Polyamines inhibit abscisic acid-induced stomatal closure by scavenging hydrogen peroxide
Abstract
Stomatal closure is regulated by plant hormones and some small molecules to reduce water loss under stress conditions. Both abscisic acid (ABA) and polyamines alone induce stomatal closure; however, whether the physiological functions of ABA and polyamines are synergistic or antagonistic with respect to inducing stomatal closure is still unknown. Here, stomatal movement in response to ABA and/or polyamines was tested in Vicia faba and Arabidopsis thaliana, and the change in the signaling components under stomatal closure was analyzed. We found that both polyamines and ABA could induce stomatal closure through similar signaling components, including the synthesis of hydrogen peroxide (H2O2) and nitric oxide (NO) and the accumulation of Ca2+. However, polyamines partially inhibited ABA-induced stomatal closure both in epidermal peels and in planta by activating antioxidant enzymes, including superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), to eliminate the ABA-induced increase in H2O2. These results strongly indicate that polyamines inhibit abscisic acid-induced stomatal closure, suggesting that polyamines could be used as potential plant growth regulators to increase photosynthesis under mild drought stress.
1 INTRODUCTION
Stomata consist of two highly specialized epidermal cells called guard cells that are located on plants' aerial organs. They always open in response to light to admit the entry of CO2 for photosynthesis and close in response to abiotic stress to reduce transpiration, thus preventing excessive loss of water to the atmosphere (Brodribb & McAdam, 2011; Henry et al., 2019). Therefore, the optimal regulation of stomatal movement is vital for the survival and growth of terrestrial plants and crop productivity (Brodribb & McAdam, 2011; Hewage et al., 2020; Zhu, 2016).
Abscisic acid (ABA), which is synthesized mainly in the leaves (McAdam & Brodribb, 2018), plays a major role in the efficient regulation of stomatal closure in response to biotic and abiotic stress, especially drought stress (Brodribb & McAdam, 2011; Gong et al., 2021; Hasan, Gong, et al., 2021). A series of signal transduction mechanisms that occur during ABA-induced stomatal closure are well known: ABA binds to its receptor (PYR, PYL, and RCAR), thereby inhibiting PROTEIN PHOSPHATASE 2C (PP2C) and releasing sucrose non-fermenting-1-related protein kinases (SnRKs) (Dittrich et al., 2019; Hasan et al., 2022; Hewage et al., 2020; Park et al., 2009). In the calcium-independent pathway, SnRKs can directly transphosphorylate slow-type anion channels (SLACs) or rapid-type anion channels, ultimately activating outwardly rectifying K+ channels and the efflux of K+ (Ache et al., 2000; Geiger et al., 2009). The massive loss in potassium ions leads to water efflux and thereby causes a decrease in guard cell turgor and volume, ultimately resulting in stomatal closure (Hosy et al., 2003). In the calcium-dependent pathway, SnRKs first activate nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase (AtrbohF), resulting in increased reactive oxygen species (ROS) (Han et al., 2018; Sirichandra et al., 2009). ROS trigger NO production, and further downstream signaling, through the activation of mitogen-activated protein kinase (MAPK), activates Ca2+-permeable channels, resulting in increased cytosolic Ca2+ (Bright et al., 2006; Pei et al., 2000; Zhang et al., 2007). Similar to SnRKs, increased cytosolic Ca2+ activates SLACs, thereby inducing stomatal closure (Geiger et al., 2010).
Polyamines, which comprise mainly putrescine (Put), spermidine (Spd), and spermine (Spm), are low-molecular-weight growth regulators that are ubiquitous in all kinds of plants and induce stomatal closure (Takahashi & Kakehi, 2010). Indeed, the Polyamines catabolic pathways of two enzymes, DIAMINE OXIDASE (DAO) and POLYAMINE OXIDASE (PAO), release H2O2 (Agurla et al., 2018; An et al., 2008; Wimalasekera et al., 2011), and the increased H2O2, in turn, promotes the synthesis of NO (Agurla et al., 2018), further activating downstream signaling such as the accumulation of cytosolic calcium and the inhibition of inwards K+ channels in the plasma membrane of guard cells, ultimately resulting in stomatal closure (Gayatri et al., 2013; Liu, 2000). Studies have shown that polyamines induced stomatal closure through the similar pathway than ABA, including the synthesis of ROS and NO, and the accumulation of cytosolic calcium (Agurla et al., 2018; Gayatri et al., 2013).
However, polyamines act as double-edged swords in plant development and stress responses (Gupta et al., 2016). Under adverse conditions, the accumulation of polyamines usually has an antioxidant role in eliminating ROS directly or by activating the ROS-scavenging enzyme system (Hasan, Alharbi, et al., 2021; Hasan, Skalicky, et al., 2021; Hassan et al., 2018; Jahan et al., 2022; Shi et al., 2010; Tanou et al., 2014). For example, exogenous polyamines were shown to significantly increase the activity of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) and decrease ROS synthesis during dehydration in citrus and in Rosa (Hassan et al., 2018; Shi et al., 2010). In this regard, no studies have investigated the crosstalk between ABA and polyamines in the regulation of stomatal movement directly.
In this study, we hypothesize that polyamines inhibit ABA-induced stomatal closure by activating ROS-scavenging enzymes, especially CAT, to eliminate hydrogen peroxide (H2O2). To test this hypothesis, stomatal aperture and conductance were measured in Vicia faba after exogenous application of ABA, Spd, or both to examine whether polyamines inhibit ABA-induced stomatal closure. Furthermore, wild-type and two mutants (adc2-4 and pao3) of Arabidopsis thaliana were used to further confirm this hypothesis. Pharmacological experiments were conducted, and confocal microscopy was used to examine whether polyamine affects ABA-induced stomatal movement by inhibiting its key signaling components, including ROS, NO, and Ca2+. Finally, the enzyme activities of SOD, POD, and CAT were measured, and the CAT inhibitor 3-amino-1,2,4-triazole (AT) was used to investigate whether antioxidant systems, especially the H2O2 scavenging enzyme CAT, were activated to eliminate ABA-induced ROS, thus inhibiting ABA-induced stomatal closure.
2 MATERIALS AND METHODS
2.1 Plant materials
Vicia faba plants were grown in a growth chamber at 22 ± 1°C under a relative humidity of 60%, a photosynthetic photon flux density (PPFD) of 300 μmol photons m−2 s−1, and a 14-h light/10-h dark photoperiod at Lanzhou University, Lanzhou, Gansu Province, China. Newly expanded leaves from approximately 5-week-old plants were used in this experiment. As Vicia faba polyamine-related mutants could not be found, we chose two A. thaliana mutants, namely, one polyamine synthesis mutant (adc2-4; ABRC number CS9660) and one polyamine degradation mutant (pao3; SALK_088387) to further test the relationship between ABA and polyamine. The expression of the polyamine synthesis gene ADC2 could not be detected, and the polyamine content decreased by more than 50% in the adc2-4 mutant (Cuevas et al., 2008). As the PAO3 gene is an early responsive gene to ABA and is expressed at relatively high levels in plant tissues to induce polyamine degradation and synthesize hydrogen peroxide (Mochou et al., 2008), we chose the polyamine degradation gene mutant pao3 (Alonso et al., 2003). These two mutants and the wild-type (ecotype Columbia) were grown in a growth chamber at 22 ± 1°C under a relative humidity of 60%, a PPFD of 200 μmol photons m−2 s−1, and a 14-h light/10-h dark photoperiod. Newly expanded leaves were selected to measure their response to exogenous ABA, Spd, and ABA plus Spd.
2.2 Viability tests
The viability of guard cells was tested following the methods described by Garcia-Mata and Lamattina (2010). Briefly, abaxial epidermal strips were pretreated with opening buffer (10 mM 2-morpholino ethane sulfonic acid (MES) 50 mM KCl, 20 μM CaCl2; pH 6.15) for at least 2 h to ensure that the stomata were fully opened and then treated with different concentrations of ABA, polyamines, and H2O2 for another 2 h to determine the proper concentration and avoid the toxic effect of these chemicals. The strips were then incubated with 5 μM fluorescein diacetate (FDA) for 5 min. After FDA loading, the strips were washed three times with the opening buffer and mounted for microscopy. Fluorescence images were obtained with a fluorescence microscope (Zeiss Z2, Germany), and cell viability was quantified via the percentage of fluorescent guard cells relative to total guard cells in the bright field. ABA, polyamines, and H2O2 did not induce a decrease in the viability of guard cells at 100 μM, 1 mM, and 100 μM, but polyamines and H2O2 induced a significant decrease in viability at concentrations of 3 mM and 200 μM, respectively, in Vicia faba (Figure S1); thus, we chose up to 100 μM, 1 mM, and 100 μM ABA, polyamines, and H2O2, respectively, to ensure that the stomatal aperture data resulted from the treatment effect but not the toxicity effect.
2.3 Stomatal aperture measurements
Stomatal bioassays were measured following the methods of Gong et al. (2021). The abaxial epidermis was peeled 2 h after the beginning of the light period, floated on the opening buffer (described above), and incubated under light conditions for approximately 2 h. All treatments were applied after the stomata were detected under a light microscope to ensure that the stomata were opened. Peels were incubated in various concentrations of ABA and polyamines for 2 h for the dose-effect test or in 50 μM ABA and 1 mM polyamines for various durations for the time-gradient effect test.
To determine how ABA and polyamines induce stomatal closure, peels were incubated with ABA and polyamine signaling pathway-related compounds for 2 h. Specifically, to test the signaling pathway of ABA-induced stomatal closure, peels were treated with 50 μM ABA, 100 μM H2O2, 100 μM sodium nitroprusside (SNP), or 1 mM Ca2+ in the opening buffer in the absence or presence of either 1 mM N-acetyl-l-cysteine (NAC), 100 U CAT, 25 μM Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME), 100 μM 2-phenyl-4,4,5,5-tetramethylimidazolinone-1-oxyl-3-oxide (cPTIO), or 1 mM methylenebis (oxyethylenenitrilo) tetraacetic acid (EGTA). To test the signaling pathway of Spd-induced stomatal closure, peels were treated with 1 mM Spd with or without 1 mM aminoguanidine (AG), 1 mM 2-hydroxyethylhydrazine (2-HEH), 1 mM NAC, 100 U CAT, 25 μM l-NAME, or 1 mM EGTA in the opening buffer.
To determine the dose-dependent effect of polyamines on stomatal closure induced by ABA, peels were incubated in 50 μM ABA with or without various concentrations of three different polyamines (Put, Spd, and Spm) for 2 h. To investigate the effect of polyamines on stomatal closure induced by different compounds of the ABA signaling pathway, peels were incubated in 50 μM ABA, 100 μM H2O2, 100 μM SNP, or 1 mM CaCl2 with or without the three different polyamines (each at 1 mM). As three kinds of polyamines induce stomatal movement in a similar way, and Spd is the most abundant polyamine in Vicia faba whether during well-watered or drought conditions (Liu, 2000), we used Spd to represent three kinds of polyamines to investigate the mechanism of how Spd inhibits ABA-induced stomatal closure.
To determine the effects of ABA and polyamines on the two Arabidopsis mutants as well as the wild-type, the abaxial epidermis was peeled 2 h after the beginning of the light period, floated on the opening buffer and incubated under light conditions for approximately another 2 h to open the stomata, after which ABA, Spd, and ABA + Spd were added to the opening buffer to a final concentration of 50 μM ABA and 1 mM Spd. Images of stomatal aperture were taken using a light microscope (Motic BA310) 2 h after treatment, and the aperture was measured using the ImageJ software (Schneider et al., 2012). Approximately 60 stomata were measured for each independent replicate, and the data are presented as the mean stomatal aperture of five independent replicates per treatment.
2.4 ABA measurements
ABA was measured by high-performance liquid chromatography (HPLC) using an OrbiTrap Fusion Lumos (Thermo Fisher) as described by Yang et al. (2021). The buffer containing 50 μM ABA with or without 1 mM polyamines was filtered through a 0.22 μm membrane and used to measure the ABA concentration to exclude the possibility that polyamines may directly affect the concentration of ABA. The ABA concentration was calculated based on a standard curve.
2.5 Gs measurements
Gs was measured according to the methods described by Brodribb and McAdam (2011). Six leafy shoots were selected for each treatment and recut under distilled water, after which the shoot base was immersed in distilled water. The Gs was measured using an infrared gas analyzer (LI-6800, LI-COR) with a leaf cuvette temperature of 22°C, a vapor pressure deficit of approximately 1.2 kPa, a CO2 concentration of 400 mmol mol−1, and a PPFD of 1000 μmol photons m−2 s−1. After approximately 1.5 h of activation, to ensure that Gs was stable, ABA or Spd or both were added to the distilled water to a final concentration of 50 μM ABA and 1 mM Spd; the chemicals were added such that they entered the transpiration stream of the excised leaf. The Gs was measured for another 2 h or more to ensure that it was stable again.
2.6 ROS, NO, and Ca2+ detection in guard cells
ROS and NO production in the guard cells was determined using the fluorescent indicator 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA, Sigma) and the specific NO dye 4,5-diaminofluorescein diacetate (DAF2-DA, Sigma), respectively, following the methods described by Bright et al. (2006). To measure and detect [Ca2+] cyt dynamics in guard cells, the Ca2+-specific fluorescence probe Fluo 3-AM was used, as described by Gong et al. (2021). In brief, after the abaxial epidermis was incubated in the opening buffer for approximately 2 h to make the stomata open, they were then incubated in the opening buffer containing 50 μM H2DCF-DA, 15 μM DAF2-DA, or 10 μM Fluo 3-AM for another 30 min in the dark, followed by a 5-min flush in the opening buffer to wash off excess dye. The strips were subsequently incubated in the opening buffer as controls or in a buffer that included 50 μM ABA, 100 μM H2O2, 100 μM SNP, and 1 mM CaCl2 with or without 1 mM Spd for 60 min. ROS and NO production or [Ca2+]cyt dynamics in the guard cells were observed with a confocal laser scanning microscope (LSM 880 CLSM; Zeiss) with a 40× objective (1% maximum laser power, excitation at 488 nm, emission at 495–550 nm, 500 gain), and all the images were analyzed using the ImageJ software (Schneider et al., 2012). To prevent photooxidation, all fluorescence images were collected with a single rapid capture. At least 30 guard cells were measured for each independent replicate, and the data are presented as the mean pixel intensities of five independent replicates.
2.7 SOD, POD, and CAT activity
The abaxial epidermis was peeled and brushed using a banister brush to remove mesophyll cells as much as possible. Then, the guard cell-enriched epidermal fractions were incubated in the opening buffer for approximately 2 h, followed by treatment with 50 μM ABA, 1 mM Spd, or both for another 2 h. After that, the fractions were collected and homogenized with a microfuge tube plastic pestle by using a small hand-held electric drill in extraction buffer composed of 50 mM phosphate buffer (pH 7.8) and 1% polyvinylpyrrolidone, followed by centrifugation for 20 min at 12,000g. The supernatants were used for different enzyme activity assays.
The SOD activity was analyzed according to the method described by Giannopolitis and Ries (1977) with some modifications. Briefly, SOD activity was assayed in a reaction mixture containing 50 μM nitro-blue tetrazolium (NBT), 13 μM riboflavin, 13 mM methionine, and enzyme extract. One unit of SOD activity was defined as the amount of enzyme that inhibited 50% of NBT photoreduction. The guaiacol-POD (EC 1.11.1.7) activity was analyzed according to the methods described by Shi et al. (2010), with modifications. For POD, 3 mL of reaction mixture contained 50 mM phosphate buffer (pH 7.0), 20 mM guaiacol, 40 mM H2O2, and 0.1 mL of enzyme extract. The activity of POD was determined by the increase in absorbance at 470 nm due to guaiacol oxidation every 20 s for 2–3 min, and 1 unit of enzyme activity was defined as an increase of 0.01 per minute in absorbance. CAT activity was measured according to the methods described by Cakmak and Horst (1991). Briefly, the CAT activity was assayed in a reaction mixture containing 50 mM phosphate buffer (pH 7.0), 10 mM H2O2, and enzyme extract. The decrease in absorbance (by decomposition of H2O2) was recorded at 240 nm, and 1 unit of enzyme activity was defined as a decrease of 0.01 per minute in the absorbance.
2.8 Statistical analysis
We used a t test to test the differences in stomatal aperture between the control group and the ABA- or polyamine-treated group at different time points. One-way anova was used to test for significant differences between the control group and different treatment groups, followed by least significant differences (lsd) tests. Two-way anova was used to test for significant differences among different treatments in different Arabidopsis mutants. In the corresponding figures, the different letters or asterisks indicate significant differences (p < 0.05) across treatments or between the treatment and control groups. All statistical analyses were performed using the SPSS version 19.0 (SPSS, Inc.).
3 RESULTS
3.1 ABA and polyamines induced stomatal closure alone
At first, we tested how ABA and three types of polyamines (Put, Spd, and Spm) alone regulated the stomatal movement of Vicia faba. The results showed that both ABA and polyamines induced gradual stomatal closure with increasing doses of ABA and polyamines and with treatment time (Figures 1 and S2). Further, the epidermis was treated with ABA, Spd, and the signaling components including H2O2, SNP, and CaCl2, and their related inhibitors, and the results showed that both ABA, Spd, and the signaling components H2O2, SNP, and CaCl2 induced stomatal closure to varying degrees. However, ABA- and Spd-induced stomatal closure was partially or totally inhibited by the H2O2 scavengers NAC and CAT, the NO synthesis inhibitor l-NAME, the NO scavenger cPTIO, and the Ca2+-chelating agent EGTA, and Spd-induced stomatal closure could also be inhibited by the degradation enzyme (PAO) inhibitors AG and 2-HEH (Figure 1C,D). In addition, H2O2-induced stomatal closure was inhibited by l-NAME and EGTA, and SNP-induced stomatal closure was inhibited by EGTA. Together, the results indicate that ABA and polyamines induced stomatal closure through the synthesis of ROS, NO, and the accumulation of Ca2+.
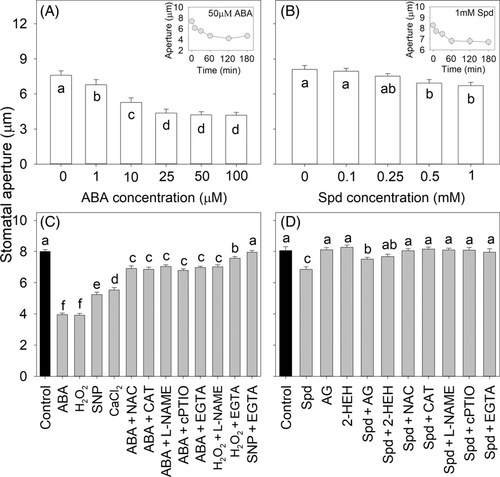
3.2 Polyamines inhibited ABA-induced stomatal closure
To investigate the interactions between ABA and polyamines in stomatal regulation, the epidermis of Vicia faba was treated with polyamines and ABA simultaneously. The results showed that Put, Spd, and Spm inhibited the ABA-induced decrease in stomatal aperture in the epidermis, even when the ABA concentration was as high as 100 μM, without affecting the concentration of ABA in the solution directly (Figures 2A, S3, S4, and S5). Then we introduced ABA, Spd, or both into the transpiration stream of the plant shoots to examine whether polyamine affected ABA-induced stomatal closure also in planta. The results showed that the stomatal conductance decreased rapidly from 0.24 to 0.06 mol m−2 s−1 at approximately 30 min after ABA treatment, but it decreased less, from 0.24 to 0.12 mol m−2 s−1, after the treatment with ABA plus Spd (Figure 2C). In addition, we tracked individual stomata through a transition of solutions from ABA to ABA plus Spd, and the results showed that Spd could reverse ABA-induced closure of individual stomata (Figure 2B). As polyamine-related mutants could not be found in Vicia faba, one polyamine synthesis mutant (adc2-4) and one polyamine degradation mutant (pao3) of Arabidopsis were chosed to further confirm that polyamine inhibited ABA-induced stomatal closure. The results showed that the stomata of adc2-4 closed to a greater degree than those of the wild-type and pao3 after ABA treatment (Figure 2D). Spd induced stomatal closure in the wild-type and adc2-4 but not in pao3, while Spd addition inhibited ABA-induced stomatal closure of the wild-type as well as the two Arabidopsis mutants (Figure 2D).
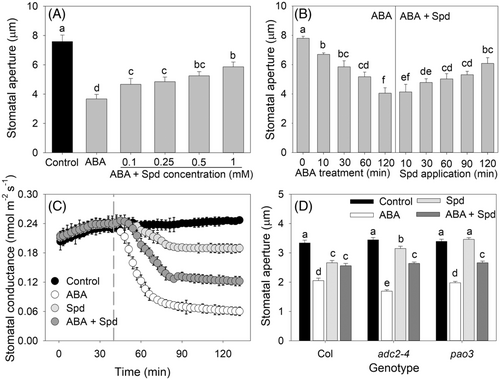
3.3 Polyamine inhibited ABA-induced stomatal closure through antioxidant enzyme-dependent ROS scavenging
Then, we investigated how polyamines affect ABA-induced stomatal closure and the downstream pathway. Both Spd and ABA alone increased the fluorescence of ROS, NO, and Ca2+ (Figure 3). However, Spd inhibited the increase in ROS, NO, and Ca2+ fluorescence induced by exogenous ABA and H2O2 and did not inhibit the NO fluorescence induced by SNP or the Ca2+ fluorescence induced by SNP and CaCl2 (Figure 3). Consequently, Put, Spd, and Spm partially inhibited the stomatal closure induced by ABA and H2O2 in a dose-dependent manner but did not affect the stomatal closure induced by exogenous SNP or CaCl2 (Figures 3 and S6). Together, polyamines inhibited ABA-induced stomatal closure through scavenging ABA-induced ROS without directly affecting the downstream signaling pathway, including NO synthesis and Ca2+ accumulation.
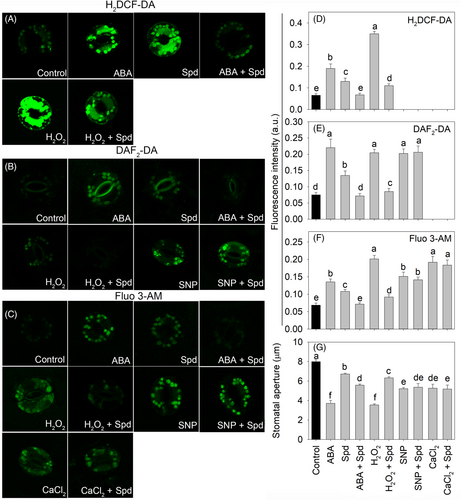
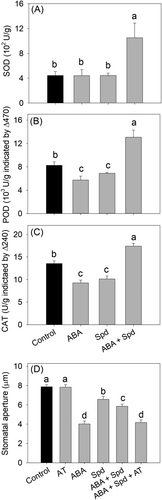
It has been shown that polyamines increase antioxidant enzymes during stress (Farooq et al., 2010; Hassan et al., 2018; Shi et al., 2010). We then investigated whether polyamines scavenged ABA-induced ROS also in the guard cells through an antioxidant enzyme-dependent way. The SOD activity in guard cell-enriched epidermal fractions was unaffected, and POD and CAT activity decreased in ABA or Spd treatment compared with that in the control (Figure 4). However, when ABA and Spd were co-treated, the activities of SOD, POD, and CAT increased (Figure 4). The CAT inhibitor 3-amino-1,2,4-triazole (AT) did not affect stomatal movement directly, but in the presence of AT, Spd failed to inhibit ABA-induced stomatal closure (Figure 4D). Furthermore, to exclude the possibility that polyamine-induced NO may also play an antioxidant role during stomatal regulation, SNP was added to test the role of NO in ABA-induced stomatal closure, and the results showed that SNP did not induce a decrease in ABA-induced H2O2 and did not inhibit ABA- or H2O2-induced stomatal closure (Figure S7). In addition, the NO synthesis inhibitor l-NAME and scavenger cPTIO did not affect the ability of polyamine to decrease the ROS induced by ABA (Figure S7). Together, polyamine inhibited ABA-induced stomatal closure through antioxidant enzyme-dependent ROS scavenging and not through polyamine degradation dependent NO synthesis.
4 DISCUSSION
4.1 Polyamines inhibit ABA-induced stomatal closure
ABA and polyamines are always synthesized simultaneously during drought stress, cold stress, or K deficiency (Li et al., 2021; Liu, 2000; Liu et al., 2005; Réthoré et al., 2021; Zhu et al., 2020). Therefore, it has been proposed that they function together to increase plant stress tolerance (Li et al., 2021; Réthoré et al., 2021; Zhu et al., 2020). ABA- and polyamine-induced stomatal closure has been reported in various studies (Agurla et al., 2018; An et al., 2008; Brodribb & McAdam, 2011; Gong et al., 2021; Hewage et al., 2020; Liu, 2000), and it has been supposed that they function synergistically to close stomata during stress to increase tolerance (Agurla et al., 2018; Liu, 2000). Here, we found that ABA and polyamines alone induce stomatal closure in Vicia faba and Arabidopsis, which is consistent with previous findings (Agurla et al., 2018; An et al., 2008; Brodribb & McAdam, 2011; Gong et al., 2021; Hewage et al., 2020; Liu, 2000), indicating that ABA- and polyamine-induced stomatal closure reduces transpiration. However, the most interesting finding was that polyamines directly inhibited ABA-induced stomatal closure in the epidermis of Vicia faba and in planta (Figures 2 and S4), and stomata could be reopened by exogenous applications of polyamine, even though the stomata were already partially closed because of ABA treatment for 2 h (Figure 2B). These results confirmed that polyamines inhibit ABA-induced stomatal closure rather than function synergistically to induce stomatal closure. In addition, we found that the stomata of the adc2-4 mutant closed more strongly in response to ABA (Figure 2D), suggesting that lacking polyamine synthesis makes the stomata more sensitive to exogenous ABA. Exogenous Spd alleviated the high sensitivity of adc2-4 to ABA, further indicating the importance of polyamines in inhibiting ABA-induced stomatal closure. Furthermore, the stomata of pao3 mutant closed similarly to that of the wild-type in response to ABA (Figure 2D), suggesting that polyamines inhibiting ABA-induced stomatal closure does not depend on polyamine degradation and ROS synthesis but may depend on its antioxidant roles.
4.2 Polyamines inhibit ABA-induced stomatal closure by scavenging H2O2
In previous studies, ABA and polyamines were shown to induce the synthesis of H2O2 through NADPH oxidase (Bright et al., 2006) and the polyamine degradation enzymes DAO and PAO, respectively (Agurla et al., 2018; An et al., 2008; Wimalasekera et al., 2011). Here, the polyamine degradation enzyme inhibitors AG and 2-HEH inhibited polyamine-induced stomatal closure, and the stomata of the polyamine degradation mutant pao3 failed to close in response to Spd, further confirming the important role of polyamine degradation enzymes in polyamine-induced stomatal closure. In addition, after ABA or polyamine treatment alone, the fluorescence of H2DCF-DA (H2O2), DAF-2DA (NO), and FLUO-3AM (Ca2+) all increased. Moreover, ABA- and polyamine-induced stomatal closure was inhibited by ROS scavengers, NO inhibitors, and Ca2+-chelating agents (Figures 1 and 3), indicating that ROS, NO, and Ca2+ are all required for ABA- and polyamine-induced stomatal closure. Furthermore, the NO synthesis inhibitor l-NAME greatly attenuated the stomatal response to H2O2, and the Ca2+-chelating agent fully inhibited SNP-induced stomatal closure, indicating that ROS act as upstream components and that Ca2+ is a downstream component of the NO signaling network involved in stomatal closure, as has been observed in previous studies (Bright et al., 2006; Pei et al., 2000). Thus, although ABA and polyamines increase H2O2 in different ways, they share a similar signaling pathway downstream of ROS, such as the synthesis of NO, the accumulation of Ca2+, and SLACs activation, to induce stomatal closure (Figure 5).
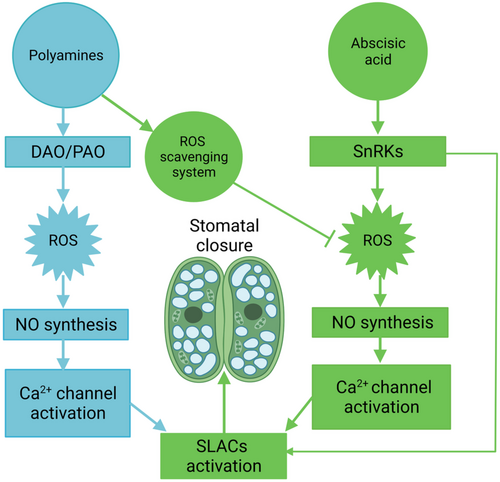
However, when ABA and polyamines were simultaneously applied, polyamines inhibited ABA- and H2O2-induced stomatal closure but did not affect SNP- or CaCl2-induced stomatal closure (Figure 3), suggesting that polyamines affect H2O2 rather than downstream pathway components. Furthermore, polyamines inhibited the fluorescence of H2DCF-DA (H2O2), DAF-2DA (NO), and FLUO-3 AM (Ca2+) induced by both exogenous ABA and H2O2 but did not affect the fluorescence of DAF-2DA (NO) induced by exogenous SNP or that of FLUO-3AM (Ca2+) induced by SNP or CaCl2 (Figure 3), further showing that polyamines inhibited ABA-induced stomatal closure by directly scavenging H2O2. Thus, we argue that although ROS production can be induced in various ways, such as via ABA or polyamine treatment, as shown in the present study, the effects are not cumulative; otherwise, increased accumulation of ROS would be harmful to cellular processes.
4.3 Polyamines eliminate H2O2 by activating antioxidant enzymes
Next, we investigated how polyamines scavenge H2O2. It has been shown that polyamines can enhance plant antioxidant capacity in leaves when plants experience several kinds of stresses, such as drought stress, high-temperature stress, cold stress, and alkali stress (Cheng et al., 2009; Gong et al., 2014; Huang et al., 2015; Mehta et al., 2002; Nambeesan et al., 2010). For example, it was reported that polyamines can increase the concentration of several nonenzymatic antioxidants, such as lycopene and ASA (He et al., 2008; Mehta et al., 2002; Nambeesan et al., 2010). More importantly, polyamine pretreatment was shown to significantly improve enzymatic antioxidants such as SOD, POD, and CAT activity to decrease the ROS content during stress (Farooq et al., 2010; Hassan et al., 2018; Shi et al., 2010), possibly by stimulating de novo synthesis of antioxidant enzymes at the translation level or by increasing the levels of the nucleoside diphosphate kinase (NDPK) protein, which is a key metabolic enzyme involved in the activation of antioxidant systems and enhanced tolerance to multiple stresses (He et al., 2008; Kim et al., 2011; Moon et al., 2003; Shi et al., 2013). Here, both POD and CAT activities decreased following ABA or Spd treatment alone, which may contribute to the accumulation of ROS induced by ABA or Spd. However, in the presence of ABA, Spd could activate the antioxidant enzymes to scavenge H2O2 induced by ABA (Figure 4). In the presence of the CAT inhibitor AT, Spd failed to inhibit ABA-induced stomatal closure, further indicating that polyamine-induced antioxidant enzymes, especially CAT, play an important role in eliminating H2O2 during Spd-induced stomatal reopening. Previous studies have shown that NO could play antioxidant roles by activating MAPK, resulting in the accumulation of several antioxidants, such as isoflavones and flavonoids, during plant development and response to stress (Dong et al., 2012; Jiao et al., 2016; Zhang et al., 2007). However, polyamine-induced NO may not play an antioxidant role during stomatal movement, as exogenous SNP did not affect ABA-induced H2O2 accumulation and stomatal closure (Figure S7). Overall, we concluded that polyamines inhibit ABA-induced stomatal closure through antioxidant enzyme-dependent H2O2 elimination (Figure 5).
4.4 Possible physiological role of polyamine inhibition of ABA-induced stomatal closure
It is well known that the rate of photosynthesis is dependent on stomatal aperture to a large extent, as stomata allow CO2 to diffuse into the leaves (Farquhar & Sharkey, 1982). If stomata close excessively or too quickly when plants experience stress, they may lose plant fitness or die because of carbon starvation. Thus, plants employ several mechanisms (such as the ability to sense low CO2 concentrations or the synthesis of ethylene) to partially induce stomatal reopening under stress, especially mild drought stress, to increase photosynthesis (Jammes et al., 2014; Merilo et al., 2014; Roelfsema et al., 2002; Tanaka et al., 2005; Watkins et al., 2017).
In many studies, both drought stress and ABA have been proven to induce polyamine synthesis (Hasan, Skalicky, et al., 2021; Liu et al., 2005; Takahashi & Kakehi., 2010; Zhu et al., 2020). In Vicia faba, Put, Spd, and Spm are approximately 0.1, 0.5, and 0.2 μmol/g under control conditions, respectively, and Spd increases to 1 μmol/g during drought (Liu, 2000). Assuming that polyamines are distributed evenly in plant cells, the concentration of polyamines in plants can be approximately converted to 0–0.5 mM under control conditions and approximately 1 mM during drought (Liu, 2000). As we have shown in our results, polyamines induced stomatal closure only at a relatively high concentration, suggesting that polyamines might not regulate stomatal movement but play other roles, such as regulating plant development under control conditions. However, they partially inhibited ABA-induced stomatal closure significantly, even at only 0.1 mM, indicating that they are more likely to inhibit drought- and ABA-induced stomatal closure as feedback to ensure the minimum supply of carbon dioxide for photosynthesis during long-term drought stress or mild drought stress. For example, consistent with greater stomatal conductance, the exogenous addition of polyamines was shown to increase the net rate of photosynthesis significantly and therefore increase the fresh weight of rice, Rosa, and chickpea plants compared with the controls during drought stress (Berahim et al., 2021; Farooq et al., 2010; Hassan et al., 2018). Exogenous application of 0.2 mM Spd or Spm significantly increased stomatal conductance and photosynthesis but did not affect the Fv/Fm ratio of Chinese dwarf cherry during 21 days of drought stress, further supporting the hypothesis that polyamines can promote photosynthesis by inhibiting stomatal closure without affecting the stability of the photosynthetic system (Yin et al., 2014).
Polyamines also have been proven to increase drought tolerance during drought stress (Farooq et al., 2010; Hasan, Skalicky, et al., 2021; Hassan et al., 2018; Yin et al., 2014). The polyamine-dependent antioxidant systems decrease the MDA content in leaves and protect the integrity of the cell membrane (Hassan et al., 2018; Yin et al., 2014). Moreover, the accumulation of polyamines induced by overexpression of polyamine synthesis genes increases the accumulation of free prolines, which are associated with abiotic stress avoidance and osmotic stress tolerance; consequently, the plant survival rate is increased after drought (Ran et al., 2022). Overall, polyamines could be used as plant growth regulators to improve both plant growth and stress resistance during drought stress.
AUTHOR CONTRIBUTIONS
Xiang-Wen Fang, Xu-Dong Liu, and Yuan-Yuan Zeng conceived and designed the experiments; Xu-Dong Liu, Xia-Yi Zhang, and Yuan-Yuan Zeng performed most of the experiments; Xue-Qian Tian, Md. Mahadi Hasan, and Guang-Qian Yao analyzed the data and assisted with the experiments; Xu-Dong Liu, Md. Mahadi Hasan, and Xiang-Wen Fang wrote and revised the manuscript.
ACKNOWLEDGMENTS
We thank the Core Facility of School of Life Sciences, Lanzhou University. The research was partially supported by the National Natural Science Foundation of China (Nos. 31971406, 32171491), Gansu Science and Technology Major Project (22ZD6FA052), the Fundamental Research Funds for the Central Unversities (lzujbky-2021-sp63), Introduction plan for high-end foreign experts (G2022175007L) and Gansu Youth Science and Technology Fund Program (Nos. 22JR5RA531).
Open Research
DATA AVAILABILITY STATEMENT
All relevant data can be found within the article and its Supporting Information.




