Alternative electron transport pathways contribute to tolerance to high light stress in lichenized algae
Abstract
The photosynthetic apparatus of lichen photobionts has been well-characterized by chlorophyll fluorescence analysis (e.g., by pulse amplitude modulation [PAM]), which provides a proxy of the activity of photosystem II (PSII) and its antenna. However, such kinetics are unable to directly characterize photosystem I (PSI) activity and the associated alternative electron pathways that may be involved in photoprotection. Instead, PSI can be probed in vivo by near-infrared absorption, measured at the same time as standard chlorophyll fluorescence (e.g., using the WALZ Dual PAM). Here, we used the Dual PAM to investigate cyclic electron flow and photoprotection in a range of mostly temperate lichens sampled from shaded to more open microhabitats. Sun species displayed lower acceptor side limitation of PSI (Y[NA]) early in illumination when compared to shade species, indicative of higher flavodiiron-mediated pseudocyclic electron flow. In response to high irradiance, some lichens accumulate melanin, and Y[NA] was lower and NAD(P)H dehydrogenase (NDH-2)-type cyclic flow was higher in melanised than pale forms. Furthermore, non-photochemical quenching (NPQ) was higher and faster relaxing in shade than sun species, while all lichens displayed high rates of photosynthetic cyclic electron flow. In conclusion, our data suggest that (1) low acceptor side limitation of PSI is important for sun-exposed lichens; (2) NPQ helps shade species tolerate brief exposure to high irradiance; and (3) cyclic electron flow is a prominent feature of lichens regardless of habitat, although NDH-2-type flow is associated with high light acclimation.
1 INTRODUCTION
Lichens often grow in microhabitats where they are exposed to severe abiotic stresses such as desiccation and temperature extremes. They are also often exposed to light levels greater than their photobionts can use in carbon fixation. Unless regulated, excess energy absorbed by the photobionts can convert ground-state oxygen to reactive oxygen species (ROS). These ROS can attack the photosynthetic apparatus, causing photoinhibition and photo-oxidative stress, reducing the ability of the photobionts to fix carbon. Theoretically, tolerance to high light can be achieved first by lowering ROS formation via synthesizing light screening pigments or by using non-photochemical quenching (NPQ) to thermally dissipate excess light energy; second, by scavenging ROS once formed; or third, by repairing ROS-induced damage (Beckett, Minibayeva, Solhaug, et al., 2021). The roles of light-screening pigments (Solhaug & Gauslaa, 2012) and NPQ (Beckett, Minibayeva, Solhaug, et al., 2021) have been relatively well studied. However, it is also known that cyclic electron flow (CEF) translocates extra protons into the thylakoid lumen, activating NPQ without producing reducing compounds in the stroma, thereby lowering ROS formation and protecting both photosystems (PSI and PSII) (Ma et al., 2021; Nawrocki et al., 2019; Roach & Krieger-Liszkay, 2019; Shi et al., 2022). In lichens, little is known about the role of CEF in photoprotection.
There are two main forms of CEF, true CEF and pseudo-CEF (PCEF) (Alboresi et al., 2019). CEF recycles electrons from PSI to the thylakoid plastoquinone (PQ) pool, decreasing NADPH formation and promoting proton pumping by cytochrome (Cyt.) b6f. In higher plants, two CEF pathways operate; the first involves PGR5/PGRL1, which directly reduces Cyt. b6f, and the second that is dependent on the activity of a chloroplast NADH dehydrogenase-like (NDH-1) complex, which reduces the PQ pool upstream of Cyt. b6f (Nawrocki et al., 2019; Peltier et al., 2016). It was recently shown that NDH-1 genes have disappeared from most Chlorophyta species, including Chlamydomonas and Chlorella (Serrano-Pérez et al., 2022). Instead, microalgae have the NDH-2 protein (Peltier et al., 2016). The current view is that the main function of NDH-2 is chlororespiration in the dark (i.e., NAD(P)H reduction of the PQ pool), whereas the PGR5/PGRL1 route is mainly responsible for CEF under light (Nawrocki et al., 2019). The main pathway for PCEF in photosynthetic organisms from cyanobacteria to gymnosperms is mediated by a class of enzymes called flavodiiron proteins (FLVs or FDPs) that accept electrons downstream of PSI to reduce oxygen to water. FLV proteins are apparently absent in angiosperms (Alboresi et al., 2019). Apart from some data suggesting FLV activity in the common lichen photobiont Trebouxia (Ilík et al., 2017), there have been few studies on CEF in lichens. This is probably at least in part because of the inability of chlorophyll fluorescence to measure PSI activity in vivo. Therefore, the potential contribution of alternative electron pathways to high light tolerance in lichens remains unknown.
The first aim of the present study was to compare CEF in a range of lichens, from woodland shade-adapted species to those that grow in open habitats. The species compared varied not only in the amount of light they receive but also in how light levels in their microhabitats vary with time. While woodland species may receive most of their PAR as “sunflecks,” during which light levels rapidly increase and decrease, sun species experience much more steady levels of high irradiance with changes only occurring as a result of variations in cloud cover. As PCEF has been suggested to be particularly effective in protecting against fluctuating light (Gerotto et al., 2016), we assessed the acceptor limitation of PSI (Y[NA]) during the first few seconds of illumination, indicative of FLV activity (Gerotto et al., 2016; Jockel et al., 2018). To quantify overall CEF, we calculated the difference in electron transport rates (ETR) of PSI and PSII under various intensities of light (Miyake et al., 2004). As CEF and NPQ have been proposed to compensate for each other in the avoidance of photoinhibition in free-living green algae (Chaux et al., 2017), the induction and relaxation of NPQ were also measured.
In an additional study, we compared NPQ and CEF between melanized and pale thalli of the two shade lichens studied here (Crocodia aurata and Lobaria pulmonaria) and between heavily and lightly pigmented thalli of the parietin-containing lichen Xanthoria parietina. More heavily pigmented thalli were collected from more sun-exposed microhabitats. The synthesis of screening pigments in the upper cortex is a particular tolerance mechanism used by some species of lichens that provides longer-term adaptation to light stress (Mafole et al., 2019a; Solhaug & Gauslaa, 2012). Furthermore, to elucidate if NDH-2-type CEF electron flow occurs in L. pulmonaria, and is associated with melanisation, we measured post-illumination-induced changes in chlorophyll fluorescence. In Chlamydomonas reinhardtii, this has been shown to be caused by the reduction of PQ by NDH-2 using stromal reductants (mainly NADPH) as part of chlororespiration (Jans et al., 2008; Krishna et al., 2019).
Our results showed that levels of CEF in photobionts are generally very high but weakly correlated with the perceived ambient light levels of the microhabitats that the lichens were growing in. Contrary to our original hypothesis, rather than being high in lichens collected from microhabitats with fluctuating rather than steady light levels, FLV activity is positively correlated with the general light level of the microhabitat of a particular lichen. Results also showed that FLV and NDH-2 activities are significantly greater in melanised thalli than in pale thalli, suggesting that melanisation on its own may offer insufficient photoprotection to Lobaria pulmonaria.
2 MATERIALS AND METHODS
2.1 Plant material
Material was collected from a variety of shaded and sunny locations as outlined in Table 1. For most species, 1 cm disks were cut 24 h before experimentation, while for Cladonia, 1 cm podetial tips were used. Thalli were hydrated by placing them on moist filter paper and kept under cool (15°C) dim light (30 μmol m−2 s−1) until measurement.
| Lichen species | Photobiont | Collection locality | Substrate | Exposure of microhabitat |
|---|---|---|---|---|
| Lobaria pulmonaria (L.) Hoffm. | Symbiochloris | Outskirts of Syktyvkar, Russian Federation | Old trees of Populus tremula | Deep shade inside a tree canopy |
| Crocodia aurata (Ach.) Link | Symbiochloris | Fort Nottingham, South Africa | Trunks of Leucosidea sericea trees | Deep shade inside a tree canopy |
| Solorina saccata (L.) Ach. | Coccomyxa | Hinterstein, Germany | Shaded wall | Wall, shaded by surrounding grasses and herbaceous higher plants |
| Peltigera leucophlebia (Nyl.) Gyeln. | Coccomyxa | Hinterstein, Germany | Soil of grassy forest clearing | Partially shaded by surrounding grasses and herbs |
| Cladonia fimbriata (L.) Fr. | Asterochloris | Großer Böhmerweiher See, Munich, Germany | Gravelly soil next to lake | Moderately exposed, limited shading by grasses and short herbs |
| Cladonia glauca Flörke | Asterochloris | Großer Böhmerweiher See, Munich, Germany | Gravelly soil next to lake | Moderately exposed, limited shading by grasses and short herbs |
| Xanthoria parietina (L.) Th. Fr. (sun) | Trebouxia | Outskirts of Gröbenzell, Munich | Rock face | No shade, exposed to full sun |
| Xanthoria parietina (L.) Th. Fr. (shade) | Trebouxia | Großer Böhmerweiher See, Munich, Germany | Branches of various small trees | Exposed, limited shading by short stubby branches |
2.2 Chlorophyll fluorescence and P700 measurement
Chlorophyll fluorescence, as an indicator of PSII activity, and changes in the redox state of the PSI primary electron donor (P700), as an indicator of PSI activity, were measured simultaneously with a Dual-PAM-100 fluorimeter (Walz) using the “red” version of the DUAL-DR measuring head. The thalli were dark-adapted for a minimum of 10 min before the experiments. The minimum fluorescence (FO) was determined before applying a saturating flash to determine maximum fluorescence (FM). While the true FO may be slightly lower than the measured FO, as some of the measured fluorescence originates from PSI, this effect is generally only considered significant in cyanobacteria and C4 plants (Anonymous, 2009), and no attempt was made to correct for this here. Small errors in the estimation of FO will not affect measurements of P700, the main focus of the present work. The maximal fluorescence in the light (FM′) was determined by applying a saturating flash of light when thalli were illuminated.
P700 was measured in the dual-wavelength mode, using the difference of absorbance at 875 and 830 nm. Briefly, light reaching the PSI centers can either lead to photochemical charge separation with quantum yield Y[I], or be non-photochemically converted to heat when P700 is either oxidized (P700+) due to donor side limitation (Y[ND]) or no charge separation is possible due to acceptor side limitation (Y[NA]).
The sum of Y[I], Y[ND], and Y[NA] = 1 (i.e., 100%).
After overnight hydration as described above, lichens were lightly blotted to remove excess superficial water and kept on moist filter paper during measurement. Tests showed that no drop-off in basic fluorimetry parameters (such as rETR or FV/FM) occurred during the 10 min needed to take a series of measurements.
2.3 Induction kinetics and rapid light curves for calculating rETR2MAX and CEF
To test for the induction of ETR and the induction and relaxation of NPQ, an actinic light at 100 μmoles m−2 s−1 was switched on and parameters were measured every 20 s for 4 min. The actinic light was then switched off, and relaxation of NPQ was measured for the following 4 min. Rapid light response curves of relative electron transport rates (rETR1 and rETR2) were measured by progressively increasing the level of the actinic light. Light levels of 0, 11, 18, 27, 42, 58, 75, 100, 131, 171, 221, and 278 μmol m−2 s−1 were used, with 10–20 s at each level, and a saturating flash was applied at the end of exposure to each light level. From the relationship between rETR2 and actinic light intensity, the equations described by Prioul and Chartier (1977) were used to estimate maximum rETR2 (rETR2MAX), and the light intensity needed for 90% rETR2MAX. The difference between rETR1 and rETR2 can be used as an approximation of CEF (Miyake et al., 2004) and was estimated for each species at the light intensity needed for 90% rETR2MAX.
2.4 Assessment of NDH-2 activity in Lobaria pulmonaria
The Ft of pale and melanised thalli of L. pulmonaria was measured during and after exposure to actinic light at 100 μmol m−2 s−1 for 5 min, using the “maxi” red version of the Imaging PAM fluorimeter (Walz). Data were normalized to the Ft value at the point the actinic light was switched off.
3 RESULTS
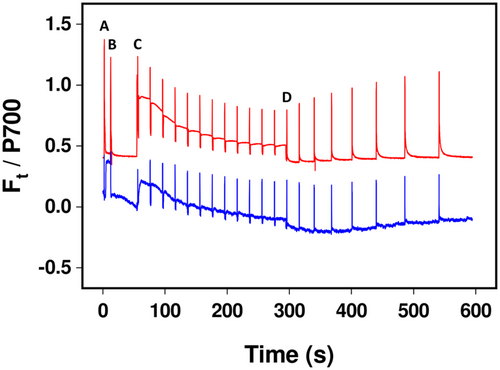
Measurements of P700 in microalgae are prone to high noise-to-signal ratios, but all lichen species tested here yielded very clean absorption measurements at 875 and 830 nm, enabling accurate assessment of the redox state of the PSI primary electron donor (Figure 1). Stable, consistent, and measurements were possible even when thalli contained a variety of pigments, such as melanin and parietin, in their upper cortex. Therefore, we felt confident to use P700 measurements to measure acceptor-side limitation of PSI (Y[NA]) as a marker of FLV activity. Y[NA] varied in accordance with the visually assessed light availability of the lichens' microhabitats, varying from values of almost 0.7 in corticolous woodland species (e.g., C. aurata) to values of around 0.2 in species that grow in exposed sunny conditions (e.g., X. parietina; Tables 1 and 2; Figure 2). In the two corticolous woodland species tested here (C. aurata, L. pulmonaria), Y[NA] was significantly lower in melanised than pale forms 1 s after illumination (P < 0.05). However, Y[NA] was similar in the two contrastingly pigmented forms of the sun species X. parietina. In species where Y[NA] was initially high, values rapidly declined to 0.2 or below within 1 min of illumination (Figure 2).
| Lichen species | Habitat | Y[NA] after 1 s of light at 100 μmol m−2 s−1 | CEF (rETR1–rETR2) at 90% light saturation | NPQ after 4 min | rETR2MAX | Light intensity needed for 90% rETR2MAX (μmol m−2 s−1) |
|---|---|---|---|---|---|---|
| Crocodia aurata (pale) | Shaded (tree branches) | 0.67 ± 0.02** | 6.2 ± 0.5 | 1.55 ± 0.22 | 13.4 ± 0.7 | 71 ± 3 |
| Crocodia aurata (melanic) | Shaded (tree branches) | 0.56 ± 0.03 | 9.6 ± 0.7 | 2.84 ± 0.31 | 14.3 ± 0.8 | 114 ± 14 |
| Lobaria pulmonaria (pale) | Shaded (tree branches) | 0.50 ± 0.03* | 7.3 ± 0.3 | 1.28 ± 0.11 | 11.5 ± 0.5 | 61 ± 5 |
| Lobaria pulmonaria (melanic) | Shaded (tree branches) | 0.36 ± 0.04 | 6.9 ± 0.5 | 1.56 ± 0.12 | 11.6 ± 0.7 | 63 ± 3 |
| Solorina saccata | Moderate shade (based of wall) | 0.40 ± 0.02 | 5.2 ± 0.4 | 3.39 ± 0.17 | 10.0 ± 0.3 | 52 ± 3 |
| Peltigera leucophlebia | Moderate shade (grassy soil) | 0.44 ± 0.03 | 6.2 ± 0.4 | 1.20 ± 0.26 | 9.8 ± 0.5 | 85 ± 13 |
| Cladonia glauca | Moderately exposed (gravel soil) | 0.37 ± 0.03 | 19.9 ± 5.8 | 0.73 ± 0.04 | 14.3 ± 1.3 | 223 ± 36 |
| Cladonia fimbriata | Moderately exposed (gravel soil) | 0.28 ± 0.04 | 9.1 ± 1.4 | 1.09 ± 0.06 | 9.7 ± 0.6 | 65 ± 10 |
| Xanthoria parietina (pale thalli) | Exposed (bare tree branches/trunks) | 0.19 ± 0.05 | 9.1 ± 0.7 | 0.76 ± 0.11 | 19.9 ± 1.2 | 114 ± 9 |
| Xanthoria parietina (dark orange thalli) | Highly exposed (rock face) | 0.23 ± 0.04 | 9.8 ± 1.0 | 0.51 ± 0.03 | 17.0 ± 0.9 | 92 ± 7 |
- Note: Comparisons were made (t-tests) between values of Y[NA] for pale and melanic forms of Crocodia aurata and Lobaria pulmonaria. Figures are given ±se, n = 12.
- * p < 0.05.
- ** p < 0.01.
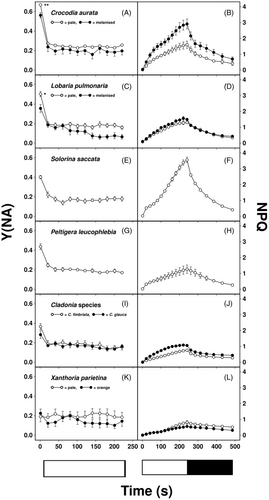
Maximum values of NPQ after 4 min illumination at 100 μmol m−2 s−1 were highest in the species from more shaded habitats, although NPQ declined quickly during darkness. In C. aurata, NPQ was higher in melanised than pale forms (Figure 2B), whereas values were similar in both forms of L. pulmonaria (Figure 1D) and in both forms of X. parietina (Figure 2L).
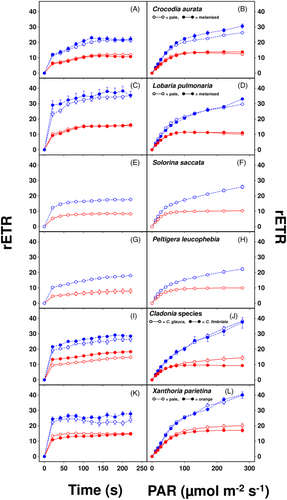
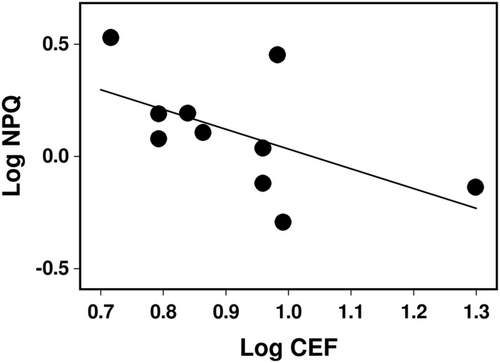
Figure 3 illustrates the induction of rETR1 and rETR2, used to calculate CEF, at either a constant intensity of 100 μmol m−2 s−1 for 4 min or during increasing light intensity (i.e., rapid light curves). In all cases, the induction of rETR1 and rETR2 at 100 μmol m−2 s−1 was rapid (Figure 3A,C,E,G,I,K), and similar in both pigmented forms of C. aurata, L. pulmonaria and X. parietina. Further analyses of the rapid light curves showed that rETR2MAX and the light intensity needed for 90% rETR2MAX (Table 2) were only weakly related to the visually assessed light availability of the lichens' microhabitats (Table 1). However, values for both parameters tended to be higher in the sun species X. parietina and Cladonia spp. than those of the shade species C. aurata and L. pulmonaria. In all species, values of CEF calculated at near-saturating light intensity (90% rETR2MAX) were >5 electrons m−2 s−1 and >40% of linear electron transport (LET), as estimated by rETR2MAX values (Table 2). However, CEF values were not consistently related to either rETR2MAX values or the visually assessed light availability of the lichens' microhabitats (Table 1). A slight negative correlation across species between NPQ capacity and CEF at high light intensity was found (p = 0.09, Figure 4).
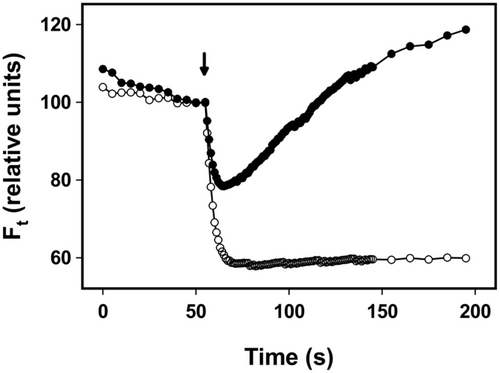
NDH-2-based CEF was assessed in pale and melanised forms of L. pulmonaria by measuring the post-illumination change in Ft (Figure 5). In the pale form, Ft declined after the actinic light was switched off, showing the typical dark-induced oxidation of the PQ pool, whereas Ft initially decreased in the melanised form before increasing to above the level observed in the light, indicative of NDH-2 activity reducing the PQ pool.
4 DISCUSSION
Lichen photobionts have long been known to have the capacity to adapt their photosynthetic apparatus to their environment (Kershaw, 1985). The introduction of standard PAM fluorimetry has simplified investigating this process; for example, understanding how lichens adapt to shaded and sunny microhabitats (Beckett, Minibayeva, & Mkhize, 2021; Mkhize et al., 2022). However, chlorophyll fluorescence measurements are unsuitable for studying PSI activity and associated alternative electron pathways, such as CEF and PCEF. Such pathways have long been known to be essential for managing the ATP:NADPH energy balance required by the Calvin-Benson cycle for carbon assimilation. It is now considered that these pathways are also very important in the adaptation of photosynthetic organisms to their light environment, particularly in challenging conditions (Gjindali et al., 2021; Shi et al., 2022). By measuring the ratio of absorptions at 830 and 875 nm, the Walz Dual PAM enables estimation of the redox state of P700, providing insights into CEF and PCEF (see Section 2 for more details). Here, we show that sun species display much lower acceptor side limitation of PSI early in illumination than shade species, which indicates that sun species display higher FLV-mediated PCEF (Gerotto et al., 2016). Compared to angiosperms, where maximum values of CEF are ca. 10% of LET (Avenson et al., 2004), CEF is high in all lichens, but tends to be slightly higher in sun than shade species. The slight negative correlation across species between NPQ capacity and CEF (Figure 4) could indicate potential compensation between these two regulatory mechanisms, as found previously in an investigation of mechanisms that prevent PSI photoinhibition in C. reinhardtii (Chaux et al., 2017). Both NPQ and CEF can potentially reduce the build-up of reduced electron carriers and thereby prevent unregulated reduction of O2 and ROS formation. One reason for the rather poor correlation between NPQ and CEF is that some lichens synthesize light screening pigments in their upper cortices, which may reduce the need for other tolerance mechanisms, such as NPQ and CEF.
4.1 PCEF activity in lichens
FLV proteins were first isolated in 1993 from the anaerobic bacterium Desulfovibrio, and were shown to have both NO and O2 reductase activity (Folgosa et al., 2018). In photosynthesis, they have been shown to be particularly active during the first few seconds following an increase in light intensity before the Calvin-Benson cycle can increase NADPH consumption. In this way, they can prevent the over-reduction of PSI and the stroma, thereby protecting both PSI and PSII (Alboresi et al., 2019). We had originally predicted that high FLV activity would be present in lichens that grow in microhabitats in which light availability fluctuates rapidly, such as Crocodia and Lobaria that derive much of their sunlight from sunflecks. We also predicted that FLV activity would be lower in sun species, such as Xanthoria that grow in more constant (although higher) illumination. However, the strong positive correlation between light availability and FLV activity found here suggests a rather more general role for FLV proteins in the tolerance of lichens to high light availability (Figure 2, Tables 1 and 2). This suggestion is consistent with the ability of FLV proteins to increase high light tolerance when transgenetically expressed in rice (Wada et al., 2018) and Arabidopsis (Yamamoto et al., 2016). Furthermore, mutants of the moss Physcomitrium patens lacking PGR5- and NDH-1-mediated CEF can still tolerate high light due to the presence of a strong PCEF mediated by FLVs (Storti et al., 2020). Thus, FLVs can functionally complement the absence of an active CEF under steady light conditions. However, when FLVs were also knocked out along with PGR5 and NDH-1, photosynthesis was drastically affected even under constant low light (Storti et al., 2020). This suggests that FLVs can have a more general role in protecting the PSI from damage caused by over-reduction of the electron transfer chain under constant light if other CEF mechanisms fail. Photosystem I is susceptible to irreparable damages because, unlike PSII, it has no repair mechanism (Sonoike, 2011; Tiwari et al., 2016).
The comparisons of FLV activity made here between sun and shade lichens are somewhat confounded by differences in the photobionts that they contain (Table 1). For the shade species, the photobionts were Symbiochloris and Coccomyxa, while those of the sun species were Trebouxia or the closely related Asterochloris. Nevertheless, although both Lobaria and Crocodia possess Symbiochloris as their photobiont, FLV activity was significantly higher in the melanised (i.e., sun) forms (Figure 2). While future surveys of FLV activity in lichens should probably include Trebouxia-containing shade lichens (e.g., some species of Lepraria), it is interesting that Nelsen et al. (2021) believe that early Trebouxia lineages were largely forest specialists or habitat generalists. Trebouxia then diversified into non-forested and more stressful habitats. It seems likely that, as Trebouxia-containing lichens emerged from shaded habitats, they enhanced their FLV activity to help them cope with the additional light.
4.2 CEF in lichens is high and only weakly associated with habitat light availability
CEF was estimated here as the difference between rETR1 and rETR2 (Huang et al., 2015; Miyake et al., 2004). In angiosperms, CEF is typically ca. 10% of LET (Avenson et al., 2004). We had originally predicted that sun species should display higher CEF than shade species, as shown for sun compared to shade leaves of higher plants (Huang et al., 2015). In general, CEF appeared to be much higher in lichen photobionts than angiosperms (Figure 3). This is consistent with the observation of Alric (2014) that under certain conditions, CEF can contribute up to 50% of the total electron flow in C. reinhardtii. CEF increased with light level (Figure 3), and values tended to be higher in sun than shade species. For example, the mean value of rETR1-rETR2 at 90% light saturation for the shade species L. pulmonaria and C. aurata was 7.5 μmol electrons m−2 s−1, corresponding to 59% of LET, while the mean value for the sun species Cladonia spp. and X. parietina was 12.0 μmol electrons m−2 s−1, corresponding to 79% of LET (Table 2). Similarly, at the highest light intensity used (271 μmol m−2 s−1), values were ca. 20 for shade and ca. μmol 30 electrons m−2 s−1 for sun species (Figure 3). While these differences were smaller than we had originally predicted, CEF has at least three important biochemical roles in photosynthesis. First, under high light, CEF can prevent the overreduction of PSI and, therefore, ROS formation and the resulting damage to PSI. Furthermore, by pumping additional protons into the thylakoid, it can increase ATP production to maintain the balance of ATP:NADPH, and also speed up the induction of NPQ, thereby protecting PSII (for review see Ma et al. (2021)). We suggest that the relatively high CEF values present in shade species facilitate lumen acidification to promote the induction of NPQ, which was clearly higher in shade species than sun species at 100 μmol photons m−2 s−1 (Figure 2, Table 2). While shade species receive much of their PAR from sunflecks, a sudden increase in light level may cause photoinhibition. They must therefore be able to rapidly dissipate the excess-absorbed light energy to prevent light stress. More work is required to understand the significance of the multiple roles that CEF plays in the adaptation of sun and shade species to the light conditions they experience in their habitat.
4.3 Differences in PSII activity between sun and shade lichens
In our earlier work, we showed that in Afromontane lichens, rETR2 saturates at lower light intensities in shade than sun species, and shade species display lower values of rETR2MAX (Beckett, Minibayeva, & Mkhize, 2021; Mkhize et al., 2022). These observations resemble the adaptation and acclimation strategies of higher plants (Greer, 2023). Results suggest that shade species are adapted to photosynthesize efficiently in low-light microhabitats but also downregulate photosynthetic capacity. The downregulation presumably occurs because high rETR2 requires high investment and maintenance costs associated with: for example, higher activities of photosynthetic cytochromes and enzymes. However, the lichens studied by Beckett, Minibayeva, and Mkhize (2021) and Mkhize et al. (2022) were from sub-tropical Afromontane vegetation, where very large differences exist between the light regimes of sun and shade species. In the present study, except for Crocodia aurata, lichens were collected from the cool temperate and alpine regions of Europe, where there are possibly less differences in light levels; therefore explaining the smaller differences in PSII activity. For example, the mean value of rETR2MAX for the shade species Lobaria and Crocodia was 12.7 μmol electrons m−2 s−1, while the mean value for the sun species Cladonia and Xanthoria was only slightly higher at 15.2 μmol electrons m−2 s−1 (Table 2). Differences were slightly greater for the light level required for 90% saturation of ETR2, being 78 for the sun and 124 μmol photons m−2 s−1 for the shade species.
Species displayed greater differences in the induction of NPQ (Figure 2), with results resembling those of Beckett, Minibayeva, and Mkhize (2021) and Mkhize et al. (2022). As discussed above, unlike results from higher plants (e.g., Demmig-Adams et al., 2020), shade lichens display higher but faster relaxing NPQ than sun species. Although values of NPQ were all measured at 100 μmol m−2 s−1, Mkhize et al. (2022) showed that, in Afromontane species at least, differences persist if higher light levels are used. High NPQ may be needed in shade or “sunfleck” species to prevent damage when light levels increase quickly, while rapid relaxation of NPQ will enable them to efficiently utilize the lower light levels available after a sunfleck has passed (Ghosh et al., 2023). Clearly, as for Afromontane lichens, in temperate lichens, large differences exist in the magnitude and the rates of induction and relaxation of NPQ.
4.4 Differences in PSI and PSII activity between lichens with and without cortical light-screening pigments
A particular mechanism for tolerance to long-term light stress in lichens is the synthesis of light-screening secondary metabolites in the upper cortex, such as brown melanins in L. pulmonaria and C. aurata and the orange pigment parietin in X. parietina; these pigments can increase tolerance to photoinhibition (Mafole et al., 2019b; Solhaug et al., 2010). In L. pulmonaria, melanic pigments apparently adjust the light received by the photobiont beneath the screening upper cortex to compensate for variations in tree canopy openness (Gauslaa & Goward, 2020). While this implies other photoprotective parameters may not differ between differently melanized lichens, the results from a range of species show that NPQ can be higher, similar or lower in melanic compared to pale thalli (Ndhlovu et al., 2022). These results suggest that melanisation may, in some instances, offer insufficient photoprotection, leading to high NPQ. Conversely, in other species, melanisation may have been excessive earlier in the growing season, thus providing excess shading leading to low NPQ. Here, the induction and relaxation of NPQ were similar in pale and melanised thalli of L. pulmonaria, but much higher in melanised than pale forms of C. aurata (Figure 1B,D), similar to results presented in Ndhlovu et al. (2022). Although, theoretically, melanisation may complicate the measurement of chlorophyll fluorescence parameters, Ndhlovu et al. (2022) used a dissection technique to show that NPQ can be satisfactorily assessed with a standard fluorimeter even in melanised thalli. It appears unnecessary to, for example, dissect away the lower cortex and medulla and take measurements from below rather than through a melanised upper cortex. Interestingly, for both species, FLV activity was significantly higher in melanised than pale thalli (Figure 1A,C), suggesting that enhanced FLV activity in melanised forms is an additional adaptation to high light. In contrast, all photosynthetic parameters were very similar in pale and orange X. parietina (Figure 2K,L, Table 2). Possibly, differences in light in the microhabitats of pale and orange thalli were less than for the melanising species tested here, and parietin on its own offers adequate photoprotection.
4.5 Acclimation of a shade species to higher light is associated with increased NDH-2-mediated CEF
In algae, there are two main forms of CEF, one based on PGRL1/PGR5 and the other on NDH-2 (Nawrocki et al., 2019). While antimycin has been suggested as a selective inhibitor of NDH-2 CEF, a less invasive way to distinguish the forms is to test for the presence of a post-illumination increase in PSII fluorescence. In C. reinhardtii, this transient has been shown to result from NDH-2 activity (Jans et al., 2008; Krishna et al., 2019). While a detailed survey of the types of CEF in each lichen was beyond the scope of the present study, we did test for the presence of a post-illumination increase in fluorescence in L. pulmonaria. Here, we found that although total CEF is only slightly higher in melanised than pale thalli (Figure 3C,D), only melanised forms possessed a post-illumination increase in fluorescence (Figure 5). While flux through NDH-2 is considered insufficient to be a major route of CEF in the light (Nawrocki et al., 2019), our observation suggests that acclimation of L. pulmonaria to higher light is correlated with a higher level of dark chlororespiration, and thus a need to consume reducing equivalents (e.g., NADPH) after experiencing a decrease in light intensity.
5 CONCLUSIONS
In lichen photobionts, the role of alternative pathways of electron flow around PSI in photoprotection has remained almost unstudied. This is somewhat surprising, as such pathways are considered very important in stress tolerance, and lichens are some of the most stress-tolerant photosynthetic organisms. Our results show that the Dual PAM can effectively measure alternative electron flows in lichen photobionts, and thereby provide useful additional information on how lichens adapt to their light microhabitats. Our data suggest that sun species display higher FLV-mediated PCEF than shade species, and, furthermore, FLV activity is much higher in melanised than pale forms of two shade species. Compared with values typically reported in higher plants, CEF is consistently high in all lichens, although there is a tendency for values to be higher in sun than shade species. It is well known that lichen photosynthesis is remarkably plastic and can change rapidly to optimize growth in particular light microhabitats (Kershaw, 1985). Future work needs to test the extent to which CEF and PCEF capacity in lichens can be adjusted as the light environment changes. Works carried out on free-living algae and cyanobacteria suggest that considerable plasticity is likely to occur. For example, Zhang et al. (2009) showed that, in the cyanobacterium Synechococcus, an increase in FLV transcripts can occur within minutes of CO2 deprivation and over reduction of the electron transport chain. We certainly plan to carry out similar transcriptomic studies with lichen photobionts in the near future. Rapid induction of FLV transcripts suggests that PCEF activity may be a rather plastic character. As many abiotic stresses reduce C fixation in poikilohydric organisms more than electron flow (Beckett, Minibayeva, Solhaug, & Roach, 2021), it seems likely that stress will increase PCEF and CEF will relieve the over-reduction of the LET chain. Gao et al. (2011) found that the ratio of PSI to PSII activity increases greatly during desiccation in the intertidal alga Ulva. Finally, the occurrence and potential benefits of the two different types of CEF in lichens need to be investigated.
AUTHOR CONTRIBUTIONS
Richard Peter Beckett designed and carried out the experiments, and wrote the first draft of the manuscript; Thomas Roach provided detailed help on using the Dual PAM, and revised the manuscript; Farida Minibayeva revised and improved the current version of the manuscript and Silke Werth provided logistical support at LMU and improved the current version of the manuscript.
ACKNOWLEDGMENTS
We thank Michael Shelyakin from the Komi Scientific Center, Russia for help colleting Lobaria pulmonaria. Richard Peter Beckett thanks the Research Fund of the University of KwaZulu-Natal and the Kazan Federal University Strategic Academic Leadership Program (PRIORITY-2030) for financial support, and the University of KwaZulu-Natal for sabbatical leave to visit Innsbruck and Munich. This work was supported by the government assignment of FRC Kazan Scientific Center of RAS for Farida Minibayeva. Serena Schwenkert and Gilbert Neuner are thanked for allowing Richard Peter Beckett to use a Walz Dual PAM at LMU and UI, respectively.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as all new created data are already contained within this article.




