Water permeability of different aerial tissues of carnations is related to cuticular wax composition
Abstract
Cuticular wax protects aerial plant tissues against uncontrolled water loss. To compare the differences among tissues, cultivars, and postharvest stages, we characterized the surface morphology, water permeability, and chemical composition of cuticular wax on the leaf, calyx, and petals of two carnation cultivars (‘Master’ and ‘Lady green’) at two postharvest stages. Obvious differences in these characteristics were found among tissues but not among cultivars or postharvest stages. The leaf surface was relatively smooth, whereas convex cells were observed on the petals. The mean minimum conductance of leaves was significantly higher than that of the calyx, followed by that of petals. It ranged between 8.8 × 10−4 m s−1 for ‘Lady green’ leaves at Stage II and 3.6 × 10−5 m s−1 for ‘Master’ petals at Stage I. Petal wax contained high concentrations of n-alkanes, whereas primary alcohols dominated in leaf wax. The weighted average chain length (ACL) was higher in petal wax than in leaf wax; it ranged from 19.6 in ‘Lady green’ leaves to 24.14 in ‘Lady green’ petals at Stage I. In conclusion, carnation petals are characterized by numerous convex cells on both the adaxial and abaxial surfaces, and their main cuticular wax components, alkanes, have a higher ACL than leaf cuticular wax, which contributes to their higher water barrier property. The results provide further evidence for the association between cuticular chemical composition and the physiological function of the cuticle in blocking water transpiration.
1 INTRODUCTION
The aboveground tissues of higher plants are covered by a thin and continuous hydrophobic cuticle (Samuels et al., 2008). The functions of the cuticle are well established; it serves as a barrier against uncontrolled water loss (Seufert et al., 2022), regulates the exchange of gas and chemicals (Ray et al., 2022), attenuates ultraviolet irradiation and protects against pathogen invasion and extreme temperatures (Aragón et al., 2021; Xue et al., 2017), and prevents organ fusion during development (Müller & Riederer, 2005). Cutin and waxes are the main constituents of the plant cuticle, and their chemical composition, relative amounts, and structural arrangement determine the various functions of the cuticle. Waxes, rather than cutin, provide the barrier against uncontrolled water loss. Cuticular wax is a mixture of very-long-chain aliphatic and cyclic compounds, such as fatty acids, primary alcohols, n-alkanes, aldehydes, alkyl esters, triterpenoids, and sterols. As a result of the chemical diversity of cuticular waxes, the cuticle permeability and, consequently, water loss properties vary among plant species, tissues, and developmental stages.
Petals, like leaves, are covered with a cuticle and must resist environmental stresses, such as desiccation. Flowers are important for sexual reproduction, and the main functions of petals are to attract and assist pollinators. The role of the petal cuticle in these functions and the structural/chemical composition–function relation of the petal cuticle remains largely unknown. Compared to leaf cuticles, the wax composition and water resistance of petal cuticles are less well studied. It has been reported that n-alkanes dominate the cuticular wax in the petals of faba bean (Griffiths et al., 1999), raspberry (Griffiths et al., 1999), potato (Goodwin et al., 2003), and snapdragon (Guo & Jetter, 2017). In other species, such as Cosmos bipinnatus (Buschhaus et al., 2015) and dandelion (Guo et al., 2017), primary alcohols have also been found in the petal wax. Buschhaus et al. (2015) examined the water barrier properties and chemical components of C. bipinnatus petal cuticles. They found that the petal cuticle showed weaker water resistance than the leaf cuticle because the chain lengths of the main components of the petal cuticle were shorter than those in the leaf cuticle. Similar findings have been made in rose and lily (Cheng et al., 2019, 2021). Goodwin et al. (2003) studied the wax composition of snapdragon petals over 12 days from flower opening to senescence and found no significant change during flower development.
Carnation (Dianthus caryophyllus L.) is one of the four major cut flowers in the world. Vase life, or longevity, is an important characteristic of cut flowers (Yagi et al., 2014), which is often restricted by adverse water relations (In et al., 2013). When transpiration is higher than water uptake, a water deficit occurs and causes wilting (Fanourakis et al., 2016). Carnation cut flowers are generally harvested at the “paintbrush” stage. After storage and/or transport, they gradually open when the flowers are placed in a vase. The transpiration barrier properties and cuticular components of carnation petals, which are the main constituents of the flower organ, have not been studied comprehensively. This study aimed to compare the morphology, water barrier properties, and wax composition among leaf, calyx, and petal cuticles of two carnation cultivars, ‘Master’ and ‘Green Lady’, at two postharvest stages.
2 MATERIALS AND METHODS
2.1 Plant materials
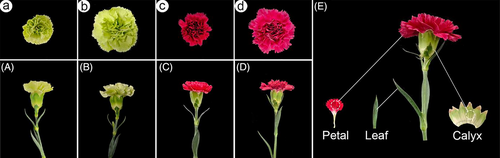
Petals, calyxes, and leaves were used to evaluate differences in water permeability and cuticular wax composition between two carnation cultivars (D. caryophyllus L. ‘Master’ and ‘Lady green’) after harvest. Carnation seedlings were grown in pots with soil under a 12/12 h light/dark cycle in a climate chamber at 20 ± 2°C and with 60 ± 2% relative humidity at Zhongkai University of Agriculture and Engineering (China). The pots were watered daily to keep the soil moist. Freshly harvested standard flowering carnation stems at the commercial (“paintbrush”) stage were harvested in the early morning and immediately placed upright in plastic buckets partially filled with tap water. After transfer to the lab, flowering stems of uniform size and color and free from defects were recut under deionized water to approximately 35 cm in length, with two pairs of leaves. Then, the stems were individually placed in 250 mL glass vases containing 100 mL deionized water. At Stages I (Figure 1a,A and c,C) and II (Figure 1b,B and d,D), the leaves and petals in the second whorls and calyxes were collected for water permeability and cuticular wax composition analyses. The classification of the carnation flower developmental stages was according to Jones (2002): Stage I, petals separated and forming a 30° angle with the axis of the calyx, and Stage II, petals fully open and forming a 90° angle with the axis of the calyx.
2.2 Scanning electron microscopy
The surface morphology of petals, calyxes, and leaves was observed using scanning electron microscopy (SEM). First, sample molds were prepared and cast using polyaddition silicone rubber (He et al., 2019). The hardened casts were coated with gold at 30 mA using a Cressington 108 sputter coater (Ted Pella), observed under an EVO-18 electron microscope (Carl Zeiss) at a 15 kV accelerating voltage, and photographed.
2.3 Water permeability analysis
The water transpiration from whole petals, calyxes, and leaves was determined to measure water loss over time. Ten fresh samples without any defects from each cultivar were collected. Before measurement, the openings of samples (such as leaf petiole) were placed in deionized water overnight and then sealed with paraffin wax (melting point, 60°C). The samples were maintained at 22 ± 2°C and 50 ± 2% relative humidity. A digital thermometer (Anymetre) was used to monitor the temperature and relative humidity of the atmosphere. The weight loss of the samples was recorded automatically every 15 min for 24 h using a digital balance with a precision of 0.1 mg (BSA-224 S; Sartorius).
The transpiration rate (flux of water vapor, F, in g m−2 s−1) was obtained from the change in fresh weight of the samples (W in g) over time (Δt in s) and the surface area (A in m2) as F = ΔW/(Δt × A). The surface area (A) was obtained by digital scanning (LaserJet Pro MFP M126nw; HP). The water permeance (P, in m s−1) was calculated as the transpiration rate (F) divided by the driving force: . The water vapor saturation concentration at the actual sample temperature (water vapor content of the air at saturation; ) was obtained from tabulated values (Nobel, 2009). The water activity in the air (aair) was the relative humidity. The water activity in the sample (asample) was assumed to be unity (Burghardt & Riederer, 2003).
2.4 Cuticular wax extraction
Parts of leaves, 1-cm-diameter discs of petals, and calyxes were dipped into chloroform for 30 s to extract the cuticular wax (Figure 1E). Each sample was extracted twice consecutively, and the extracts were combined. n-Tetracosane was added to the extracts as an internal standard. The solvent was evaporated under a gentle stream of nitrogen.
2.5 Wax composition analysis
The dried samples were derivatized with N,O-bis(trimethylsilyl)trifluoroacetamide in pyridine at 70°C for 30 min and then used to quantify the wax components. The samples were analyzed using a capillary gas chromatograph (GC; 7820A; Agilent Technologies) equipped with a capillary column (30 m × 0.32 mm, DB-1 ms, 0.1 μm film; J&W Scientific; Agilent Technologies). The GC oven was held at 50°C for 2 min, raised to 200°C at 40°C min−1, held at 200°C for 2 min, then raised to 320°C at 3°C min−1 and held at 320°C for 30 min. The carrier gas was hydrogen. The area of the peaks was compared with that of the internal standard to quantify the wax components.
The wax components were analyzed using a temperature-controlled capillary GC equipped with a mass spectrometric detector (m/z 50–750, MSD 5975; Agilent Technologies) under the same gas chromatographic conditions but using helium as carrier gas. Single compounds were identified based on their electron ionization mass spectra using authentic standards, the Wiley 10th/NIST 2014 mass spectral library (W10N14; John Wiley & Sons), or by interpretation of the spectra according to their retention times and/or by comparison with literature data.
2.6 Statistical analysis
Statistical analyses were performed using SPSS (v.23, IBM) and SigmaPlot 10 (Systat Software). The normal distribution of the data was tested using the Shapiro–Wilk or Kolmogorov–Smirnov normality test. Means were compared using one-way analysis of variance (ANOVA). SigmaPlot 10 was used to depict the graphs. p < 0.05 was considered significant.
3 RESULTS
3.1 Micrographic features of different aerial tissues of carnation
SEM was used to investigate the microrelief of the leaf, calyx, and petal surfaces in the two carnation cultivars (Figures 2 and 3). The shapes of epidermal cells varied among the tissues. Regular, wavy cuticle folds, obvious stomata, and some crystals were observed on the adaxial and abaxial leaf surfaces and the abaxial calyx surface of ‘Master’ carnations at Stages I and II (Figure 2). The folds were more obvious at Stage II. The abaxial calyx surface had fewer stomata than the leaf surface. The adaxial and abaxial petal surfaces had smooth, convex papilla cells (Figure 2). Stomata were not observed on both petal surfaces (Figure 2). Similar morphological observations were made for ‘Lady green’ carnations (Figure 3).
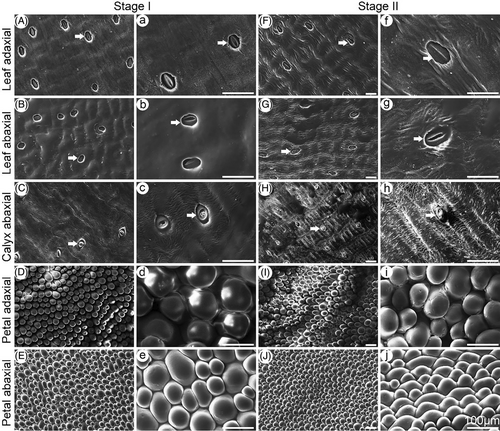
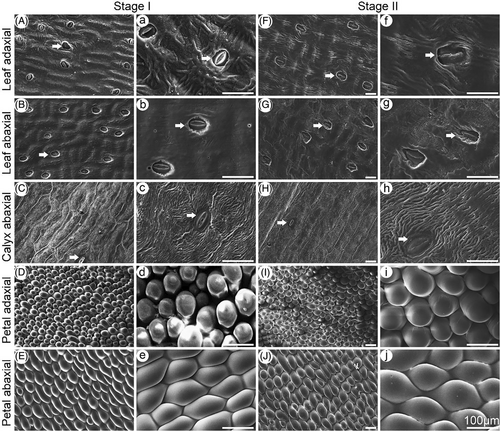
3.2 Water permeance across different aerial tissues of carnation
The minimum leaf conductance, which corresponds to cuticle transpiration, varied among the leaf, calyx, and petal. The minimum conductance of the leaf was significantly higher than that of the calyx, followed by that of the petal, in both cultivars (Figure 4A). For ‘Lady green’, the mean minimum conductance of the leaf was 88.8 × 10−5 m s−1 at Stage II and 46.1 × 10−5 m s−1 at Stage I, whereas that of the petal was lower, being 10.7 × 10−5 m s−1 at Stage II and 5.0 × 10−5 m s−1 at Stage I (Figure 4A). Similar trends were observed for the three tissues in the cultivar ‘Master’. The water loss rate generally increased during flower development (Figure 4A).
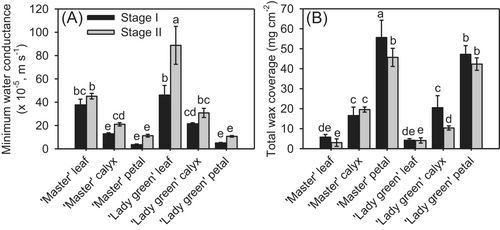
3.3 Wax coverage of different aerial tissues of carnation
The wax coverage ranged from 3.03 ± 1.89 μg cm−2 (leaves of ‘Master’ at Stage II) to 55.61 ± 8.65 μg cm−2 (petals of ‘Master’ at Stage I) (Figure 4B). In both ‘Master’ and ‘Lady green’, the overall wax coverage on leaves was significantly lower than that on petals (Figure 4B). No obvious differences in wax coverage existed between the two cultivars and stages (Figure 4B).
3.4 Wax composition of different aerial tissues of carnation
The chemical composition of the cuticular wax on leaves, calyxes, and petals of ‘Master’ and ‘Lady green’ at the two developmental stages was analyzed (Figure 5). Overall, the cuticle wax on the various tissues mainly contained typical VLCFAs and their derivatives, including fatty acids, primary alcohols, aldehydes, n-alkanes, n-alkenes, acetates, and methyl esters (Figure 5; Table S1). Methyl esters and enols were found only in ‘Master’ petal cuticle wax (Figure 5; Table S1). The major components of “Master” leaf cuticle wax were primary alcohols (40.9–53.8%), n-alkanes (16.5–17.4%), and aldehydes (7.94–15.3%). Similarly, the major components of ‘Lady Green’ leaf cuticle wax were primary alcohols (33.1–61.3%), n-alkanes (9.2–11.6%), and aldehydes (2.8–10.9%). The contents of the main components in the leaf cuticle fluctuated between the postharvest stages, with similar trends in ‘Master’ and ‘Lady Green’ (Figure 5A,D).
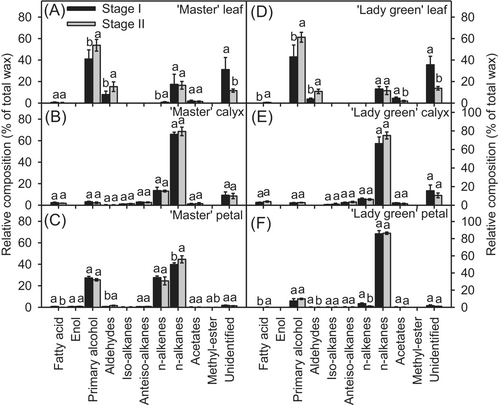
In the calyx cuticle, n-alkanes and n-alkenes were the dominant components, with n-alkanes showing a nearly threefold higher concentration than n-alkenes (Figure 5B,E). The concentrations of n-alkanes and n-alkenes were similar in ‘Master’ and ‘Green lady’ calyx cuticles at different developmental stages (Figure 5B,E).
In petal cuticle wax, n-alkanes were the major components, constituting 39.7–44.6% in ‘Master’ and 85.6–86.3% in ‘Lady green’ petal cuticle wax (Figure 5C,F). Notably, the relative contents of n-alkenes and primary alcohols were higher in ‘Master’ petal cuticles than in ‘Lady green’ (Figure 5C,F).
3.5 Chain length distribution in the cuticular wax on different aerial tissues of carnation
The cuticular wax on the leaves, calyx, and petals of carnations consisted predominantly of a homologous series of acyclic VLCFAs and their derivatives. The chain length distributions among compound classes were similar in the two cultivars and at the two developmental stages, whereas the dominant carbon chain lengths differed among tissues. The longest chain length in fatty acids, alkanes, alcohols, and aldehydes was C30, C33, C32, and C30, whereas the shortest chains had 20, 23, 22, and 20 carbons, respectively (Figures 6 and 7).
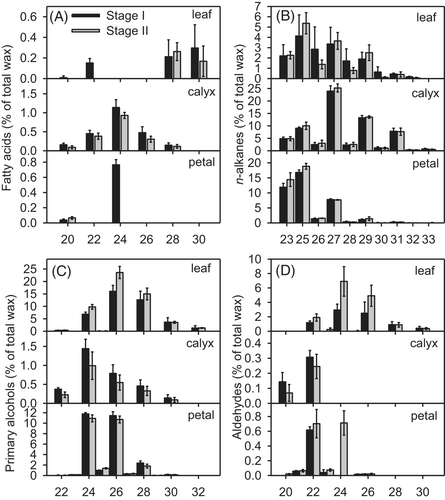
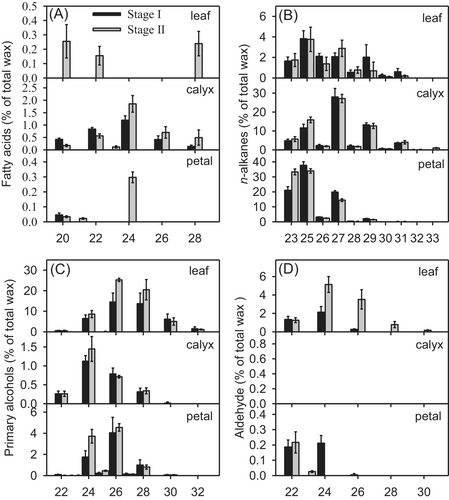
n-Alkanes, the most abundant fraction in the cuticular waxes on the calyx and petals, were present in all tissues of ‘Master’ and ‘Lady green’ carnations at both stages. The chain length of n-alkanes, predominantly odd number carbon chains, ranged from C23 to C33, with C25 and C27 alkanes dominating in leaves, C27 alkanes in calyxes, and C23 and C25 alkanes in petals (Figures 6 and 7).
The primary alcohol fraction of leaf wax, comprising mainly even-numbered chains, ranged in length from C22 to C32, with C26 chains being predominant, whereas that of wax on the calyx and petals ranged from C22 to C30 in length, with a prominent accumulation of C24 alcohols in the calyx and of C24 and C26 alcohols in the petals (Figures 6 and 7).
3.6 Weighted ACL of the cuticular wax on different aerial tissues of carnation
The ACL of acyclic compounds, calculated based on the molar concentrations of VLCFAs, ranged from 19.6 for ‘Lady green’ leaves at Stage I to 24.1 for petal Stage I. ACLs were higher in petals than in leaves and calyxes (Table 1).
| Very-long-chain acyclic fraction | Average chain length | |
|---|---|---|
| ‘Master’ | ||
| Leaf | ||
| Stage I | 5.76 ± 1.35 | 21.61 ± 1.17 |
| Stage II | 3.04 ± 1.89 | 22.29 ± 0.82 |
| Calyx | ||
| Stage I | 16.54 ± 4.28 | 23.59 ± 1.18 |
| Stage II | 19.57 ± 1.27 | 24.02 ± 1.07 |
| Petal | ||
| Stage I | 55.61 ± 8.65 | 24.04 ± 0.24 |
| Stage II | 45.68 ± 4.54 | 24.02 ± 0.23 |
| ‘Lady green’ | ||
| Leaf | ||
| Stage I | 6.01 ± 1.20 | 19.63 ± 0.75 |
| Stage II | 4.11 ± 1.33 | 22.53 ± 1.23 |
| Calyx | ||
| Stage I | 20.52 ± 6.03 | 21.50 ± 0.25 |
| Stage II | 10.38 ± 1.06 | 23.01 ± 1.10 |
| Petal | ||
| Stage I | 47.24 ± 4.31 | 24.14 ± 0.40 |
| Stage II | 42.34 ± 3.11 | 24.09 ± 0.06 |
- Note: Data are given as means ± standard deviations (n = 5).
4 DISCUSSION
The present study aimed to characterize the water permeability and cuticular wax composition of the leaves, calyxes, and petals of ‘Master’ and ‘Lady green’ carnations at two developmental stages. Few studies have characterized water transpiration in petals or calyxes. Schuster et al. (2017) reported that the water permeance of leaves of 160 species investigated ranged from 2.6 × 10−7 m s−1 (Zamioculcas zamiifolia (Lodd.) Engl.) to 4 × 10−3 m s−1 (Ipomoea batatas (L.) Lam.). In line with this, in the present study, the mean minimum conductance in carnation ranged from 3.59 × 10−5 m s−1 (‘Master’ petal at Stage I) to 88.8 × 10−5 m s−1 (‘Lady green’ leaf at Stage II).
The water resistance of petal cuticles is less well studied than that of leaf cuticles. Buschhaus et al. (2015) found that the effectiveness of the C. bipinnatus petal cuticle in blocking water transport was only one-tenth of that of the leaf cuticle. Similarly, in our previous studies on rose and lily, the water permeance of the petals was higher than that of leaves (Cheng et al., 2019, 2021). The water permeance of lily petals was 5.5 × 10−5 m s−1 (‘Huang tianba’) and 1.3 × 10−4 m s−1 (‘Tiber’), whereas the minimum conductance of the leaves was lower, at 2.2 × 10−5 m s−1 (‘Huang tianba’) and 1.5 × 10−5 m s−1 (‘Tiber’). The water permeability was also higher in the whole petals of Rosa ‘Movie star’ (4.1 × 10−5 m s−1) and ‘Tineke’ (8.9 × 10−4 m s−1) than in the leaves (1.9 × 10−5 m s−1 for ‘Movie star’ and 1.8 × 10−5 m s−1 for ‘Tineke’). Buschhaus et al. (2015) speculated that the water resistance of petals is lower than that of leaves because the main functions of petals are to attract and assist pollinators, which may be at the detriment of their water barrier properties. In the present study, we show that the minimum conductance of the leaves was significantly higher than that of the calyx and petals of ‘Master’ and ‘Lady green’ carnations (Figure 4A). For ‘Lady green’, the mean minimum leaf conductance was nearly four times that of the petals. The variation in water barrier properties among carnation tissues may be due to differences in cuticle morphology, cuticular wax quality, and quantity.
The minimum water conductance of the plant tissues was little influenced by the epidermal cell shape and wax crystal morphology. Compared with that of the leaves and calyx tissues, the petal surface was covered with convex cells and was highly undulated (Figures 2 and 3), which may enhance the effect of the unstirred boundary layer on the surface and contribute substantially to its water resistance (Buschhaus et al., 2015). This explains, to some extent, why the water barrier properties of the petals were better than those of the leaves (Figure 4A).
Cuticle barrier properties are strongly affected by the quality and quantity of cuticular wax components (Huang et al., 2022). In the present study, waxes on carnation petals and leaves showed major differences in total wax coverage, relative composition, and chain length distributions in component classes. First, the overall wax coverage on leaves was significantly lower than that on petals in both ‘Master’ and ‘Lady green’ cultivars (Figure 4B). This is the opposite of the findings in C. bipinnatus (Buschhaus et al., 2015), rose (Cheng et al., 2019), and lily (Cheng et al., 2021). Studies have shown that there is no significant relationship between cuticular water permeability and total wax amount (Jetter & Riederer, 2016; Riederer & Schreiber, 2001). Therefore, their relationship will not be discussed here.
Second, the main components of carnation petals and calyxes were n-alkanes, whereas leaf waxes were dominated by primary alcohols (Figure 5). Alkanes, with only hydrocarbon chains and no hydrophilic ends, are strongly hydrophilic. Water loss from plant tissues was shown to be negatively correlated with the alkane content in several studies. The alkane content was increased in the CER1 overexpression lines of Arabidopsis (Bourdenx et al., 2011) and wheat (Li et al., 2019); concurrently, the water barrier properties were also increased. Similar results were also reported in citrus (Yang et al., 2022). Moreover, Yang et al. (2022) found that coating C28 alkane on fruits could reduce water loss in six citrus varieties. Alkanes are also reportedly the main wax constituents on petals in lily (Cheng et al., 2021), rose (Cheng et al., 2019), and snapdragon (Guo & Jetter, 2017). In contrast, in C. bipinnatus, petal wax is mostly dominated by primary alcohols and leaf and stem waxes by alkanes (Buschhaus et al., 2015). Primary alcohols have been found in the petal wax layer in other species, such as dandelion (Guo et al., 2017) and petunia (Ray et al., 2022), primary alcohols have been found in the petal wax layer. Besides alkanes and primary alcohols, other wax constituents may affect the water barrier properties of plant cuticles. For example, C24 and C26 aliphatic aldehydes significantly reduce the water loss rate of citrus fruits (Zou et al., 2022).
Third, the chain length distribution in cuticular waxes was organ-specific. In the petal and leaf waxes of both ‘Master’ and ‘Green lady’, fatty acids and aldehydes had relatively shorter chain lengths (mainly C22/C24), whereas primary alcohols and alkanes had longer chain lengths (mainly C24/C26 and C25/C27, respectively) (Figures 6 and 7). ACLs were higher in petals than in leaves and calyxes (Table 1). The ACLs of acyclic compounds were calculated based on the molar concentrations of VLCFAs, such as fatty acids, alkanes, alkenes, primary alcohols, and aldehydes. As a parameter of overall chain length distribution, ACL is widely used as an indicator of wax quality in plant tissues; the higher the ACL value, the better the water barrier properties of the cuticle. Cuticle wax molecules have been suggested to be aligned in parallel and form crystalline domains to hinder water penetration (Barthlott et al., 2017; Riederer, 1995; Riederer & Schreiber, 2001; Schönherr et al., 1984). Accordingly, the relatively short-chain-length compounds in carnation leaves may lower the crystalline fraction, resulting in high water permeance.
5 CONCLUSIONS
In conclusion, the morphology and water barrier properties of cuticles and the chemical composition of cuticular waxes on the leaves, calyxes, and petals of ‘Master’ and ‘Lady green’ carnations at two postharvest stages were characterized in detail in the present study. Major differences existed among tissues but not between cultivars and among stages. Unlike in other species, the leaf cuticular barrier was less effective than those of the petal and calyx. The leaf wax contained high concentrations of primary alcohols, whereas alkanes dominated petal wax. In addition, the ACL of acyclic compounds was lower in leaves than in petals. Furthermore, the large number of convex cells on the petal surface also contributes to its higher water barrier properties. Taken together, the results substantiate the relationships among plant tissue surface morphology, cuticular wax chemical composition, and water barrier properties of tissues.
AUTHOR CONTRIBUTIONS
Guiping Cheng and Xuewu Duan designed the study. Guiping Cheng and Changchun Ye performed most of the experiments and prepared the manuscript. Jiajun Zhang and Hongmei Li contributed to part of the experiments and to data analysis. Xuewu Duan and Yueming Jiang improved the manuscript and obtained the funds for this study.
ACKNOWLEDGMENTS
This work was supported by the Characteristic Innovation Project of the Education Department of Guangdong Province (Natural Science 2020KTSCX096) and the Foundation of Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences (PCU202002).
Open Research
DATA AVAILABILITY STATEMENT
The data that support this study are available from the corresponding author upon reasonable request.




