Genome-wide identification and characterization of tomato 14-3-3 (SlTFT) genes and functional analysis of SlTFT6 under heat stress
Abstract
The plant 14-3-3 proteins are essential for many biological processes and responses to abiotic stress. We performed genome-wide identification and analysis of the 14-3-3 family genes in tomato. To explore the properties of the thirteen Sl14-3-3 found in the tomato genome, their chromosomal location, phylogenetic, and syntenic relationships were analyzed. The Sl14-3-3 promoters were found to have a number of growth-, hormone-, and stress-responsive cis-regulatory elements. Moreover, the qRT-PCR assay revealed that Sl14-3-3 genes are responsive to heat and osmotic stress. Subcellular localization experiments evidenced that the SlTFT3/6/10 proteins occur in the nucleus and cytoplasm Additional analysis on Sl14-3-3 putative interactor proteins revealed a number of prospective clients that potentially participate in stress reactions and developmental processes. Furthermore, overexpression of an Sl14-3-3 family gene, SlTFT6, improved tomato plants thermotolerance. Taken together, the study provides basic information on tomato 14-3-3 family genes in plant growth and abiotic stress response (high temperature stress), which can be helpful to further study the underlying molecular mechanisms.
1 INTRODUCTION
In eukaryotes, 14-3-3 proteins are ubiquitous with relatively conserved amino acid sequences. The 14-3-3 protein was first isolated from the bovine brain in 1967 (Ferl et al., 1994). Plant 14-3-3 proteins are also called general regulatory factors (GRFs) as they act as plant-specific proteins to regulate growth, development and stress response. Epsilon (ε) and non-epsilon are two different classes of plant 14-3-3 genes that are separated based on the gene structure, with ε class containing more exons and introns (Denison et al., 2011). 14-3-3 proteins can recognize specific sequences of phosphorylated serine/threonine residues, where it can bind to target proteins (Muslin et al., 1996; Yaffe et al., 1997). In addition to the main phosphorylation-dependent binding action, 14-3-3 proteins can also bind target proteins independently of phosphorylation (Petosa et al., 1998). So far, multiple members of plant 14-3-3 family genes were identified in Arabidopsis (15), rice (8), tobacco (17), wheat (17), orange (9), and grape (11), respectively (Cheng et al., 2018; DeLille et al., 2001; Konagaya et al., 2004; Lyu et al., 2021; Shao et al., 2021; Yao et al., 2007).
Plant 14-3-3 proteins participate in gene regulation and signal transduction for a variety of biological activities. Using affinity chromatography, the tomato 14-3-3 protein was found to participate in the de-etiolation process by the cytokinin signaling pathway (Hloušková et al., 2019). In rice, 14-3-3 proteins interact with proteins involved in root development, indicating that these 14-3-3 proteins are potentially involved in the control of root growth (Zhang et al., 2017). Furthermore, RICE CENTRORADIALIS, a protein similar to TERMINAL FLOWER 1, prevents rice from blooming by competing with Hd3a (a FLOWERING LOCUS T homolog protein) for its ability to bind to the 14-3-3 protein (Kaneko-Suzuki et al., 2018). Moreover, the amount of seed oil increased by overexpressing 14-3-3κ and 14-3-3λ in Arabidopsis; these 14-3-3 proteins interact with AtWRI1, a crucial transcription factor that controls the biosynthesis of plant oils (Ma et al., 2016). The 14-3-3λ/14-3-3κ double mutant displayed delayed leaf senescence and increased starvation-induced autophagic vesicles as compared to the wild type (WT; Qi et al., 2022). In GF14f-RNAi rice plants, a decrease in GF14f expression in the endosperm led to a considerable rise in grain length and weight, which enhanced grain yield (Zhang, Zhao, et al., 2019).
Plant 14-3-3 proteins play also important roles in plant defense against biotic stresses such as pathogenic diseases and insect pests. For example, CaTFT4 silencing in pepper delays the onset of effector-triggered immunity-related cell death and promotes the growth of both virulent and avirulent Xcv (bacterium Xanthomonas euvesicatoria) (Teper et al., 2014). SlTFT7 contributes to tomato aphid resistance by interacting with Me10, which is a salivary protein that enhances aphid reproduction (Chaudhary et al., 2019). Likewise, 14-3-3 proteins also play a role in plant response to abiotic stress such as drought, salinity, and cold. For instance, 14-3-3 proteins bind to SALT OVERLY SENSITIVE 2 (SOS2) and suppress its kinase activity, these interactions cause 14-3-3 to negatively regulate salt tolerance in Arabidopsis (Zhou et al., 2014). Moreover, salt stress stimulates the interaction between SOS2-LIKE PROTEIN KINASE5 (PKS5) and 14-3-3 proteins, which releases the inhibition of SOS2 and represses PKS5 kinase activity (Yang et al., 2019). Ectopic expression of MdGRF11 increased the transgenic tobacco resistance to salt and drought stresses (Ren et al., 2019). The osgf14b mutant showed enhanced tolerance to osmotic stress and drought compared with the WT, partially in an abscisic acid (ABA)-dependent way (Liu et al., 2019). However, the ectopically expressed TaGF14b conferred increased tolerance to drought and salt stresses in tobacco via the ABA signaling pathway (Zhang et al., 2018). Cold acclimation and freezing tolerance are adversely influenced by RCI1A (14-3-3ψ) in Arabidopsis (Catalá et al., 2014). The ability of plants to withstand cold temperatures is negatively influenced by the translocation of 14-3-3λ into the nucleus, where it mediates CBF1/3 degradation via the 26S proteasome pathway (Liu et al., 2017). Moreover, overexpression of At14-3-3ε and At14-3-3ω leads to improved cold tolerance (Visconti et al., 2019). Studies have also revealed that the 14-3-3 genes are potentially involved in the response to nutrient deficiency. As such, plant growth is promoted by overexpression of tomato TFT6/7 under low phosphorus conditions (Xu et al., 2012). Apple 14-3-3 protein MdGRF11 interacts with MdBT2 and affects nitrate-responsive anthocyanin accumulation (Ren et al., 2021). Under sugar shortage, MYBS2 is retained in the cytoplasm through interactions with rice 14-3-3 proteins, which rescues sugar starvation (Chen et al., 2019). Arabidopsis 14-3-3κ/λ plays a critical role in plant response to cadmium (Cd) stress by modifying the accumulation of glutathione and thus changing hydrogen peroxide (H2O2) content (Zhang, Guo, et al., 2019).
Heat stress is a major abiotic stress that threatens plant production worldwide (Ahammed et al., 2021). Therefore, mining high temperature-related genes is of great significance for developing heat-resistant germplasm resources (Liu et al., 2022). High temperature generates excessive reactive oxygen species (ROS), which caused oxidative damage to cells, and increased malondialdehyde (MDA) content and ion leakage (Zang et al., 2017). The main forms of ROS in plants are H2O2 and superoxide (O2−). To survive heat stress, plants have evolved a variety of strategies, including the induction of HSPs, the activation of antioxidant enzymes and altered stomatal movement (Lamers et al., 2020). Although the roles of 14-3-3 in abiotic stress responses such as salt, drought, and cold have achieved great progress, the role of 14-3-3 in plant heat stress still remains poorly elucidated. In this study, the 14-3-3 gene family from tomato (Solanum lycopersicum L.) was examined to explore the family members, phylogenetic tree, conserved motif, subcellular localization, and expression level of gene family members under abiotic stress. Through qRT-PCR analysis, we found that SlTFT6 responded to a variety of abiotic stresses, and SlTFT6 was strongly downregulated under heat treatment. We then hypothesized that SlTFT6 might participate in the response to heat stress. Moreover, we examined the function of a tomato 14-3-3 family gene, SlTFT6, through a reverse-genetic approach. Our results showed that overexpression of SlTFT6 could enhance heat tolerance, which unveiled crucial mechanisms of plant 14-3-3-mediated response to heat stress. Overall, the study provided valuable information and a theoretical framework for the functional investigation of tomato 14-3-3 family members.
2 MATERIALS AND METHODS
2.1 Identification, characterization, and phylogenetic analysis of 14-3-3
The 14-3-3 protein sequence was identified by searching the 14-3-3 motif (Pfam database (Finn et al., 2015) PF00244) in the HMMER3.2 software. Subsequently, the 14-3-3 protein domains were further verified through SMART (Letunic & Bork, 2018; http://smart.emblheidelberg.de/smart) and CDD (conserved domain database; https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cg) to remove nonconserved miscellaneous proteins. In addition, the basic physical and chemical properties were predicted by the ExPASy database (http://www.expasy.org/tools/). Furthermore, we utilized MEGA7.0 software (Kumar et al., 2016) to construct the phylogenetic tree using the neighbor-joining (NJ) method (1000 replicates for computing the bootstrap values).
2.2 Conserved motifs and gene structure analysis of 14-3-3
MEME (Bailey et al., 2015; https://meme-suite.org/meme/tools/meme) was applied to predict motifs with a width set between 5 and 50. The 14-3-3 gene structure visualization was obtained from GSDS2.0 (Hu et al., 2014; http://gsds.gao-lab.org/). PlantCARE (https://bioinformatics.psb.ugent.be/webtools/plantcare/html) was used to predict the cis-regulatory elements of the 14-3-3 gene promoters (2000 bp).
2.3 Chromosomal location and syntenic analysis
The genome sequence data of numerous species were uploaded to Tbtools (Chen et al., 2020), so that the Multiple MCScanX software could do syntenic analysis. Tbtools was also used to explore the chromosomal location and syntenic gene pairs in the tomato, pepper, potato, and eggplant as previously described (Manzoor et al., 2021).
2.4 Abiotic stress treatments and qRT-PCR analysis
For gene expression analysis under abiotic stress such as heat, chilling, and osmotic stress, 7-day-old tomato (S. lycopersicum L. cv. Alisa Craig, AC) plants were used as previously described (Liang et al., 2022). The transformation of tomato plants with the SlTFT6 overexpression vector was performed as previously described (Liang et al., 2022). For functional analysis of SlTFT6 under heat stress, 30-day-old SlTFT6 overexpression T2 lines and WT plants were exposed to 42°C for 48 h in the artificial climate incubator (16 h day/8 h night). The total RNA from leaves was extracted with TriQuick Reagent (Solarbio), then used to generate the first-strand cDNA via M-MuLV Reverse Transcriptase (GenScript). The Top Green qPCR SuperMix (TransGen) was used to conduct qRT-PCR experiments. As a baseline gene, SlACTIN7 was selected. The specific primer sequences utilized for expression analyses are provided in Table S3.
2.5 Protein interaction network analysis
We utilized STRING (Szklarczyk et al., 2020; https://cn.string-db.org/) to predict the interaction network of the 14-3-3 protein, then further processed and visualized using cytoscape3.8 software (Shannon et al., 2003).
2.6 Subcellular localization of SlTFT3/6/10
The full-length CDS of SlTFT3/6/10 lacking the stop codon was inserted into pCAMBIA2300-GFP (35S:: SlTFT3/6/10-GFP). Subcellular localization analyses were performed as previously described (Liang et al., 2022).
2.7 Yeast transactivation assay
The pGBKT7-SlTFT6 was created by PCR amplification of the full-length SlTFT6 CDS using primers described in Table S3. The vectors were then introduced into Y2H-gold using the yeast transformation kit (Coolaber). Finally, selective plate media (SD/-Trp and SD/-Trp/-His/-Ade) was used to cultivate the transformants for 4 days.
2.8 Measurement of physiological indicators
For in situ visualizations of ROS, detached tomato leaves were soaked with NBT (Solarbio) and DAB (Deeyee) for 3 and 10 h to perform histochemical staining for O2− and H2O2, respectively. The relative electrolyte leakage (REL), MDA content, proline content, and activities of superoxide dismutase (SOD) and peroxidase (POD) were performed as previously described (Liang et al., 2022).
3 RESULTS
3.1 Identification and phylogenetic analysis of tomato 14-3-3 genes
By using BLAST analysis, Hidden Markov Model search, and recognition of the conserved domains, thirteen 14-3-3 genes were identified from the tomato genome (ITAG4.0). There were 249 to 285 encoded amino acids, with predicted molecular weight ranging from 28.2 to 32.2 kDa. Additionally, the theoretical isoelectric points ranged from 4.61 to 4.97 (Table S1).
To study the evolutionary relationships of 14-3-3 orthologs, a phylogenetic tree was built using the NJ method based on the proteins from five plant species, including tomato, potato, pepper, eggplant, and Arabidopsis (Figure 1). In short, 28 were clustered into ε group, while 44 were grouped into the non-ε group. As for tomato, only SlTFT7, SlTFT8, SlTFT9, SlTFT12, and SlTFT13 were placed into the ε group, while the other SlTFTs were grouped into the non-ε group. The strong closeness of the proteins from tomato, potato, pepper, and eggplant suggests that the 14-3-3s have been conserved throughout the evolution of Solanaceae plants.
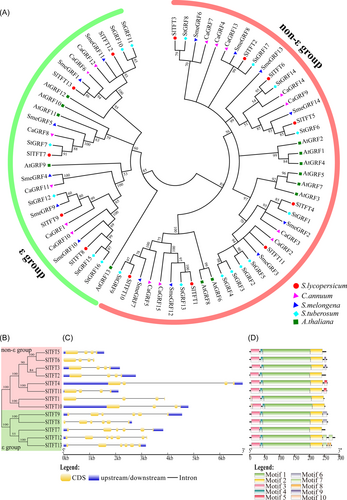
3.2 Gene structure and motif analysis of tomato 14-3-3 genes
A 14-3-3 gene structure map was created to systematically analyze the gene structures of Sl14-3-3 genes (Figure 2). All non-ε class Sl14-3-3 genes had three introns, whereas SlTFT12 had six introns and the last gene of ε class, Sl14-3-3, contained five introns. Furthermore, a highly conserved 14-3-3 domain was revealed by the multiple sequence alignment of Sl14-3-3 proteins (Figure S1). The MEME program predicted 10 conserved motifs of Sl14-3-3 proteins, of which motifs 1–4 were conserved in Sl14-3-3 from both ε class and non-ε class. Additionally, the remaining Sl14-3-3 members have at least five motifs, with the exception of SlTFT5, SlTFT2 and SlTFT7, which only contained four motifs (motifs 1–4). However, according to predictions of transmembrane helices, all tomato 14-3-3 proteins were found to lack a transmembrane structure (Figure S3).
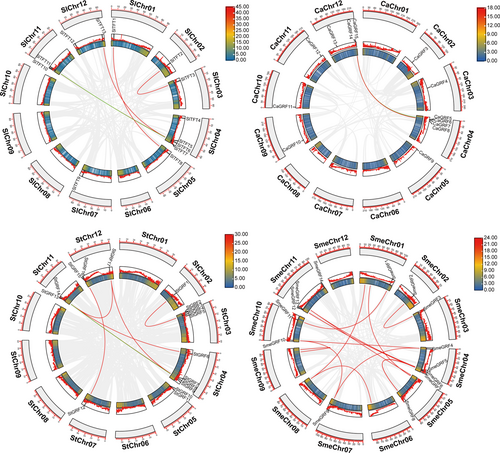
3.3 Chromosomal location and syntenic analysis of 14-3-3 genes
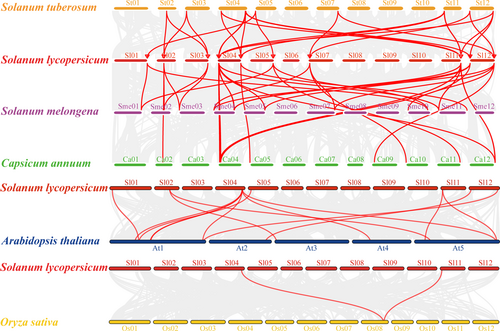
The 14-3-3 genes were mapped onto the chromosomes of the tomato, potato, pepper, and eggplant genomes. A total of 13, 15, 14, and 17 14-3-3 genes were distributed on 8, 9, 8, and 6 tomato, pepper, eggplant, and potato chromosomes, respectively (Figure 2). In tomato, three Sl14-3-3 genes were found on chromosome 4, two were found on chromosomes 11 and 12, and one on chromosomes 1, 2, 3, 5, and 7. This study also found intragenomic synteny blocks for each species; tomato, pepper, potato, and eggplant contained 5, 2, 6, and 13 syntenic pairs, respectively. Moreover, we created a tomato comparative syntenic map with other Solanaceae species, Arabidopsis, and rice. In Solanaceae plants, there are 27, 26, and 19 orthologous gene pairs between tomato and potato, eggplant, and pepper, respectively (Figure 3). As for comparisons with distantly related plants, total orthologous gene pairs between tomato and Arabidopsis was 14, and 2 between tomato and rice. These findings suggested that, compared to Arabidopsis and rice, tomato was closely related to the other Solanaceae species.
3.4 Cis-regulatory elements analysis of tomato 14-3-3 genes
We scanned the 2 kb region upstream of each gene's start codon (ATG) to look for cis-regulatory elements in the Sl14-3-3 promoter sequences. These elements were divided into three main groups: plant growth and development, phytohormone responsiveness, and stress responsiveness (Figure S4). For instance, the most prevalent components in the phytohormone responsiveness group were the EREs for ethylene responsiveness and the ABREs for ABA responsiveness. The biggest number of elements in the three categories was those involved in stress response, indicating that Sl14-3-3 may have a role in response to abiotic stress. The plant growth and development group included Circadian, O2-site, GCN4_motif, RY-element, and HD-Zip 1; GCN4 motif being the most frequent. In comparison to plant growth and development, the number of components involved in phytohormone responsiveness and stress response was much larger. Overall, our study revealed that the Sl14-3-3 genes may be involved in a number of biological activities in tomato.
3.5 Tomato 14-3-3 protein interaction prediction
To explore the protein interaction network of the tomato 14-3-3 family, the STRING database was used for protein interaction analysis, with Sl14-3-3s as queries and tomato as the target alignment species. As shown in Figure S5, Sl14-3-3 protein members can interact with each other to form heterodimers. Moreover, the results show that Sl14-3-3 can interact with a variety of important proteins, including the following: nitrate reductase (NR), plasma membrane H+-ATPase (LHA1/2/4), HSP70, and Ser/Thr protein phosphatase (BSL1, Solyc05g018300, Solyc06g073960, Solyc01g009280). These results suggest that tomato 14-3-3 protein may participate in various growth and development pathways through interaction networks.
3.6 Tissue expression patterns of tomato 14-3-3 genes
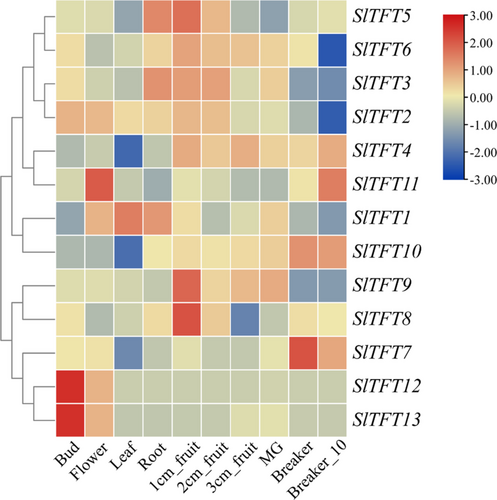
The expression patterns of the thirteen 14-3-3 genes were analyzed in diverse tomato tissues via the TFGD website (http://ted.bti.cornell.edu). With the exception of the breaker fruit, all organs exhibited high levels of overall expression for three genes (SlTFT6, SlTFT3, and SlTFT2), indicating that these Sl14-3-3 genes are crucial for the function of various tissues (Figure 4). Additionally, the patterns of a homologous gene pair (SlTFT1 and SlTFT10) showed variation. SlTFT1 was highly expressed in the flower, leaf, and root, while SlTFT10 expression was low in these tissues. Remarkably, the patterns displayed by another homologous gene pair (SlTFT12 and SlTFT13) were identical. The transcript abundances of SlTFT12 and SlTFT13 were highest in the bud, while their expression levels were very low in other tissues.
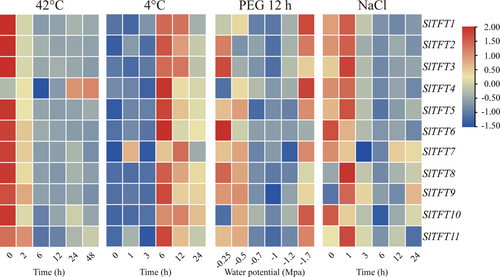
3.7 Expression patterns of tomato 14-3-3 genes under different abiotic stresses
To investigate how tomato 14-3-3 genes respond to various abiotic stresses, qRT-PCR was used to analyze Sl14-3-3 expression patterns under various stresses (Figure 5). With the exception of SlTFT4, all Sl14-3-3 genes were downregulated when exposed to heat. Additionally, most genes maintained very low expression after 48 h of heat treatment. In contrast to high-temperature treatment, chilling led to almost opposite responses in Sl14-3-3, wherein all Sl14-3-3 genes were moderately upregulated by chilling for 24 h with relatively strong upregulation at 6 h of chilling. As for simulated drought stress treatment, the expression levels of SlTFT3, SlTFT6, and SlTFT8 were downregulated, and the other Sl14-3-3 were slightly altered. When tomato plants were exposed to NaCl treatment, the transcripts of most Sl14-3-3 genes upregulated initially at 1 h, followed by gradual downregulation. Especially, SlTFT6 showed a strong gradual downregulated expression pattern from 0 to 24 h. Taken together, these results suggest that Sl14-3-3 may play important roles in abiotic stress responses.
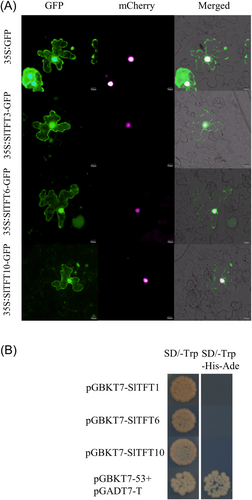
3.8 Subcellular localization and transcriptional activation assay of Sl14-3-3 proteins
For the analyses of subcellular localization, the stress-responsive proteins SlTFT3, SlTFT6 and SlTFT10 were chosen. We observed that the signals of the fusion proteins SlTFT3-GFP, SlTFT6-GFP, and SlTFT10-GFP were dispersed in the cytoplasm and the nucleus (Figure 6). Our results are consistent with previous reports on apple and wheat (Ren et al., 2019; Zhang et al., 2018). Additionally, SlTFT1/6/10 proteins did not show any transcriptional activation activity in the yeast-two-hybrid assay (Figure 6). Our findings suggest that the SlTFT1/6/10 proteins lack transcriptional activation capability.
3.9 Overexpression of SlTFT6 increases heat tolerance in tomato
To reveal the biological function of SlTFT6 under high-temperature stress, we performed genetic transformation by overexpressing SlTFT6 in tomato (Alisa Craig background). The qRT-PCR results revealed that the transcript abundance of SlTFT6 in the OE-3 and OE-5 plants was about 17-fold and 30-fold, respectively (Figure S6) but had similar growth and phenotype under optimal growth conditions. When plants were 4-week-old, WT and transgenic plants were exposed to 42°C for 2 days (Figure 7A). After heat stress for 2 days, the SlTFT6 overexpression plants showed less stem bending, the electric conductivity and MDA content in SlTFT6 overexpression plants were about 28 and 43% lower than those in the WT plants, indicating that the overexpression of SlTFT6 resulted in decreased damage under heat stress. After recovery for 2 days, the survival rate of SlTFT6 OE plants was about 50% higher than that of WT plants.
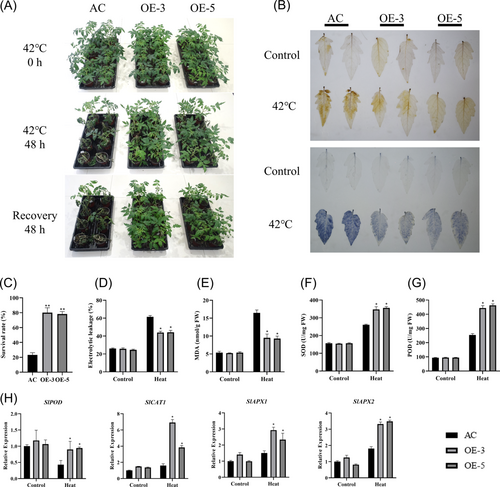
We used DAB and NBT staining to determine the H2O2 and O2− contents. Between the WT and the SlTFT6 overexpression plants, there were no discernible variations in the accumulations of H2O2 and O2− under control conditions. However, SlTFT6-overexpressing plants showed lower O2− and H2O2 levels during heat stress than the AC plants (Figure 7B). After exposure to heat stress, it was found that the SOD and POD activities in OE plants were approximately 35% and 80% higher than those in the AC plants. Furthermore, in comparison to AC plants under heat stress, SlTFT6 overexpression plants showed considerably higher levels of transcripts for genes encoding for antioxidant enzymes such as SlPOD, SlCAT1, SlAPX1, and SlAPX2. These results demonstrate that overexpression of SlTFT6 improves ROS detoxification under heat stress.
3.10 Expression profiles of heat stress-related genes in the SlTFT6 overexpression plants
In addition to the antioxidant enzyme system, the expression of important heat stress response-related genes such as HSPs and Hsfs plays a vital role in plant tolerance to high-temperature stress (Xu, Zhao, et al., 2016; Zhuang et al., 2018). After 2 h heat treatment, SlHsfA1a, SlHsfA2, SlHsfB1, SlHSP70-5, SlHSP90-1, and SlHSP100 showed higher induction in SlTFT6-overexpressing plants than in AC plants, whereas the expression levels of SlHsfA3, SlHSP17.7, and SlHSP90-4 were strongly induced in SlTFT6-overexpressing plants at multiple time points (Figure 8). However, SlHsfA1b, and SlHSP70-3 exhibited no significant difference between overexpressing plants and AC plants under heat stress. These results suggest that the high-temperature resistance of tomato plants overexpressing SlTFT6 is partly mediated by enhanced expression of some SlHsfs and SlHSPs.

4 DISCUSSION
The growth, development, and stress responses of plants are significantly influenced by 14-3-3 proteins (Huang et al., 2022). Thus, we studied Sl14-3-3 genes across the whole genome of tomato. We discovered 13 members of the Sl14-3-3 gene family using bioinformatic. There was an uneven distribution of the Sl14-3-3 family genes on the chromosomes. Using the data of five species, 72 14-3-3 proteins were grouped into two main categories (ε and non-ε) by phylogenetic analysis. In tomato, similar to many species, non-ε class 14-3-3 proteins are more prevalent than ε class 14-3-3 proteins and the secondary structure of 14-3-3 proteins was conserved except for the C-terminal region. It has been previously shown that the C-terminal motifs vary and have a direct impact on how 14-3-3 proteins interact with other proteins (Börnke, 2005).
The response of plant 14-3-3 to various abiotic stresses has been reported in earlier studies. In our study, all tomato 14-3-3 members except SlTFT4 were strongly suppressed under heat treatment. However, all 14-3-3 genes were induced by chilling stress to some extent. Under NaCl and PEG treatments, eight Sl14-3-3 genes exhibited reduced transcript levels, whereas three Sl14-3-3 genes showed higher transcript levels. Interestingly, under heat, NaCl and PEG stresses, there were more downregulated Sl14-3-3 members than upregulated genes. Moreover, SlTFT6 was highly downregulated under heat, NaCl and PEG stresses, indicating that this gene might be the key gene responsible for regulating stress tolerance. Thus, the biological function of SlTFT6 under heat stress was studied and we found that overexpression of SlTFT6 enhanced heat resistance in tomato, but the role of SlTFT6 in other abiotic stress needs to be further elucidated in future studies.
Although the role of plant 14-3-3 in abiotic stress tolerance has been extensively researched in recent decades, plant 14-3-3 was not substantially characterized under heat stress, in terms of its function and regulatory mechanism. Lipid peroxidation is induced by high-temperature stress and it can be characterized by ROS accumulation and MDA content. Furthermore, antioxidant enzymes, such as POD, SOD, and APX, are crucial for scavenging the overflow of ROS. In our experiment, heat stress caused a rise in H2O2 and O2•− accumulation, but plants overexpressing SlTFT6 had much lower ROS, MDA, and REL levels than AC plants. Furthermore, WT plants had a lower survival rate and antioxidant enzyme activity compared with SlTFT6-overexpressing plants. These findings strongly suggest that SlTFT6 overexpression may confer thermotolerance to tomato plants, thus minimizing ROS-induced cellular damages.
Most frequently, 14-3-3 proteins accomplish their functions through interactions with other proteins. A series of possible target proteins of 14-3-3 proteins, such as NR, PLASMA MEMBRANE H+-ATPase (LHA1/2/4), HSP70, Ser/Thr PROTEIN PHOSPHATASE, were predicted by bioinformatics analysis. NR is the rate-limiting enzyme for nitrogen assimilation in higher plants (Solomonson & Spehar, 1977). It has the ability to directly control nitrate reduction, which in turn regulates nitrogen metabolism and influences photosynthetic carbon metabolism. In Arabidopsis, the 14-3-3-mediated inhibition of NR is considerably facilitated by a novel 14-3-3 binding location inside the NR N-terminus (Chi et al., 2015). Importantly, magnesium (Mg) enhances the interaction between the 14-3-3 protein from Panax notoginseng and NR under Cadmium (Cd) stress, thereby reducing NR activity and NO generation (Li et al., 2020). Plasma membrane H+-ATPases (PM H+-ATPases) are important proton pumps that transport protons from the cytoplasm to the apoplast. Under nitrate treatment, the 14-3-3 interaction with phosphorylated H+-ATPase was improved in spinach roots. At the germination and young seedling stage, transgenic tobacco plants overexpressing So14-3-3 exhibited increased tolerance to nitrate treatment (Xu, Cai, et al., 2016). Depending on the time of the cold treatment, the activity of PM H+-ATPase in Arabidopsis is affected differently. These alterations in activity are related to the degree of 14-3-3 and PM H+-ATPase interaction (Muzi et al., 2016). The HSPs are essential for improving plant thermotolerance and reducing heat stress damage to plants. Several HSP70s and HSP90s were identified to interact with SlTFT6 by LC/MS analysis in tomato young plants exposed to blue light (Hloušková et al., 2019).
Furthermore, Ser/Thr protein phosphatases can interact with 14-3-3s to perform important functions. Ser/Thr protein phosphatases can selectively dephosphorylate the Tyr or Ser/Thr residues of intracellular proteins, thereby cooperating with protein kinases to regulate the phosphorylation level and degree of intracellular proteins. In mammalian cells, 14-3-3ζ modulates the nuclear trafficking of PP1α (Ser/Thr phosphatases) by binding to it (Jérôme & Paudel, 2014). The biogenesis of microRNAs is facilitated by PP4R3A (Ser/Thr protein phosphatase) in association with PROTEIN PHOSPHATASE X1 (PPX1) and PPX2 (Wang et al., 2019). These findings imply that 14-3-3 may play critical functions via their interaction network.
Our findings suggested that overexpression of SlTFT6 is sufficient to give heat stress tolerance in tomato, but the function of SlTFT6 in the regulation of heat-stress-related genes is currently unknown. HsfA1, HsfA2, and HsfB1 are well known crucial transcription factors that control the transcription of heat stress-related genes, including HSPs in tomato (Baniwal et al., 2004; Xu et al., 2022). Plant heat tolerance is improved by HSPs, which are the direct target genes of Hsfs. In the present study, several Hsfs and HSPs gene were obviously increased in SlTFT6 overexpression plants following heat stress. These findings suggest that an increase in HSPs and Hsfs expression might be linked to SlTFT6-mediated heat stress response.
AUTHOR CONTRIBUTIONS
Yunfei Liang and Xiangqiang Zhan conceived the experiments. Yunfei Liang and Fang Ma performed the experiments. Ruili Zhang, Wenyu Li, Jiao Dang, Boyu Li, and Huai Su analyzed the data. Tixu Hu, Mingke Zhang, and Yan Liang participated in the production of the experiment materials. Yunfei Liang wrote the original paper. Yunfei Liang and Xiangqiang Zhan revised the paper.
ACKNOWLEDGMENTS
This work was supported by the National Agriculture Science and Technology Major Program (No. NK20220904), Key Research and Development Plan of Shaanxi Province (No. 2021LLRH-07), Key Research and Development Projects of Ningxia Hui Autonomous Region (No. 2022BBF02008-03), Xi'an Urban Agriculture Key Technology Research Project, and the 100 Talents Plan of Shaanxi Province.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary material of this article.




