Effects of drought, subsequent waterlogging and redrying on growth, physiology and metabolism of wheat
Abstract
With climate change, longer periods without precipitation but also heavy rains will become more frequent. Thus, understanding and predicting the implications of drought–waterlogging–redrying cycles for plants is essential. We examined the effects of such events on wheat (Triticum aestivum). We measured the impacts of subsequent water treatments (drought–waterlogging–redrying) on plant shoot and root biomass, photosynthesis and transpiration, as well as on primary metabolites and transcripts of leaves. Drought and drought followed by waterlogging severely reduced shoot and root biomass. Chlorophyll fluorescence parameters and the CO2 assimilation rate per unit leaf area were not affected by the treatments but, after the redrying phase, plants grown under the stress treatments showed a higher transpiration rate per unit leaf area and a lower instantaneous water use efficiency. Many organic acids of the citrate cycle were less concentrated in leaves of stressed plants, while most amino acids were more concentrated. Transcript analysis of genes involved in signalling and metabolism revealed different expression patterns. While some genes responded only to drought or drought followed by waterlogging, several genes were induced upon both treatments and some were still upregulated at the end of the redrying phase. We provide insights into how wheat responds to changes in water regimes, with some of the changes probably allowing the plants to cope with these stressors, at least to a certain degree.
1 INTRODUCTION
Global climate change scenarios predict increases in the frequencies of extreme climate events, such as droughts followed by heavy rains leading to flooding (IPCC, 2014). In light of these challenging scenarios, it is important to understand how they affect plants, including crops. Numerous studies have been performed to test the effects of drought on plant growth, physiology and metabolism (Fàbregas & Fernie, 2019; Farooq et al., 2009; Shanker et al., 2014). Likewise, plant responses to waterlogging are increasingly studied in some species (Pedersen et al., 2021; Zhou et al., 2020). However, there is a substantial research gap in understanding how plants respond to a sequence of extreme weather events, such as long droughts followed by sudden waterlogging due to heavy rainfalls and subsequent redrying of the soil.
Under drought, resources may be rather allocated to generative plant parts, leading to a higher harvest index (Stallmann et al., 2020) and allocated rather to root than shoot biomass (Kleine & Müller, 2014). Moreover, plants often show reductions in the net carbon dioxide (CO2) assimilation rate (A), turgor and nutrient uptake under water shortage (Chaves et al., 2003; Nezhadahmadi et al., 2013) and close their stomata to reduce the transpiration rate (E), affecting water use efficiencies (WUE). The instantaneous WUE (A/E; WUEinst) and the intrinsic WUE (A divided by stomatal conductance to water vapour) are often enhanced, at least during mild drought (Chaves et al., 2003; Kang & Kang, 2019). Likewise, drought-exposed plants can exhibit a more efficient use of water for biomass production (applied WUE, WUEapl) compared with well-watered plants, as shown, for example, in common wheat (Triticum aestivum L., Poaceae; Stallmann et al., 2020). In addition, many primary metabolites that function as osmoprotectants accumulate in plant tissues under drought, reducing the osmotic potential of plant cells, facilitating the absorption of water and preventing damage from dehydration (Fàbregas & Fernie, 2019; Shanker et al., 2014; Singh et al., 2015). The amino acid proline is a particularly important osmoprotectant, which acts as an osmolyte, stabilises membranes and proteins and is involved in protection against radicals (Hayat et al., 2012). Furthermore, concentrations of other amino acids such as γ-aminobutyrate (GABA), organic acids, sugars and sugar alcohols are often higher in leaves of drought-exposed compared with well-watered plants (Obata et al., 2015; Wenzel et al., 2015).
The sudden availability of huge water amounts after long drought periods is increasingly becoming a problem for agriculture. For example, higher summer rainfall intensities in Europe over the last decades have caused numerous floods, which can and will lead to soil erosion, leaching of compounds from the soil and yield losses (Olesen et al., 2011). Flooding disrupts the movement of molecular oxygen (O2) from the air to the flooded plant parts (Lee et al., 2011), resulting in hypoxia or even anoxia. As a consequence, a core set of hypoxic genes is induced, for example in Arabidopsis thaliana, including genes involved in fermentation and members of the ERFVII transcription factor family, which play central roles in hypoxia signalling (Licausi et al., 2011; Mustroph et al., 2009; Schmidt et al., 2018). In wheat, TaERFVII.1 contributes to waterlogging tolerance (Wei et al., 2019). Moreover, the root architecture often changes in response to waterlogging (Pedersen et al., 2021). Adventitious roots partly replace damaged roots and the degradation of root parts and formation of aerenchyma can lead to lower root biomass and higher shoot-to-root ratios under waterlogging (Colmer & Greenway, 2011). Furthermore, waterlogging can hamper the activities of nitrifying microbes in the soil, resulting in reduced soil nitrogen (N) availability (Nguyen et al., 2018). In wheat, waterlogging led to lower uptake and transport of nutrients to the shoots (Trought & Drew, 1980). Due to reduced shoot photosynthesis and lower aerobic respiration in flooded plant parts, plants experience carbohydrate and energy deficiencies, respectively. Although the effects of O2 shortage are more pronounced in the roots, systemic plant parts can also be affected due to the impacts of flooding on the transport of water, minerals and hormones to the shoots and due to impacts on photosynthesis (Vartapetian & Jackson, 1997). For example, higher proline concentrations have been found in leaves of barley after waterlogging (Yordanova & Popova, 2001).
The redrying of the soil after waterlogging may also be challenging for plants, depending on the strength and sequence of previous stresses and the length of the redrying phase. Plants may survive a period of root anoxia but perish upon re-exposure to air due to oxidative damage (Scandalios, 1993). During re-aeration, plants need to metabolically adjust to reach a normal homeostatic state (Tsai et al., 2016). Proline can aid in recovery from drought and waterlogging by acting as N and carbon (C) source for growth and resumption of metabolism (Aloni & Rosenshtein, 1982). However, information is scarce regarding changes in photosynthetic capacity, their associations with plant growth and metabolic responses to redrying after waterlogging.
Next to rice and maize, common wheat is one of the world's most important crop species and is cultivated in many temperate regions (Shewry, 2009). Both drought and waterlogging are known to have negative effects on the shoot biomass and yield of wheat (Ding et al., 2018). In this study, the effects of a drought phase followed by a phase of waterlogging and a redrying phase on shoot and root biomass, physiological traits (photosynthesis and transpiration per unit leaf area), as well as primary metabolites and transcripts (enzymes of primary metabolism, transcription factors and signalling genes) of leaves of T. aestivum were investigated. For each time point (i.e., at the end of the drought, waterlogging and redrying phases, respectively), traits of stress-treated plants were compared with those of control plants. We hypothesised that wheat plants subjected to drought have a lower shoot and root biomass, a higher ratio of inflorescence biomass to total shoot biomass, a lower shoot-to-root ratio, a higher WUEapl, lower photosynthesis and transpiration, a higher WUEinst, enhanced concentrations of several primary metabolites, in particular proline, as well as a modulated expression pattern of genes from carbohydrate and amino acid metabolism compared with control plants. Plants subjected to drought and subsequent waterlogging were expected to show similar patterns as drought-exposed plants but with a higher shoot-to-root ratio due to the degradation of roots and aerenchyma formation under waterlogging. Moreover, in leaves of these plants, a higher expression of fermentation genes and ERFVII genes was expected than in leaves of control plants. At the end of the redrying phase, when soil water contents (SWC) were back to normal allowing stomatal opening and regular metabolism, wheat plants were still expected to be smaller but to show similar photosynthesis, transpiration, foliar metabolite concentrations and transcript levels as the control plants.
2 MATERIALS AND METHODS
2.1 Plant cultivation
The experiment was performed in Bielefeld (Germany) from October to December 2020 in a climate chamber at 20°C, a relative humidity (r.h.) of 60% and a photoperiod of 16-h light:8-h dark. Grains of spring wheat (T. aestivum L., cv. Tybalt; W. von Borries-Eckendorf, Leopoldshöhe, Germany) were germinated in a steamed (≥90°C) 1:2 mixture of soil (Fruhstorfer special substrate type P; Hawita Group) and river sand. Seven days post-sowing (dps), seedlings were planted into 2-L pots (11.3 × 11.3 × 21.5 cm). Each pot (N = 60) received 2107 g wet substrate and two seedlings (distance: 10 cm) to simulate intraspecific competition under field conditions. The initial SWC (mass of water per dry mass of substrate) was 26.8%, determined gravimetrically by drying three subsamples according to ISO 11465 (1993). The pots had holes at the bottom and were placed on dishes to prevent water loss and enable the uptake of drained water. Pots were placed in a randomised block design and positions were weekly changed within blocks. Fertiliser [Floranid Twin NPK2O (+MgO + S) 14019 (+3 + 11) with traces of B, Cu, Fe, Mn and Zn; Compo Expert] was added, before watering, into the soil at 18 dps (2 g per pot) and 35 dps (1.2 g per pot).
2.2 Water treatments and harvest
Initially, all plants were kept well-watered by watering the pots with tap water to a SWC of 26.8% every other day (determined gravimetrically by averaging over 20 randomly chosen pots) until 29 dps. At this time, stem elongation started and pots were randomly assigned to one of two water treatment groups (control or stress treatment; 30 pots each). Control pots were adjusted to a SWC of 26.8% every other day and maintained at constant irrigation. Plants of the stress treatment groups were subjected to a sequence of drought, waterlogging and redrying phases (Figure 1). These plants were not watered until the soil reached a mean SWC of 3.3% (at 35 dps). Then, pots were adjusted to a SWC of 8.9% every other day. At 50 dps (T1), when the first ears were visible, but not yet emerged from the sheath, plants of 10 control and 10 drought-stressed pots were harvested. All remaining pots were transferred into 5 L canisters (Purania Stilles Quellwasser; TSI Consumer Goods) which had been cut at the top. Canisters with pots subjected to the stress treatment were carefully filled with water up to 5.4 cm above the substrate and adjusted to that level every other day. After 8 days of waterlogging, at 58 dps (T2), when ears were fully emerged from the sheath in nearly all plants, again plants of 10 control and 10 stressed pots were harvested. All remaining pots were taken out of the canisters and placed back on dishes to enter the redrying phase of 6 days. Pots of the previously water-stressed plants were watered to 26.8% SWC, when the SWC had fallen below this value (i.e., once at 63 dps). All remaining plants (10 pots per treatment) were harvested at 64 dps (T3) when all plants had fully emerged ears and anthesis was half completed. Thus, T3 represents the time point when the original SWC was reached again, that is, the end of the redrying phase.
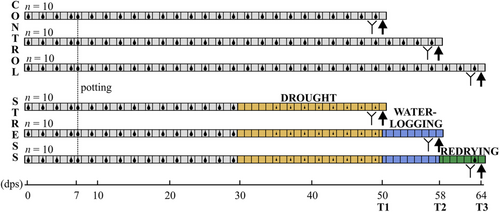
In the pots that had been assigned to the corresponding harvest time point, one plant was used for non-invasive measurements of chlorophyll fluorescence and gas exchange (n = 9 instead of n = 10 per treatment group due to time limitations, Figure 1) and for counting the number of ears (n = 10). The other plant was used for analyses of metabolites (n = 10) and transcripts (n = 5) of leaves. Harvests were done at noon (12–2 p.m.). The blades of the second youngest fully developed leaves (counted without the flag leaves) of the main stems were cut, frozen in liquid N2 and stored at −80°C for later analyses of metabolites. Blades of the next younger leaves were harvested and stored in the same way for later analyses of transcripts. For assessment of the biomass, the total (remaining) shoots (both plants per pot) were cut. Additionally, when ears had emerged (T2, T3), shoots were separated into vegetative and generative parts. Roots were washed from the substrate. All plant parts were dried at 45°C for 6 days, dry biomass was determined (including the leaf biomass harvested for metabolome analysis, determined after lyophilisation) and the ratio of inflorescence biomass to total shoot biomass (for T2, T3) as well as the shoot-to-root ratio were calculated. The WUEapl was calculated according to Boyle et al. (2016) as the ratio of the shoot or root dry mass per pot to the cumulative amount of water received per pot until T1. At T2 and T3, the WUEapl could not be calculated because no exact cumulative water volume could be defined during waterlogging.
2.3 Measurements of chlorophyll fluorescence and gas exchange
Chlorophyll fluorescence was measured 2 days before harvest (i.e., 1 day after watering all plants, Figure 1) between 1 and 3 p.m. with a pulse-amplitude-modulated photosynthesis yield analyser (MINI-PAM, Walz) at the second youngest completely developed leaf of the main stem of each respective plant. The maximum photochemical quantum yield of photosystem (PS) II, Fv/Fm, was measured after 15 min dark adaptation. The effective photochemical quantum yield of PS II, ∆F/Fm′, was measured using actinic light at a photosynthetically active photon flux density (PPFD) of 342 μmol m−2 s−1 (similar to PPFD at plant stem height in the climate chamber) and used for calculating the apparent electron transport rate (ETR).
Gas exchange was measured at two consecutive days (n = 4 pots per group at first day, n = 5 at second day) before harvest (Figure 1) between 1 and 5 p.m. using an infrared gas analyser (GFS-3000, Walz) on the same leaves that had been used for chlorophyll fluorescence measurements. A cuvette covering 3 cm2 leaf area was used. If a leaf did not cover the whole area, the respective leaf area within the cuvette was determined in a non-destructive manner. Leaf areas were used for the calculation of the net CO2 assimilation rate (A) and the transpiration rate (E), both given per unit leaf area. The conditions in the cuvette were set to 20°C, 400 ppm CO2, 60% r.h., 750 μmol s−1 flow and a PPFD of 340 μmol m−2 s−1. The WUEinst was calculated as WUEinst = A/E (Kang & Kang, 2019).
2.4 Analyses of primary metabolites of leaves
Metabolite profiling of primary metabolites was done using lyophilised leaves. The leaf material were weighed and the lower halves of the leaf blades were homogenised. To analyse carbohydrates, organic acids, myo-inositol and amines, leaf material was processed according to Schweiger et al. (2014) with modifications. Dry leaf powder (5 mg) was extracted in a mixture of 1:2.5:1 (vol:vol:vol) chloroform, methanol and Millipore water (chloroform: high-performance liquid chromatography [HPLC] grade; AppliChem; methanol: liquid chromatography mass spectrometry (LC MS) grade; Fisher Scientific) with ribitol (99%, AppliChem) as internal standard. After phase separation by adding water, subsamples of the methanol/water phase were dried and derivatised at 37°C using methoximation with O-methylhydroxylamine hydrochloride (>97%; Sigma Aldrich-Merck; >98%; Alfa Aesar) in pyridine (≥99.9%, HPLC grade; Honeywell) for 90 min followed by silylation with N-methyl-N-trimethylsilyl-trifluoroacetamide (>98%; Macherey-Nagel) for 30 min. Samples were diluted with pyridine and analysed using gas chromatography coupled to mass spectrometry (gas chromatography mass spectrometry [GC MS]; GC-2010 Plus coupled to QP2020; Shimadzu) with a VF-5 ms column (Agilent Technologies; 30 m × 0.25 mm × 0.25 μm, with circa 10 m guard column) at 250°C injection temperature in split mode (1:10) at a helium flow of 1.14 mL min−1. The oven temperature was 80°C for 3 min, followed by a ramp of 5°C min−1 to 310°C held for 5 min. The transfer line temperature was 250°C, the ion source temperature 230°C. Measurements were performed in electron impact positive ionisation mode at 70 eV. A set of n-alkanes (C7 C40; Sigma-Aldrich) was measured to calculate Kováts retention indices (RI; Kováts, 1958). In addition, three blanks without biological material were measured. Data were analysed using GCMS Postrun Analysis 4.45 (Shimadzu). Analytes were identified based on their RI, mass spectra and qualifier and quantifier mass-to-charge ratios (m/z) by comparing these parameters with entries in the GOLM Metabolome database (Hummel et al., 2010; Kopka et al., 2005), mass spectral and retention time (RT) index libraries (Schauer et al., 2005) and with standards measured with the same method. The analytes were quantified based on their peak heights (total ion counts; summed for metabolites with two analyte peaks: fructose, galactose, glucose and malate). Peak intensities were related to ribitol and sample dry mass. Only peaks that were present in at least half of the replicates of at least one treatment group (i.e., water treatment x harvest time point) were retained in the dataset. The sample size of the stress treatment group at T3 was reduced to n = 9, as one sample failed.
To analyse amino acids, dry leaf powder (5 mg) was extracted threefold with 80% (vol:vol) methanol (LC MS grade; Thermo Fisher Scientific) with L-norvaline and sarcosine (Agilent Technologies) as internal standards and extracts were filtered (0.2 μm syringe filters; Phenomenex). Samples were analysed using HPLC coupled with fluorescence detection (HPLC-FLD; 1260/1290 Infinity HPLC und FLD; Agilent Technologies) with a ZORBAX Eclipse Plus C18 column (250 × 4.6 mm, 5 μm particle size, with guard column; Agilent Technologies), modified after Jakobs and Müller (2018). Samples were derivatised at 6°C in the autosampler with borate buffer (0.4 M, pH = 10.2; Agilent Technologies), ortho-phthaldialdehyde (in borate buffer, with 3-mercaptopropionic acid; Agilent Technologies), 9-fluorenyl-methyl chloroformate (in acetonitrile; Agilent Technologies) and an injection diluent, consisting of eluent A (1.4 g Na2HPO4 [AppliChem], 3.8 g Na2B4O7 × 10 H2O [Sigma-Aldrich] and 32 mg NaN3 [Carl Roth] in 1 L Millipore water; pH = 8.2) and 85% phosphoric acid (AppliChem) in a ratio of 1:0.004 (vol:vol). The chromatographic separation was performed using a gradient from eluent A to eluent B, starting at 2% B (4.5 acetonitrile [LC MS grade; Thermo Fisher Scientific]: 4.5 methanol: 1 Millipore water, vol:vol:vol), held for 0.84 min and then increased linearly to 57% B (reached at 68.4 min), followed by a cleaning and equilibration cycle. The flow rate was 1.5 mL min−1 and the column temperature 40°C. Amino acid derivatives were detected with excitation wavelengths of 340 nm for primary and 260 nm for secondary amino acids and emission wavelengths of 450 and 325 nm, respectively. In addition, eight blanks without plant material as well as mixtures of standards were measured. Data were analysed using OpenLab ChemStation C.01.07 (Agilent Technologies). Amino acids were identified by RT matching using the standard mixes and quantified by their peak heights in relation to the internal standards (L-norvaline for primary, sarcosine for secondary amino acids). Only peaks with mean intensities of at least five times the mean intensity in the blanks in at least one treatment group and which were present in at least half of the replicates of at least one treatment group were retained in the dataset. Peak intensities were then related to sample dry mass. Because concentrations of asparagine were above the saturation threshold, this amino acid was quantified via GC MS instead of HPLC-FLD.
2.5 RNA extraction and qRT-PCR analysis of transcripts of leaves
To investigate transcriptional responses to the drought waterlogging redrying cycle, several drought-responsive and/or flooding-responsive genes involved in signalling and genes encoding enzymes involved in the metabolism of the measured metabolites were selected for quantitative real time polymerase chain reaction (qRT-PCR) analysis. Extraction of total RNA was performed using TRI Reagent (Invitrogen) with small adjustments to the manufacturer's instructions: the phase separation with chloroform and the washing step with ethanol were each repeated once. Potentially contaminating DNA was removed using TURBO DNA-free™ Kit (Invitrogen) before the cDNA was synthesised (RevertAid First Strand cDNA Synthesis Kit; Thermo Fisher Scientific). For qRT-PCR, SYBR Green (Applied Biosystems) was used as described previously (Schmidt et al., 2018). Used oligonucleotide sequences are listed in Table S1. TaACTIN was used as reference gene.
2.6 Statistical analyses
If not otherwise stated, all statistical analyses were performed with R 4.0.3 (R Core Team, 2020) using the packages car, lattice (Sarkar, 2008) and vegan (Oksanen et al., 2013). Significance thresholds were set to α = 0.05. Data were compared between samples of the control and stress treatment within time points. Data were tested for normal distribution using the Shapiro Wilk test; homoscedasticity was assessed using the Levene test. Depending on the data distribution, a Mann Whitney U-test, Welch t-test, or t-test was applied, using the same test for all three time points for each parameter.
Metabolite data are given as metabolite concentrations, that is, peak heights that were normalised using the corresponding internal standard and sample dry mass (see above). Fold changes (FC) of metabolites were computed within time points as mean intensities in stress-exposed plants divided by mean intensities in controls and are presented on a log2 scale. FC were only calculated for metabolites that were present in at least half of the replicates in the control or stress treatment group of the corresponding time point. Metabolites were considered to be “modulated” by the treatment based on two criteria, that is, a significant difference between the treatment groups and FC of <0.67 (−0.58 on log2 scale; lower concentration in stress treatment) or >1.5 (>0.58 on log2 scale; higher concentration in stress treatment). For transcripts, the method was used for the calculation of relative transcript abundances (Livak & Schmittgen, 2001). Positive ΔΔCT values [ΔΔCT = ΔCT (control) − ΔCT (stress); ΔCT = CT (gene of interest) − CT (TaACTIN)] indicate upregulation, whereas negative values indicate downregulation of the gene upon stress in comparison to control plants within time points. Cluster heatmaps were constructed for FC (metabolites) and ΔΔCT values (transcripts; separate for genes related to signalling and for those encoding enzymes of primary metabolism), respectively, using average linkage hierarchical clustering relying on Euclidean correlation distance matrices and clustering both over harvest time points and metabolites/transcripts with Cluster 3.0 (de Hoon et al., 2004) and JavaTreeView (Saldanha, 2004). A metabolic map was constructed following Schweiger et al. (2014) using the Kyoto Encyclopedia of Genes and Genomes (KEGG) reference pathways (Kanehisa & Goto, 2000).
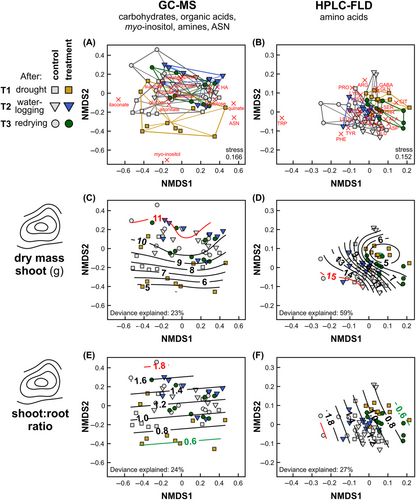
To compare the metabolite profiles, non-metric multidimensional scaling (NMDS) analyses were performed separately for the GC MS and the HPLC-FLD data, using Wisconsin double standardisation of square root-transformed data and Kulczynski distances. The total dry mass of shoots as well as the shoot-to-root ratio were plotted as contour lines into the NMDS plots via the ordisurf function using generalised additive models based on restricted maximum likelihood estimations with Gaussian error distributions, identity link functions and thin plate regression splines.
3 RESULTS
3.1 Plant biomass
At all time points, the vegetative and, at T2 and T3, also the generative shoot dry biomass were significantly lower in wheat plants of the stress treatments (drought-exposed, waterlogged or redried) compared with the respective control plants (Figure 2A,B). The emergence of ears was slightly delayed in plants of the stress treatments. At 57 dps, control plants had one to four fully emerged ears, while only 85% of the plants showed just one fully emerged ear in the stress treatment group. Root dry biomass was significantly lower in plants at the end of the drought (T1) and waterlogging (T2) phase compared with control plants, while at the end of the redrying phase (T3) there was only a trend for lower biomass in stressed plants but no significant difference (Figure 2C). At T1, drought-exposed plants had a significantly lower shoot-to-root ratio than control plants, while neither the WUEapl of the shoots nor that of the roots differed significantly (Figure 2E G). At T2, the ratio of inflorescence biomass to total shoot biomass and the shoot-to-root ratio were not significantly affected by the treatment and differences between groups were small, while at T3 both traits were significantly lower for the previously stress-exposed than for the control plants (Figure 2D,E). There was a quite high variation in some of the plant traits, for example, the root dry mass, the ratio of inflorescence biomass to total shoot biomass (at T2), the shoot-to-root ratio (at T2 and T3) and the shoot WUEapl (in plants of the stress treatment).
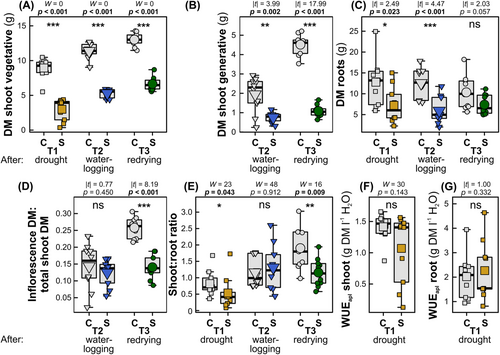
3.2 Plant physiology
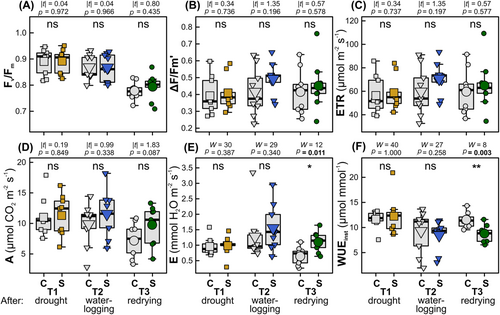
At the end of the drought phase (T1) and at the end of the drought and subsequent waterlogging phase (T2), there were no significant differences between control and stress-exposed plants in any of the photosynthesis- and transpiration-related traits measured per unit leaf area (Figure 3). At the end of the redrying phase (T3), plants of the stress treatment had significantly higher E and lower WUEinst than control plants (Figure 3E,F).
3.3 Metabolic composition of leaves
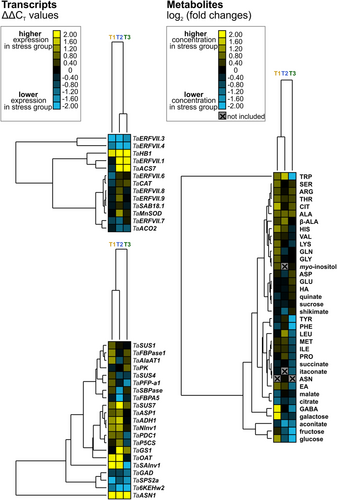
In total, 36 primary metabolites detected in wheat leaves were retained in the data set (Table S2). Hierarchical clustering and pathway mapping revealed that many metabolites were modulated (based on significance and FC) by the stress treatment in a time point/treatment-specific manner, with the metabolite responses differing between compound classes and the corresponding pathways of primary metabolism (Figures 4 and 5). At the end of the drought phase (T1), aconitate had a lower concentration in leaves of drought-exposed compared with control plants, while galactose, glucose and various amino acids had higher concentrations (Figures 4, 5 and S1). At the end of the waterlogging phase (T2), fructose, aconitate and shikimate had lower concentrations, while few amino acids had higher concentrations in leaves of the stress-exposed compared with the control plants. Proline had significantly (1.5-fold) higher concentrations in leaves of plants that had been subjected to the drought waterlogging cycle than in those of control plants. At the end of the redrying phase (T3), the responses of many metabolites were quite distinct from those at T1 and/or T2, leading to a grouping of T1 and T2, with T3 as an outgroup in the corresponding cluster tree (Figure 4). Fructose, glucose, succinate, aconitate and citrate, as well as the amino acids GABA, tyrosine, tryptophan, phenylalanine and leucine, had lower concentrations, while citrulline and alanine had higher concentrations in leaves of plants of the stress treatment than in leaves of control plants. Proline concentrations were not modulated at T3.

In the NMDS analyses, metabolic patterns, particularly of amino acids, were well separated between plants of the stress treatment and control plants, most pronouncedly at the end of the redrying phase (T3; Figure S2). Metabolic patterns could be linked to the shoot dry mass and the shoot-to-root ratio, as indicated by p < 0.05 for the approximate significances of the corresponding smooth terms (Figure 6). The dry biomass of shoots and the shoot-to-root ratio correlated with the two-dimensional NMDS ordinations of the samples, being lowest in the regions where mostly samples of the drought-exposed plants (T1) clustered (Figure 6C F).
3.4 Transcript levels in leaves
Several drought-responsive and/or flooding-responsive genes involved in signalling and genes encoding enzymes involved in the metabolism of the measured metabolites showed significant upregulation. At the end of the drought phase (T1), the gene TaHB1 and many of the genes involved in carbohydrate and amino acid metabolism were slightly upregulated; within the latter group, TaOAT, TaSAInv1 and TaASN1 showed the strongest responses (Figures 4 and 5). These genes stand exemplary for many genes that were induced at the end of the waterlogging phase as well, also including typical hypoxia-responsive genes such as TaADH1 and TaSUS7. Moreover, at the end of waterlogging (T2), TaERFVII.1, a member of the ERFVII transcription factors (Wei et al., 2019), and TaACS7, involved in ethylene biosynthesis (Lin et al., 2009), were strongly upregulated, both not showing a response to the prior drought period. At the end of the redrying phase (T3), the expression of several genes that responded to drought followed by waterlogging like TaHB1, TaERFVII.1/3/4, TaACS7, TaASN1 and Ta6KEHw2 did not revert to the basal non-stressed level but remained as high or low as under stress conditions (Figures 4 and 5).
4 DISCUSSION
4.1 Effects of drought
At the end of the drought phase, shoot and root biomass of drought-exposed wheat plants were lower than those of the respective control plants, as expected. Negative effects of drought on biomass production, including vegetative and generative plant parts, are commonly found (Hu & Schmidhalter, 2005). Photosynthesis is often limited under drought because of stomatal closure to prevent loss of water, leading to a reduced CO2 supply and/or because of drought-induced damage of the photosynthetic machinery (Okamoto et al., 2013; Sharma & Dubey, 2005). Reductions in stomatal gas exchange rates as well as in Fv/Fm, ΔF/Fm′ and ETR indicate that plants are stressed (Meng et al., 2016). However, chlorophyll fluorescence and gas exchange parameters can be resistant to water deficits, being either unaffected (Shangguan et al., 2000) or affected only under severe drought (Souza et al., 2004). In this study, contrary to our hypothesis, neither traits related to chlorophyll fluorescence (Fv/Fm, ΔF/Fm′, ETR) nor to gas exchange per unit leaf area (A, E) were affected by drought. These findings suggest that in plants of the present experiment, the PSII complexes and the remaining photosynthetic machinery were not chronically damaged and that drought did not or only transiently induce stomatal closure. Indeed, in wheat, the bulk leaf water potential threshold for stomatal closure is, compared with the dicot lupin (Lupinus cosentinii, Fabaceae), quite low (wheat: −1.4 to −1.5 MPa; lupin: −0.85 to −0.9 MPa), meaning that wheat plants tend to keep their stomata open under water deficit (Henson et al., 1989). However, although the leaf area-based CO2 assimilation and transpiration rates were unaffected by drought in our experimental plants, the absolute gas exchange rates of the whole plant individuals were probably lower under drought, as less photosynthetic active area was available. The plants may have maintained the photosynthetic functioning of the existing leaves at the expense of biomass production. In line with the findings described above, WUEapl and WUEinst were not significantly affected by drought in our experiment. However, some plants showed a quite low shoot WUEapl at the end of the drought phase. Such low WUEapl may lead to a lower plant performance if another drought period follows. Next to photosynthesis-mediated effects of drought on plant biomass, a reduced nutrient uptake and/or transport may affect plant biomass (Vesala et al., 2017). Under drought, allocation of resources within plants can be affected due to increases in hormone concentrations (Farooq et al., 2009). Accordingly, we found a lower shoot-to-root ratio in drought-exposed compared with well-watered plants. Such an enhanced relative investment into root growth allows the plants to better take up water and nutrients under drought.
Another mechanism to maintain plant water relations under drought is the accumulation of osmolytes through synthesis, release from other compounds and/or translocation. These changes promote an influx of water, allowing stabilisation of cell volumes, and delaying turgor decay in the leaf mesophyll, which benefits the CO2 acquisition (Hayat et al., 2012; Turner, 1986). As expected, we found enhanced concentrations of several primary metabolites at the end of the drought phase, with several of them possibly acting as osmolytes. Higher concentrations of glucose and galactose may indicate an adaptation to ensure high grain yield despite drought stress. Responsiveness of SUS and soluble acid INV genes to drought has been shown previously (Xue et al., 2008). The moderate or strong upregulation of TaSAInv1, TaNInv1 and TaSUS7, found in this study, probably contributed to higher glucose concentrations, together with the downregulation of TaSPS2a. The organic acids related to the tricarboxylic acid (TCA) cycle were not, or negatively, modulated by drought. In contrast, general increases of primary metabolites, including these organic acids, have been reported, for example in maize (Witt et al., 2012). The findings of this study may be due to a higher usage of the mentioned organic acids as precursors for amino acids and, thus, a higher flux to amino acid metabolism in wheat. Indeed, many amino acids showed higher concentrations in leaves of drought-exposed plants. However, proline, known for its functions in enabling plants to cope with various stresses (Hayat et al., 2012), only tended to be highly concentrated at the end of the drought phase. In plants, proline is synthesised mainly from glutamate (Kavi Kishor et al., 2005). Both higher proline biosynthesis and reduced proline catabolism under drought have been described (Kavi Kishor et al., 2005). Several other amino acids, including citrulline and GABA, had a pronounced response to drought in our study. Citrulline can act as a radical scavenger (Akashi et al., 2001), while GABA is involved in protection against different stresses, including drought (Shelp et al., 1999). Interestingly, GABA accumulation was not accompanied by higher transcript levels of TaGAD, which encodes the enzyme catalysing the decarboxylation of glutamate to GABA (Shelp et al., 2012). However, glutamate decarboxylase (GAD) is a Ca2+-dependent enzyme (Snedden et al., 1996) and Ca2+ influxes under drought have been reported (Knight et al., 1997). Thus, the higher GABA concentrations might be due to GAD activation upon drought. Additionally, expression upregulation of genes coding for metabolic enzymes did not necessarily lead to higher concentrations of the metabolic products of the catalysed reactions, as seen for TaOAT and proline. This suggests that important regulatory steps occur between transcription and metabolite synthesis. The higher leaf concentrations of several primary metabolites at the end of the drought phase in this study may have helped the wheat plants to survive under drought.
4.2 Effects of drought and subsequent waterlogging
The lower shoot and root biomass and delayed ear emergence in stress-exposed compared with control wheat plants at the end of the waterlogging phase (T2) may largely reflect the drought-induced reduction in biomass that occurred during the first stress phase in our experimental plants. Waterlogging probably attenuated the negative effects of the preceding drought stress during the first hours or 1 2 days after flooding, as the plants may have used the water for previously water-limited processes. However, upon saturation of the substrate with water, the soil O2 pools may have been rapidly depleted by respiratory processes and the soil pH may have dropped. Under such conditions, the N availability can be lower, whereas the solubility of toxic metal ions can increase (Nguyen et al., 2018; Setter et al., 2009); the latter effect was probably less relevant with the substrate we used in our experiment. Moreover, the energy balance of the roots was probably affected by the waterlogging, as the slow diffusion of O2 may have limited aerobic respiration. Upon complete O2 starvation in flooded plant parts, aerobic mitochondrial respiration switches to anaerobic pathways (Alam et al., 2010). Besides the formation of aerenchyma, prolonged flooding can cause injury and death of roots (Colmer & Greenway, 2011). The facts that chlorophyll fluorescence and gas exchange per unit leaf area were not reduced after drought and subsequent waterlogging in the present experiment indicate that the photosynthetic machinery was still intact and that the stomata were not closed, although gas exchange was probably reduced at the whole-plant level due to the lower shoot biomass.
In contrast to our expectation, the ratio of inflorescence biomass to total shoot biomass was only slightly negatively affected at the end of the waterlogging phase, with a high variation in the data. Potentially, more investment into generative parts during drought, as observed previously in wheat (Stallmann et al., 2020), but a decrease in wheat yield under severe cyclic drought and waterlogging (Ding et al., 2018), may have levelled off in this study. Likewise, the shoot-to-root ratio was not higher in stress-exposed compared with control plants. Since the preceding drought phase led to lower shoot-to-root ratios in stressed plants, the effects of waterlogging on this trait may be partially masked.
Drought followed by waterlogging modulated various primary metabolites in the leaves. Although some effects of waterlogging are restricted to the flooded roots, systemic tissues can likewise be affected, for example, due to the transport of compounds between plant parts. The increased demand for C to drive glycolysis during root hypoxia/anoxia can lead to a decrease in the starch pool. Lower concentrations of the initial intermediates of glycolysis, such as glucose and fructose, may be explained by an enhanced transport of sucrose to the roots and, therefore, less cleavage of sucrose to glucose and fructose. With the upregulation of TaSAInv1 and TaSUS7, plants may prepare for higher glycolysis activity in leaves as well. Especially, SUS genes are responsive to hypoxia and waterlogging (Mustroph et al., 2009; Wei et al., 2019; Xue et al., 2008). Binding of C18:1-CoA, by a binding protein, to the plasma membrane causes a release and nuclear accumulation of the transcription factor ERFVII RELATED TO APETALA 2.12 (RAP2.12; Schmidt & van Dongen, 2019). In the absence of O2, N-end rule-dependent degradation is prevented, leading to the stabilisation of RAP2.12 (Licausi et al., 2011). This transcription factor is central to the activation of anaerobic metabolism under stress. In addition to effects being still visible due to the preceding drought, such processes may explain the lower concentrations of some organic acids related to the TCA cycle in the leaves after waterlogging in this study. TaERFVII.1 was demonstrated to contribute to waterlogging tolerance, also highlighted by a higher transcript level after waterlogging in a waterlogging-tolerant wheat cultivar (Wei et al., 2019). Similar findings were made in this study. Other members of the wheat ERFVII family only showed a weak upregulation or were even downregulated after drought with subsequent waterlogging, further underlining that TaERFVII.1 might be a crucial regulator upon waterlogging in wheat. Moreover, ethylene concentrations rise under hypoxia, especially due to ethylene entrapment upon waterlogging, leading to the activation of its signalling pathway (Perata, 2020). In line with this, ethylene biosynthesis genes were responsive to stress in this study. Pyruvate production is increased by activation of glycolysis and should be prevented during hypoxia, as this activates respiratory O2 consumption (Zabalza et al., 2009). To keep glycolysis going, cytosolic NAD+ must be continuously regenerated from NADH via fermentation reactions leading to the accumulation of lactate and ethanol (Zabalza et al., 2009). This is achieved by upregulating the fermentation genes TaADH1 and TaPDC1, whose orthologues in A. thaliana represent hypoxia core genes (Mustroph et al., 2009) and which indeed showed higher transcript levels at the end of the waterlogging phase in our study. Further investigations are needed to understand the role of these genes under drought stress, as they also showed higher expression at the end of the drought phase. The amount of pyruvate can be reduced via the aminotransferase that produces alanine, of which significantly higher concentrations were found at the end of the waterlogging phase.
The effects of drought followed by waterlogging on the amino acids in wheat leaves were less pronounced than after the drought phase, but some amino acids, including proline, showed higher concentrations in leaves of the waterlogged plants. In tomato, a lower accumulation of proline was found in leaves of a waterlogging-sensitive than in those of a tolerant cultivar (Aloni & Rosenshtein, 1982). Further studies using different wheat cultivars may reveal how drought- and waterlogging-tolerant the variety used in this study is and whether the proline accumulation as well as the induction of the proline synthesis genes TaOAT and TaP5C5 contribute to this tolerance. Such studies may contribute to breeding wheat cultivars with a higher performance under climate change. Particularly high concentrations of alanine and GABA were found in the roots of flooded plants of Medicago truncatula (Fabaceae), whereas tyrosine, tryptophan and phenylalanine, among others, had higher concentrations in the leaves (Lothier et al., 2020). Of the latter three amino acids, only tryptophan showed higher concentrations under waterlogging in the present experiment with wheat, highlighting that responses are stress- and species-specific.
4.3 Effects of drought, subsequent waterlogging and redrying
At the end of the redrying phase, plants subjected to stress treatment had a lower biomass, as expected. Long-term effects of the previous drought, with pronounced negative effects on the biomass as well as potentially also long-term effects of the waterlogging phase, probably contributed to this outcome. In addition, the redrying process may have induced a stress. In post-flooding phases, a sudden increase of O2 can lead to the formation of reactive oxygen species (Scandalios, 1993). If these are not buffered by antioxidants, cells collapse due to membrane injury (Croft et al., 1990), which could contribute to the low biomass found in stressed plants in this study. Such processes may also have hampered the investment of resources into generative plant parts, leading to a lower ratio of inflorescence biomass to total shoot biomass in stress-treated plants. The fact that the root dry mass was much lower in plants of the stress treatment compared with controls at T2 but not at T3, whereas the shoot-to-root ratio in the plants of the stress treatment was lower at T3 but not at T2, may indicate that plants were able to restore parts of the likely damaged root systems during the redrying phase. As expected, most physiological parameters did not differ between plants of the stress treatment and control plants at T3. In contrast, the transpiration rate per unit leaf area was higher in the previously stressed plants and the WUEinst was lower, maybe indicating an imbalance in water transport and/or stomatal regulation. Such imbalances may be decisive for the ability of plants to cope with potential subsequent drought periods.
At the end of the redrying phase, some metabolites showed higher concentrations in leaves of the stress treatment plants compared with the controls, while some others had lower concentrations. Likewise, several of the responsive genes at T1 and T2 remained as expressed/repressed as under the previous stress conditions. These results contradict our hypothesis that the effects of the stresses will level out in the redrying phase, as found by others (Rocha et al., 2010). This study's finding that several amino acids had lower concentrations at the end of the redrying phase may indicate that amino acids were needed as building blocks and energy for other functions that were limited during the stress periods and resumed during the redrying phase. Further studies should investigate the plant status at different time points during redrying phases and subsequent recovery phases from single and (simultaneously or subsequently applied) combined stresses.
5 CONCLUSION
This study revealed that various wheat traits, including morphological, physiological and metabolic ones, were affected by drought and/or drought followed by waterlogging and that many of them were still affected at the end of the redrying phase. Effects of drought followed by waterlogging have rarely been studied yet. Thus, this study contributes to the understanding of whether and how plants can cope with different subsequent stresses. Post-stress phases, such as redrying phases, likely have a stronger influence on plants than thought before. Indeed, it appears from the results that the aftermath of the previous stresses was still present and/or that re-aeration induced further stress in the redrying phase. The fact that we found a high variation in some plant traits in our study shows that even highly cultivated plants show individual responses. In conclusion, this study highlights that drastic changes in precipitation patterns will have negative influences on the growth and yield of wheat. Understanding the complexity of plant responses to different water regimes may help to improve breeding for tolerant varieties.
AUTHOR CONTRIBUTIONS
Rabea Schweiger, Alena-Maria Maidel, Romy Schmidt-Schippers and Caroline Müller conceived the idea and designed the experiment; Rabea Schweiger, Alena-Maria Maidel and Tilo Renziehausen performed the experiment, collected and analysed the data. Rabea Schweiger, Alena-Maria Maidel, Tilo Renziehausen, Romy Schmidt-Schippers and Caroline Müller interpreted the data. Caroline Müller acquired funding. Alena-Maria Maidel and Caroline Müller drafted a first version of the article. All authors contributed to the final version.
ACKNOWLEDGEMENTS
This work was funded by a grant of the Deutsche Forschungsgemeinschaft to CM [MU 1829/23-1]. We thank the gardeners of Bielefeld University for their help with steaming the substrate, Jonas Hippler for helping with the amino acid analyses and Isabel Schnülle for her help with the chlorophyll fluorescence measurements. Moreover, we thank Isabel Schnülle, Alessa Barber, Marika Böhm and Hendrik Schweiger for help in washing the roots and Stephan Unger for providing us with the portable infrared gas analyser. Open Access funding enabled and organized by Projekt DEAL.
Open Research
DATA AVAILABILITY STATEMENT
Further data are presented in the Supporting information. The raw data that support the findings of this study are given in Table S3.




