Serotonin: A frontline player in plant growth and stress responses
Abstract
Serotonin is a well-studied pineal hormone that functions as a neurotransmitter in mammals and is found in varying amounts in diverse plant species. By modulating gene and phytohormonal crosstalk, serotonin has a significant role in plant growth and stress response, including root, shoot, flowering, morphogenesis, and adaptability responses to numerous environmental signals. Despite its prevalence and importance in plant growth and development, its molecular action, regulation and signalling processes remain unknown. Here, we highlight the current knowledge of the role of serotonin-mediated regulation of plant growth and stress response. We focus on serotonin and its regulatory connections with phytohormonal crosstalk and address their possible functions in coordinating diverse phytohormonal responses during distinct developmental phases, correlating with melatonin. Additionally, we have also discussed the possible role of microRNAs (miRNAs) in the regulation of serotonin biosynthesis. In summary, serotonin may act as a node molecule to coordinate the balance between plant growth and stress response, which may shed light on finding its key regulatory pathways for uncovering its mysterious molecular network.
1 INTRODUCTION
Plants and animals constantly communicate with neighbouring and nonadjacent cells via numerous signalling molecules. These signalling molecules communicate and govern numerous elements of plant development, such as the plant's growth cycle, root, shoot, flowering and fruiting. These growth and development processes are affected by both external and internal factors in response to changes in the genetic structure and environmental cues (Feng et al., 2020). Plants produce a variety of signalling molecules to regulate their growth and development throughout their life cycle. They act as ligands and have a high affinity for specific receptors. These signalling molecules, such as auxin, cytokinin (CK), gibberellins and abscisic acid (ABA), function as growth regulators and are classified as phytohormones.
Serotonin (5-hydroxytryptamine) has been implicated in several aspects of plant growth and development. Serotonin is an indoleamine neurotransmitter first identified in the mammalian central nervous system (Frazer & Hensler, 1999). Although serotonin has received much attention in the study of human behaviour, its role in plant growth and development remains poorly understood. In plants, serotonin is detected in roots, leaves, fruits and seeds in more than 90 species and 37 plant families (Erland et al., 2016). The biosynthesis of serotonin is a multistep process coupled with two or more biocatalytic reactions. In vertebrates, serotonin is produced from tryptophan, which first involves the transfer of a hydroxide by tryptophan hydroxylase (TPH) into the neuroactive compound 5-hydroxytryptophan (5-HTP) and then decarboxylated into serotonin by aromatic amino acid decarboxylase (AADC) (Tanis et al., 2008). However, in plants, tryptophan is decarboxylated into tryptamine by tryptophan decarboxylase (TDC) to generate bioactive tryptamine, which is subsequently hydroxylated to form serotonin by tryptamine-5-hydroxylase (T5H) (Figure 1A) (Berlin et al., 1993; Kang et al., 2008; Murch et al., 2000). Furthermore, serotonin N-acetyltransferase (SNAT) and acetyl serotonin methyltransferase (ASMT) convert serotonin to melatonin through a series of enzymatic reactions (Bhowal et al., 2021; Negri et al., 2021).
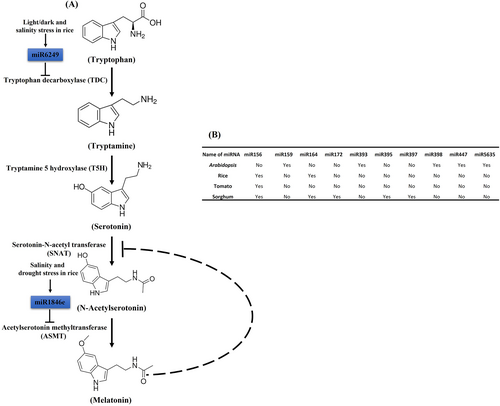
Serotonin works as a morphogen in plants, modulating plant growth and development from embryos to growing seedlings, including germination, shoot branching, root architecture, flowering, senescence and subsequent growth and survival under various environmental circumstances and pathogen attacks (Box 1) (Pelagio-Flores et al., 2016; Pelagio-Flores & López-Bucio, 2016). In Arabidopsis, serotonin inhibits primary root growth while promoting root hair formation during lateral and adventitious root development (Pelagio-Flores et al., 2011). Furthermore, serotonin regulates root growth by modulating reactive oxygen species (ROS) and jasmonic acid (JA)–ethylene (ET) crosstalk during Arabidopsis development (Pelagio-Flores et al., 2016). Serotonin can either be beneficial or harmful to the growth of plants. It has been previously documented that overproduction of serotonin causes stunted growth and lower fertility in rice plants, implying that serotonin has a negative effect on plant growth and development (Kanjanaphachoat et al., 2012). Serotonin, together with melatonin, promotes cell division and ethylene and isoflavone synthesis under temperature stress in Glycine max. Both serotonin and melatonin serve as master regulators of plant growth and development. In sunflowers, both serotonin and melatonin have been shown to regulate root architecture under salt stress. Furthermore, it has been shown that high concentrations of serotonin inhibit root development in sunflower seedlings by regulating auxin crosstalk under salt stress (Mukherjee et al., 2014). In addition to normal growth and development, serotonin has been implicated in the response to various abiotic and biotic stressors (Hayashi et al., 2016). Such evidence suggests a role of serotonin in various aspects of plant biology. The current review article focuses on recent studies that help us better understand serotonin and its role in plant growth and stress response.
2 SEROTONIN ACCUMULATION AND ITS INVOLVEMENT IN PLANT GROWTH AND DEVELOPMENT
Serotonin is found in varying amounts in all plant organs. Serotonin concentrations in the reproductive organs of some species, such as Juglandaceae seeds, reach 300–400 μg g−1 fresh weight, well beyond the usual levels in plants. Serotonin buildup aids in fruit abscission and embryo protection by detoxifying free ammonium ions produced by proteolysis (Negri et al., 2021). In addition to the Juglandaceae family, it has also been reported in pineapple, tomatoes, bananas, cranberries, kiwifruit, and a variety of other fruits and vegetables (Negri et al., 2021). It has been observed that the level of serotonin increases during fruit ripening, indicating that it plays a key role in fruit development (Ramakrishna et al., 2012; Rayne, 2010). The serotonin accumulation in edible fruits reflects the coevolution of angiosperms with frugivorous animals.
However, detailed molecular and functional studies are necessary to understand the purpose of serotonin accumulation in fruits and seeds. Initial genetic screening and classical gain- and loss-of-function techniques, as well as misregulation analyses of serotonin and its biosynthetic components, revealed insight into serotonin's role in plant growth and stress response. Serotonin regulates various physiological and developmental processes ranging from lower to higher plants, such as Arabidopsis, Oryza sativa and Zea mays, under normal and varying environmental conditions (Negri et al., 2021). The activity of serotonin is regulated by various distinct mechanisms. Recently, miRNAs have been shown to regulate serotonin biosynthesis (Bhowal et al., 2021).
3 MIRNAS AS IMPORTANT REGULATORS OF SEROTONIN BIOSYNTHESIS
Previously, miRNAs have been implicated in various aspects of plant growth and development, including several abiotic and biotic stresses (Gautam et al., 2017, 2021; Mishra et al., 2022, 2023; Mishra & Maurya, 2023; Panda et al., 2022; Singh et al., 2018, 2020, 2021; Vivek & Kumar, 2021; Yadav et al., 2020). In animal systems, it is well known that miRNAs regulate the accumulation of serotonin by modulating various genes (Arai et al., 2013; Kassan et al., 2022; McKibben & Dwivedi, 2021; Zhao et al., 2019). However, there have been no such reports in plants to date. Recently, it has been reported that miRNAs affect the accumulation of serotonin and melatonin by targeting their biosynthetic genes (Bhowal et al., 2021). They identified 40, 51, 57 and 7 probable miRNAs in Arabidopsis, rice, sorghum and tomato, respectively, that can target enzymatic genes involved in serotonin and melatonin biosynthesis. Furthermore, they predicted that conserved miR156 could target the ASMT gene family in rice, sorghum and tomato but not in Arabidopsis. They also predicted that other miRNAs, including miR159, miR393a, miR398a, miR447 and miR5635, can precisely target Arabidopsis genes but not the homologous rice, sorghum or tomato genes due to a lack of target sequence homology (Bhowal et al., 2021) (Figure 1B). Furthermore, they predicted and validated that osa-miR6249 can target OsTDC5 under light/dark and salinity stress. osa-miR1846e preferentially targets OsASMT18 under salinity and drought stress (Bhowal et al., 2021). Both miRNAs, however, had no effect on their targets in response to heat stress.
4 SEROTONIN RECEPTOR AND MODE OF ACTION MECHANISM
Investigations into serotonin receptors suggest that they played a key role during the early course of evolution in both vertebrates and invertebrates (Erland et al., 2015, 2016, 2018; Erland, Turi, & Saxena, 2019). Serotonin receptors such as 5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5, 5-HT6 and 5-HT7 have been implicated in serotonin signalling in both vertebrates and invertebrates (Erland et al., 2016). However, the serotonin receptors in plants have yet to be discovered. As a result, identifying and characterizing serotonin receptors in plants are an exciting field for future research. It is possible that auxin receptors might be substituted by the serotonin receptor in plants due to the structural similarity of auxin with serotonin and thus regulate plant architecture (Mukherjee, 2020). The following sections will explain how serotonin affects plant growth and development by modulating phytohormonal crosstalk.
5 SEROTONIN–AUXIN CROSSTALK IN PLANT GROWTH AND ROOT DEVELOPMENT
Auxin is a crucial regulator of plant growth and development and acts as a versatile coordinator of these processes. Both auxin and serotonin have functional similarities with a common biosynthetic pathway. Serotonin, like auxin, has been implicated in plant growth and root development, exhibiting basic functional similarities. Furthermore, both auxin and serotonin are primarily synthesized in plant roots (Kaur et al., 2015). It has been demonstrated by using gas chromatography–mass spectrometry (GC–MS) that plants can absorb serotonin from the growth media.
Previously, it was shown that serotonin promotes plant growth and root development in various plant species, such as Hypericum perforatum, Mimosa pudica, Hordeum vulgare, Juglans nigra × Juglans regia, Helianthus annus and Arabidopsis thaliana (Csaba & Pál, 1982; Gatineau et al., 1997; Giridhar & Ravishankar, 2009; Murch et al., 2001; Pelagio-Flores et al., 2011). Recently, auxin has been shown to modulate indoleamine biosynthesis and affect the ratio of serotonin:melatonin to modulate plant growth and morphogenesis (Erland & Saxena, 2019). Serotonin (100 μM) promotes root induction and shoot multiplication by 60% and 70%, respectively, in M. pudica (Giridhar & Ravishankar, 2009). Moreover, it has been shown that lower doses of serotonin (10–160 μM) enhanced lateral root (LR) growth in Arabidopsis. However, at high doses (150–600 μM), serotonin inhibits primary and LR growth, as well as root hair formation, but it promotes adventitious root development, indicating that serotonin acts in a dose-dependent manner during LR development (Pelagio-Flores et al., 2011).
Further research showed that the expression of both DR5: uidA and BA3: uidA (auxin reporter) are inhibited in primary and adventitious roots, as well as in LR primordia (LRP), upon serotonin treatment. Serotonin, in combination with indole-3-acetic acid (IAA) or naphthaleneacetic acid (NAA), inhibited the expression of DR5: uidA and BA3: uidA, suggesting that serotonin has an inhibitory effect on auxin-responsive genes. This finding demonstrates that serotonin promotes LR initiation in an auxin-independent manner (Pelagio-Flores et al., 2011). This inhibitory effect of serotonin provides the possibility of serotonin-auxin crosstalk during plant growth and development through altering auxin signalling.
Further research showed that salt stress (120 mM NaCl) modifies the endogenous level of serotonin in H. annuus L. (sunflower) seedlings (Mukherjee et al., 2014). It has been demonstrated that serotonin is primarily localized in the symplastic region of root cells and is differentially accumulated in different zones of primary roots, such as the pericycle, endodermis and vascular bundle, in response to salt stress (Mukherjee et al., 2014). Furthermore, the level of serotonin increases during the progression of seed germination. However, salt stress affects the differential accumulation of serotonin in the oil body-containing cells of sunflower cotyledons, implying that serotonin is involved in long-distance signalling from roots to cotyledons (Mukherjee, 2018). Both serotonin and auxin are important signalling molecules that play crucial roles in primary root development. High salt stress (100 mM) has been shown to suppress the growth of primary root length and LRP due to the inhibition of auxin accumulation in the emerging LRP (Zolla et al., 2010). Furthermore, NaCl (120 mM) treatment inhibited primary root growth and hypocotyl elongation in sunflower seedlings (Mukherjee et al., 2014). Further investigation showed that the exogenous application of serotonin restored primary root growth and hypocotyl elongation of sunflower seedlings subjected to salt stress (Mukherjee et al., 2014). The inhibition of root growth by salt stress is thus due to a partial impairment of auxin activities caused by increased serotonin biosynthesis. Thus, the distribution pattern of serotonin affects the auxin gradient between roots and cotyledons in sunflower seedlings under salt stress (Mukherjee, 2018). However, further research is needed to fully understand the mechanisms underlying this relationship.
According to a recent study, serotonin suppresses primary root elongation in Arabidopsis by regulating meristem and elongation zones (Wan, Zhang, Sun, et al., 2018). Furthermore, serotonin promotes the accumulation of H2O2 in the elongation zone and inhibits the accumulation of O2− in the meristematic zone via the pathway controlled by UPBEAT1 (UPB1), a transcription (TF) with a basic helix–loop–helix (bHLH) domain (Tsukagoshi et al., 2010). It has been shown that UPB1 directly modulates the expression of a group of peroxidases that regulate the balance of ROS between the zones of cell proliferation and the zone of cell elongation (Tsukagoshi et al., 2010). Furthermore, it has been suggested that the UPB1 pathway is independent of auxin and cytokinin. Thus, UPB1 regulates the ROS signalling in Arabidopsis root to control the transition from proliferation to differentiation. The disruption in UPB1 activity affects this ROS equilibrium, which delays the onset of differentiation (Tsukagoshi et al., 2010). Thus, serotonin disrupts the ROS balance in the root tips and inhibits primary root growth (Wan, Zhang, Sun, et al., 2018). Moreover, serotonin has been demonstrated to influence auxin distribution in root tips by decreasing the expression of auxin-related genes and decreasing auxin transport by modulating the abundance of AUXIN TRANSPORTER PROTEIN 1 (AUX1) and PIN-FORMED 2 (PIN2) (Wan, Zhang, Sun, et al., 2018). These findings suggest that high levels of serotonin inhibit primary root elongation by regulating H2O2 and O2− distribution in primary root tips in an auxin-dependent manner (Wan, Zhang, Sun, et al., 2018; Wan, Zhang, Wang, et al., 2018).
6 SEROTONIN-JA AND ET CROSSTALK IN ROOT GROWTH DEVELOPMENT
Jasmonic acid and ethylene are two important phytohormones that have been implicated in plant defense and root architecture. In plants, JA and ET work together to transactivate defense-related genes. Exogenous application of methyl jasmonate (MeJA) has been found to improve serotonin biosynthesis (Ishihara et al., 2008). It has been shown that serotonin inhibits primary root growth by modulating JA-ET crosstalk and ROS signalling (Pelagio-Flores et al., 2016). Previously, it was shown that the ozone-sensitive radical-induced cell death1 (rcd1) mutant is resistant to chloroplastic ROS and has a longer primary root length at 300 μM serotonin than Col-0, demonstrating resistance to serotonin. Furthermore, coronatine-Insensitive 1 (coi1-1) and jasmonate resistant 1 (jar1) mutants have been demonstrated to be resistant to serotonin (300 μM) and to have longer primary root and meristem sizes than the control Col-0 (Pelagio-Flores et al., 2016). Under normal conditions, coi1 and jar1 mutants accumulate more ROS than serotonin-treated plants, indicating that coi1 and jar1 mutants are resistant to serotonin-induced ROS accumulation (Pelagio-Flores et al., 2016). As a result, serotonin-mediated ROS redistribution at the root tip hampers primary root growth. This evidence points to the importance of COI1 and JAR1-mediated regulation of primary root growth in response to serotonin via ROS-JA crosstalk in Arabidopsis.
Moreover, recent research suggests that ET inhibits primary root growth in rice and Arabidopsis by modulating auxin, ABA, gibberellic acid (GA), CK, JA and brassinosteroid (BR) crosstalk (Qin et al., 2019). The ET signalling mutants ethylene response 1 (etr1), ethylene insensitive 2 (ein2) and ethylene insensitive 3 (ein3) were recently found to have longer primary root lengths than wild-type (WT) Arabidopsis upon treatment with 300 μM serotonin, implying ET-mediated regulation of primary root length in response to serotonin (Pelagio-Flores et al., 2016). Previously, silver ions have been shown to inhibit ethylene responses and affect downstream signalling by binding to its receptors (Beyer, 1976; Rodrı́guez et al., 1999). Furthermore, the silver nitrate (AgNO3)-treated seedlings had longer roots than the WT and serotonin (300 μM)-treated Arabidopsis seedlings. The shortened primary root length of serotonin-treated seedlings was restored by AgNO3, indicating the involvement of ET crosstalk in primary root growth and development (Pelagio-Flores et al., 2016). Furthermore, compared to WT seedlings, jar1, etr1, ein2 and ein3 mutants accumulated less ROS at the root tip in response to paraquat (Pelagio-Flores et al., 2016). Paraquat is generally used to generate ROS. These findings suggest that the accumulation of ROS in meristematic and elongation zones may be implicated in serotonin-mediated suppression of primary root growth development (Pelagio-Flores et al., 2016). Thus, serotonin-JA-ET crosstalk, together with ROS, plays an important role in Arabidopsis root growth and development.
7 SEROTONIN-CK AND ABA CROSSTALK IN PLANT MORPHOGENESIS
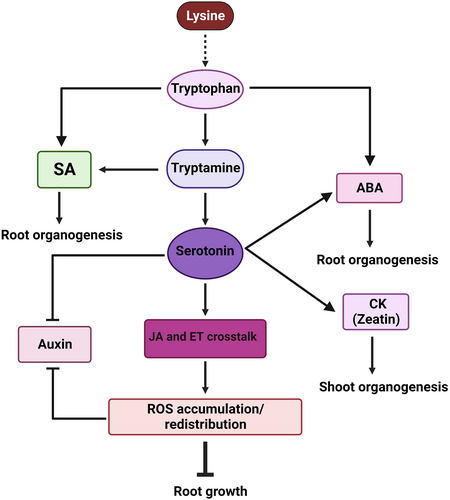
Plants respond to a variety of environmental and internal stimuli to maximize their growth and development for survival. ABA and CK are two important plant hormones that function antagonistically to regulate various developmental processes as well as environmental stress in plants. However, the molecular evidence behind this antagonism is poorly understood (Huang et al., 2018). The RiceXPro database of 7-day-old seedlings suggests that ABA and JA are the primary regulators of serotonin production (Sato et al., 2011). Recently, serotonin-treated plants have been shown to perform better under salt stress by upregulating ZINC FINGER OF ARABIDOPSIS THALIANA12 (ZAT12) and ROS signalling (Shukla et al., 2021). ZAT12 is a C2H2 zinc finger protein that is induced by various abiotic stress, such as drought, salinity, osmotic, oxidative, and ABA treatment (Ciftci-Yilmaz & Mittler, 2008; Davletova, Rizhsky, et al., 2005; Davletova, Schlauch, et al., 2005; Koussevitzky et al., 2007; Miller et al., 2008; Mittler et al., 2006; Rizhsky et al., 2004; Rossel et al., 2007; Sakamoto et al., 2004; Zhang et al., 2012). It has been recently shown that, when exposed to high salt concentrations (50 mM NaCl), plants pre-treated with 10 μM serotonin activated the expression of the ROS signalling transcription factor (TF) ZAT12. However, it has been previously demonstrated that the gene AT3G02480 (ABR), an ABA-response protein (ABR), was upregulated in plants overexpressing ZAT12 (Davletova, Schlauch, et al., 2005). Recently, ABA Insensitive 5 (ABI5) has been demonstrated to regulate ABRs expression by directly binding to their promoter (Su et al., 2016). Thus, the upregulation of ZAT12 by serotonin pre-treatment and high salt concentration ultimately activates the ABA signalling, which plays a vital role in plant growth and morphogenesis. The serotonin-treated Arabidopsis plants have a better response than that of non-treated Arabidopsis seedlings in terms of fresh weight, root number, and root length when exposed to high salt concentrations (50 mM NaCl) (Shukla et al., 2021). As a result, serotonin lessened the inhibitory effect of salt stress by increasing organogenesis. On the other hand, in H. perforatum (L.), serotonin treatment dramatically enhanced the production of zeatin (cytokinin) and ABA during root culture, suggesting that serotonin, together with cytokinin and ABA, plays an important role in plant morphogenesis (Erland et al., 2018). Based on these findings, we developed a hypothetical model of serotonin and phytohormonal crosstalk in plant growth and root development (Figure 2).
8 SEROTONIN-ABA CROSSTALK IN ROOT SUBERIZATION
Suberin has been shown to act as a physical barrier, preventing water and nutrient losses from tissues and providing protection against various abiotic and biotic stresses (Chen et al., 2022). As a result, root suberization is critical in response to various abiotic and biotic stressors (Chen et al., 2022). Previous research has demonstrated that serotonin can regulate osmotic pressure in plants under salt stress by altering ion transport into roots (Roshchina, 1990). According to a recent study, serotonin is more produced in rice roots than in other vegetative tissue but has minimal effect on root architecture (Lu et al., 2022). Furthermore, Lu and his colleagues demonstrated that serotonin acts downstream of ABA in regulating suberization in rice and Arabidopsis roots and adversely controls the suberization in rice roots under salinity stress (Lu et al., 2022). Thus, the ABA-serotonin module could be used to develop new crop varieties resistant to various abiotic and biotic challenges. Overall, serotonin and phytohormone crosstalk play an important role in plant growth and stress responses (Table 1). Therefore, serotonin and phytohormone crosstalk is an active research areas and may have important implications for plant breeding and crop improvement.
| Phytohormone | Function | Species | References |
|---|---|---|---|
| Auxin | Root growth development | Arabidopsis | (Erland et al., 2018; Erland & Saxena, 2019; Mukherjee, 2018; Pelagio-Flores et al., 2011; Wan, Zhang, Sun, et al., 2018; Wan, Zhang, Wang, et al., 2018). |
| ABA | Plant growth and morphogenesis (root and shoot organogenesis), tissue detoxification and free radical homeostasis. | O. sativa, H. perforatum L. | (Erland et al., 2018; Mukherjee, 2018; Sato et al., 2011). |
| Root suberization | O. sativa and Arabidopsis | (Lu et al., 2022). | |
| CK (Zeatin) | Plant growth and morphogenesis (root and shoot organogenesis) | H. perforatum L. | (Erland et al., 2018). |
| ET | Root growth development together with JA and serotonin | Arabidopsis | (Mukherjee, 2018; Pelagio-Flores et al., 2016). |
| JA | Root growth development together with ET and improve nutritional values in rice grains and defense responses | Arabidopsis O. sativa |
(Sato et al., 2011; Pelagio-Flores et al., 2016; Mukherjee, 2018; Yang et al. 2018). |
- Abbreviations: ABA, abscisic acid; CK, cytokinin; ET, ethylene; JA, jasmonic acid.
9 SEROTONIN IN PLANT FITNESS AND STRESS TOLERANCE
Plant growth and development are regulated by both external and internal factors. The normal expression of serotonin is crucial for growth and fitness. Previous studies have shown that serotonin promotes plant growth and development and maintains plant fitness under both normal and stress conditions by modulating various genes and phytohormones (Roychoudhury, 2021).
9.1 Serotonin: senescence and pollen development
Senescence is a well-regulated and genetically controlled developmental process characterized by leaf yellowing due to chlorophyll and protein degradation during nutrition reallocation from old to new organs (Distelfeld et al., 2014; Kang, Kim, et al., 2009; Roychoudhury, 2021). In rice, the content of serotonin is increased up to 700-fold in senescent leaves (Kang, Park, et al., 2009; Negri et al., 2021). Thus, serotonin functions as a natural antioxidant in senescent tissue and may postpone senescence-induced oxidative damage in plants as well as protect the integrity of vascular components by permitting nutrient mobilization (Erland & Saxena, 2017; Kang, Kim, et al., 2009). Furthermore, serotonin has been reported to play a crucial role in bud maturity and ovule development in Datura metel (Erland & Saxena, 2017). Moreover, serotonin has been shown to play a crucial role in stage transition in H. perforatum during the early stages of reproduction. However, in Hippeastrum hybridum and Equisetum arvense, exogenous serotonin promotes pollen germination, highlighting the significance of serotonin in pollen development (Negri et al., 2021; Roychoudhury, 2021).
9.2 Serotonin: light and circadian clock
The circadian clock regulates various aspects of plant growth and development in diverse plant species (Eckstein et al., 2021; Sakuraba, 2021; Yakir et al., 2007). Serotonin has been proposed as a crucial messenger and a potent modulator of phytochrome signalling (Erland & Saxena, 2017; Roychoudhury, 2021). Compared to red light-exposed protoplasts, serotonin treatment improves calcium uptake in dark-grown protoplasts (Das & Sopory, 1985; Erland et al., 2016). In addition, serotonin-soaked maize leaves had increased nitrate reductase activity compared to red light-exposed leaves. These findings demonstrated the role of serotonin as a promoter of phosphoinositide turnover and a putative primary messenger induced by the activation of phytochrome far-red (Pfr), which is located upstream of nitrate reductase in maize (Erland et al., 2016; Raghuram & Sopory, 1995). Serotonin treatment replicated the red-light response, promoted the synthesis of nitrate reductase and inhibited the accumulation of phytochrome I (PhyI). These findings suggest that serotonin acts as an activator of phytochrome signalling (Roychoudhury, 2021).
Furthermore, serotonin and its precursors have been observed to differentially accumulate in response to different wavelengths of light (Erland et al., 2016). Recently, Sun and colleagues have identified a basic leucine zipper 18 (OsbZIP18) TF as a positive regulator of serotonin biosynthesis in rice (Sun et al., 2022). The authors have showed that the expression of OsbZIP18 was upregulated to ~10-fold at 3 h of ultraviolet B (UV-B) light treatment (Sun et al., 2022). Further investigation showed that OsbZIP18 overexpression lines accumulated more serotonin and its early precursors (tryptophan and tryptamine), resulting in stunted growth, dark-brown, and UV-B sensitive phenotypes in rice (Sun et al., 2022). Furthermore, using luciferase and electrophoretic mobility shift (EMSA) assays, they further validated that OsbZIP18 promotes serotonin biosynthesis genes by directly interacting with the promoters of OsTDC1, OsTDC3 and OsT5H (Sun et al., 2022). In Dunaliella bardawil, serotonin has been shown to accumulate differently in response to light. Recently, it has been shown that the accumulation of serotonin in Cordyline australis is regulated by seasonal variations and the circadian clock, suggesting the involvement of the circadian clock in serotonin biosynthesis (Beilby et al., 2015; Ramakrishna et al., 2011). Furthermore, circadian clock has been shown to modulate the endogenous concentrations of indole-3-acetic acid (IAA), serotonin, melatonin, ABA, and JA in both summer and winter, in Characeae (Chara australis Brown) (Beilby et al., 2015). Moreover, Beilby et al. (2015) showed the close synchronization of IAA and serotonin concentration maxima in both summer and winter plants, indicating that IAA is synthesized via the tryptamine pathway, which interacts with the serotonin/melatonin synthesis pathway (Beilby et al., 2015). In rice, recent research has shown that the diurnal/circadian clock regulates the genes that code for the enzymes involved in serotonin and melatonin biosynthesis, such as TDC, T5H, SNAT, ASMT and so forth. (Bhowal et al., 2021). However, the detailed mechanism of serotonin biosynthesis regulation by the circadian clock is yet to uncover. In addition, by modulating various phytohormones, light and serotonin crosstalk may regulate flowering time. However, there is no direct evidence that light and serotonin crosstalk regulates flowering time in plants. Earlier, it has been shown that serotonin directly alters phytochrome by direct activation and modification of phytochrome I transcripts (Erland et al., 2016; Erland, Turi, & Saxena, 2019; Roychoudhury, 2021). In plants, phytochromes have been shown to play an essential role in regulating the flowering time by sensing the presence of light and activating the genes involved in the flowering process (Yuan et al., 2023). Thus, it is possible that serotonin could control flowering time through interactions with photoreceptors. However, more research is required to support this hypothesis.
9.3 Serotonin in gamete compatibility and bud dormancy
Gamete interactions can have a large impact on species speciation and hybridization. As a result, the component responsible for gamete compatibility is essential for the early development of reproductive incompatibilities across identical or similar species (Harper & Hart, 2005). Pollen allelopathy has been previously shown to play an important role in fertilization success and gamete compatibility (Erland et al., 2016; Erland & Saxena, 2017; Erland, Turi, & Saxena, 2019; Roshchina, 2001, 2005, 2006; Roshchina & Melnikova, 1998). Serotonin has been implicated in pollen allelopathy, and the evidence suggests that it could play a role in microspore formation by modulating cyclic adenosine monophosphate (cAMP) signalling (Erland et al., 2016; Roshchina, 2001). Further research indicates that inoculating microspores with serotonin promotes germination in Hippeastrum spp. (Erland et al., 2016; Roshchina, 2001). Moreover, it has been demonstrated that serotonin promotes somatic embryogenesis in coffee (Erland et al., 2016).
Serotonin has been found to have a crucial role in bud dormancy, a phenomenon in which most physiological actions are delayed or slowed (Erland et al., 2016; Gianella et al., 2021). It has been observed that serotonin and other biogenic amines, such as proline, tyramine and histamine, are substantially accumulated during dormancy. Similarly, in D. metel L., the level of serotonin is strongly induced at the beginning of the various stages of ontogenesis, implying a role of serotonin and other biogenic amines in bud dormancy (Erland et al., 2016).
9.4 Serotonin in abiotic stress resistance
The role of serotonin in abiotic stress is a new and exciting area of study. Both serotonin and melatonin are tryptophan-based indole compounds that regulate various biological and developmental responses related to plant growth and defense (Kaur et al., 2015). Researchers have shown that both serotonin and melatonin play important roles in abiotic stress tolerance (Erland et al., 2016; Kaur et al., 2015). Previously, serotonin has been shown to modulate ion influx into chloroplasts, implying the importance of serotonin in salt/salinity stress (Erland et al., 2016). Serotonin-treated sunflower seedlings grown on NaCl demonstrated longer primary roots than untreated seedlings (Erland et al., 2016). Recently, salinity stress has been shown to inhibit LR growth and have a negative impact on Arabidopsis development (Xu et al., 2020; Zhang et al., 2023). It has been previously demonstrated that both serotonin and melatonin induce the expression of LATERAL ORGAN BOUNDARIES DOMAIN 16 (LBD16) and XYLOGLUCAN ENDOTRANSGLYCOSYLASE RELATED 6 (XTR6), which promote LR growth in Arabidopsis (Wan, Zhang, Wang, et al., 2018). Both LBD16 and XYLOGLUCAN ENDOTRANSGLUCOSYLASE 23 (XTH23)/XTR6 play essential roles during salinity stress (Xu et al., 2020; Zhang et al., 2023). Furthermore, ZINC FINGER OF ARABIDOPSIS THALIANA 6 (ZAT6) has been shown to regulate LBD16 activity, which contributes to downstream cell wall remodelling and promotes LR development under salinity stress (Zhang et al., 2023). XTH23/XTR6, on the other hand, are involved in LR development via the BRI1-EMS-SUPPRESSOR 1 (BES1)-dependent pathway and contribute to LR adaptation to salinity stress (Xu et al., 2020). Thus, the plants pre-treated with serotonin and melatonin may perform better when subjected to salinity stress due to the activation of the LBD16 and XTH23/XTR6 genes.
Stresses like temperature, heavy metals, such as cadmium (Cd), and x-ray radiation are some of the most severe abiotic stresses (Erland et al., 2016). It has been suggested that serotonin plays an important role in Cd stress (Erland et al., 2016). Furthermore, Cd has been shown to induce the serotonin biosynthesis genes TDC and T5H in rice, implying a putative function of serotonin in plants under Cd stress (Erland et al., 2016). On the other hand, x-ray radiation can induce DNA damage, mutations and alteration of gene expression in plants. These effects can severely affect plant growth and fitness (Erland et al., 2016). It has been demonstrated that Vicia faba L., exposed to 400R x-ray radiation, exhibited a dose-dependent suppression of secondary root growth. Intriguingly, the administration of serotonin in combination with 400R x-ray radiation induced secondary root growth after a lag phase (Erland et al., 2016; Lozeron et al., 1964). Thus, these results suggest the importance of serotonin in connection with heavy metals and x-ray radiation. However, the specific role of serotonin in plant growth and stress response to heavy metals and x-ray radiation is still not well known. Similarly, temperature stress can significantly impact plant growth and fitness. Recently, it was observed that serotonin and melatonin act together to promote cell proliferation, ethylene, and isoflavone production in G. max under temperature stress (Kumar et al., 2021). It has been further demonstrated that the ratio of serotonin and melatonin regulates the isoflavone content and cell biomass in G. max under temperature stress (Kumar et al., 2021). Both serotonin and melatonin cause the co-expression of isoflavone and ethylene biosynthesis genes, suggesting that ethylene is possibly involved in increasing the expression of isoflavone biosynthesis genes and, as a result, increasing the isoflavone content under temperature stress (Kumar et al., 2021).
10 SEROTONIN IN BIOTIC STRESS RESISTANCE
Serotonin is essential for biotic stress resistance and acts as a structural barrier against fungal infections (Hayashi et al., 2016). Pathogen elicitors strongly stimulate serotonin in Anabaena variabilis, Camptotheca acuminata and Capsicum annuum (Negri et al., 2021). The accumulation of serotonin in response to pathogen stress has demonstrated that serotonin protects various members of the Poaceae family against fungi and nematodes (Erland et al., 2015). Although the precise mechanism of serotonin in the response to pathogen stress is unknown, it has been demonstrated that serotonin accumulation in plant cells acts as a mechanical barrier against invading pathogens (Hayashi et al., 2016; Negri et al., 2021). Recently, the accumulation of serotonin dehydrodimer (5,5′-dihydroxy-2,4′-bitryptamine) in barley, rice and foxtail millet has been demonstrated to protect against the necrotrophic fungus Bipolaris sorokiniana (Ishihara et al., 2017).
Similarly, together with biogenic amines, serotonin acts as a barrier against various herbivores (e.g., acetylcholine and histamine) (Negri et al., 2021). Setaria viridis accumulates the tryptophan-derived chemicals tryptamine and serotonin, which play a crucial role in pest defense responses (Dangol et al., 2022). To investigate the defensive role of serotonin, Rhophalosiphum padi (bird cherry-oat aphids) were either exogenously applied to the plant media or subjected to artificial diet bioassays (Dangol et al., 2022). Interestingly, serotonin exposure enhanced aphid mortality in both situations (Dangol et al., 2022). As a result of this study, serotonin could be a promising biochemical tool for generating pest-tolerant verities or cultivars.
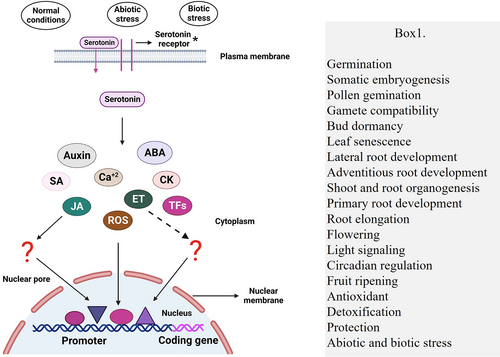
A study in rice showed that serotonin reduces biotic stress by producing halo lesions (a lesion with a brown ring) against M. oryzae (Hayashi et al., 2016). Furthermore, it has been shown that halo formation requires both light and ABA-mediated synthesis of tryptamine and serotonin (Hayashi et al., 2016). In rice, TDC1-mediated serotonin accumulation has been shown to delay leaf senescence and provide protection against Bipolaris oryzae (Ishihara et al., 2008; Kang, Kim, et al., 2009). Moreover, in rice, ABA and methanol have been found to work synergistically to enhance N-acetylserotonin (NAS) production and regulate leaf senescence (Kang et al., 2011). It has been shown that melatonin is synthesized via NAS from serotonin by the sequential action of two enzymes, SNAT and ASMT (Bhowal et al., 2021; Negri et al., 2021). Previous research has shown that both NAS and melatonin equally induced defense-related genes, such as ISOCHORISMATE SYNTHASE 1 (ICS1), PATHOGENESIS-RELATED PROTEIN 1 (PR1) and PATHOGENESIS-RELATED PROTEIN 5 (PR5) (Lee et al., 2014). However, NAS could also activate the MITOGEN-ACTIVATED PROTEIN KINASES (MAPKs) to confer biotic stress tolerance, but to a lesser extent than melatonin (Lee & Back, 2016; Yu et al., 2018). Furthermore, high light intensity causes ROS production, implying that ROS production, together with serotonin biosynthesis and oxidation, is implicated in halo formation. Thus, light and ABA-dependent serotonin synthesis promote scavenging mechanisms adjacent to non-infected tissue, which is implicated in disease resistance. As a result, serotonin has been demonstrated to be regulated by both environmental and internal factors, which then activate different signalling pathways to govern plant growth and stress response either directly or indirectly (Figure 3).
11 FUNCTIONAL CORRELATIONS OF SEROTONIN AND MELATONIN
Serotonin and melatonin are both important signalling molecules in plants, and they have been shown to have functional correlations in various plant processes. Both serotonin and melatonin synthesis pathways initially start with the same precursor, l-tryptophan, and have structural similarities to IAA (Mukherjee, 2018). The serotonin and melatonin pathways are highly coordinated and well-regulated (Mukherjee, 2018). In plants, serotonin is synthesized from the tryptophan amino acid through a series of enzymatic reactions. The tryptophan is converted into tryptamine, by the enzyme known as TDC. Further, the enzyme T5H converts tryptamine into serotonin. On the other hand, melatonin is synthesized from serotonin via a series of enzymatic reactions. The first step involves the conversion of serotonin to NAS by the enzyme SNAT. NAS is then converted to melatonin by the enzyme ASMT (Mukherjee, 2018; Negri et al., 2021). It has been proven that serotonin is substantially more abundant than melatonin. However, serotonin appears to be less dynamic than melatonin in generating a wide range of physiological responses. As serotonin, melatonin also modulates various plant physiological pathways, including growth and reproduction, senescence, chrono-regulation, root and shoot organogenesis, and complex hormonal interactions, as well as stress responses (Bhowal et al., 2021; Liu et al., 2022; Mukherjee, 2018; Zhang et al., 2022). Both serotonin and melation have been shown to control root system architecture (Mukherjee, 2018). However, recently, the comparative physiological responses and transcriptome analysis showed that medium concentrations of serotonin and melatonin considerably promote LR growth but have no effect on primary root growth (Wan, Zhang, Wang, et al., 2018).
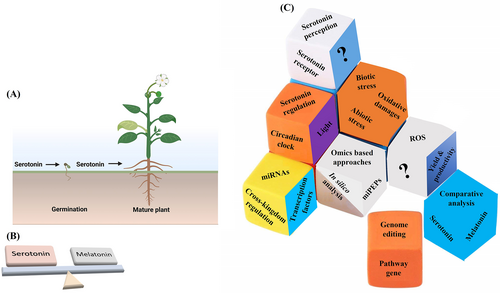
Recently, quantum dot nanoparticles have been developed to directly visualize melatonin and serotonin, as well as their redistribution in living plant tissue (Erland, Yasunaga, et al., 2019). Further investigation showed that both serotonin and melatonin promote the expression of the cell wall remodelling-related genes LBD16 and XTR6. Previously, it was shown that both LBD16 and XTR6 regulate LR formation by modulating the auxin gradient (Goh et al., 2019; Vilches Barro et al., 2019). Thus, these data support the idea that serotonin and melatonin are devoid of auxin-like activity (Abbasi et al., 2020). Additionally, both serotonin and melatonin promote tolerance under iron (Fe) deficiency in Arabidopsis by activating the Fe-responsive genes (Wan, Zhang, Wang, et al., 2018). Both serotonin and melatonin have been demonstrated to be important for H. performatum L. morphogenesis (Erland & Saxena, 2019). Furthermore, a balanced ratio of melatonin and serotonin in plants has been found to alter growth and morphogenesis (Figure 4A,B) (Erland & Saxena, 2019; Murch et al., 2001; Pelagio-Flores & López-Bucio, 2016). It has recently been discovered that serotonin and melatonin act synergistically and promote cell division, ethylene production and isoflavone biosynthesis in G. max during temperature stress (Kumar et al., 2021).
Melatonin has been shown to promote vegetative development in dark-grown Lupinus albus L., increase cotyledon extension, and stimulate adventitious and LR formation in a manner similar to IAA, suggesting that melatonin has auxinic-like activity on plant growth (Pelagio-Flores & López-Bucio, 2016). It has been shown that both serotonin and melatonin regulate root system architecture and plant growth in a very specific and dose-dependent manner by modulating phytohormonal crosstalk (Tables 1 and 2) (Pelagio-Flores & López-Bucio, 2016). In contrast, melatonin treatments promote LR growth without affecting auxin-responsive genes (Pelagio-Flores et al., 2012). Previously, it was shown that melatonin treatment reduced ethylene accumulation in etiolated lupin hypocotyls (Arnao & Hernández-Ruiz, 2007). However, Weeda et al. (2014) recently identified that most genes of the ABA, ET, SA and JA pathways were upregulated in response to melatonin treatment, whereas genes involved in auxin responses and signalling were mostly downregulated. As a result of their amphipathic and pleotropic nature, both serotonin and melatonin exhibit characteristics of classic plant hormones due to their diverse nature and abilities to regulate genetic functions. They have been classified as master regulators in plants due to their following functions: (i) Both acts as a bio-stimulator under abiotic and biotic stresses with the ability to activate signalling pathways pertaining to SA, ABA, ET, and JA (Khanna et al., 2023; Mukherjee, 2018). (ii) Both serotonin and melatonin serve as regulatory molecules in the antioxidant defense network, playing a role in maintaining redox homeostasis. Additionally, they also regulate oxidative stress and ROS level (Khanna et al., 2023; Mukherjee, 2018). (iii) Both serotonin and melatonin play a key role in regulating enzymes that encode various phytohormones, transcripts, enzymes and co-factors involved in signalling crosstalk (Erland et al., 2018; Kaur et al., 2015; Khanna et al., 2023; Mukherjee, 2018, 2020; Mukherjee et al., 2014). (iv) They both regulate plant growth and stress response through their interaction with various phytohormones (Tables 1 and 2) (v) They both play key role in delaying senescence by modulating senescence-related marker genes (Kang, Kim, et al., 2009; Khanna et al., 2023).
| Phytohormone | Function | Species | References |
|---|---|---|---|
| Auxin | Delaying leaf senescence, adventitious root development | Arabidopsis, Solanum lycopersicum L. | (Shi et al. 2016; Wen et al. 2016; Mukherjee, 2018). |
| ABA | Induced cold tolerance in drought primed wild type Barley, regulates ABA metabolism under drought stress, provides cold tolerance by modulating both ABA dependent and ABA independent, cold tolerance in Melon | Barley, Malus species Elymus nutans Citrullus lanatus L. |
(Li et al. 2015, 2017; Fu et al. 2017; Mukherjee, 2018). |
| CK | Delaying heat stress-induced leaf senescence by modulating ABA and CK biosynthesis and signalling pathways | Lolium perenne L. | (Zhang et al. 2017; Mukherjee, 2018). |
| ET | Regulates fruit ripening in tomato and some other plants | Solanum lycopersicum L | (Weeda et al. 2014; Sun et al. 2015, 2016; Mukherjee, 2018). |
| JA | Biotic stress tolerance against various pathogens and regulates anthocyanin biosynthesis | O. sativa, Arabidopsis | (Wei et al. 2016; Ai and Zhu 2018; Mukherjee, 2018). |
| SA | Biotic stress tolerance against various pathogens | Arabidopsis Nicotiana benthamiana, Malus prunifolia |
(Yin et al. 2013; Lee et al., 2014; Arnao and Hernández-Ruiz, 2015; Mukherjee, 2018). |
| GA | Promotes seed germination by modulating GA and ABA under high salinity | Cucumis sativus L. | (Zhang et al. 2014; Mukherjee, 2018). |
| BR | Regulating seedling development | O. sativa | (Hwang and Back 2018; Mukherjee, 2018). |
- Abbreviations: ABA, abscisic acid; BR, brassinosteroid; CK, cytokinin; ET, ethylene; GA, gibberellic acid; JA, jasmonic acid; SA, salicylic acid.
12 FUTURE PERSPECTIVES
Climate change is a major challenge for agricultural production across the world. To fulfil the food demands of world populations, we are highly dependent on agriculture. However, agricultural augmentation promotes plant stress via abiotic and biotic stresses, such as cold, heat, temperature, salinity, drought, herbivores and pathogens. Understanding the functional and molecular mechanisms of serotonin in normal and stressful conditions can be a useful tool for plant breeding, especially in the context of climatic and human-induced environmental changes. Plant pathogens are one of the most significant challenges for plants to survive and perform better. However, serotonin has been shown to play a crucial role in plant development and defense by modulating phytohormonal crosstalk. The application of serotonin biosynthesis genes such as tryptophan decarboxylase (TDC), aromatic L-amino acid decarboxylase (AADC), and tryptamine 5 hydroxylase (Trp 5H), together with modern technology and genome editing tools such as zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), the CRISPR/Cpf1 system and CRISPR/Cas9 in agriculture, can potentially improve the resistance of plants to pathogens. Recently, the first melatonin receptor (CAND2/PMTR1) was discovered in Arabidopsis. However, the serotonin receptor in plants has yet to be discovered. Clearly, more research is required on receptor-mediated serotonin signalling in other aspects of plant growth and development that might be regulated by serotonin signalling in plants. As a result, the identification and characterization of the serotonin receptor could be an interesting area for future research (Figure 4C). Furthermore, the balanced activity of serotonin and phytohormonal crosstalk are required under normal and stressful conditions for a sustainable approach to crop production. To investigate the serotonin-mediated antagonistic or synergistic regulation of phytohormones, more experimental research is needed, such as crystallographic studies coupled with genetic techniques. The identification of cis-elements and receptors of specific genes could also be an important topic. Moreover, investigations of serotonin-mediated regulation of phytohormonal crosstalk and their involvement in plant growth and root development by using omics-based approaches such as genomics, transcriptomics, miRNAomics, interactome, proteomics, metabolomics and metagenomics, are required under normal and stress conditions (Figure 4C).
AUTHOR CONTRIBUTIONS
Vishnu Mishra designed the outline of the article and composed the manuscript and figures. Ananda K. Sarkar provided scientific feedback and critical comments and revised the content. All of the authors read and approved the manuscript.
ACKNOWLEDGEMENTS
Vishnu Mishra and Ananda K. Sarkar acknowledge DBT (DBT/PR41136/AGIII/103/1271/2020) for funding and support. AKS acknowledges support from DST-FIST (SR/FST/LSII/-046/2016) to SLS, JNU, Ananda K. Sarkar also thank Science and Engineering Research Board (SERB) (CRG/2020/005763) for financial support and funding. We also thank JNU for providing excellent facilities and workplace. We also acknowledge https://biorender.com/ for creating the figures.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.




