes, g31563 (log2FC + 5.19) and g41182 (log2FC + 4.38), which encode proteins with similarity to 1,4-alpha-glucan-branching enzymes/alpha/maltogenic amylase and beta amylase, were enriched in nodal cells. While it is known that streptophyte algae use starch as a storage product (García, 1994), the upregulation of both genes in the nodal cell sample indicates active carbohydrate storage molecule metabolism in the respective cells of Chara braunii.
| Gene ID | Description | log2FC |
|---|---|---|
| g31563 | Putative alpha-amylase, 1,4-alpha-glucan-branching protein | 5.19 |
| g29739 | Caffeoylshikimate esterase | 4.93 |
| g48048 | Mediator of RNA polymerase II transcription subunit 19a-like | 4.61 |
| g19216 | Spastin | 4.59 |
| g41182 | Beta-amylase 3, chloroplast | 4.38 |
| g23946 | Protein TRIGALACTOSYLDIACYLGLYCEROL 3 (TGD3), chloroplast | 4.07 |
| g19355 | Serine/threonine-protein kinase EDR1 isoform X1 | 2.60 |
| g55470 | Serine/threonine-protein kinase EDR1-like isoform X1 | 2.59 |
| g17647 | ETHYLENE INSENSITIVE 3-like 1 protein | 2.38 |
| g197 | Probable ADP-ribosylation factor GTPase-activating protein AGD11 | 2.34 |
| g84830 | START-like domain homeobox protein Wuschel-like | 2.33 |
| g30098 | ETHYLENE INSENSITIVE2 | 2.11 |
| g32224 | PAP11, MurE-like_UDP-N-acetylmuramoyl-l-alanyl-d-glutamate-2, 6-diaminopimelate ligase | 1.17 |
| g19139 | Chlorophyll a-b binding protein 50, chloroplast | −1.44 |
| g51450 | PAP10, thioredoxin-like protein CITRX, chloroplast | −1.45 |
| g40841 | Chlorophyll a-b binding protein CP26, chloroplast | −1.91 |
| g20154 | Major chlorophyll binding protein | −2.10 |
| g72716 | Chlorophyll a-b binding protein P4, chloroplast | −2.27 |
| g19118 | Chlorophyll a-b binding protein 151, chloroplast | −2.43 |
| g17032 | PAP9, Superoxide Dismutase, FeSOD | −2.57 |
| g19140 | Chlorophyll a-b binding protein 50, chloroplast | −2.58 |
| g31512 | Photosystem I reaction center subunit VI, chloroplast | −2.89 |
| g19133 | Major chlorophyll binding protein | −3.20 |
| g20157 | Chlorophyll a-b binding protein 151, chloroplast | −3.26 |
| g50764 | Photosystem II repair protein PSB27-H1, chloroplast | −3.30 |
| g61495 | Peroxiredoxin-2B | −3.40 |
| g48045 | PAP5 | −3.63 |
| g20151 | Chlorophyll a-b binding protein 151, chloroplast | −3.64 |
| g602 | Protein LHCP TRANSLOCATION DEFECT | −3.93 |
| g19141 | Chlorophyll a-b binding protein 3C, chloroplast | −5.16 |
| g20155 | Major chlorophyll binding protein | −5.37 |
| g19126 | Chlorophyll a-b binding protein 50, chloroplast | −6.79 |
- Note: The gene ID in the first column is followed by the annotation and the calculated differential expression (log2FC). A positive value for the log2FC indicates higher read count in the nodal sample (shaded in light brown), while a negative value indicates a higher read count in the control (shaded in light green).
With their publication of the Chara draft genome, Nishiyama et al. (2018) predicted the presence of certain factors involved in phytohormone biosynthesis and signaling pathways. Within our data set, we detected several upregulated genes belonging to these pathways. Related to ethylene signaling, we detected three upregulated genes in the nodal samples. The genes g30098 (log2FC +2.1) and g17647 (log2FC +2.4) encode putative homologs of the ETHYLENE INSENSITIVE 2 and 3 proteins (EIN2 and EIN3) (Nishiyama et al., 2018), proteins that are central in the regulation of ethylene-mediated responses (Chao et al., 1997; Solano et al., 1998). Furthermore, we detected putative serine/threonine-protein kinases EDR1, g19355 (log2FC +2.6) and g55470 (log2FC +2.59), homologs of two proteins involved in crosstalk between ethylene-, ABA- and salicylic acid signaling in A. thaliana (Tang et al., 2005; Wawrzynska et al., 2008). Additionally, we detected with a log2FC of +4.1 enrichment of g23946, encoding a homolog of the trigalactosyldiacylglycerol 3 (TGD3) protein, involved in chloroplast lipid import (Lu et al., 2007), jasmonic acid signaling and plant defense responses via phosphatidic acid signaling (Tagami et al., 2021).
Regarding further upregulated, signaling-related genes, g197 (log2FC +2.3), an mRNA encoding a putative ADP-ribosylation factor GTPase-activating protein (ARF), was detected. Another upregulated gene, g48048 (log2FC +4.6), encoding a putative mediator of RNA polymerase II transcription subunit 19a-like, points towards an increase in transcriptional activity for the actively dividing nodal cells. Another potential regulatory factor enriched in the nodal sample (log2FC +4.6) is a putative spastin-like protein encoded by g19216.
Within our data set, we detected several genes encoding proteins involved in photosynthetic processes expressed at lower levels in nodal cells. Among them, the genes g19126, g20155, g19141, g20151, g20157 and g19133 (log2FC −6.8, −5.4, −5.2, −3.6, −3.3, −3.2) encode chlorophyll a/b binding proteins, apoproteins of the light-harvesting complex of photosystem II (Liu et al., 2013). Additionally, g31512 (log2FC −2.9; encoding the PSI reaction center subunit VI), g50764 (log2FC −3.3; encoding the putative PSII repair protein PSB27) and g602 (log2FC −3.9; encoding a putative LHCP translocation defect protein) point at less active photosynthetic processes in central and nodal cells. Interestingly, g61495 (log2FC −3.4), a putative peroxiredoxin involved in cellular protection from reactive oxygen species (ROS), was downregulated as well. Besides their crucial scavenging capabilities, however, peroxiredoxins also function as modulators of stress response pathways with their interaction with transcription factors (Bréhélin et al., 2003; Hopkins & Neumann, 2019).
The primary cell walls of streptophyte algae contain pectin, hemicellulose and cellulose, and resemble a basal version of those in land plants (Domozych & Bagdan, 2022). Within our data set, we detected mRNAs of several cell wall-associated genes. We found putative cellulose synthase catalytic subunits (g8592 and g8591) upregulated in the nodal sample, as well as an expansin-like protein (g49779) and an mRNA encoding a putative polygalacturonate 4-alpha-galacturonosyltransferase-like protein (g11196). Additionally, a putative cellulose synthase catalytic subunit (g31308) was downregulated, as well as an expansin like protein (g3302), and a putative polygalacturonate 4-alpha-galacturonosyltransferase-like protein (g3195). Furthermore, we detected six putative pectine esterases; Four of which were downregulated (g9040, g11990, g12130, and g17556) and one upregulated (g20314), while one putative pectinesterase/pectinesterase inhibitor 24-like protein was upregulated (g32217). Finally, we detected g29739 (log2FC +4.9), a putative caffeoylshikimate esterase (CSE) involved in the biosynthesis of lignin as enriched in the nodal material (Table 4).
3.3 Divergent phylogenies for paralogous genes highlight evolutionary innovations
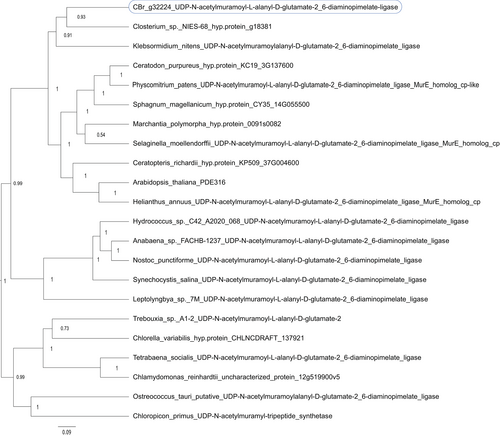
Another set of proteins with central functions in the chloroplast are the PEP-associated proteins (PAPs), which control the activity of the plastid-encoded RNA polymerase (PEP) (Pfalz & Pfannschmidt, 2013). Here, we detected a lower expression for several of these PAPs in the nodal sample compared to the control, with log2FC of −3.63, −2.57, and −1.45 for PAP5, PAP9 and PAP10 (genes g48045, g17032, and g51450), which hence is consistent with their role in photosynthetically active cells. However, we detected for one of these proteins, the MurE-like PAP11 (gene g32224), enrichment in the nodal material (log2FC +1.17). Phylogenetic analysis of the putative MurE-like PAP11 gene (g32224) indicated that it is most closely related to homologs from Closterium sp. NIES-68 (67/83% identical/similar amino acids in a 531-residue overlap between g32224 and protein GJP33877.1) and K. nitens (60/75% identical/similar amino acids in a 586-residue overlap with protein GAQ90408.1), which form a shallow sister group to mosses, ferns and land plant PAPs (Figure 4) and, therefore, do not contradict a divergent functionality of the PAP11 homolog in Chara braunii. Surprisingly, we detected a deep divergence among the homologs in some green algae. These homologs, found in Chloropicon primus, Trebouxia, Chlorella variabilis, Tetrabaena socialis, Chlamydomonas reinhardtii, and Ostreococcus tauri form a distinct group against homologs from all other organisms (Figure 4, 43/56% identical/similar amino acids in a 589-residue overlap between g32224 and protein QDZ19900.1 of Chloropicon primus).
The putative 1,4-alpha-glucan-branching enzyme/alpha amylase g31563 and the beta amylase g41182 highly activated in nodal cells also showed an interesting phylogenetic divergence (Figures 5 and 6). g31563 encodes a protein with high sequence conservation to homologs from other algae (51% identical and 68% similar amino acids in a 572-residues overlap with the homolog in the Klebsormidiophyceae alga K. nitens (accession no. GAQ89829) and 47% identical and 65% similar amino acids in a 585 residues-long overlap with the homolog in the Zygnematophyceae algae Closterium sp. NIES-68 (accession no. GJP44716)). The next-closely related proteins are mainly from Apicomplexa, such as Toxoplasma gondii, and other Alveolata, the Chlamydomonadales and other green algae.

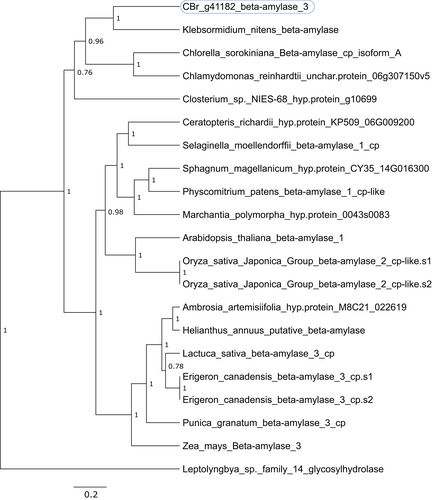
The most closely related protein to the protein encoded by g41182 is, again, a homolog from K. nitens (52/69% identical/similar amino acids in a 485-residues overlap with protein GAQ79925) and homologs from Chlorella vulgaris, Chlamydomonas reinhardtii, and Closterium sp. NIES-68 (Figure 6). However, these form a shallow sister group to proteins from land plants, indicating a closer relationship to homologs in land plants (e.g., 49/61% identical/similar amino acids in a 505 residues-long overlap between the Chara braunii g41182 gene product and protein KAJ0630873 from sunflower (Helianthus annuus)).
4 DISCUSSION
Transcriptome profiling has provided comprehensive and detailed insight into the stress responses of streptophyte alga, including Chara globularis (de Vries et al., 2018; Liang et al., 2020). Using this powerful approach, we aimed to obtain a first insight into possible cell-type dependent differences in gene expression in Chara braunii by studying the transcriptome of Chara nodal cells under optimized, basal growth conditions. We found that the two transcriptome samples differed enormously with 3145 differentially regulated genes. The fact that most of the differentially regulated genes were upregulated in the nodal cell sample compared to the control indicates active transcription of genes across a broader functional spectrum in these cells.
In the control sample, the enriched mRNAs were mostly associated with photosynthesis and protein synthesis, consistent with the photosynthetic lifestyle and fast growth of the Chara braunii algae. Accordingly, chloroplasts are of fundamental physiological relevance. Regarding regulation in the chloroplast, it is noteworthy that we detected the enrichment of mRNAs encoding several PEP-associated proteins (PAPs) in the total thallus material, consistent with their control function over the activity of the plastid-encoded RNA polymerase (PEP) (Pfalz & Pfannschmidt, 2013). In land plants such as Arabidopsis thaliana, 12 PAPs were described (Pfalz & Pfannschmidt, 2013). These PAPs are associated with the development of chloroplasts in photosynthetically active cells and therefore constitute highly selective cell type-specific markers. Ten PAP orthologs were previously detected in C. braunii, in contrast to only 5 or 8 in C. reinhardtii and Klebsormidium nitens, respectively (Nishiyama et al., 2018). Here, we detected the transcription of PAP5, PAP9, PAP10, and PAP11. Transcripts for the first three were more abundant in the whole thallus sample, consistent with their role in PEP regulation in the chloroplast. In contrast, PAP11 was upregulated in the nodal cells, pointing at its divergent function in these cells. This is consistent with its phylogenetic separation, along with homologs from other algae, from land plants homologs (Figure 4). The essential role of PAP11 in chloroplast development in Arabidopsis has been established (Garcia et al., 2008). However, PAP11 possesses a MurE ligase domain (Garcia et al., 2008; Pfalz & Pfannschmidt, 2013; Pfannschmidt et al., 2015), suggesting involvement in the biosynthesis of peptidoglycans. Its divergent regulation suggests that PAP11 in Chara braunii may be less relevant in the regulation of PEP in the chloroplasts, and may rather represent a non-PAP version (Nishiyama et al., 2018).
We detected high transcript levels for several ribosomal proteins or proteins associated to ribosomes. Some of the ribosomal proteins encoded by the detected mRNAs are universally found throughout the tree of life (Lan et al., 2022), and some also facilitate extraribosomal functions (reviewed in Xiong et al., 2021). RpL6 has been shown to promote growth, productivity and tolerance towards salt stress in rice (Moin et al., 2021). RpL35a in humans has been shown to inhibit cell death (Lopez et al., 2002). Finally, RpL10 is not only highly conserved due to its critical function in joining the 40S and 60S ribosomal subunits into a functional 80S ribosome, but is also involved in cell growth and resistance to non-host disease and reactive oxygen species in plants (Ferreyra et al., 2010; Ramu et al., 2020). As reviewed in (Li & Wang, 2020), ribosomes can be highly heterogenous and able to preferentially translate specific subsets of mRNAs. Paralogues of ribosomal protein are seldom found in mammals but exist widely in plants, yeast and bacteria (Li & Wang, 2020). Variations in the building blocks and assembly of ribosomal subunits could therefore enable ribosomes to function as key players in the regulation of translational control and an organism's broader development. Our data are an indication that these mechanisms are also active in Chara braunii.
The high transcript level of g46838, encoding a DNA-binding factor-like protein with similarity to the PAX3- and PAX7-binding proteins, in both samples indicates a likely more general role of this factor. Homologs of this protein in animals are involved in epigenetic regulation (Diao et al., 2012), but there is no information on putative plant homologs.
The enrichment of g19216, encoding a putative spastin-like protein, in the nodal sample has probably broader implications for its stated function in the coordination of microtubule and endoplasmatic reticulum dynamics. While spastins modulate the lipid droplet network and their dispersion throughout the cell in animals, this has been speculated to be an ancestral process in eukaryotes, as antecedent ER elongation along microtubules is a conserved mechanism also described in plants (Arribat et al., 2020; Hamada et al., 2014). Hence, this view is supported here.
A substantial part of a plant genome is devoted to genes encoding enzymes for the biosynthesis of cell wall components. The primary cell walls of streptophyte algae and their mode of non-enzymatic expansion, as well as the precise wall composition, have been extensively studied (Anderson & King, 1961a, b; Boyer, 2009, 2016; Domozych & Bagdan, 2022; Höfte et al., 2012; Pfeifer et al., 2023). They consist of cellulose embedded within a pectin and hemicellulose matrix and therefore resemble those of land plants in many aspects (Sørensen et al., 2011). We detected mRNAs for several enzymes associated with cell wall synthesis in our data set. While most of these genes were down-regulated in the nodal sample, some were upregulated, pointing at possibly divergent roles of the respective proteins in nodal compared to other cells. Among these upregulated genes was g29739, a putative caffeoylshikimate esterase (CSE) involved in the biosynthesis of lignin, which was already confirmed to be present in Chara through biochemical analysis (Rekha & Sujathamma, 2020).
The precise functional characterization of the mentioned upregulated gene functions in nodal cells is of great interest for future analyses.
Overall, the analysis of the nodal cell sample indicated the enrichment of transcripts associated with signaling, protein biosynthesis, starch metabolism and ethylene signaling pathways, while the whole thallus sample showed enrichment of transcripts associated with photosynthesis, chloroplasts and primary metabolism.
AUTHOR CONTRIBUTIONS
Wolfgang R. Hess conceptualized the study. Anja Holzhausen established the dissecting method, Daniel Heß and Anja Holzhausen dissected nodal cells, Daniel Heß generated RNA samples and performed all bioinformatic analyses. Daniel Heß and Wolfgang R. Hess drafted and Anja Holzhausen edited the manuscript and all authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We thank the Freiburg Galaxy team for running this great infrastructure, Madeleine Tarika Maier for maintaining vegetative cultures of Chara braunii, Manuel Brenes-Álvarez for assistance with bioinformatic analyses and the research group of Annegret Wilde (all University of Freiburg) for use of their mixer mill. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) priority program 2237 “MAdLand”, http://madland.science, grant HE2544/18-1 to Wolfgang R. Hess. The Galaxy server that was used for some calculations is in part funded by the Collaborative Research Centre 992 Medical Epigenetics (DFG grant SFB 992/1 2012) and the German Federal Ministry of Education and Research (BMBF grants 031 A538A/A538C RBC, 031L0101B/031L0101C de.NBI-epi, and 031L0106 de.STAIR (de.NBI)). Open Access funding enabled and organized by Projekt DEAL.
Open Research
DATA AVAILABILITY STATEMENT
Raw Illumina RNA-seq data produced in this study have been deposited in the Sequence Read Archive (SRA) with the accession numbers SRX19048645 and SRX19048646 under BioProject PRJNA924555 at https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA924555.




