RETRACTED: CRISPR/Cas genome editing perspectives for barley breeding
Abstract
The CRISPR/Cas9 technology shows potential to improve crop breeding efficiency and antiviral defense. The interest in DNA editing in crops has grown due to the possibility of increasing the resistance of different plants to many viruses. Our aim was to create an elite disease-resistant local barley cultivar using CRISPR/Cas9 biotechnology. For this purpose, we used CRISPR/Cas 9-eIF4E with the eukaryotic translation initiation factor 4E (eIF4E) barley gene to edit the genomes of five local Kazakhstan barley cultivars. After identifying the single guide RNA (sgRNA) target sequences, they were synthesized and cloned into the CRISPR-plant vector before being introduced into barley cells via our own patented Agrobacterium germ-line transformation technique. Barley plants eIF4E-modified were successfully obtained and were resistant to virus infection. Based on our research, the CRISPR/Cas9 system for plant genome editing could be a prospect for applying this breakthrough biotechnology in barley breeding.
1 INTRODUCTION
Clustered regularly interspaced palindromic repeats (CRISPRs) is an RNA-regulated protection mechanism in bacteria and archaea that is the base of the new popular technology of gene editing in humans, animals, and higher plants. The CRISPR/Cas9 system is a simple, inexpensive, and versatile tool for genome editing, resulting in a groundswell of research (Chandrasekaran et al., 2016; Gerasimova et al., 2017; Gonatopoulos-Pournatzis et al., 2020; Khlestkina & Shumny, 2016; Mushtaq et al., 2019). A tool as powerful as CRISPR is currently very useful in improving the quality of crops. It is estimated that up to 40% of the harvest is lost worldwide to pests/diseases threatening our food supply. Viral and fungal diseases often affect major crops, such as rice, barley, and wheat, which can be the main reason for the drop in yield and loss of grain quality.
Genome editing with the use of CRISPR/Cas is the most efficient technology that has been introduced in biological research over the past decade. CRISPR/Cas nucleases (an enzyme capable of cleaving the phosphodiester bonds between nucleotides of nucleic acid chains) are easily programed. The programable nature of CRISPR/Cas facilitates the creation of transgenic organisms through site-specific gene mutations, knock-ins, or large chromosomal rearrangements (Fourth annual CRISPR…, 2020; Koeppel et al., 2019; Robb, 2019).
CRISPR involves the creation and destruction of bonds using nucleic acids and their associated proteins. With the help of nucleic acid, the cleavage site is recognized and precisely separated. Genes are then not transferred from either alien or close-related species but mutations are created at predetermined points. CRISPR integrates metabolic and genomic models to identify genes (Chandrasekaran et al., 2016; Decaestecker et al., 2019; Kumlehn et al., 2018). This also applies to RNase Cas12 (formerly known as Cpf1). These Nicase and DNases Cas9 have been applied to obtain new traits, such as powdery mildew resistance in wheat or improved pathogen resistance in barley, maize, and other crops (Barakate & Stephens, 2016; Koeppel et al., 2019; Schindele et al., 2018).
Many scientists have been studying how plasmids behave in different plants (Koeppel et al., 2019; Park & Choe, 2019; Robb, 2019). Recently, the off-targeting of CRISPR-Cas9 was shown to be reduced in a study using pre-assembled complexes of purified Cas9 protein and guide RNA (ribonucleoproteins complexes or RNPs) into plant protoplasts (Woo et al., 2015). RNPs enhance the specificity of CRISPR-Cas9 in plants and reduces the chimera or mosaic modifications in progeny plants (Liang et al., 2017; Svitashev et al., 2016). In order to detect potential off-targets and to design sgRNA in plants, scientists used different bioinformatic tools, such as CasOT, CRISPRMultiTargeter (Chen et al., 2019), and Cas-OFFinder (Bae et al., 2020). CRISPR-Cas9 edited plants may contain insertions of plasmid DNA and harboring insertions, deletions at the different sites of chromosome (Kim et al., 2019); these unwanted deletions/insertions are a problem that may limit the acceptance of these plants agronomically, though gene-editing in plants could provide a sustainable agriculture (Sauer et al., 2016). However, off-targeting is commonly low in plants compared with animals and humans and can be eliminated by backcrossing (Bortesi et al., 2016). Although CRIPSR-modified plants are often considered genetically modified organisms (GMOs) and may be subjected to GM laws in European countries, the CRISP technique offers new possibilities in plant research and selection, as it minimizes the GMO regulatory burden (in countries not considering it as GMO), the ecological challenges, and promotes public acceptance of genome-edited plants (Pyott, Sheehan & Molnar, 2016). New techniques associated with genome editing will complement farmers' tools and allow breeders to achieve their desired goals with greater speed and accuracy, thereby expanding the range of genotypic variation to increase crop diversity (Kumlehn et al., 2018; Schindele et al., 2018; Wolter & Puchta, 2017). Consumers will have the opportunity to receive higher quality food through high-quality editing of crop genomes.
Barley being economically important, it is important to have an efficient gene-editing method for this crop (Zhao-Gui et al., 2017). In this study, we aimed to explore the perspectives of antiviral defense in barley through CRISPR/Cas9 genome editing – knockout and modification of the eIF4E gene. eIF4E is a plant cellular translation initiation factor essential for potyvirus infection, and mutations in the eIF4E gene can confer resistance to potyviruses (Doman et al., 2020; Schindele et al., 2018). In this research, five varieties of barley with different productivity and stress resistance (Arna, Shashbaulym, Jan, Symbat, and Sever 1) that grow in Kazakhstan were selected as plant material. We successfully transformed all of them, showing the efficacity of our protocol, obtaining gene-edited barley with higher resistance to potyvituses.
2 MATERIALS AND METHODS
2.1 Plant materials
Five varieties of barley that grow in Kazakhstan were selected: Arna, Shashbaulym, Jan, Symbat, and Sever 1. The first two are mostly used for brewing, whereas the last three are used for feeding. Seeds for the experiment were provided free of charge by farmers specialized in growing these varieties of barley. All plants were planted in pots and grown in the laboratory with a photosynthetic photon flux density of 200 μmol m−2 s−1, humidity level of 65%, and soil temperature of 25°C. Soil type: moderately heavy loam with a neutral or slightly alkaline reaction.
2.2 Cloning of CRISPR/Cas9—eif4e
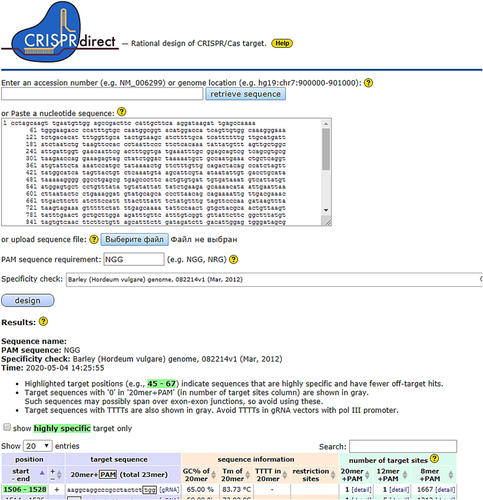
The creation of a genetic construct for editing the barley genome to modify the eif4e gene (CRISPR/Cas9 – eif4e) was carried out by restriction and ligation methods. The nucleotide sequence of this gene from the NCBI Bank (GenBank: AJ704454.1. Hordeum vulgare partial eif4e gene for putative eukaryotic translation initiation factor 4E, exons 2–5, cultivar Franka. FASTA Graphics) was used to search for the sgRNA sequence, edited to construct the barley genome CRISPR/Cas9 – eif4e, by in silico method using the following software: CRISPRdirect, CRISPR-PLANT, CRISPR-P, E-CRISP, and CRISPR RGEN. As a result of the analysis, the following sgRNA sequences were selected: (1) AAGGCAGGCCCGCCTACTCT (20 bp + 3 bp PAM for Cas9 nuclease recognition) with 65% GC, Tm 83.73°C, PAM – TGG, aka guide sgRNA, as well as (2) CAAAACCCTAGAGGACGTGG, PAM – CGG (Figure 1). Next, we performed the synthesis and molecular cloning of the guide sgRNA for the eif4e gene into the CRISPR/Cas9 plasmid (Sigma-Aldrich) containing the U6 promoter for nRNA-eif4e, nuclease Cas9 with the 35S promoter, antibiotic resistance marker genes to hygromycin (hptII) and kanamycin (nhtII) as well as all necessary restriction sites.
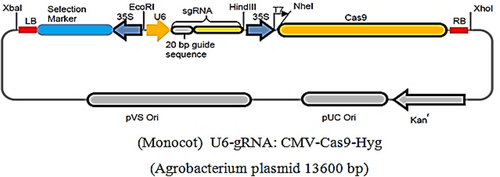
The plasmid system CRISPR-plant (Sigma-Aldrich, Germany) was used for the stable Agrobacterium-mediated transformation. sgRNA was synthesized and cloned into the CRISPR-plant Sigma-Aldrich vector for agrobacterial transformation at EcoRi and HindIII sites under the WhU6 promoter for monocots from wheat. Cas9 nuclease under the 35S promoter is from maize. The hptII and nptII genes were used as marker genes in the construct, imparting the resistance of the construct to the antibiotics hygromycin and kanamycin. The above-mentioned CRISPR/Cas9-eif4e plasmid was used for Agrobacterium transformation. Primers for sgRNA of eIF4E genes were selected with WhU6 promoter region for amplification of a 362-base pair fragment: F 5′-GACCAAGCCCGTTATTCTGAC-3′, R 5′-AAGTCTGATGCAGCAAGCGAG-3′; for the region including Cas9 with the promoter: F 5′-GCTCCTGGTCCATCCACG-3′, R 5′-CGTG-GATGGACCAGGAGC-3′; for hptII: F 5′-GCTGCGCCGATGGTTTCTACA-3′, R 5′-GCCCAAAGCATCAGCTCATCG. The recommended amplification mixture contained 5 mg of the DNA template (Applied Biosystems); 2.5 mM MgCl2; 250 μM dNTPs (Beijing SBS Genetech Co., Ltd.); 10 pmol of each primer (Applied Biosystems), a 1xPCR buffer 1 unit (Thermo Fisher Scientific). Positive colonies were detected by PCR using the following PCR conditions for U6 amplification: 95°C–2 min; 30 cycles (95 C – 30 s, 60°C – 30 s, 72°C – 30 s). A positive colony was grown in expplan medium with antibiotic to a OD600 of XX before centrifugation at 4500g for 10 min. The supernatant was decanted, and 3 ml of LB medium without antibiotic was added to the pellet and further diluted with (LB? no adjuvant?) until an optimum density at 600 nm of 0.8–1.0 (1010 cells/ml according to [Gerasimova et al., 2017]) was obtained. The resulting suspension was used for barley transformation. Isolation of plasmid and genomic DNA was carried out using the Biolabmix Kit according to the manufacturer's instructions.
The resulting CRISPR/plant expression vector containing the U6-sgRNA fragment of the eIF4E gene (CRISPR/Cas9-eIF4E) was introduced into the competent Agrobacterium tumefaciens cells. The Agrobacterium suspension was used to transform barley embryo lines with the Agrobacterium tumefaciens strain EHA105 using the expression cassette editing instruments for barley cells developed by Kershanskaya et al. (2019).
2.3 Genotype determination – Sequencing
Edited plant DNA was sequenced using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems), followed by fragment separation using the 3730xl DNA Analyzer (Applied Biosystems) and analysis using the Serial Cloner computer software. The purification of PCR products from unbound primers was performed by an enzymatic method using Exonuclease I (Fermentas, SibEnzyme) and alkaline phosphatase (Shrimp Alkaline Phosphatase, Fermentas) (Bae et al., 2020). The sequencing reaction was carried out using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) according to the manufacturer's instructions, followed by separation of the fragments on an automatic genetic analyzer 3730xl DNA Analyzer (Applied Biosystems) at the NCB KN MES RK and at Syntol, Moscow, Russia. The analysis of the obtained sequences and the sequence of the original gene was carried out using the Serial Cloner program.
3 RESULTS
Viruses use the cellular mechanisms of a host to generate offspring because they do not encode in their genomes all the components necessary for replication. eIF4E is a key protein of the host genome that belongs to the initiation complex of virus translation and regulation of translation and is involved in various cellular processes, such as proliferation, cancer, and plant diseases. Hence, the mutation of this protein was investigated using CRISPR tool, which is very advantageous compared with other editing methods. In monocotyledon, the following stages of the genomic editing process are distinguished: target sequencing and sgRNA generation; creation of genetic vectors for the distribution of Cas9 nuclease, sgRNA; supply of “structure-changing instruments” to plant cells; identification of progressive development in genomic DNA.
CRISPR/Cas9-eIF4E gene-editing system was engineered to target and introduce InDel mutations in eIF4E, which can be deleterious, via double-strand breaks at specific DNA sequences by the error-prone non-homologous recombination end-joining pathway (Figure 2). The size of one sgRNA expression cassette was 362 kb (Figure 3). The total size of the plasmid was 13,600 kb.
Agrobacterium cells were plated on agar standard LB medium, with the addition of the antibiotic kanamycin at a concentration of 50 μg/ml (Figure 4). Colonies appeared on the second day of cultivation at 28°C.
The expression cassette carrying sgRNA and Cas9 nuclease was delivered to the stigma of a barley flower pistil exactly before anthesis. Pollen tubes were used as a delivery vector of the targeted construct into a mature, but not divided, zygote. By the developed method (see material and methods), 353 ears of 5 local barley cultivars were transformed with the CRISPR/Cas9-eIF4E plasmid with Agrobacterium tumefaciens strain. The greatest number of modified ears were produced in varieties Arna and Symbat (87 and 85), which corresponded to the largest number of obtained seeds (1170 and 1278 seeds, respectively). The lowest number of modified ears (51 ears of barley) and the number of seeds obtained (640 grains) were in the variety Jan. The “seed weight per ear” was the highest in the variety Shashbaulym (0.65 g, data is not shown). The seed crop T0 was harvested in the middle of July. The putative genetic-engineered barley plants T0 were sown into unique pots to produce T1 plants for gene editing confirmation. The detection of edited events in barley was carried out by restriction analysis, PCR, and DNA sequencing, using Serial Cloner Program (Figure 5).
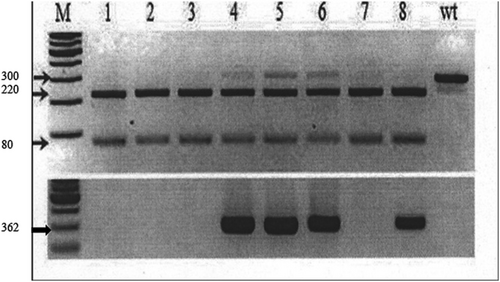
In 50 Agrobacterium-transformed T1 lines with CRISPR/Cas9-eIF4E target gene, deletions in 8 lines were found. In the transgenic lines 4–6, progeny from the T1 generations showed partial cleavage activity of Cas9 (Figure 6). The control was the nucleotide sequence of the wild type. The largest number of deletions were shown at the DNA double-strand break in plants 4 and 6, which ranged from 1 to 15 nucleotides: 4 and 6 – T1 plants of the Shashbaulym variety, 5–8 – T1 plants of the Sever1 variety.

4 DISCUSSION
Barley is one of the major cereal crops with wheat, rice, and maize and is important for beer brewing and as livestock feed culture. With its genome of 5.1 Gbp and its economic importance, it is a convenient and necessary object for CRISPR/Cas 9 editing. Moreover, the investigation of knockout or modification of the eIF4E gene or other negative factors of virus or micro-pathogens contamination is not widely known yet. Our investigation tries to feel a gap in this knowledge and to elaborate and establish biotechnologies of CRISPR/Cas9 genetic engineering for biotic stress resistance and germ-line genetic transformation in barley local Kazakhstan cultivars, not only in the worldwide known Golden Promise cultivar. The eIF4E gene editing was successful in the five local barley cultivars.
The potential efficiency of CRISPR/Cas9 technology is much higher than conventional breeding. The time for the creation of a stable genotype by CRISPR/Cas9 technology is 2 years, which is 3–4 times faster than using the backcross methods of traditional breeding, or 2–3 times faster than modern methods of marker-assisted selection (MAS) + marker double haploid (MDG) + backcross (BC) or double haploid hybrids (Gerasimova et al., 2017). The practical advantage of CRISPR/Cas9 is the ease of multiplexing (Khlestkina & Shumny, 2016). The multiplexed CRISPR technology is the use of a large number of unique primers in a single PCR reaction to obtain multiple PCR products of different lengths. They have facilitated powerful biological engineering applications, vastly enhancing the scope and efficiencies of genetic editing and transcriptional regulation. The advantage of CRISPR is in its ability to force a DSB event. For genome editing purposes, the generation of a targeted double-strand DNA break (DSB) is the crucial event that opens up multiple repair options both for the cell and the genome engineer. Mechanism of repairing includes one of two pathways: homology-directed repair (HDR) or non-homologous end joining (NHEJ). Cells use NHEJs more often than HDR. This is because HDR requires a pattern homologous to the regions flanking the gap and rates or deletions (INDEL) at the current position, resulting in functional knockout or modification of a gene. These change the targeted gene function. In all phases of the cell cycle other than the S phase, NHEJ acts as a stop-gap and repairs DSB and repair chromosomal integrity. NHEJ is a restorative process using ligases, nucleases, and polymerases to reseal a break and result in indels. If the break occurs in protein-coding indels, it will result in abrogating the protein's function (Bae et al., 2020; Carvajal-Garcia et al., 2020; Svitashev et al., 2016; Woo et al., 2015). Published researchers show the possibility of creating an edited plant system with specific editions stably inherited in generations using CRISPR/Cas9.
Viruses use the host's cellular mechanisms to generate offspring because they do not encode in their genomes all the components necessary for replication. Eukaryotic virus translation initiation factor (eIF4E) is a regulatory factor essential in the complex of initiation and regulation of virus translation (Wang et al., 2021). It is involved in various cellular processes, such as proliferation, viral diseases, and cancer. Recent studies have shown that eIF4E is a common target for some viruses that regulate it to improve their replication cycles, where eIF4E not only plays an important role in the translation of viral mRNAs but also participates in the proliferation of infected cells (associated with the development of cancer) and the reactivation of viral latency (Yu et al., 2020; Montero et al., 2015).
The interest in editing it in monocotyledonous and dicotyledonous cultures has now grown due to the possibility of increasing the resistance of plants to many viruses. Among studies on the engineering of a wide range of resistance of agricultural plants over the past 4 years, 20% is devoted to editing the eIF4F complex (Mushtaq et al., 2019). Plant RNA viruses, particularly potyviruses, attack the eukaryotic translation initiation factor 4E (eIF4E), resulting in replication (Chandrasekaran et al., 2016). The virus-encoded protein (VPg) provides the binding of viruses with eIF4E. Simultaneously, mutations in the eIF4E gene disrupt the interactions of virus molecules and the host gene, thereby starting a quantitative variation in resistance phenotype (Chandrasekaran et al., 2016). Kis et al. (2019) used the CRISPR/Cas9 system to inhibit an economically significant, phloem-limited, insect-transmitted virus belonging to the Geminiviridae family in Barley—Wheat dwarf virus (WDV).
Generally, the CRISPR/Cas9 system for plant genome editing is a breakthrough technology in breeding and a prospect for creating elite high-yielding crops today and in the nearest future with great social and economic benefits. Successful results of using CRISPR/Cas9 technology in agriculture that has been achieved up to this day represent only the first initial uses of this exciting biotechnology. Incredible opportunities for crop breeding and agriculture in the nearest future can be expected.
5 CONCLUSIONS
Crops are extra sensitive against pathogens because they are weakened by poor nutrition and abiotic stress. Crops that can withstand a variety of diseases bring significant industry benefits; this requires an efficient genome editing method to efficiently and quickly modify target genes conferring superior resistance to crops. Therefore, in this work, we developed the CRISPR/Cas9 editing technology for barley, modifying the eIF4E gene that encodes a key host protein responsible for virus's multiplication. The modification of this gene was successful in the five barley cultivars that grow in Kazakhstan. We now have a functional protocol to transform/edit barley plants. Testing the edited plants for virus and micro-pathogen resistance is the next step. We will then see if editing eIF4E is a good strategy to fight against potyviruses or if another target should be tackled.
ACKNOWLEDGMENTS
This research has been funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan (Grant No. AP05132774, 2018-2020).
AUTHOR CONTRIBUTIONS
All authors designed the work, collected information from literature, and wrote the paper.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.




