Analysis of the histidine kinase gene family and the role of GhHK8 in response to drought tolerance in cotton
Abstract
As an important member of the two-component system (TCS), histidine kinases (HKs) play important roles in various plant developmental processes and signal transduction in response to a wide range of biotic and abiotic stresses. So far, the HK gene family has not been investigated in Gossypium. In this study, a total of 177 HK gene family members were identified in cotton. They were further divided into seven groups, and the protein characteristics, genetic relationship, gene structure, chromosome location, collinearity, and cis-elements identification were comprehensively analyzed. Whole genome duplication (WGD) / segmental duplication may be the reason why the number of HK genes doubled in tetraploid Gossypium species. Expression analysis revealed that most cotton HK genes were mainly expressed in the reproductive organs and the fiber at initial stage. Gene expression analysis revealed that HK family genes are involved in cotton abiotic stress, especially drought stress and salt stress. In addition, gene interaction networks showed that HKs were involved in the regulation of cotton abiotic stress, especially drought stress. VIGS experiments have shown that GhHK8 is a negative regulatory factor in response to drought stress. Our systematic analysis provided insights into the characteristics of the HK genes in cotton and laid a foundation for further exploring their potential in drought stress resistance in cotton.
1 INTRODUCTION
The two-component system (TCS) performs a crucial function in the signal transduction of plant responses to environmental stimuli and growth regulators. The TCS comprises HKs, histidine photosensitives (HPs), and response regulator proteins (RRs). These components work in harmony to sense and relay signals, enabling plants to adapt and thrive in their ever-changing surroundings. Recent research has shed light on the intricate interplay between these proteins, uncovering novel insights into the molecular mechanisms underlying plant signaling pathways and offering potential targets for enhancing crop productivity and stress tolerance. Conservative histidine residues in the kinase region of HK phosphorylate themselves after receiving a cytokinin signal, and they then transfer phosphate groups to aspartic acid residues in the receptor area. The histidine phosphate transfer protein (HP) in the cytoplasm receives the phosphate group. Once phosphorylated, HP enters the nucleus and adds the phosphate group to the aspartic acid residue of the class B-cytokinin response regulator. This phosphorylation overrides the negative regulation imposed by the receptor region of class B-RR, leading to the activation of class A-ARR and other response factors, orchestrating an elaborate cytokinin signaling pathway (Müller & Sheen, 2007).
The TCS route is one of the key signaling systems for abiotic stress, such as drought. The HKs are present in a variety of plant species, including maize (Yonekura-Sakakibara et al., 2004), Arabidopsis (Hwang et al., 2002), rice (Pareek et al., 2006), soybean (Mochida et al., 2010), poplar (Gurpreet & Ruchi, 2012), chickpea (Ahmad et al., 2020; Tiwari et al., 2021), Sorghum bicolor (Zameer et al., 2021), banana (Dhar et al., 2019), Lotus japonicus (Ishida et al., 2009), and Cucumis melo L. (Liu et al., 2020). Almost all Arabidopsis HK have negative or positive responses to cold, drought, and salt stress (Kim et al., 2013; Kumar et al., 2013; Pham et al., 2012; Tran et al., 2007; Tran et al., 2010; Wohlbach et al., 2008). Arabidopsis has a total of 17 HK genes, of which five encode ethylene receptors and 12 encode non-ethylene receptor kinases (AHK1, AHK2, AHK3, CRE1/AHK4, CKI1, CKI2/AHK5, PHYA, PHYB, PHYC, PHYD, PHYE, and PDK) (Huo et al., 2020; Hwang et al., 2002; Nongpiur et al., 2012). The AHK1/ATHK1, AHK2, AHK3, and CRE1 control how plants react to abiotic stress. The rice genome contains 11 HK genes, comprising six non-ethylene receptor genes (OsHK1-6) and five ethylene receptor genes (Nongpiur et al., 2012; Pareek et al., 2006; Tsai et al., 2012). According to research, the signal transduction of ABA, salt, drought, and osmotic stress is positively regulated by AHK1 (Tran et al., 2007; Urao et al., 1999; Wohlbach et al., 2008), while AHK2, AHK3, and AHK4/CRE1 play a negative regulatory role in salt, ABA, cold, and dry stress signal transduction (Jeon et al., 2010; Nongpiur et al., 2012; Tran et al., 2007; Tran et al., 2010). The ABA and ethylene signaling pathways, which are reliant on the AHK5 gene, and the H2O2-dependent pathway in stomatal guard cells may both be mediated by the AHK5 gene (Iwama et al., 2007; Desikan et al., 2008).
Within the intricate landscape of plant responses to environmental challenges, emerging research has provided insightful glimpses into the role of histidine kinases (HKs) as key regulators. Notably, the homologous ZmHK9 of AHK5 in maize has been revealed to wield positive control over root elongation, drought resilience, and stomatal development, guided by ABA-dependent signaling pathways (Wang et al., 2012). Furthermore, in the face of cold, salt, and drought stress, the up-regulation of OsHK3 (AHK3 homologous in rice), as evidenced by microarray studies, highlights the broader impact of HKs in stress adaptation (Jain et al., 2008). The significance of HKs in signal transduction during salt, cold, ABA, and drought stress has thus been underscored. While over five HK genes in Cyanobacterium synechocystis are linked to osmotic stress (Paithoonrangsarid et al., 2004; Wang et al., 2012), the unexplored territory lies in deciphering the identity and contribution of HK family genes in cotton's response to drought stress. This uncharted avenue beckons for exploration to unravel the intricate web of plant stress responses and potential avenues for enhancing crop resilience.
As the impact of global warming reverberates across our planet, the indispensability of cotton as a vital natural fiber crop remains undiminished, sustaining national economies. However, the intensifying onslaught of extreme weather events, exemplified by drought and elevated temperatures, poses an existential threat to cotton production. The shrinking cultivation expanse further underscores the imperative to unearth drought-resistant genes that can fortify cotton's resilience. Despite the comprehensive exploration of histidine kinases' (HKs) modus operandi in drought stress within other plant species, their distinct role and precise functions in cotton, especially amidst drought conditions, continue to elude comprehension. Notably, this endeavor delved into the discovery and examination of the HK gene family within cotton, delving into their expression profiles in upland cotton tissues and their responses under abiotic stress conditions. Employing cutting-edge virus-induced gene silencing (VIGS) technology, a profound exploration into the drought stress response mechanism of GhHK8 was undertaken. The findings revealed that GhHK8 assumes a negative regulatory role in the context of cotton's drought stress response. This study emerges as a beacon, proffering vital theoretical guidance and a treasure trove of genetic resources for navigating abiotic stresses within the realm of cotton cultivation.
2 MATERIALS AND METHODS
2.1 Identification of cotton HK gene members
The sequences of the Arabidopsis HK protein were acquired from TAIR (http://www.arabidopsis.org/). Gossypium arboreum (Du et al., 2018), G. raimondii (Paterson et al., 2012), G. barbadense (Liu et al., 2015), and G. hirsutum (Hu et al., 2019) HK protein sequences were downloaded from CottonFGD (https://cottonfgd.net/) (Zhu et al., 2017). The HK protein sequences of other plant species, including Z. mays, B. rapa, S. bicolor, O. sativa, G. max, T. cacao and V. vinifera, G. max, and C. arietinum were downloaded from Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html). The proteins containing the HK domain (IPR005467; e-value <0.1) were identified through InterProScan 5 website (http://www.ebi.ac.uk/interpro/) (Wang et al., 2021a; Zhang et al., 2020). Using the protein sequence of the HK gene in Arabidopsis as the search sequence, all HK genes in cotton were obtained through Blastp alignment (Zhang et al., 2020). Using Cello v2.5 server to predict the subcellular location of HK protein (Yu et al., 2004).
2.2 Sequence alignment and phylogenetic tree analysis
HK protein sequences from 11 plants were aligned using the MEGA-X and NJ methods with 1000 bootstrap values to illustrate the evolutionary relationships between all classes of plants (Kumar et al., 2016).
2.3 Analysis of chromosome position, gene structure, and conserved motifs
Finding conserved motifs of HKs using multiple Em (expectation maximization algorithm) for the motif elicitation website (http://meme-suite.org/) with p-values <1 × e−5 (Bailey et al., 2009). The gene structure of GhHKs was analyzed with the Gene Structure Display Server (Guo et al., 2007). We use TBtools to draw images of conserved chromosomal positions and gene structures for each gene (Chen et al., 2020). The TBtools program was used to examine gene duplication and collinearity (Chen et al., 2020).
2.4 Analysis of HK gene promoter and expression profile
The 2000-bp upstream column of the HK family gene promoter from CottonFGD was used to search the PlantCARE database for cis-elements with functional associations (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). The TBtools software was used for graph analysis (Yu et al., 2014). All RNA seq data used in this study were obtained from the Cotton Omics Database (http://cotton.zju.edu.cn/10) (Hu et al., 2019). Using TBtools software, the heatmap, cis-acting elements, and phylogenetic tree were created based on the fragments per kilobase of exon per million mapped (FPKM) data.
2.5 Quantitative RT-PCR of GhHKs
The cotton varieties TM-1, ZhongS9612, ZhongJ0102, Zhong9807, and ZhongH177 were obtained from Stress Resistance Identification Laboratories (Institute of Cotton Research, CAAS, China). The ZhongH177, ZhongS9612, Zhong9807, and ZhongJ0102 seedlings were cultivated in an illuminated incubator (25°C, 16 h/8 h day/night), and, beginning in the 4th week, they were treated with 12% PEG 6000 or 400 mM NaCl (expect for control plants). The TM-1 seeds were grown at 28°C. Then, samples were taken from the leaves, stems, and roots of plant seedlings cultured in water for 2 weeks. Similarly, during the peak flowering period in the field, samples were taken from TM-1 plants, including petals, stamens, pistils, sepals, stipules, bracts, stigmas, 0 DPA ovules, and fibers from 3, 6, 9, 12, 15, 18, 21, and 27 days after flowering, and store them at −80°C after liquid nitrogen treatment for later use. The Bio-Rad CFX96 fluorescent quantitative PCR system in combination with TransStart Green qPCR SuperMix was used to measure the relative expression of GhHK gene, with three independent replicates, by qRT-PCR analysis using a 2−ΔΔCT system (Schmittgen & Livak, 2008). GhUBQ7 (GenBank accession No. DQ116441) was used as the internal reference gene. The exact primers for qRT-PCR of GhHK are listed in Table S8.
2.6 GhHK protein family network regulation
To forecast how the GhHK family of genes would interact with other genes in cotton, the STRING database (https://string-db.org/) was used to investigate the HK protein interaction network based on Arabidopsis orthologs using the confidence level of 0.4.
2.7 Construction of GhHK8 gene silencing vector
Zhong9807 seedlings were cultured in small pot soil under controlled conditions (25°C, 16 h/8 h light/dark period, humidity above 60), and VIGS experiments were conducted during the flat spread period of cotton cotyledons. The purified 224-bp fragment was inserted into the pYL156 vector through the restriction sites BamHI and SacI. The recombinant plasmid TRV: GhHK8 was transformed into Agrobacterium GV3101 chemocompetent cells through electroporation. The empty vector TRV: 00 was employed as control. As a positive control, the TRV: CLA1 (control albino phenotype would manifest following successful infection of cotton leaves) was employed. For the complete VIGS procedure, we followed Wang et al. (2021a). The silencing efficiency of GhHK8 in the VIGS silencing experiment was tested by qRT-PCR. Table S3 provides gene-specific primers for VIGS.
2.8 Analysis of drought resistance of GhHK gene function
After the positive control shows albino seedlings, 20% PEG6000 was applied to the silenced plants in the trefoil stage and the control plants for 24 h. There were three replications examined, with 15 plants in each replication.
2.9 Measurement of physiological parameters related to drought stress
The SOD and POD content in cotton leaves were determined using peroxidase (Solarbio, BC0090) and superoxide dismutase detection kits (Solarbio, BC0170). CAT activity was determined utilizing a CAT test kit on 100 mg of cotton leaves (Zhang et al., 2020). Using a standard curve with known H2O2 values, the H2O2 concentration was determined in 100 mg of cotton leaves using spectrophotometry (Alexieva et al., 2001).
3 RESULTS
3.1 Identification of HK genes
The discovery of a total of 59, 59, 30, and 29 HK genes from G. hirsutum, G. barbadense, G. arboreum, and G. raimondii was made possible via a BLASTp search of HK genes from Arabidopsis (At). These genes were given in increasing order based on their chromosomal locations and domains: GhHK1-31, GhHKL1-28, GbHK1-30, GbHKL-29, GaHK1-15, GaHKL1-15, GrHK1-14, and GrHKL-15. The HK family genes were also identified in Oryza sativa, Brassica rapa, Glycine max, Cicer arietinum, Theobroma cacao, Vitis vinifera, and other plants. It was discovered that the two allotetraploid cotton species, G. hirsutum and G. barbadense, have roughly twice as many HK genes than the two diploid cotton species (G. arboreum and G. raimondii). Additionally, they were substantially more HK gene members than in other plant species, proving that cotton's HK Gene family has grown through time. Detailed information is presented in Table 1.
| Species | Cytokinin receptor | Ethylene receptor | PHY-like | CKI1-like | CKI2-like | AHK1-like | PDK-like | Total | References |
|---|---|---|---|---|---|---|---|---|---|
| A. thaliana | 3 | 5 | 5 | 1 | 1 | 1 | 1 | 17 | Hwang et al. (2002) |
| O. sativa | 4 | 5 | 3 | 1 | 1 | 0 | 0 | 14 | Schaller et al. (2007) |
| B. rapa | 4 | 6 | 5 | 1 | 3 | 1 | 0 | 20 | Liu et al. (2014) |
| G. max | 0 | 7 | 8 | 0 | 0 | 0 | 0 | 15 | Mochida et al. (2010) |
| C. arietinum | 4 | 5 | 4 | 1 | 2 | 2 | 0 | 18 | Ahmad et al. (2020) |
| T. cacao | 3 | 4 | 4 | 2 | 1 | 1 | 1 | 16 | (This work) |
| V. vinifera | 4 | 5 | 4 | 1 | 1 | 1 | 1 | 17 | (This work) |
| Gossypiumhirsutum | 18 | 14 | 10 | 5 | 4 | 4 | 4 | 59 | (This work) |
| Gossypium barbadense | 18 | 15 | 10 | 4 | 4 | 4 | 4 | 59 | (This work) |
| Gossypium arboreum | 9 | 8 | 5 | 2 | 2 | 2 | 2 | 30 | (This work) |
| Gossypium raimondii | 7 | 8 | 5 | 3 | 2 | 2 | 2 | 29 | (This work) |
We analyzed the biophysical characteristics of cotton HK genes through genome characteristics, protein length, CDS, subcellular localization, Isoelectric point (pI) and molecular weight. Among the 177 identified cotton HK members, they all had <122 amino acids, GrHKL3 being the smallest HK protein, and GaHK14 the largest. The molecular weight ranges from 13.088 to 234.891 kDa, while the pI value ranges from 4.747 to 9.014. The predicted subcellular localization results suggested that most genes were localized in the plasmid; while a small amount of HK genes were located in the nucleus, cytoplasm, and chloroplast. It is speculated that the HK family proteins may be crucial to the formation and expansion of cotton cell proliferation. Detailed information is presented in Tables S1 and S2.
3.2 Evolutionary tree analysis of HK gene family in different plants
To better understand the evolutionary connection of the HK genes in the four Gossypium species, we constructed a phylogenetic tree using the neighbor-joining (NJ) technique with monocotyledonous plants (A. thaliana, O. sativa, V. vinifera), and dicotyledonous plants (T. cacao, G. max, B. rapa, C. arietinum). Using 294 homologous and collateral HK protein sequences from G. hirsutum, G. barbadense, G. raimondii and G. arboreum, we were able to identify the evolutionary connection (Figure 1). The findings indicated that the seven following subfamilies might be formed within the plant HK gene family: ethylene receptor subfamily, cytokinin receptor subfamily, PHY-like subfamily, PDK-like subfamily, AHK1-like subfamily, CKI1-like subfamily and CKI2-like subfamily (Figure 1). Cluster analysis showed that the ethylene receptor subfamily was the largest, containing 79 genes, and the cytokinin receptor subfamily and PHY-like subfamily were second with 73 and 63 genes, respectively. The members of the CKI1-like subfamily and CKI2-like subfamily both contained 21 gene members, while the AHK1-like subfamily contained 18 gene members. The PDK subfamily was the smallest, with only 15 genes.
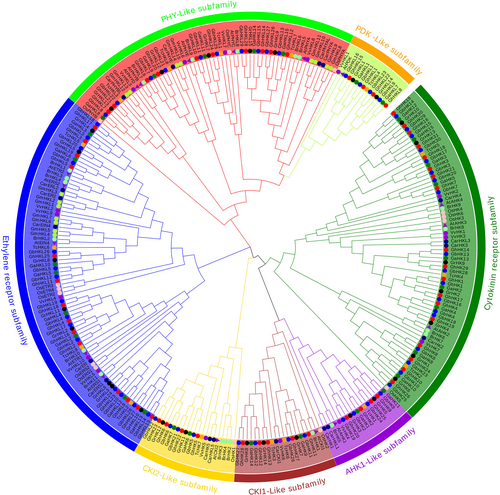
Understanding the evolutionary and orthologous connections between several cotton species, we constructed a NJ phylogenetic tree of the cotton HK and AtHK family (Figure S1). The evolutionary tree results further confirm that ancestral hybridization and doubling of diploid cotton G. arboreum and G. raimondii produced allotetraploid cotton G. hirsutum and G. barbadense (Hu et al., 2019). Noteworthy is the fact that in terms of the cytokinin receiver domain, G. hirsutum has one less than G. barbadense, and G. raimondii has two less than G. arboreum. In the CKI1-like subfamily, G. raimondii has one more than G. arboreum, and G. hirsutum has one more than G. barbadense, which may be related to the replication and loss of genes during evolution.
3.3 Analysis of HK gene structure and conservative genetics in cotton
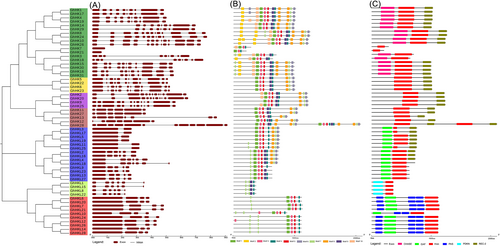
The distribution of exons and introns in the HK family genes was predicted and examined using the GSDS-2.0 website, and the conservative motif of the HK protein was identified using the online resource MEME (Figure 2) to investigate the sequence structure, motif, and conservative domain of ChHKs. Analysis of the HK family protein domain was conducted using the InterPro website, and schematic pictures of the outcomes are shown (Figure 2C). Genes in the same group shared structural traits and conserved motifs, according to the examination of the phylogenetic tree, gene structure, and conserved motifs. It is noteworthy that most cotton HK genes contained intron and exon structures, while only ChHK7 and ChHK21 possessed no introns and only one HisK domain and the gene structures and conserved motifs of HKs among different cotton species were conserved (Figure 2A–C). Ten different motifs were identified for the protein encoded by HK genes (Figure 2B) and had a similar motif composition in the same clade. It is believed that the different composition of motifs is an indicator of gene function diversity (Fan et al., 2021). More than half of the proteins in the cytokinin receptor subfamily contained three domains, and only two HK genes encoded a protein containing one domain (ChHK7 and ChHK21), and two HK genes encoded a protein containing two domains (ChHK3 and ChHK18). The CKI1-like subfamily, CKI2-like subfamily, and AHK1-like subfamily contained the HisK and REC-2 domains. The protein encoded by the ChHK21 gene in the CKI1-like subfamily contained two HisK domains and two REC-2 domains. According to the location of the domain, the ChHK21 gene is thought to have experienced gene fragment duplication. The ethylene receptor subfamily genes contain GAF and His domains, most of which contain REC-2 domains. Only the ChHKL9, ChHKL13, ChHKL18, ChHKL23, and ChHKL27 proteins do not contain REC-2 domain, which indicates that HK proteins may have different functions in different forms. The PDK-like subfamily contained a typical PDK-N domain and HisK domain. The PHY-like subfamily contained PAS domains, GAF domains, and HisK domains. The difference is that the ChHKL10 protein only contained two PS domains, while the other proteins contained three PS domains.
3.4 Chromosomal distribution, collinear analysis and selection pressure analysis of HK among A. thaliana and G. hirsutum
In order to research how HKs are distributed genetically in cotton subgenomes, genomes, and chromosomes, we produced a histogram of the distribution of HK genes on the chromosome (Figure S2) and constructed chromosomal maps of the 177 HK genes from G. hirsutum, G. barbadense, G. arboreum, and G. raimondii (Figure S3). Of the 177 HK genes, 176 were assigned to distinct chromosomes, while one GrHK gene was assigned to an unmapped scaffold. A11 and D11 possessed the most genes in G. hirsutum and G. barbadense, while the most genes were found in chr11 in the G. arboreum variety. In addition, A6, A8, D6, D8, chr06, and chr08 did not have HK genes. Among G. raimondii, the highest distribution of genes was found on chromosome chr07, while there were no HK genes on chromosomes chr10 and chr12.
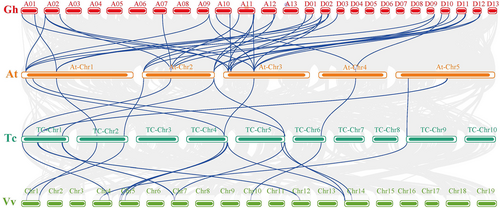
Gene replication stands as a cornerstone in the perpetual dance of species evolution, weaving its influence across diverse biological taxa and multiple epochs of life's journey. The intricacies of this process, manifesting through three distinct avenues—whole-genome duplication (WGD), segment replication, and tandem replication—orchestrate the remarkable evolution of gene families, each thread in this evolutionary tapestry contributing to the grand narrative of life's unfolding complexity (Xu et al., 2012). In order to understand the process of the growth of the HK gene family in cotton, synteny analysis was carried out in a twofold comparison of the four species of cotton using multiple and pairwise alignments of HKs (Figure S4). Both the tetraploid cotton species G. arboreum (A2) and G. raimondii (D5) include orthologous/paralogous gene pairs for the G. hirsutum (AD1) and G. barbadense (AD2) subgenomes (Table S3). Most of the HKs were found to be well conserved throughout the various cotton species, according to the synteny study. A total of 740 orthologous/paralogous gene pairs were found, of which 580 orthologous gene pairs underwent WGD and 160 pairings were expected to undergo segmental duplication to generate paralogous gene pairs in the GhAD1At/GhAD1Dt, GbAD2At/GbAD2Dt, A2/A2, and D5/D5 subgenomes. For the HK gene family, no tandem duplication gene pairs were discovered. These findings led us to the conclusion that before polyploidization entered the evolutionary process, orthologous/paralogous gene pairs often formed from WGD (Figure S4). According to Table S3, we calculated that the retention rate of the HK gene in G. arboreum is 90% in G. barbadense and 87.6% in G. hirsutum. The retention rate of G. raimondii HK gene in G. barbadense is 93.1%, and in G. hirsutum is 93.1%.
Before monocotyledonous and dicotyledonous plants differentiated, their common ancestors underwent second and third genome-wide doubling events that occurred 319 and 192 million years ago, respectively, referred to as ζ and ε whole-genome doubling events. To further investigate potential evolutionary relationships between them and assess the conservation and diversification of cotton species throughout evolution, we also looked at the collinearity between orthologues of G. hirsutum, A. thaliana, T. cacao, and V. vinifera (Figure 3). Collinear gene pairs were discovered in 35 pairs in GhHK and AtHK, 12 pairs in AtHK and TcHK, and 16 pairs in TcHK and VvHK, suggesting that these genes were already present during whole-genome doubling and had a significant impact on plant evolution (Table S4).
To investigate the effects of Darwin's positive selection and selection pressure on HK evolution, the synonymous substitution rate (Ks) and non-synonymous substitution rate (Ka) of 35 pairs of homologous genes were calculated between genomes and within genomes/subgenomes pairs of A. thaliana and G. hirsutum. A total of 15 pairs of HK genes did not have a Ka/Ks ratio, according to the Ka/Ks ratio analysis. For 20 pairs of homologous genes, the Ka/Ks ratio was <1 in 20 pairs (57.1%), 0.5 or greater in 10 pairs, and between 0.5 and 1 in 10 pairs (Table 2). This shows that the evolution of these genes included purifying selection.
| HK gene of A. thaliana | HK gene of G. hirsutum | Ka | Ks | Ka/Ks |
|---|---|---|---|---|
| AT2G47430.1 | GH_A12G1420.1 | 0.489787 | 2.919807 | 0.167746 |
| AT2G47430.1 | GH_D11G3084.1 | 0.578177 | 3.435579 | 0.168291 |
| AT2G47430.1 | GH_D12G1435.1 | 0.491155 | 3.22425 | 0.152332 |
| AT2G47430.1 | GH_A11G3055.1 | 0.587739 | 3.284569 | 0.178939 |
| AT2G17820.1 | GH_D03G0081.1 | 0.219243 | 2.414157 | 0.090816 |
| AT2G17820.1 | GH_D11G0473.1 | 0.224713 | 1.682852 | 0.133531 |
| AT2G17820.1 | GH_A11G0453.1 | 0.219427 | 1.624812 | 0.135048 |
| AT4G18130.1 | GH_D10G0413.1 | 0.222951 | 1.866778 | 0.119431 |
| AT4G18130.1 | GH_A10G0393.1 | 0.225704 | 1.820013 | 0.124013 |
| AT3G06483.1 | GH_A10G1925.1 | 0.104928 | 1.960674 | 0.053516 |
| AT3G06483.1 | GH_D01G0301.1 | 0.088774 | 1.590143 | 0.055828 |
| AT3G06483.1 | GH_D10G2023.1 | 0.106812 | 2.243922 | 0.047601 |
| AT3G06483.1 | GH_A01G0316.1 | 0.084716 | 1.601073 | 0.052912 |
| AT3G04580.1 | GH_A11G2822.1 | 0.228714 | 2.659154 | 0.08601 |
| AT3G04580.1 | GH_D02G0410.1 | 0.323554 | 6.329099 | 0.051122 |
| AT3G04580.1 | GH_D11G2854.1 | 0.227017 | 2.683428 | 0.084599 |
| AT1G27320.1 | GH_D01G1759.1 | 0.189613 | 2.491395 | 0.076107 |
| AT1G27320.1 | GH_D02G1622.1 | 0.173046 | 1.985748 | 0.087144 |
| AT1G27320.1 | GH_A01G1664.1 | 0.192149 | 2.40705 | 0.079828 |
3.5 Expression patterns of GhHK gene in tissues and fibers
We created a heatmap of the expression patterns of HK family genes in various organs and fibers at various stages of cotton development using RNA-seq data (Table S5). The findings demonstrated that the majority of the cytokinin receptor subfamily genes were expressed at various phases of cotton tissue and fiber development (Figure S5). The ethylene acceptor subfamily gene had a significant expression advantage, which indicates that the ethylene acceptor subfamily gene plays a significant function in cotton's development cycle. The CKI1-like subfamily, PHY-like subfamily, PDK-like subfamily, and AHK1-like subfamily were discovered not to be expressed in cotton tissue and fiber development.
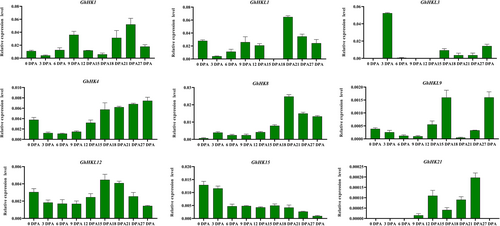
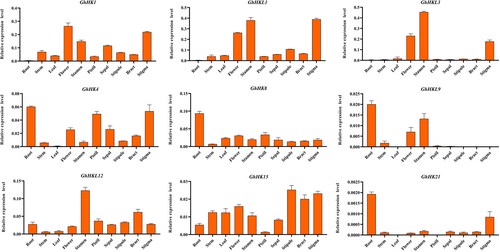
To explore how the HK family genes are expressed in fibers at various development stages and tissues, we used genetics standard TM-1 root, stem, leaf, flower, stamen, pistil, sepal, stipule, bract, stigma, 0 DPA ovule, and fiber cDNA (3, 6, 9, 12, 15, 18, 21, 24, and 27 DPA) at different stages as templates. Referring to the heatmap obtained from the RNA-seq data, we selected nine genes, ChHK1, ChHKL1, ChHKL3, ChHK4, ChHK8, ChHKL9, ChHKL12, ChHK15, and ChHK21, for further verification. Analysis of fiber expression at different stages of cotton showed that the nine genes showed constitutive expression during several phases of the growth of cotton fiber (Figure 4). Among these nine genes, the expression trend of ChHK4 was first decreased and then increased, reaching the highest at 27 DPA, suggesting that ChHK4 played an important role in fiber development. ChHK8 expression trend grew first and then dropped, reaching the highest at 18 DPA, indicating that ChHK8 possibly has a significant impact on the growth and elongation of cotton fiber. The expression of ChHKL12 decreased at first and then increased, and began to decline after peaking at 15 DPA. ChHKL12 is thought to have a role in the start and elongation of cotton fiber formation. The RNA-seq findings are compatible with these genes' patterns of expression. Expression analysis in different tissues shows that ChHK1, ChHKL1, ChHKL3, ChHK4, ChHK8, ChHKL12, and ChHK15 are all expressed in cotton tissues (Figure 5). In particular, ChHKL1 and ChHKL3 were dominantly expressed in cotton reproductive organs such as the flower, stamen, and stigma, and ChHKL12 was dominantly expressed in the stamen. ChHK8 and ChHK21 were significantly expressed in the roots.
3.6 Cis-elements analysis of GhHKs genes promotors and stress heatmap
Using the PlantCARE database, we found and examined a 2000-bp 5′-flanking region upstream of the HK gene's transcription start site in order to better understand the transcriptional regulatory mechanism of HK genes. In the meanwhile, we examined the differentially expressed genes using RNA-seq under various levels of cold, heat, salt, and polyethylene glycol (PEG) stress for lengths of time (1, 3, 6, 9, 12, and 24 h) (Figure S6). In the 59 HK gene promoter regions, there were common regulatory elements in all HK genes, such as CAAT-box and TATA-box. Nine cis-elements related to abiotic or biotic stress were discovered, including MBS (MYB binding site involved in drought-inducibility), MYC (dehydration-responsive elements), LTR (cis-acting elements involved in low-temperature responsiveness), STRE (pressure-responsive member), W-box (wounding and pathogen response site), wound-responsive element (WRE3), and GC-motif GC-motif (enhancer-like element involved in anoxic specific inducibility). The MYB binding site was discovered to be one of the 9 cis-elements under abiotic or biotic stress and to be associated with drought inducibility by examination of the cis-elements of 21 HK gene promoters. In the RNA-seq expression data of these 21 genes, only GhHK24, GhHKL6, GhHKL13, GhHKL18, GhHKL20, and GhHKL27 were differentially expressed under drought stress. However, GhHK21, GhHK9, GhHK25, GhHKL14, GhHKL28, GhHKL11, and GhHKL27 were hardly expressed. The expression stress heatmap results of RNA-seq are compatible with the cis-elements software analysis.
3.7 Analysis of the expression level of HK genes under salt and drought stress
To analyze the function of the HK family genes in cotton under abiotic stress, a total of 10 genes, GhHK1, GhHK3, GhHK4, GhHK8, GhHKL9, GhHKL12, GhHKL13, GhHK15, GhHK21, and GhHK26, were selected as the research subjects based on the heat maps generated by RNA-seq data. Cotton leaves were treated with 12% PEG6000 (Figure 6) and 400 mM NaCl (Figure S7) at different time points, and reverse transcriptional cDNA was used to detect the relative expression levels of the above-mentioned 10 genes. Four cotton cultivars were used: salt-tolerant Zhong9807, salt-sensitive ZhongJ0102, drought-tolerant ZhongH177, and drought-sensitive ZhongS9612.
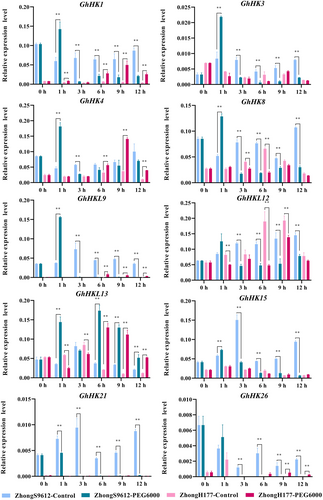
Under salt stress, the expression of GhHK1, GhHK21, and GhHK26 in the salt-tolerant cultivar (Zhong9807) was significantly lower than that in the salt-sensitive one (ZhongJ0102), and the expression of GhHK1 in the different stress periods of ZhongJ0102 was significantly higher than the control at 6, 9, and 12 h after stress. It is speculated that GhHK1 participated in stress regulation at about 12 h after salt stress in cotton. After ZhongJ0102 was subjected to salt stress, the expression of GhHK26 was significantly higher than in the non-treated control at 1 and 6 h. The expression of GhHK21 was higher at 6 h than in the control, but significantly lower at 12 h, indicating that these two genes may be involved in the regulation of cotton salt stress. The expression of GhHK3 and GhHKL13 in Zhong9807 and ZhongJ0102 was lower than in the control at the same time when they were treated with salt stress for 3 h, suggesting that GhHK3 and GhHKL13 play an important role in cotton salt stress. The expression of GhHK8 changed significantly following salt stress. In Zhong9807, the expression of GhHK8 decreased first, increased, and then decreased after salt stress; being noticeably lower than the control at 3 and 12 h. After salt stress was applied to Zhong9807, the expression of GhHK15 decreased, being below control expression only 3 h after the beginning of the treatment. It is hypothesized that GhHK15 has a significant impact on the way cotton salt stress is regulated.
Under drought stress, the expression of the 10 genes examined was significantly different in both the drought-tolerant ZhongH177 and drought-sensitive ZhongS9612 compared to the control. At 1 h following the application of drought stress to ZhongH177 and ZhongS9612, the expression of GhHK1 was significantly higher than that of the control; however, at 3, 6, 9, and 12 h, the expression of GhHK1 in displayed the opposite pattern in both cultivars, indicating that GhHK1 was crucial in the regulation of cotton drought resistance. GhHK26's expression pattern matched that of GhHK1, another gene involved in the drought stress of cotton. The patterns of expression of GhHK3, GhHKL9, GhHK15, and GhHK21 were similar. The expression of GhHK3, GhHKL9, GhHK15, and GhHK21 in the drought-sensitive cultivar 3 h after stress treatment was substantially lower than that in the control group. The expression of GhHK4 increased first and then decreased, peaking at 9 h, and the expression at 6 and 9 h after stress was substantially greater than the control. This indicates that GhHK4 may participate in cotton drought stress response. The expression trends of GhHK8 and GhHKL12 were similar: after 3 h of drought stress, their expression levels were significantly lower than that of the control, which may be related to the similarity in the structure of these two genes. One hour after ZhongH177 and ZhongS9612 were subjected to drought stress, the expression of GhHKL13 in ZhongS9612 was considerably greater compared to the control group, and the expression of GhHKL13 in ZhongH177 was considerably lower than the control. At different time periods after stress, the expression pattern of GhHKL13 in these two cultivars was the same and was considerably higher than the control. It was inferred that GhHKL13 played a significant role in how cotton reacts to drought stress.
3.8 Interaction network of GhHK proteins
Network analysis indicated that the HK protein family genes interact with the signal transduction histidine kinase (COG0642) regulation pathway and light-regulated signal transduction histidine kinase (COG4251) regulation pathway. Most of the proteins that interacted with the HK family genes belong to photosensitive pigments and regulatory factors related to diurnal regulation (Figure 7C).

Through promoter analysis and stress expression analysis, we selected GhHK8 as the further research object. The GhHK8 protein sequence had the highest similarity with Arabidopsis HK2 based on the protein sequence search and comparison using STRING software (Figure 7A). Through the prediction of protein interactions, it was found that almost all of the proteins that interacted with HK8 were HP proteins, with the exception of the A-RR5 and RR1 proteins, indicating HK participated in the regulation of the TCS system with HP (Figure 7B). CKI1 and AHPs 1, 2, and 3 have interactions that were first discovered by Urao et al. (2000). Additional research revealed interactions between ETR1 and AHPs 1, 2, 3, and 5 (Zdarska et al., 2019). The findings demonstrated for the first time that AHK4 was capable of interacting with AHP1, 2, 3, and 5 via a close phosphorelay reaction (Suzuki et al., 2001). Studies in Arabidopsis showed that the reaction to drought is negatively impacted by the histidine phosphotransfer AHP4 (Ha et al., 2022), AHP1, AHP2, AHP3, and AHP5 can communicate with AHK2, AHK3, and AHK4 (Dortay et al., 2006; Mähönen et al., 2006), and histidine phosphotransfer proteins from Arabidopsis AHP2, AHP3, and AHP5 serve as redundant negative regulators of the drought stress response (Nishiyama et al., 2013). This indicates that GhHK8 may participate in cotton drought control
3.9 Silencing of GhHK8 compromises cotton tolerance to drought
We used VIGS to investigate the GhHK8 gene function. Using the interaction networks and gene expression pattern, we speculate that GhHK8 has a potential important role in regulating stress responses. Two weeks following the VIGS experiment, plants transformed with the positive control TRV: GhCLA1 had an albino phenotype visible on their leaves (Figure 8A). Comparing TRV: GhHK8 to the control TRV: 00, we discovered that the expression level of HK8 was much lower in the latter (Figure 8B), indicating that GhHK8 gene was indeed turned off. The silenced plants were more resistant to water depletion (by applying 20% PEG 6000) than the control plants, which had the top tender leaves becoming extremely dry and the cotyledons drooping significantly (Figure 8C, D). Hence, GhHK8 seems to negatively regulate cotton drought stress resistance.
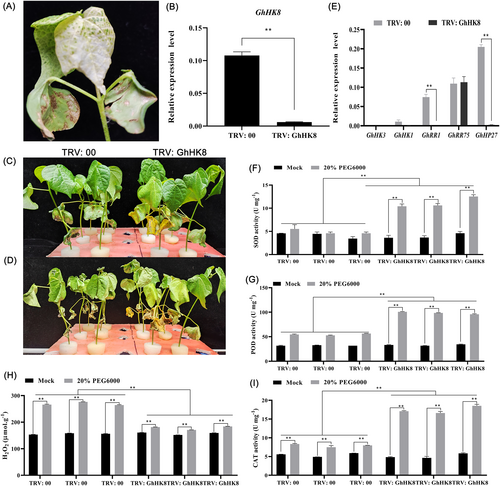
Under stress, the activity of antioxidant enzymes in plants increases to clear the excess ROS, thereby preventing membrane lipid peroxidation and reducing damage to plants (Tan et al., 2008). In this study, the superoxide catalase (CAT) activities, dismutase (SOD), and peroxidase (POD) activity levels were recorded. In the drought environment, the CAT, POD, and SOD activities were higher in GhHK8-silenced plants than control plants (Figure 8I, G, F), and the H2O2 concentration dramatically decreased in leaves (Figure 8H). These findings suggest that GhHK8 gene silencing might improve cotton plants' ability to withstand drought stress.
Analysis of the GhHK8 protein regulatory network suggests that GhHK8 may be in the same regulatory pathway as GhHK3, GhHK1, GhRR1, GhRR75, and GhHP27. We hence examined their expression in GhHK8-silenced plants by RT-PCR (Figure 8E). GhHK3 exhibited almost no expression in the control and silenced plants, and the levels of GhHK1 and GhRR75 expression did not alter significantly. It is worth noting that, compared to the control TRV: 00 plant, the expression levels of GhRR1 and GhHP27 genes in TRV: GhHK8 were significantly reduced. The AHK1 gene is considered to positively regulate abiotic stress. On the contrary, cytokinin receptors AHK2 and AHK3 (ahk2 ahk3) or B-type ARRs (arr1 arr12) are negative regulators, GhRR1 belongs to B-type ARRs, and GhHP27 has been proven to play a negative regulatory role in cotton response to drought stress (Mason et al., 2010; Tran et al., 2007; Zhao et al., 2022). Presumably, the interaction between GhHK8, GhHP27, and GhRR1 negatively regulates cotton drought stress.
4 DISCUSSION
One of the most evolutionary conserved signaling components found in all species is TCS. Studies suggest that this signaling is crucial for various stress responses (Dhar et al., 2019; Lohrmann & Harter, 2002). As an important member of TCS, HK has been identified in Arabidopsis (Hwang et al., 2002), rice (Pareek et al., 2006), maize (Yonekura-Sakakibara et al., 2004), soybeans (Mochida et al., 2010), poplar (Gurpreet & Ruchi, 2012), Lotus japonicus (Ishida et al., 2009), chickpea (Ahmad et al., 2020; Tiwari et al., 2021), S. bicolor (Zameer et al., 2021), banana (Dhar et al., 2019), and C. melo L. (Liu et al., 2020). In the present study, we identified HK genes in G. hirsutum (59), G. barbadense (59), G. arboreum (30), and G. raimondii (29). Compared to other crops, cotton contains more members of the HK gene family, possibly due to its larger genome and the occurrence of replication events during evolution. It is important to note that G. arboreum and G. raimondii have different numbers of HK family genes. Through analysis, we found that there were more than two HK genes in the cytokinin receptor subfamily in G. arboreum than in G. raimondii. In the CKI1-like subfamily, G. raimondii was missing one HK gene, which might result from the duplication of genes and loss of gene fragments that occurred during the development of diploid cotton cultivars.
Analyzing the intricate branches of evolutionary lineage, the meticulously constructed tree of the HK gene family intricately segregates dicotyledonous and monocotyledonous plants into distinct clusters, reflecting the ancient divisions within the botanical realm. Notably, the kinship between cotton's HK gene family and that of T. cacao resonates with the echoes of shared plant evolutionary narratives, as captured in the annals of science (Soltis & Soltis, 2016). The essential role of gene replication emerges as a timeless conductor orchestrating the amplification of TCS genes, as attested by the scientific chorus (Liu et al., 2014; Mochida et al., 2010). Within the cotton saga, the grand narrative of HK gene duplication within their genomes finds its driving force in the WGD. The cross-species collinearity analysis reveals these genes as ancestral witnesses to the whole-genome doubling.
The cytokinin receptor subfamily not only upholds the stalwart architecture of the conservative HK-domain but also showcases the distinct CHASE conservative structural domains, purpose-built for cytokinin binding (Dhar et al., 2019). Across this evolutionary canvas, the ethylene receiver subfamily and the PHY-like subfamily stand distinct, decorated with their unique GAF domains. The GAF domains bind molecules such as cAMP and cGM (Aravind & Ponting, 1997). The PAS domains, borne by the PHY-like subfamily, are present across the realms of archaea, bacteria, and eukaryotes, embodying the role of signal sentinels in biological responses (Hefti et al., 2004). The CKI1-like subfamily, CKI2-like subfamily, and AHK1-like subfamily contained HK and REC domains, and the phosphate receptor sites contain the REC domain.
The qRT-PCR expression analysis of ChHKs in different tissues and different stages of fiber development showed that ethylene receiver subfamily genes and cytokinin receptor subfamily genes are vital for the development of cotton nutrient tissues. The ChHK4, ChHKL12, and ChHK15 play important roles in fiber development. The ChHKL1 and ChHKL3 genes are particularly important in floral reproductive development, and ChHK21 may be a part of the regulatory network for growth and development. According to RNA-seq data, the ChHK8 and ChHKL12 genes are involved in different phases of cotton growth and development. This research lays the groundwork for investigating genes involved in cotton fiber production as well as growth and development.
Gene expression is mostly accomplished by attaching transcription factors to cis-elements upstream of the promoter region (Malik et al., 2020). In the promoter region of GhHKs, we predicted many cis-elements related to abiotic stress, growth and development, and plant hormone responses. It was discovered that cis-acting components linked to abiotic stress, including MBS, TC-rich repeats, LTR, MYC, STRE, wound-responsive element, W-box, and GC-motif, were in the promoter regions of various GhHKs (Figure S3). The majority of GhHK genes are predicted to have a function in plant growth, development, and phytohormone response, the most crucial of which is abiotic stress, according to earlier studies. For instance, Arabidopsis AHK1/ATHK1, AHK2, AHK3, and CRE1 respond to plant stress. Previous studies on the promoter region of the banana HK gene family showed that this region also contains similar cis-elements, which are functionally related (Dhar et al., 2019).
Transcriptome data analysis showed that the expression patterns of homologous genes in GhHK family genes were similar. The qRT-PCR results indicate that under salt and drought stress, the genes of the GhHK family are involved in regulating abiotic stress in cotton. The GhHK1, GhHK3, GhHK8, GhHK4, and GhHKL13 genes play a significant part in reducing cotton's sensitivity to drought and salt stress. In particular, GhHK8 showed significant differences at different time periods after drought stress in the drought-sensitive and drought-resistant cultivars. Multiple sequence network analysis of protein families indicated that the signal transduction of the histidine kinase (COG0642) regulatory pathway is mediated by genes of the HK protein family. It was also found that the majority of the proteins that engage the HK family genes belong to photosensitive pigments and regulatory factors related to diurnal regulation. For example, in Arabidopsis, PIF4 forms the FLS2-RBOHD-PIF4 template with FLS2 and RBOHD in reaction to drought stress (Liu et al., 2022). In maize, ZmPIF3 improves drought stress tolerance in rice (Gao et al., 2015), while COP1 plays a significant role in Arabidopsis and pea drought stress tolerance (Moazzam-Jazi et al., 2018). In Arabidopsis, the EDR1 (ENHANCED DISEASE RESISTANCE 1) protein controls cell death, ethylene signaling, disease resistance, and stress responses (Tang et al., 2005). It is speculated that the HK family proteins in cotton play a significant part in controlling abiotic stress, particularly drought stress.
According to research, the TRV virus affects cotton in a negligible way and is frequently employed to examine the functional genes that control how the plant reacts to biotic and abiotic stress. By utilizing the TRV virus, scientists can pinpoint the functional genes that control cotton's adaptive mechanisms, shedding light on its ability to withstand diverse environmental challenges (Cai et al., 2019) (Wang et al., 2021b). After 20% PEG6000 drought stress, we found that control plants were more water-deficient than GhHK8-silenced plants, with the top tender leaves being extremely dry and the cotyledons drooping significantly (Figure 8C/D). Catalytic enzymes or superoxide dismutases can remove ROS accumulated in plants under drought stress (Miller et al., 2010). Indeed, compared to the control, the SOD, POD and CAT activities were significantly higher (Figure 8F, G, I), but the H2O2 contents in the leaves were significantly lower (Figure 8H). The expression of GhRR1 and GhHP27 was considerably lower in both the control and silenced plants. Research has found that GhHP27 has a negative regulatory effect on cotton drought stress (Zhao et al., 2022). One theory suggests that the interaction between GhHK8, GhHP27, and GhRR1 has a negative regulatory effect on cotton drought stress response.
5 CONCLUSIONS
This study comprehensively and systematically analyzed the HK gene family comprising four Gossypium species. Four species of Gossypium were used in the current work to provide a thorough examination of the HK gene family. Through systematic bioinformatics methods, including analysis of gene structure, phylogeny, collinearity, and promoter substructure, the cotton HK family genes could be properly assesse. Using transcriptome data, the expression patterns of GhHKs in diverse tissues under various abiotic stressors were examined, indicating that they may predict cotton reproductive growth under abiotic stresses, dryness and salt in particular. At the same time, VIGS analysis and validation have demonstrated the role of the GhHK8 gene in drought stress mechanisms. The GhHK8 gene may interact with genes in the GhRR and GhHP gene family, thereby regulating the content of ROS and negatively regulating cotton drought stress.
AUTHOR CONTRIBUTIONS
Wuwei Ye, Lanjie Zhao: conceived and designed the experiments. Lanjie Zhao: writing original draft, methodology, Writing-review & editing. Yongbo Wang, Ruifeng Cui, Yupeng Cui and Xuke Lu: Writing-review & editing. Xiugui Chen and Junjuan Wang: Methodology. Denglong Wang, Zujun Yin and Shuai Wang: Bioinformatic analysis. Fanjia Peng, Lixue Guo and Chao Chen: Methodology. Wuwei Ye: Concept of study, Supervision and revised the manuscript. The published version of the manuscript has been read and approved for publication by all authors.
ACKNOWLEDGMENT
Thank you very much to the editor and reviewers for their review of the manuscript and their constructive comments.
FUNDING INFORMATION
This work was supported by National Engineering Research Center of Cotton Biology Breeding and Industrial Technology / Institute of Cotton Research of CAA (NERC010103), China Agriculture Research System of MOF and MARA (CARS-15-41).
Open Research
DATA AVAILABILITY STATEMENT
The cotton genome data in the article can be downloaded from Cotton FGD (https://cottonfgd.org/), while the Arabidopsis genome data can be downloaded from TAIR (https://www.arabidopsis.org/). O. sativa, V. vinifera, T. cacao, G. max, B. rapa, and C. arietinum are examples of other plant species. Sequences for HK proteins were acquired from phytozome (https://phytozome.jgi.doe.gov/pz/portal.html). The dataset can be obtained from the corresponding author according to reasonable requirements.




