Enhanced nodulation and phosphorus acquisition from sparingly-soluble iron phosphate upon treatment with arbuscular mycorrhizal fungi in chickpea
Abstract
The coordination/trade-off among below-ground strategies for phosphorus (P) acquisition, including root morphology, carboxylate exudation and colonisation by arbuscular mycorrhizal fungi (AMF), is not well understood. This is the first study investigating the relationships between root nodulation, morphology, carboxylates and colonisation by an indigenous community of AMF under varying P levels and source. Two chickpea genotypes with contrasting amounts of rhizosheath carboxylates were grown in pots at six P levels (from 0 to 160 μg g−1) as KH2PO4 (KP, highly soluble) or FePO4 (FeP, sparingly soluble), with or without AMF (±AMF) treatment. Under both FeP and KP, the presence of AMF inhibited shoot growth and shoot branching, decreased total root length and specific root length, increased mean root diameter and root tissue density and reduced carboxylates. However, the role of AMF in acquiring P differed between the two P sources, with the enhanced P acquisition under FeP while not under KP. Co-inoculation of AMF and rhizobia enhanced nodulation under FeP, but not under KP. Our results suggest that the effects of AMF on shoot branching were mediated by cytokinins as the reduced shoot branching in FeP40 and KP40 under +AMF relative to −AMF coincided with a decreased concentration of cytokinins in xylem sap for both genotypes.
1 INTRODUCTION
Plants have evolved the capability to adjust their root characteristics to acclimate to different soil niches. For example, thanks to root plasticity, they can enhance phosphorus (P) acquisition in P-impoverished environments through: (1) root foraging strategies to explore larger volumes of soil, such as an increase in root mass ratio, lateral root branching, root hair length and density and specific root length/surface area (Lynch & Brown, 2008; Wen et al., 2019); (2) enhanced carboxylate exudation, which mobilises P from sorbed mineral or organic soil P fractions (Lambers, 2022; Lambers et al., 2006, 2015); and (3) symbioses with arbuscular mycorrhizal fungi (AMF) to access labile inorganic soil P in the soil solution beyond root-depletion zones (Sawers et al., 2017; Smith & Read, 2008). These root adjustments incur carbon costs and thus require coordination of internal resource allocation to balance costs and benefits, according to the principle of carbon economy (Bloom et al., 1985).
Previous results on the effects of AMF on crop growth and P acquisition are somewhat controversial, partially because different experiments were often undertaken under varying conditions and also because most studies were undertaken in a glasshouse where AMF have great impact, but results may often not be applicable to field conditions (Ryan & Graham, 2018). For example, Bazghaleh et al. (2018) found that AMF treatment increased the biomass of seven chickpea (Cicer arietinum) genotypes grown in pots, relative to the control. Klironomos (2003) studied the response of 64 plant species grown in pots in response to 10 species of AMF, and found substantial variation in plant growth responses to AMF, ranging from strongly negative to strongly positive. Soil P level often affects the impact of AMF on P acquisition; often, AMF affect crop growth more under low-P than under high-P conditions. For example, McLachlan et al. (2021) found that the apparent benefit of AMF was most significant when soil P supply was below half of the critical external P requirement for five annual pasture legumes.
The coordination/trade-off of colonisation by AMF with root morphology and carboxylate exudation in response to soil P availability is largely unknown. Tisserant et al. (1996) showed that upon inoculation of Platanus acerifolia with Glomus fasciculatum, the development of fungal biomass in all lateral root orders coincided with an increase in root branching. Plant species with thicker roots often have more colonisation by AMF than species with thinner roots (Li et al., 2017; McCormack et al., 2015). McLachlan et al. (2021) found that AMF modified the acclimation of root morphology to P stress, particularly a lower proportion of root biomass allocated to topsoil in five annual pasture legumes. Ryan et al. (2012) found that AMF treatment reduced the amount of rhizosheath carboxylates for 10 Kennedia species under low-P conditions. Within chickpea genotypes, Wen et al. (2020) reported that genotypes with more carboxylates based on root dry weight (DW) had thinner roots, higher specific root length, and higher colonisation by AMF at a low P level. Lambers et al. (2015) suggested that AMF acquire P from the soil solution by accessing orthosphosphate (Pi) beyond the root depletion zone, and AM strategies are less effective in P acquisition than P-mobilising exudates at very low P or high P availability. Therefore, the P source/level would likely affect colonisation by AMF and their coordination/trade-off with root morphology and carboxylate exudation together with their role in P acquisition. The limited studies on the effects of AMF on root morphology and carboxylate exudation were often restricted to a single P level (Ryan et al., 2012; Tisserant et al., 1996) or involved AMF without a −AMF control (Wen et al., 2020). Therefore, in their review, Ryan and Graham (2018) advocated using a response curve to allow a thorough assessment of root traits and mycorrhizal effects in response to P levels.
The rhizosphere is a region of intense microbial activity, with bacteria and fungi being dominant soil microbes associated with plants (Pang et al., 2021). For legumes, the coordination of the tripartite symbiosis between plants, AMF and rhizobia is essential for plant growth in low-P environments (Foo & Davies, 2011). Mixed inoculation with bacteria and AMF creates synergistic interactions that may significantly increase growth and mineral nutrient uptake (Bonfante & Anca, 2009; Nautiyal et al., 2010). Tajini et al. (2012) reported that the combined inoculation of AMF and rhizobia significantly increased plant growth under moderate P deficiency in common bean (Phaseolus vulgaris) relative to plants inoculated with rhizobia only. The formation of rhizobial and fungal symbionts is energetically costly, and the extent of these symbioses is controlled by plants (Wang et al., 2021). Legumes have evolved negative regulatory systems to maintain nodule formation and AM colonisation, termed autoregulation, and thus limit the costs for symbiose establishment (Catford et al., 2003). Autoregulation enables plants to restrain the extent of symbioses via negative feedback (Wang et al., 2018). Catford et al. (2003) showed that pre-colonisation of roots with AMF not only systemically inhibits further mycorrhization but also nodule formation, and nodulation also systemically suppresses AM colonisation and further nodulation. However, the effects of P source/level in soil are not clear. The effects of AMF treatment in combination with rhizobia on nodulation under different P sources and P levels have rarely been studied.
Phytohormones regulate the activity of secondary shoot meristems. Three hormonal signals are involved in shoot branching: auxins, strigolactones and cytokinins (Leyser, 2009; Waldie & Leyser, 2018). Jones et al. (2015) found that the presence of fungal micro-symbionts severely affected endogenous plant cytokinin levels in pea, as young mycorrhizal roots had significantly lower cytokinin levels than non-inoculated roots. Few studies have investigated the effects of AM colonisation on shoot branching and phytohormones as long-distance signals in the process of shoot branching.
We selected two chickpea genotypes, ICC4814 and ICC14799 with ICC4814 having more rhizosheath carboxylates, thinner roots and a higher specific root length than ICC14799 (Pang et al., 2018). We aimed to investigate the coordination/trade-off between an indigenous community of AMF from a low-P soil, nodulation, root morphology and carboxylate exudation under different P levels/sources, using six P levels as KH2PO4 (KP, highly soluble) or FePO4 (FeP, sparingly soluble), under +AMF or −AMF. We hypothesised that inoculation of a community of AMF from a low-P soil would: (1) significantly change root morphology (i.e., increase root diameter, reduce total root length and specific root length) and carboxylate exudation; (2) play an important role in P acquisition compared with −AMF under sparingly soluble FeP, but not under KP; (3) enhance nodulation under FeP but not under KP; (4) affect shoot branching by affecting the level of cytokinins.
2 MATERIALS AND METHODS
2.1 Plant material and growth conditions
We used two chickpea genotypes (ICC4814 and ICC14799) with different amounts of rhizosheath carboxylates (106 vs. 32 μmol g−1 root DW, respectively) when grown at low P supply, according to Pang et al. (2018), who investigated 100 chickpea genotypes for complex root traits. Seeds of ICC4814 and ICC14799 contained 4.51 ± 0.15 mg and 4.85 ± 0.06 mg N per seed (n = 3), and 0.651 ± 0.03 and 0.665 ± 0.05 mg P per seed (n = 3), respectively. The experiment was conducted during autumn/winter, the chickpea growing season, in a temperature-controlled glasshouse at The University of Western Australia, Perth, Australia (31.57°S, 115.47°E), with an average maximum air temperature of 22°C, minimum temperature of 15°C, and mean relative humidity of 65%. The daily maximum light intensity at plant height in the glasshouse mostly varied from 735 to 1225 μmol m−2 s−1, but having a maximum value of less than 700 μmol m−2 s−1 in 9 out of 54 days (Figure S1).
We used a 1:9 (w:w) mixture of sieved, dried loamy soil and river sand. The brown, sandy loam soil was collected from the upper 0.15 m layer of unfertilised native vegetation sites at The University of Western Australia Farm, Pingelly, Western Australia (32.51°S, 116.99°E). The field soil was air-dried, sieved through a 2-mm sieve, and mixed with washed river sand. Soil analyses were undertaken on subsamples by CSBP FutureFarm analytical laboratories (Bibra Lake). The soil mixture contained 1 μg g−1 nitrate-N, 2.5 μg g−1 ammonium-N, 3 μg g−1 Colwell-P, and 55 μg g−1 Colwell-K, 0.5% organic carbon, had a pH (CaCl2) of 5.7 and a P-retention index of 17.2 (a measure of a soil's capacity to sorb P, as described by Allen and Jeffery [1990]).
Each plastic pot (8.5 × 8.5 × 18 cm) was filled with a mixture of 1170 g of washed and sterilised river sand and either 130 g of field soil (+AMF) or pasteurised field soil (−AMF). For the −AMF treatment, the soil mixture was steam pasteurised at 80°C for 1 h on consecutive 2 days to remove AMF and then dried in a sterile environment for 3 days. To retain most other soil microbial biota for the −AMF treatment, a filtrate of the field soil was made by passing a mixture of 1.5 L deionised water and 500 g unpasteurised field soil through three sieves with pore sizes of 900, 250 and 50 μm, respectively, as described in Nazeri et al. (2014). The filtrate was assumed to have no mycorrhizal hyphae or spores (Hetrick et al., 1988; Nazeri et al., 2013, 2014). For the −AMF treatment, we added 20 mL of field soil filtrate to the top of the soil in each pot before planting. Note that for soil from the Western Australian cropping zone, similar to that in this experiment, Nazeri et al. (2013) found the addition of microbial filtrate had no impact on plant growth or P uptake compared with a pasteurised soil control. The soil mixture contained sufficient basal nutrients, except N. A small amount of extra N (15 μg N g−1 soil mixture) was supplied as Ca(NO3)2·4H2O in solution before planting to provide starter N during early growth, before the initiation of nodulation by rhizobium.
For +AMF and −AMF treatments, there were two P sources, that is, KH2PO4 (abbreviated as KP, water-soluble) or FePO4 (abbreviated as FeP, extra pure, sparingly soluble; Acros Organics). Both P sources were used at six P levels with final concentrations of 0, 10, 20, 40, 80 and 160 μg P g−1 dry soil. The bulk soil resin P concentration was very low, ranging from 1.0 to 3.2 μg g−1 under all Fe-P levels, while it increased from 1.0 to 61.5 μg g−1 when soil KP level increased from 0 to 160 μg P g−1 (Figure S2). To balance K, we supplied appropriate concentrations of KCl to the P treatments. In each pot, we planted three seeds at 25 mm depth and inoculated these with ~1 g of peat-based Group N rhizobium (New Edge Microbials). Eleven days after sowing, seedlings were thinned to one plant per pot, and the surface of each pot was covered with a 10-mm layer of plastic beads to minimise soil evaporation. Pots were watered every second day to 80% pot capacity making sure no water leached from the bottom of the pots. There were four replicates for each treatment combination, for a total of 192 pots (2 genotypes × 2 AMF treatments × 2 P sources × 6 P levels × 4 replicates).
2.2 Plant measurements
Plants were harvested 7 weeks after planting. The number of shoot branches, including the main stem, was counted. Shoots were severed from the roots. The soil was carefully tipped out of the pots, and the root systems gently shaken to remove excess soil. The entire root system with rhizosheath soil (soil attached to roots) was transferred to a beaker containing a known volume of 0.2 mM CaCl2 to ensure cell integrity, and then gently shaken to remove the rhizosheath soil. The pH of the rhizosheath solution was measured with a pH metre. To analyse rhizosheath carboxylates, a subsample of the rhizosheath extract was filtered through a 0.45-μm syringe filter into a 1 mL high-performance liquid chromatography vial, with a drop of orthophosphoric acid added to acidify samples. The samples were stored at −20°C until being analysed following the protocols described elsewhere (Cawthray, 2003; Pang et al., 2015).
To analyse the concentration of cytokinins in xylem sap, plants under two P levels (0 and 40 μg g−1) were cut at about 2 cm above the soil surface, and placed into a pressure chamber (Soil Moisture Equipment Corp.) for the collection of xylem sap. The cut surfaces were blotted with a 14:1:2 (volume) mixture of methanol, formic acid and water to remove contaminating cell debris and also prevent enzymatic reactions from phytohormone breakdown. The first few drops of xylem sap were discarded to avoid contamination. About 100–200 μL xylem sap from each sample was collected into microcentrifuge tubes containing 25 μL concentrated formic acid using a micropipette placed on ice. Collected samples were stored at −80°C until analysis. Samples within each treatment were pooled due to low volumes of xylem sap collected and split into two sets of analysis using ultra-performance liquid chromatography-electrospray ionisation-tandem mass (UPLC-ESI-MS/MS) in ESI positive mode for cytokinins, following the same protocol described in Wong et al. (2022). The following cytokinins were analysed including N6-benzyladenine, N6-benzyladenosine, cis-zeatin, dihydrozeatin, dihydrozeatin-O-glucoside, dihydrozeatin riboside, N6-isopentenyladenine, N6-isopentenyladenosine, kinetin, trans-zeatin, trans-zeatin-O-glucoside and trans-zeatin riboside.
After the carboxylate extraction, roots were washed and separated from shoots. All nodules were removed from the root system. Shortly after harvest, roots with nodules removed were spread out on a transparent plastic tray with water to obtain root images using an Epson Perfection V700 scanner at 300 dpi. To obtain total root length, root surface area, root volume and mean root diameter, root images were analysed using WinRhizo 2009 software (Regent Instructions). Root samples after scanning, nodules, and shoot samples were dried at 70°C for 72 h and then weighed separately for DW. Root mass ratio was calculated as the ratio of root DW (excluding nodules) to total plant DW. Specific root length was calculated as root length per unit root DW (Pang et al., 2015), and root tissue density was calculated as the ratio of root DW to root volume. The percentage of nodule DW to total below-ground DW was calculated as ([nodule DW]/[nodule DW + root DW]) × 100. Root hair length was determined on the first-order lateral roots at two P levels (0 and 40 μg g−1). Five root fragments with representative root hair length and density were photographed under a microscope along the root mature zones (20 mm from the root tip to the basal root area). Root hair length was analysed using the Image J programme (Schneider et al., 2012), with three randomly selected and homogenously distributed root hairs in each graph measured, for a total of 15 (3 × 5) root hair length measured per replicate (Pang et al., 2018).
The determination of mycorrhizal colonisation followed a modified method of Phillips and Hayman (1970). Briefly, root subsamples were cleared with 10% (w/v) KOH in a water bath at 80°C for 26 min, acidified with 2% (v/v) HCl for 30 s at room temperature, before being stained with 0.05% (w/v) aniline blue at 80°C for 8 min. Stained root samples were placed in a 70% (v/v) glycerol solution overnight to remove excess stain. The line-intersect method was used to assess the percentage of root length colonised by AMF (Giovannetti & Mosse, 1980).
Dried shoot samples were ground to a fine powder using Geno/Grinder 2010 (Spex SamplePrep). Subsamples of ~100 mg were digested using a hot concentrated nitric-perchloric (3:1) acid mixture. Total shoot P concentration ([P]) was determined by the malachite green method (Motomizu et al., 1983) with a UV–VIS spectrophotometer (Shimadzu Corporation). To determine shoot N concentration ([N]), ~100 mg subsamples were analysed using a Vario Macro combustion analyser (Elementar Analysensysteme GmbH). Shoot P content was calculated as the product of shoot [P] and shoot DW. Shoot N:P ratio was calculated as the ratio of [N] to [P] in shoots.
2.3 Statistics
The experiment was a four-factorial (species, AM treatment, P source and P level) completely randomised block design. All parameters were analysed by ANOVA in Genstat v.19.1 (VSN International, Hemel Hempstead, UK, 2018). When there was significant interaction of genotype × AMF treatment × P source × P level, the least significant difference at p = 0.05 is presented in figure captions.
3 RESULTS
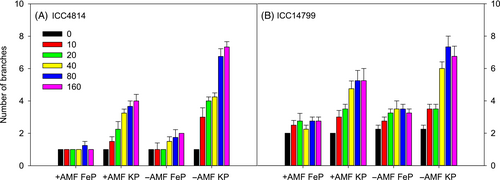
3.1 Number of shoot branches
A significant genotype × AMF treatment × P source × P level interaction occurred for the number of shoot branches (p < 0.05; Figure 1, Table 1). In most treatments, ICC14799 had more branches than ICC4814. For both genotypes under FeP, the number of branches varied little among P levels for +AMF, but slightly increased with increasing P level for −AMF. Under FeP, uninoculated plants tended to have slightly more branches, but this was not significant, compared with their inoculated counterparts when P levels ≥40 μg g−1 for ICC4814 and ≥20 μg g−1 for ICC14799; the only significant increase occurred for ICC4814 at 160 μg g−1. For both genotypes under KP, the number of branches significantly increased with increasing P level, with the highest values observed at P levels ≥40 μg g−1 (+AMF) and ≥80 μg g−1 (−AMF). Under KP, the −AMF treatments had significantly more branches than the +AMF treatments at P levels ≥10 μg g−1 for ICC4814 and ≥40 μg g−1 for ICC14799.
| Variable | G | AMF | PS | PL | G × AMF | G × PS | AMF × PS | G × PL | AMF × PL | PS × PL | G × AMF × PS | G × AMF × PL | G × PS × PL | AMF × PS × PL | G × AMF × PS × PL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Branch number | *** | *** | *** | *** | * | * | *** | ns | *** | *** | ns | ns | *** | * | * |
| Shoot dry weight (DW) | *** | *** | *** | *** | * | *** | *** | *** | *** | *** | ns | * | ** | *** | ns |
| Root DW | *** | ns | *** | *** | ns | ns | *** | ns | *** | *** | ns | ns | ns | * | ns |
| Nodule DW | *** | * | *** | *** | ** | *** | *** | *** | ns | *** | ns | ns | *** | ** | ns |
| % nodule DW to total below-ground DW | * | ns | *** | *** | *** | ns | *** | * | *** | *** | *** | ns | ns | ** | ns |
| Root mass ratio | *** | *** | *** | *** | *** | ns | ns | *** | ns | *** | * | * | ns | ns | ns |
| Shoot [N] | * | *** | *** | *** | *** | *** | ns | ns | ns | *** | ns | ns | ns | *** | ns |
| Shoot [P] | *** | ns | *** | *** | *** | *** | *** | *** | *** | *** | ns | ns | *** | *** | ns |
| Shoot N:P | *** | *** | *** | *** | ns | *** | *** | *** | *** | *** | ns | ** | * | *** | ns |
| Log10 shoot P content | *** | *** | *** | *** | ns | *** | *** | * | *** | *** | ns | * | ns | *** | ns |
| Total root length | *** | *** | *** | *** | * | ** | *** | ns | * | *** | ns | * | ns | ** | ns |
| Mean root diameter | *** | *** | ns | ns | * | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Specific root length | *** | *** | ns | *** | *** | ** | ns | *** | ** | ns | ns | ** | ns | ns | ns |
| Root tissue density | *** | *** | ns | * | ns | * | ns | *** | ns | ns | ns | ns | ns | ns | ns |
| Root hair length (0 and 40 mg P kg−1) | *** | ns | ns | * | ns | ns | ns | ns | ns | ns | ns | * | ns | ns | ns |
| Root length colonised by AMF (%) | ns | *** | *** | ns | *** | *** | ns | ||||||||
| Rhizosheath carboxylates per g root DW | *** | *** | *** | *** | *** | ** | *** | *** | *** | *** | ns | *** | ** | *** | ** |
- Note: G, AMF, PS and PL represent genotype, AMF treatment, P source and P level, respectively. Four-way ANOVA statistical analysis was undertaken (n = 4).
- Abbreviation: ns, not significant.
- * p < 0.05;
- ** p < 0.01;
- *** p < 0.001.
3.2 Shoot DW
For shoot DW, significant three-way interactions occurred for genotype × AMF × P level (p < 0.01), genotype × P source × P level (p < 0.01), and AMF × P source × P level (p < 0.001) (Figure 2A,B, Table 1). The interaction of genotype × AMF × P level reflects that in both genotypes, shoot DW for +AMF was 7–40% less than that for −AMF at all P levels, except nil-P. ICC4814 produced significantly more shoot DW than ICC14799 at P levels ≥20 μg g−1 (+AMF) and ≥10 μg g−1 (−AMF). The interaction of genotype × P source × P level shows that the KP treatments produced 1.7–3.4 times more shoot DW for ICC4814 and 1.6–3.6 times more shoot DW for ICC14799 than the FeP treatments. Shoot DW varied little at FeP levels ≤40 μg g−1 and ≤80 μg g−1 for ICC4814 and ICC14799, respectively, but increased by ~20% at 80 and 160 μg g−1 for ICC4814 and at 160 μg g−1 for ICC14799. The interaction of AMF × P source × P level reflects that −AMF under KP (≥10 μg g−1) produced significantly more shoot DW than +AMF, while shoot DW in the FeP treatments showed little response to P levels, and no difference between +AMF and −AMF.
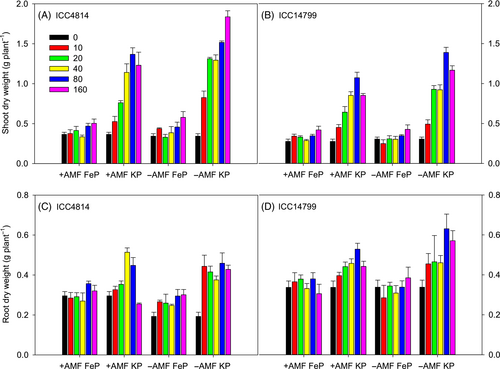
3.3 Root DW
A three-way AMF × P source × P level interaction occurred for root DW (p < 0.05; Figure 2C,D, Table 1). The interaction reflects that root DW under FeP did not significantly differ among P levels for the +AMF and −AMF treatments. Root DW of +AMF plants under KP increased gradually with increasing soil P level from 0 to 80 μg g−1, but dramatically decreased to the value close to that of nil-P at 160 μg g−1, while that of −AMF plants under KP increased by 70% at soil P levels of 10, 20 and 40 μg g−1, relative to nil-P, and increased further at 80 and 160 μg g−1. A significant genotype × P source interaction occurred for root DW (p < 0.05): ICC14799 had more root DW than ICC4814 under FeP and KP, and both genotypes under KP had more root DW than under FeP.
3.4 Nodule DW and percentage of nodule DW to total below-ground DW
A significant three-way genotype × P source × P level and AMF × P source × P level interaction occurred for nodule DW (both p < 0.001, Figure 3A,B, Table 1). These interactions reflect that nodule DW under FeP was very low for both +AMF and −AMF plants and did not significantly differ among P levels; it was significantly greater under KP than under FeP, increasing with increasing P level to reach maximum values at 80 and 160 μg g−1 for both ICC4814 and ICC14799. ICC14799 under KP had 15–57% greater nodule DW than ICC4814 at soil P levels from 40 to 160 μg g−1, but no such difference was found at soil P levels ≤20 μg g−1. The estimated means for the interaction of AMF × P source × P level showed that under KP, −AMF had significantly more nodule DW than +AMF at P levels ≥20 μg g−1.
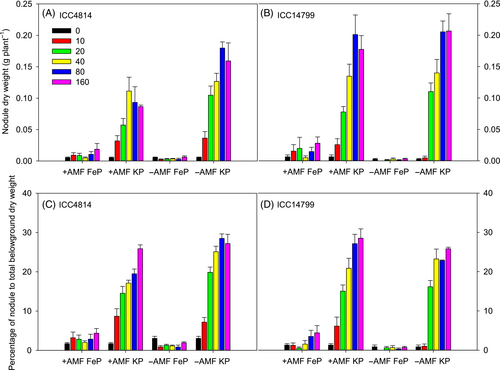
The percentage of nodule DW to total below-ground DW followed a similar trend to nodule DW, with three-way interactions of genotype × AMF × P source (p < 0.001) and AMF × P source × P level (p < 0.01) (Figure 3C,D, Table 1).
3.5 Root mass ratio
Three-way interactions for genotype × AMF × P source (p < 0.05) and genotype × AMF × P level (p < 0.05) occurred for root mass ratio (Figure 4, Table 1). Root mass ratio was significantly greater in ICC14799 than in ICC4814, regardless of P source or inoculation treatment, more so under FeP than under KP. The estimated means for the interaction of genotype × AMF × P level reflect a decreased root mass ratio with increasing soil P level from 0 to 160 μg P g−1 for both genotypes; slightly lower root mass ratios for −AMF at soil P levels of 0, 20, 40 and 80 μg g−1 than +AMF but no differences at 10 and 160 μg g−1 in ICC4814, and no differences between +AMF and −AMF at any P level for ICC14799.

3.6 Shoot N concentration
A significant AMF × P source × P level interaction occurred for shoot [N] (p < 0.001, Figure 5A,B, Table 1). The interaction reflects that for both ICC4814 and ICC14799, shoot [N] for the −AMF plants was significantly greater than that for +AMF plants at P levels ≤80 μg g−1, while similar at 160 μg g−1 under FeP, and at P levels ≥20 μg g−1 under KP. Shoot [N] under FeP was significantly greater than that under KP when P levels ranged from 20 to 160 μg g−1 under +AMF, and from 10 to 80 μg g−1 under −AMF. A significant genotype × AMF interaction occurred for leaf [N] (p < 0.001), which reflects similar shoot [N] for ICC4814 and ICC14799 under +AMF, and significantly greater leaf [N] under −AMF, and more so for ICC14799 (24%) than for ICC4814 (14%). A significant genotype × P source interaction for shoot [N] reflects similar values for the two genotypes under KP, while they were significantly greater under FeP than under KP, more so in ICC14799 (24%) than in ICC4814 (10%).
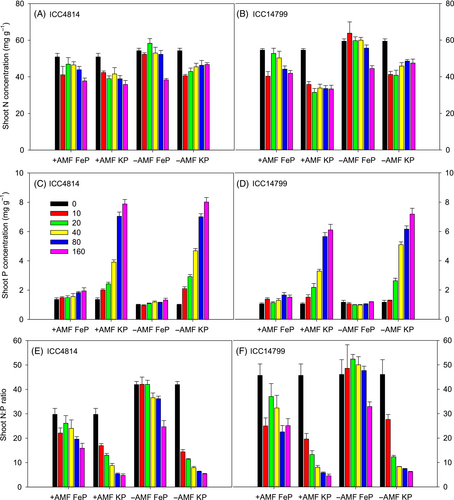
3.7 Shoot P concentration
Three-way interactions for genotype × P source × P level (p < 0.001) and AMF × P source × P level (p < 0.001) occurred for shoot [P] (Figure 5C,D, Table 1). The interaction of genotype × P source × P level reflects that shoot [P] under FeP increased slightly from 1.20 to 1.63 mg g−1 for ICC4814 and 1.11 to 1.35 mg g−1 for ICC14799 with increasing soil P level from 0 to 160 μg g−1, while shoot [P] under KP increased seven-fold for ICC4814 and six-fold for ICC14799 with increasing soil P level from 0 to 160 μg g−1. No significant differences were monitored between genotypes at P levels ≤40 μg g−1 but ICC4814 had significantly higher shoot [P] than ICC14799 at 80 and 160 μg g−1. The interaction of AMF × P source × P level reflects that shoot [P] under FeP remained low and increased only slightly from 1.21 to 1.73 mg g−1 (+AMF) and 1.08–1.26 mg g−1 (−AMF) with increasing soil P level from 0 to 160 μg g−1. Interestingly, +AMF had 26–60% higher shoot [P] than −AMF across different soil P levels. In contrast, −AMF had significantly higher shoot [P] than +AMF at 20, 40 and 160 μg P g−1 under KP.
3.8 Shoot N:P ratio
Three-way interactions occurred for genotype × AMF × P level (p < 0.01), genotype × P source × P level (p < 0.05), and AMF × P source × P level (p < 0.001) for shoot N:P ratio (Figure 5E,F, Table 1). The interaction of genotype × P source × P level reflects that shoot N:P ratio under FeP varied little at soil P levels ≤40 μg g−1 and decreased significantly at 80 and 160 μg g−1 in both genotypes, while it dramatically declined from 36 to 5.1 in ICC4814 and from 49 to 5.6 in ICC14799 under KP with increasing soil P levels from 0 to 160 μg g−1. ICC14799 had a significantly higher shoot N:P ratios than ICC4814 at all P levels under FeP and at 0 and 10 μg P g−1 under KP. The interaction of AMF × P source × P level reflects significantly higher shoot N:P ratios under −AMF than +AMF at all P levels when supplied as FeP, but similar values (except at nil-P) to +AMF when supplied as KP.
3.9 Shoot P content
Three-way interactions of genotype × AMF × P level (p < 0.05), and AMF × P source × P level (p < 0.001) occurred for shoot P content (Figure S3, Table 1). The genotype × AMF × P level interaction reflects that shoot P content under −AMF was 26–81% and 26–53% greater than that +AMF for ICC4814 and ICC14799, respectively, at soil P levels from 20 to 160 μg g−1; the shoot P content was significantly greater in ICC4814 than in ICC14799 at all P levels for +AMF and −AMF, except under −AMF at nil-P.
Shoot P content under FeP was always very low (<1 mg plant−1) and showed little response to P levels. The AMF × P source × P level interaction reflects that shoot P content under KP increased from 0.4 to 7.8 mg plant−1 for + AMF and 0.4 to 11.3 mg plant−1 for −AMF with increasing soil P level from 0 to 160 μg g−1; −AMF had significantly greater shoot P content than +AMF under KP at soil P levels from 10 to 160 μg g−1, but the opposite was true under FeP at soil P levels from 10 to 80 μg g−1.
3.10 Root morphology
For total root length, significant three-way interactions occurred for genotype × AMF × P level (p < 0.05) and AMF × P source × P level (p < 0.01) (Figure 6A,B, Table 1). The genotype × AMF × P level interaction reflects that total root length for +AMF varied slightly among P levels for both genotypes, while for −AMF, it almost doubled compared with that of nil-P for ICC4814 at 10 and 20 μg P g−1, declining slightly at higher P levels, and increasing at soil P levels ≥20 μg g−1 for ICC14799, relative to that of nil-P and 10 μg P g−1. Total root length of −AMF increased by 18–80% and 19–55% for ICCC2884 and ICC14799, respectively, relative to +AMF at different P levels. The AMF × P source × P level interaction reflects that +AMF and −AMF did not respond to different P levels under FeP; however, −AMF under KP had 30–122% more total root length than −AMF under FeP at soil P levels ≥10 μg g−1; a similar response occurred for +AMF at 20, 40 and 80 μg P g−1 (by 29–74%).
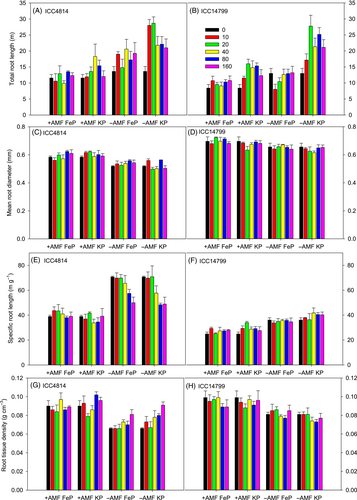
For mean root diameter, a significant genotype and AMF interaction occurred (p < 0.001), but no other interactions were found (Figure 6C,D, Table 1). The interaction reflects that the mean root diameter for +AMF increased by 13% and 6% in ICC4814 and ICC14799, respectively, relative to −AMF. ICC14799 had a significantly higher mean root diameter than ICC4814 for +AMF and −AMF.
For specific root length, a three-way interaction occurred for genotype × AMF × P level (p < 0.01) and a two-way interaction occurred for genotype × P source (p < 0.01) (Figure 6E,F, Table 1). The three-way interaction reflects that specific root length for +AMF varied little among P levels for ICC4814 (36.3–41.7 m g−1) and ICC14799 (27.9–29.6 m g−1). In contrast, for −AMF, it differed between soil P levels with the highest values at ≤20 μg g−1 and significant reductions at higher P levels in ICC4814, but no such response in ICC14799. The specific root length for −AMF was 27–82% greater for ICC4814 and 22–45% greater for ICC14799 under different P levels than −AMF.
For root length density, significant effects occurred for the AMF treatment (p < 0.001) and two-way interactions of genotype × P source (p < 0.05) and genotype × P level (p < 0.001) (Figure 6G,H, Table 1). The estimated mean for root tissue density under +AMF was 0.090 g cm−3, 21% greater than that of −AMF (0.076 g cm−3). The two-way interactions reflect that ICC4814 had a slightly higher root tissue density under KP than under FeP, while the opposite was true for ICC14799. ICC4814 had the lowest root tissue densities at 0, 10 and 20 μg P g−1, increasing slightly at P levels ≥40 μg g−1, while ICC14799 had the lowest root tissue densities at 80 μg P g−1, which did not significantly differ from those at 20, 40 and 160 μg P g−1, but was significantly lower than those at 0 and 10 μg P g−1.
For root hair length, a significant genotype × AMF × P level interaction occurred (p < 0.05; Figure S4, Table 1). The interaction reflects that root hair length for −AMF in ICC4814 significantly differed with P levels, with values being 50% higher at 40 μg P g−1 than at nil-P, but this did not occur in ICC14799. Root hair length for +AMF did not significantly differ between 0 and 40 μg P g−1 for either genotype. ICC14799 only had significantly greater root hair length than ICC4814 under −AMF at nil-P.
3.11 Root colonisation by AMF
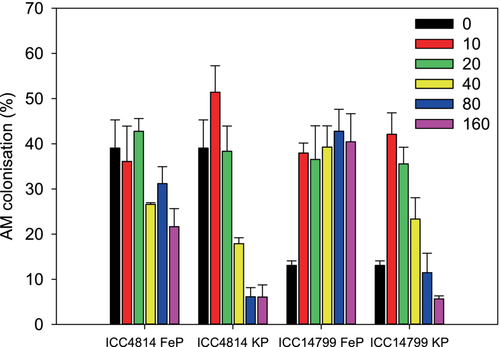
Significant interactions occurred for genotype × P level (p < 0.001) and P source × P level (p < 0.001) for AM colonisation (Figure 7, Table 1). The genotype × P level interaction reflects that ICC4814 had the highest percentage of AM colonisation (~40%) at 0, 10 and 20 μg P g−1, decreasing significantly to 15%–22% at P levels ≥40 μg g−1. ICC14799 had a relatively low percentage of AMF at nil-P (13%), significantly increasing to 40% at 10 μg g−1 before gradually declining at higher P levels to only 15% at 160 μg g−1. The P source × P level interaction reflects that the percentage of AM colonisation under FeP was 26% at nil-P, increasing slightly to 32–40% at soil P levels from 10 to 160 μg g−1. The percentage of AM colonisation under KP at 10 μg P g−1 was almost double that at nil-P, gradually decreasing at higher soil P levels to 6% at 160 μg g−1.
3.12 Rhizosheath carboxylates
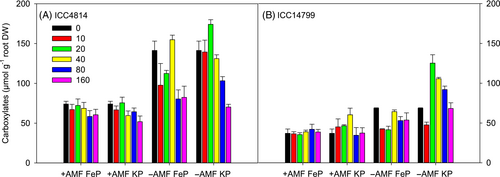
A significant genotype × AMF × P source × P level interaction occurred for the amount of carboxylates per gram of root DW (p < 0.01, Figure 8, Table 1). ICC4814 had significantly more carboxylates per g root DW than ICC14799 in most treatments. For both genotypes under all P levels and P sources, +AMF had significantly less carboxylates per g root DW than −AMF. For both genotypes under FeP, the amount of carboxylates per g root DW varied little across most P levels for +AMF, but decreased significantly for −AMF at 10 and 20 μg P g−1, relative to nil-P, before increasing at 40 μg P g−1 to a similar level to nil-P, and then decreasing at P levels ≥80 μg g−1 by 48% for ICC4814 and 18% for ICC14799. Both genotypes under KP had the most carboxylates per g root DW at 20 μg P g−1, which then decreased with increasing P level under −AMF. Under +AMF, the amount of carboxylates per g root DW varied little between P levels, except for a significant decline in ICC4814 at 160 μg g−1 and significant increase in ICC14799 at 40 μg g−1, relative to nil-P.
3.13 Cytokinins in xylem sap
Detected cytokinins in the ribosylated form included N6-isopentenyladenosine (iPR), dihydrozeatin-O-glucoside (DZOG) and trans-zeatin-O-glucoside (tZOG) in the xylem sap pooled within each treatment (Table 2). Both genotypes had relatively low iPR concentrations under +AMF and −AMF. The concentrations of DZOG, tZOG and, therefore, total detected cytokinins for −AMF were significantly higher than those with +AMF. Total detected cytokinin concentrations under −AMF at nil-P, FeP40 and KP40 were 8.0, 3.6 and 3.9-fold greater than those under +AMF in ICC4814, and 1.3, 2.3 and 8.6-fold greater in ICC14799. The concentrations of total detected cytokinins in ICC14799 were 1.4–3.8 fold greater than those in ICC4814 under 0 and 40 μg g−1, except under −AMF at nil-P, when they were 3.4-fold lower instead.
| P0 | FeP40 | KP40 | |||||
|---|---|---|---|---|---|---|---|
| +AMF | −AMF | +AMF | −AMF | +AMF | −AMF | ||
| ICC4814 | iPR | 0.28 | 0.18 | 0.28 | 0.52 | 0.16 | 0.13 |
| DZOG | 0.67 | 8.44 | 1.13 | 4.97 | 0.34 | 1.59 | |
| tZOG | 0.52 | 3.13 | 0.88 | 2.68 | 0.27 | 1.30 | |
| Total | 1.46 | 11.75 | 2.29 | 8.17 | 0.77 | 3.02 | |
| ICC14799 | iPR | 0.52 | 0.46 | 0.29 | 0.53 | 0.69 | 0.06 |
| DZOG | 0.98 | 1.70 | 3.51 | 8.77 | 0.36 | 4.14 | |
| tZOG | 1.15 | 1.25 | 4.79 | 10.42 | <LOQ | 4.84 | |
| Total | 2.66 | 3.41 | 8.59 | 19.72 | 1.06 | 9.04 | |
- Note: Xylem sap samples from four replicates were pooled into one sample for each treatment, which was analysed twice. iPR, DZOG and tZOG represent N6-isopentenyladenosine, dihydrozeatin-O-glucoside and trans-zeatin-O-glucoside. <LOQ indicates concentration below the limits of quantification.
4 DISCUSSION
4.1 AMF had a negative impact on chickpea growth
For both genotypes, the introduction of an indigenous community of AMF resulted in 7–40% less shoot DW than that of the non-mycorrhizal treatment at all P levels, except nil-P. This was somewhat unexpected, as there are reports in the literature of positive impacts of AMF on chickpea (e.g., Pellegrino & Bedini, 2014). However, there are no reports of positive impacts from high AMF colonisation for pulses (e.g., field peas) and other crops expected to be highly-responsive to AMF (e.g., linseed) in field experiments in southern Australia where crops are sown in autumn and harvested in late spring or early summer (Ryan & Graham, 2018; Ryan & Kirkegaard, 2012). Indeed, AMF may reduce the growth of wheat in these systems (Angus et al., 2015; Ryan et al., 2005). It has been speculated that low temperatures, low light intensities and a low phosphorus demand due to slow crop growth may all contribute to this situation; poorly-responsive crop cultivars may also contribute (Ryan & Kirkegaard, 2012). While the glasshouse was maintained at 15 − 22°C and did not reach the low temperature that a crop might experience in the field at this time of year, low temperatures may have restrained plant growth sufficiently to negate a need for AMF to P supply. Interestingly, Pellegrino and Bedini (2014), in a field experiment in Italy, found that chickpea growth and yield were improved more by +AMF treatment when sown in spring than when sown in autumn. Konvalinková and Jansa (2016) found that when AM plants are exposed to intensive shading on a long-time scale (i.e., weeks to months), positive mycorrhizal growth responses decrease and may eventually become negative. The daily maximum light intensity during the growth period in the present glasshouse study varied mostly from 735 to 1225 μmol m−2 s−1, which was higher than those in most other studies (Konvalinková & Jansa, 2016; Püschel et al., 2017). For example, a reduction to 65% of the light intensity (unshaded control was 690 μmol m−2 s−1) had almost no effects on shoot biomass or shoot P content (Konvalinková & Jansa, 2016). Therefore, the light received for chickpea plants under AMF treatment in the present study would not be a reason for reduced plant biomass relative to the −AMF treatment.
4.2 AMF treatment affected root morphology and carboxylate exudation and played an important role in accessing P from sparingly soluble iron phosphate
Our hypothesis that AMF significantly change root morphology and carboxylate exudation was supported for both ICC4814 and ICC14799; therefore, the contribution of root morphology and root exudates to P acquisition would have changed significantly following AMF treatment. Our results show that, regardless of P source, AMF had significant effects on root morphology: AMF-colonised plants of both genotypes had lower total root length and specific root length and greater mean root diameter and root tissue density than uninoculated plants. AMF had little effect on root mass ratio under FeP for both genotypes and under KP for ICC14799. In the literature, the effects of colonisation by AMF on root morphology are inconsistent. Ryan et al. (2012) found that the impact of AMF treatment on root DW varied greatly among Kennedia species, ranging from a 69% increase in K. lateritia to a >50% reduction in K. prostrata and K. microphylla. Root mass ratio of many Kennedia species was little affected by AMF treatment, while it greatly increased in K. stirlingii and K. lateritia and greatly decreased in K. prostrata. Hooker et al. (1992) found that root colonisation by AMF in poplar (Populus trichocarpa × P. deltoides) did not affect total root length, but significantly increased the length of individual secondary and tertiary roots and the number of laterals per unit length of colonised roots, compared with non-colonised roots. Chen et al. (2020) reported that mycorrhizal seedlings of Catalpa bungei grown under low P supply significantly increased root morphological parameters (including total root length, total root surface area, projected root area, total root volume, root tips, root branching number, length and surface area of fine roots), but significantly decreased mean root diameter (30%) and root mass ratio, relative to non-mycorrhizal seedlings.
Species with thicker roots, such as chickpea, may have limited root morphological plasticity in response to soil P availability (Eissenstat et al., 2015; Wen et al., 2019). For mean root diameter, both ICC4814 and ICC14799 varied little among P levels, regardless of P source or AMF treatment; a similar response occurred for specific root length (except a reduction at P levels ≥40 μg g−1 under KP in ICC4814). The lack of response to P levels in mean root diameter and specific root length in the present study was consistent with the study by Wen et al. (2020) that included two groups of chickpea genotypes inoculated with AMF—a group of genotypes with abundant rhizosheath carboxylates accompanied by lower root diameter, root mass ratio, root mass density and higher specific root length, relative to another group with few rhizosheath carboxylates—both groups showed little response in root morphology (including root diameter, root mass density, specific root length and specific root surface area) to three levels of P (10 μg g−1 as FePO4, 10 and 50 μg g−1 as KH2PO4). However, as Wen et al. (2020) did not have a −AMF treatment, our finding of reduced variation in specific root length in response to P levels under +AMF relative to −AMF further advances our understanding of the response of root morphology to varying P levels.
Consistent with the findings of Wen et al. (2020), our results show that for all treatments, ICC4814 with substantial rhizosheath carboxylates (based on root DW) had thinner roots, lower root mass ratio and root mass density, and higher specific root length, than ICC14799 with less rhizosheath carboxylates. While both genotypes followed a similar trend in root morphology in response to AMF treatment, the morphological change in response to AMF treatment (increase in mean root diameter and root tissue density, and reduction in mean root diameter) was more pronounced for the genotype with greater amounts of carboxylates (ICC4814) than for the genotype with fewer carboxylates (ICC14799); the detailed mechanisms underlying this response need to be explored.
Under nil-P, ICC4814, with thinner roots, had a significantly higher percentage of root length colonised by AMF than ICC14799, which had thicker roots. This contrasts with previous findings at the species level showing that species with thicker roots tend to have higher AMF colonisation (Li et al., 2017; Wen et al., 2019). As a carboxylate-releasing P-mining strategy is superior in severely P-impoverished soils (Lambers et al., 2015), the higher AMF colonisation in ICC4814 than in ICC14799 at nil-P might be due to the mobilisation of more P via carboxylates in ICC 4814, enhancing P availability in the rhizosheath and, in turn, enhancing AM colonisation. It is commonly reported, in similar P-response glasshouse experiments with annual pasture legumes, that colonisation by AMF is maximised at moderate P availability and then becomes very low at both very high and very low P availability (Abbott et al., 1984; Bolan et al., 1984; Jeffery et al., 2018). The present study further advanced this by showing that chickpea genotypes respond differently to different levels of FePO4 in terms of the percentage of root length colonised by AMF; Wen et al. (2020) only included one P level as FePO4 and did not assess the response to different FePO4 levels. Under FeP, ICC4814 maintained AMF colonisation at P levels from 0 to 20 μg g−1, decreasing at higher P supply, which might be due to the increased leaf P concentration with increasing P supply, thus systemically suppressing AMF colonisation as found previously (Abbott et al., 1984; Wen et al., 2020). In contrast with ICC4814, AMF colonisation significantly increased in ICC14799 under FeP, relative to nil-P, with little variation at FeP levels from 10 to 160 μg g−1. The reason for the maintenance of a high level of AMF colonisation in ICC14799 under FeP is not clear and warrants further exploration. Under KP, both genotypes followed a similar pattern, with the highest AMF colonisation at 10 μg P g−1, declining at increasing P levels, with little difference between the genotypes. The pattern of AMF colonisation in response to KP in this study agrees with others studies showing that AMF colonisation is suppressed under both very low and high P in Trifolium subterraneum (Abbott et al., 1984; Jeffery et al., 2017). Using three P sources (KH2PO4, colloidal iron phosphate and crystalline iron phosphate, i.e., strengite) spanning low to high levels in subclover and ryegrass (Lolium rigidum), Bolan et al. (1987) found that the percentage of AMF colonisation increased at low P levels and decreased at higher P levels. While ICC4814 and ICC14799 differed in their response to FeP, neither followed the pattern of subclover and ryegrass in response to colloidal iron phosphate or strengite. Bolan et al. (1987) showed that the relationship between percentage of AMF colonisation and P level varied with P source and species (subclover and ryegrass); similarly, in our study, AMF colonisation differed between genotypes and depended on the P source.
AMF treatment reduced the rhizosheath carboxylates of both genotypes under FeP and KP, which agrees with previous studies in a range of host species (Nazeri et al., 2014; Ryan et al., 2012). To our knowledge, this is the first study to show that AMF treatment suppressed variation in carboxylates among the different P levels under both FeP and KP. For −AMF, the greatest amount of carboxylates was found at 20 μg P g−1 as KP and 40 μg P g−1 as FeP for both genotypes, which then gradually decreased with increasing P levels. Studies on chickpea have reported contrasting results in the response of carboxylate release to P level, for example, an increase in response to P deficiency in a single genotype grown in hydroponics without AMF treatment (Neumann & Römheld, 1999) and little change in response to varying P concentrations in two genotypes with no AMF treatment (Wouterlood et al., 2004, 2005). Wen et al. (2020) found that low soil P availability does not induce carboxylate exudation in chickpea, suggesting that carboxylate exudation under low P is not determined by plant P status and not a specific P-mining strategy. This is in sharp contrast with the typical roles of carboxylate in a range of species such as Lupinus, Proteaceae and Cyperaceae (Lambers, 2022; Lambers et al., 2015). Instead, carboxylate exudation in chickpea, although mobilising P, may actually serve as a mechanism to dispose of surplus carbon, as proposed by Prescott et al. (2020). Carboxylates may also provide critical substances to shape the composition and function of microbial communities in the rhizosphere (Zhalnina et al., 2018). Although we found fewer carboxylates in the rhizosheath for +AMF relative to −AMF, under both P sources in both genotypes, our results suggest that more abundant carboxylates per gram of root DW is not necessarily more effective at using sparingly soluble phosphate (FePO4), as indicated by the lower shoot P concentration under −AMF. We should bear in mind, however, that FePO4 is not a major P compound in soil, and that P is actually sorbed onto oxides and hydroxides of Fe (Celi et al., 2020; Geelhoed et al., 1998), and carboxylates may have a different effect on these.
Our results support the hypothesis that AMF play an important role for P acquisition compared with −AMF under sparingly soluble FeP, but not under KP. We showed that for both genotypes under FeP, AMF-colonised plants had higher shoot [P] and shoot P contents than uninoculated plants; this was not the case under KP. While the root morphological changes (increased root diameter and root tissue density, reduced specific root length and total root length) and reduced amounts of carboxylates in response to AMF colonisation was unfavourable for P acquisition, +AMF plants had more shoot [P] under FeP than −AMF plants. This implies that the increased shoot [P] in response to AMF colonisation under FeP could not be attributed to a change in root morphology and carboxylates, but AMF colonisation allowed chickpea to acquire P more than carboxylate exudation and root morphology did under sparingly soluble iron phosphate. However, the importance of root morphology and carboxylates in P acquisition should not be discounted. In our previous study, shoot P content was strongly correlated with root morphology, including total root length and root surface area, and total carboxylates per plant in 100 chickpea genotypes supplied with 10 μg P g−1 as FePO4, but without AMF treatment (Pang et al., 2018). In 20 chickpea genotypes colonised by AMF and supplied with 10 μg P g−1 as FePO4, shoot P content was also positively correlated with total root length, root surface area and total carboxylates per plant, in addition to AMF colonisation (Wen et al., 2020). The inclusion of +AMF and −AMF treatments in the present study demonstrates that AMF play a more important role than root morphology and rhizosheath carboxylates in acquiring P from FePO4, a sparingly soluble form of P, in chickpea plants colonised by AMF. We did not observe this under KP, implying a less important role of AMF in P acquisition in the presence of a more soluble P source, as suggested previously (Lambers et al., 2015).
4.3 AMF treatment enhanced nodulation under FeP, while the reverse was true under KP
- Improved nodulation in +AMF might be due to improved P acquisition under FeP, as observed in this study. AMF can improve plant P uptake, resulting in more P available for nodulation by rhizobia when both N and P are limiting (Fitter & Garbaye, 1994). Similar results by Püschel et al. (2017) show that AMF treatment enhances biological nitrogen fixation efficiency under low P supply, most likely mediated through improved P acquisition in the AMF treatment relative to the −AMF treatment in both Medicago truncatula and M. sativa. In addition, flavonoids and isoflavonoids are exuded by host plant roots and these play a role as early signals for both nodulation and mycorrhization in legumes (Xie et al., 1995).
- Reduced nodulation for −AMF might be partially attributed to higher leaf [N] than for +AMF in both genotypes under FeP. Higher leaf [N] for −AMF may provide a feedback signal to inhibit nodule formation (Hartwig, 1998; Lodwig et al., 2003). Almeida et al. (2000) reported that increased organic [N] in the phloem at low P supply acts as N feedback to downregulate or even prevent nodulation and nodule growth, leading to a reduction in the proportion of whole-plant N derived from symbiotic N2 fixation in white clover (Trifolium repens).
- Enhanced nodulation for +AMF might be regulated partially by lower cytokinin concentrations relative to −AMF. We found that +AMF had lower cytokinin concentrations in xylem sap than −AMF for both genotypes, consistent with the findings of Jones et al. (2015) in pea (Pisum sativum), who reported that young mycorrhizal roots had significantly lower cytokinin concentrations than uninoculated roots, suggesting that the presence of the fungal micro-symbiont affects cytokinin concentration in plants. Those authors suggested that cytokinins play an essential role in mediating the entry of both rhizobia and fungi into the root cortex; cytokinins had a differential effect, inhibiting the entry of rhizobia while promoting entry of fungi (Jones et al., 2015). In addition, strigolactones positively affect nodulation in legumes: application of a synthetic strigolactone (GR24) increased nodulation in alfalfa (Medicago sativa), pea and soybean (Glycine max), and strigolactone-biosynthesis mutants had fewer nodules than wild-type plants in Lotus japonica, pea and soybean (Foo & Davies, 2011; Foo et al., 2013; Haq et al., 2017; Liu et al., 2013; Rehman et al., 2018; Soto et al., 2010). The question of whether enhanced nodulation in +AMF plants under FeP, relative to that in −AMF plants, might be partially explained by the difference in strigolactones requires further investigation.
4.4 AMF treatment reduced shoot branching under FeP and KP, and reduced shoot growth under KP but not under FeP
Shoot growth in response to AMF treatment differed under FeP and KP for both genotypes; under KP, shoot DW for +AMF significantly decreased at soil P levels ≥10 μg g−1, relative to −AMF, while under FeP, shoot DW did not differ between +AMF and −AMF treatments. Bolan et al. (1987) showed that mycorrhizal colonisation increased the growth of subterranean clover for three P sources with different solubility (KH2PO4, colloidal FePO4 and strengite). The greatest benefit from AMF occurred under strengite, the least soluble P source; AMF significantly increased the growth of ryegrass with strengite but had no effect with KH2PO4 or colloidal FePO4. The authors suggested that mycorrhizas can locate and use the point sources of P from insoluble FePO4 by exploring the soil more thoroughly. The inconsistency of our results with those of Bolan et al. (1987) might be due to differences in plant species, fungus species and/or soil conditions. Plant growth in response to AMF colonisation strongly depends on plant species, ranging from negative and neutral to highly positive; no significant negative response was found for 15 crop species including cereals, legumes and vegetables grown in pots filled with a low-P soil in the glasshouse (Tran et al., 2019). In addition, Klironomos (2003) showed that the direction and magnitude of the growth response to AMF colonisation depend on the interaction of plant and fungal species using a combination of 64 plant species and 10 fungal species.
Our hypothesis that AMF colonisation affects shoot branching was partly supported, as AMF treatment reduced the number of shoot branches in both genotypes but varied for genotype and P level, that is, at P levels ≥10 μg g−1 for ICC4814, and ≥40 μg g−1 for ICC14799 under KPand ≥40 μg g−1 for ICC4814 and ≥20 μg g−1 for ICC14799 under FeP. Our results show a decreased concentration of cytokinins in FeP40 and KP40 under +AMF relative to −AMF coincided with decreased shoot branching for both genotypes, suggesting that cytokinins might regulate the branching process in response to AMF treatment. This is consistent with earlier findings on the stimulative effects of cytokinins on shoot branching in other plant species (Leyser, 2009; Waldie & Leyser, 2018). However, no such relationship was observed under nil-P for either genotype; the xylem sap cytokinin concentrations decreased under +AMF in both genotypes, particularly in ICC4814, but there was no difference in the number of shoot branches, relative to −AMF. This might be due to the involvement of other phytohormones such as strigolactones and auxins, as many studies showed that auxin and strigolactones often inhibit shoot branching and tiller numbers (Guo et al., 2020; Leyser, 2009; Waldie & Leyser, 2018). Strigolactones act as rhizosphere signalling molecules that enhance symbioses with AMF, and have been widely reported to suppress shoot branching (Chesterfield et al., 2020; Gomez-Roldan et al., 2008; Kohlen et al., 2011). Therefore, auxin and strigolactones might also be involved in reduced shoot branching in response to +AMF in the present study. The involvement of cytokinins, auxins, strigolactones and their interacting effects in regulating shoot branching in response to +AMF in chickpea is unclear and requires further investigation.
In summary, this study shows that AMF treatment reduced shoot DW, inhibited shoot branching, significantly changed root morphology (decrease in total root length and specific root length, increase in mean root diameter and root tissue density), and reduced the amount of rhizosheath carboxylates for two chickpea genotypes (ICC4814 and ICC14799) under both FePO4 and KH2PO4. However, AMF play different roles when acquiring P from different P sources. Our results demonstrate that under FePO4, a sparingly soluble form of P, AMF likely played a more important role than root morphology and rhizosheath carboxylates in acquiring P in chickpea, while we did not observe this under KH2PO4. Interestingly, co-inoculation with AMF and rhizobia enhanced nodulation under FePO4.
AUTHOR CONTRIBUTIONS
Jiayin Pang, Megan H. Ryan, Hans Lambers and Kadambot H. M. Siddique designed the study. Jiayin Pang, Zhihui Wen, Guillaume Tueux, Yifei Liu, Yi Zhang, Wei San Wong and Jean Wan Hong Yong performed the experiments and collected the data. Jiayin Pang, Bede Mickan, Sasha Jenkins and Wei San Wong analysed and interpreted the data. Jiayin Pang led the writing of the manuscript. Hans Lambers, Kadambot H. M. Siddique, and Megan H. Ryan contributed critically to the drafts and all authors gave final approval for publication.
ACKNOWLEDGEMENTS
The work was funded by the Australian Research Council (LP200100341) and the UWA Institute of Agriculture. We thank Mr Rob Creasy and Mr Bill Piasini for helping to maintain the plants in the glasshouse, Drs Jun Zhou, Hongtao Zhong, Daihua Ye, Feng Wang and Jingwen Gao for help with the glasshouse harvest, Mr Greg Cawthray for help with the analysis of carboxylates. Open access publishing facilitated by The University of Western Australia, as part of the Wiley - The University of Western Australia agreement via the Council of Australian University Librarians.
Open Research
DATA AVAILABILITY STATEMENT
The authors state that the data generated within the paper are available.




