De novo transcriptome sequencing, assembly, and gene expression profiling of a salt-stressed halophyte (Salsola drummondii) from a saline habitat
Funding information: Office of VC for Research and Graduate Studies at the University of Sharjah, Grant/Award Number: 1602145041-P
Abstract
Salsola drummondii is a perennial habitat-indifferent halophyte growing in saline and nonsaline habitats of the Arabian hyperarid deserts. It offers an invaluable opportunity to examine the molecular mechanisms of salt tolerance. The present study was conducted to elucidate these mechanisms through transcriptome profiling of seedlings grown from seeds collected in a saline habitat. The Illumina Hiseq 2500 platform was employed to sequence cDNA libraries prepared from shoots and roots of nonsaline-treated plants (controls) and plants treated with 1200 mM NaCl. Transcriptomic comparison between salt-treated and control samples resulted in 17,363 differentially expressed genes (DEGs), including 12,000 upregulated genes (7870 in roots, 4130 in shoots) and 5363 downregulated genes (4258 in roots and 1105 in shoots). The majority of identified DEGs are known to be involved in transcription regulation (79), signal transduction (82), defense metabolism (101), transportation (410), cell wall metabolism (27), regulatory processes (392), respiration (85), chaperoning (9), and ubiquitination (98) during salt tolerance. This study identified potential genes associated with the salt tolerance of S. drummondii and demonstrated that this tolerance may depend on the induction of certain genes in shoot and root tissues. These gene expressions were validated using reverse-transcription quantitative PCR, the results of which were consistent with transcriptomics results. To the best of our knowledge, this is the first study providing genetic information on salt tolerance mechanisms in S. drummondii.
1 INTRODUCTION
Agricultural land is continuously decreasing due to global climate change and adverse environmental processes, in addition to increasing occurrence of natural disasters and population pressure (Hasanuzzaman, Nahar, Alam, et al., 2013; Hasanuzzaman, Nahar, & Fujita, 2013). Soil salinization is a major limiting factor that causes significant losses in the productivity of cultivated arid and desert lands. Saline soils occur mainly in arid and semi-arid regions (FAO, I., 2015). Approximately 10% of the total arable land is currently affected by salinity and sodicity, with further increases due to global changes and human activities (Becerra et al., 2019; FAO, I., 2015; Ruiz-Lozano et al., 2012; Shahid et al., 2018). Halophytes can potentially be used for desalination and restoration of saline soils in a process known as phyto-desalination (Rabhi et al., 2009; Riadh et al., 2010; Saddhe et al., 2020). The succulent Salsola drummondii is one of the most Na+-hyperaccumulating plants with a Na+ concentrations in roots, stems, and leaves of 7.01, 24.7, and 98.4 mg/g dry weight, respectively, when grown in saline habitats. However, it did not affect the concentrations of other essential nutrients (Hussain et al., 2020).
Halophytes are characterized by their high salinity tolerance owing to effective physiological and metabolic processes and pathways supported by a distinctive network of genes and proteins (Kumari et al., 2015). Different mechanisms enable halophytes to prevent salt stress, such as salt exclusion, salt secretion, shedding of old leaves (including the salt content therein), stomatal responses, and succulence (Aslamsup et al., 2011; Munss & Tester, 2008). Moreover, several other physiological mechanisms enhance salt tolerance, such as ion homeostasis, salt accumulation, and compartmentalization (translocation of salt to specialized organs). The key to salt tolerance of halophytes is their ability to regulate Na+ and Cl− uptake while preserving the cytoplasmic K+ and Mg2+ levels required for the activation of essential enzymes (Bose et al., 2015; Gupta & Huang, 2014). The superior antioxidant capacity of halophytes enables them to tolerate the high oxidative stress caused by excessive reactive oxygen species (ROS) and high salt concentrations (Flowers & Colmer, 2008; Ozgur et al., 2013). Furthermore, plants in saline habitats produce several major osmolytes such as proline, glycine betaine, polyols, and sugars to adjust their osmotic potential and provide protection against ROS (Jithesh et al., 2006). A recent study showed a high activity of antioxidant enzymes that scavenge ROS in S. drummondii plants from saline habitats (Hussain et al., 2020). Identification of the molecular and biochemical mechanisms affecting the viability of halophytes is crucial for developing phyto-desalinators, thus imparting valuable knowledge which may help produce salt-tolerant crops.
Regulation of gene expression during salt stress involves different plant mechanisms, including upregulation or downregulation of specific gene products (i.e., RNA or proteins). Through transcriptomic analysis, a comprehensive knowledge of gene expression can be obtained to screen candidate genes involved in stress tolerance responses (Mosa et al., 2017). As such, several transcriptomics studies on halophytes under salt stress conditions provided insights into halophyte salt tolerance mechanisms at a molecular level, including differential expression of several genes associated with growth, cell wall and carbohydrate metabolism, transporters, the ROS-scavenging system, vacuolar compartmentalization, photosynthetic electron transport, carbon fixation, transcription factors, phosphoinositide, and hormone signaling (Dang et al., 2013; Diray-Arce et al., 2015; Gharat et al., 2016; J. Huang, Lu, et al., 2012; Jin et al., 2016; Ma et al., 2016; Wang et al., 2015; Yamanaka et al., 2009).
The United Arab Emirates (UAE) is one of the Arabian Gulf countries challenged by extreme environmental conditions such as hyperarid hot climates, high temperatures, and high salinity. Plants growing in this region may possess specific mechanisms and produce unique compounds that help tolerate such extreme conditions (Cybulska et al., 2014). S. drummondii Ulbr. is a leaf succulent, perennial habitat-indifferent halophyte. This evergreen shrub grows equally well in both nonsaline and saline soils of the UAE. Intensive plant surveys indicated that plants associated with S. drummondii on saline soils include euhalophytes such as Halocnemum strobilaceum (Pall.) Bieb., Halopeplis perfoliata (Forssk.) Bunge, and Aeluropus lagopoides (L.) Thwaites. However, plants associated with S. drummondii in nonsaline habitats were classified as glycophytes (salt-sensitive plants), including Indigofera oblongifolia Forssk., Pennisetum divisum (Gmel.) Henr., Cornulaca monacantha Delile, and Launaea capitata (Spreng.) Dandy (Elnaggar et al., 2020; El-Keblawy et al., 2019). Other habitat-indifferent halophytes such as Zygophyllum qatarense Hadidi and Suaeda vermiculata Forssk. ex. J.F. Gmel. were also co-occurring with S. drummondii in both saline and nonsaline habitats (Jongbloed et al., 2003). Moreover, S. drummondii may be commercially used; for example, leaves can be burned to produce soda ash, different parts of the plant have medicinal uses (Gilani et al., 2010), and its leaves have been utilized as animal feed (Qureshi et al., 1993). Furthermore, S. drummondii is an important ecological plant for restoring salt-affected or degraded habitats (Dagar & Minhas, 2016).
In a previous study, we demonstrated different biochemical and physiological adaptations of S. drummondii in response to different salinity levels (Elnaggar et al., 2020). Therefore, we hypothesized that S. drummondii plants have developed biochemical, physiological, and molecular adaptation mechanisms that enable them to thrive under higher salinity levels. The current study aimed to investigate the responses of S. drummondii to salinity at a molecular level using de novo transcriptome assembly. To gain a specific understanding of the involved tolerance mechanisms, we identified and investigated the expression of genes involved in salt stress regulation in S. drummondii using transcriptome analysis and reverse-transcription quantitative PCR (RT-qPCR). Elucidating the molecular basis of tolerance mechanisms facilitating the growth of habitat-indifferent halophytes under the hypersaline conditions of the hyper-arid climate of the UAE desert may assist in conserving this species in such harsh habitats. We assume that a habitat-indifferent halophyte may possess potential salinity tolerance gene(s), which may be up- or downregulated, depending on soil salinity. Such gene regulation likely enables habitat-indifferent halophytes to thrive in both saline and nonsaline habitats. To the best of our knowledge, this is the first study assessing gene regulation in response to salt stress in a plant that grows equally well in saline and nonsaline habitats (i.e., a habitat-indifferent halophyte). Understanding the molecular mechanisms of salinity tolerance in a habitat-indifferent halophyte may provide new insights into enhancing plant tolerance to salinity stress and developing salt-tolerant crops.
2 MATERIALS AND METHODS
Fruits of S. drummondii were collected from a population growing in a salt marsh in Kalba city, UAE, and the fruits' wings were removed. Seeds were sterilized and planted in plastic pots containing autoclaved sand. The pots were placed under 12:12 h light: dark cycles (at 30°C and 25°C, respectively) with light intensity of approximately 300 μmol m−2 S−1. The plants were irrigated with a 10% Hoagland No. 2 Basal Salt Mixture (Sigma-Aldrich) twice per week. Two-month-old seedlings were used for salinity treatments (0 and 1200 mM sodium chloride [NaCl]) and samples were harvested after 0, 6, 12, and 24 h. At each time point, three replicates of 10 seedlings were used. Treated seedlings were carefully removed from the soil to obtain the whole root system, and the plants were rinsed with distilled water at least three times to remove all sand granules. The harvested seedlings were separated into roots (whole root system) and shoots (all leaves and stem), and the samples were stored separately at −80°C until RNA isolation.
2.1 RNA isolation and cDNA library preparation
Total RNA of controls and NaCl-treated shoot and root samples (0 and 24 h, three replicates) was isolated using the RNeasy Plant Mini kit (Qiagen) following the manufacturer's instructions. To remove genomic DNA contamination, on-column DNase treatment was performed using the RNase-Free DNase Set (Qiagen) following the manufacturer's instructions. The quality of RNA samples was determined using a Bioanalyzer (Agilent Technologies). A standard TrueSeq RNA Library Prep Kit (Illumina) was used for library preparation. Paired-end sequencing (2 × 100 bp) was performed on a HiSeq 2500 platform at AgriGenome Labs Pvt, Ltd (India).
2.2 RNA sequencing data processing and de novo transcriptome assembly
Adaptor sequences were removed from all reads using Cutadapt software (v1.8.1) (Martin, 2011). Reads with average quality scores <20 in any of the merged paired-end reads were removed using Sickle software (v1.33) (Joshi & Fass, 2011). High-quality reads were aligned using Silva (high-quality ribosomal RNA databases) (Quast et al., 2012) with the program bowtie2 (v2.2.9) (Langmead et al., 2009). High-quality reads were used for the final de novo assembly using the Trinity assembler (version: v2.4.0) (Garber et al., 2011). Unigenes were identified using “cd-hit-est” (version 4.6), which removes redundant transcripts. The unigene expression levels were estimated using DESEQ2 (Love et al., 2014) and various other in-house pipeline tools.
2.3 Functional annotation
Fragments per kilobase per million mapped reads (FPKM) values of unigenes were calculated to identify salt response transcripts. De novo assemblies of transcriptomes were produced using high-quality sequence reads. The unigenes (at an FPKM value ≥1) were annotated, and a sequence-based homology search was performed using NCBI, Swiss-Prot, and various other major databases (Altschul et al., 1997) with the Blast2GO tool (Götz et al., 2008). Plant metabolic network analysis was carried out using BLAST, along with a PMN database search and annotations (Schläpfer et al., 2017).
2.4 Identification of differentially expressed genes (DEGs)
DESeq (version 1.16.0) was used to identify differentially expressed sequences from the de novo assembly (Anders & Huber, 2010). The false discovery rate (FDR) was used to identify significant DEGs (FDR <0.05). The log2(fold change) was calculated based on a cutoff value of 0.05. FPKM were calculated from the number of reads mapping to each specified gene sequence, considering gene length. The candidate DEG contigs were individually searched in plant genomic databases and in relevant publications for further insights into their functions and association with salinity stress.
2.5 RT-qPCR analysis
For RT-qPCR experiments, total RNA was isolated from 100 mg of control and salt-treated root and shoot tissues at different time points (0, 6, 12, and 24 h). Subsequently, total RNA was used to synthesize cDNA using the Superscript™ III First-Strand Synthesis kit (Invitrogen) according to the manufacturer's instructions. The RT-qPCR reaction was performed using the SensiFAST™ SYBR® No-ROX Kit (Bioline), with 200 ng cDNA and 10 pmol of each forward and reverse primer in a final reaction volume of 20 μl using a CFX96TM multiplex PCR device (Bio-Rad) with the following settings: 95°C for 2 min and 40 cycles of 95°C for 5 s, 56°C for 10 s, and 72°C for 20 s. A melting curve was produced immediately after the RT-qPCR reaction from 65 to 95°C in increments of 0.5°C to confirm the absence of DNA contamination, primer dimers, and secondary products. Three biological replicates of each treatment time point were tested for some of the candidate transcripts (Table S1), and ELONGATION FACTOR 1 ALPHA was used as a housekeeping gene. The salt-response transcripts were identified based on our differentially expressed transcripts compared with the available literature and sequences retrieved from raw files. PCR primers were designed using the Primer 3 tool (NCBI) (Table S1). The 2−ΔΔCt method (Livak & Schmittgen, 2001) was used to calculate the relative gene expression levels of the candidate genes. The calculated relative gene expression was converted to log2(fold-change). The values of the estimated log2(fold change) were expressed as mean values of three measurements.
3 RESULTS
3.1 RNA sequencing and de novo transcriptome assembly
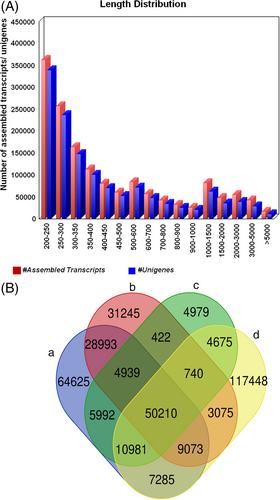
RNA sequencing and bioinformatics analysis resulted in 69,000,000–137,000,000 sequence reads (Table 1), with an average base quality >Q30 (error probability ≥0.001) in 92.25% of the base calls. The numbers of assembled reads before and after cd-hit-est are summarized in Table S2. The length distributions of transcripts and unigenes of the de novo assembly are shown in Figure 1A. The guanine–cytosine (GC) summary of the number of unigenes and assembled transcripts in S. drummondii is shown in Figure S1. The de novo assembly was produced using 1,509,047 transcripts from all data files, and their mean GC content was 43.05% (Table S2). The numbers of transcripts shared between treatments and tissues (control and treated roots and shoots) of S. drummondii are illustrated in a comprehensive Venn diagram (Figure 1B).
| Sample name | Raw reads | Clean reads | GC_perc (%) of clean reads | Base quality score Q_30 of clean reads |
|---|---|---|---|---|
| Control-Shoot 1 | 112,052,706 | 108,729,456 | 41.925 | 96.435 |
| Control-Shoot 2 | 89,803,448 | 87,931,968 | 41.815 | 96.01 |
| Control-Shoot 3 | 110,644,316 | 107,521,028 | 41.935 | 95.705 |
| Control-Root 1 | 95,624,532 | 92,562,572 | 45.5 | 96.545 |
| Control-Root 2 | 79,500,996 | 77,893,790 | 44.855 | 96.585 |
| Control-Root 3 | 88,264,288 | 85,406,850 | 46.5 | 95.36 |
| Treated-Shoot 1 | 69,646,476 | 67,761,214 | 42.145 | 95.8 |
| Treated-Shoot 2 | 128,798,320 | 124,097,624 | 42.025 | 95.68 |
| Treated-Shoot 3 | 92,992,834 | 90,388,068 | 41.845 | 94.48 |
| Treated-Root 1 | 111,473,604 | 97,194,942 | 47.3 | 95.895 |
| Treated-Root 2 | 82,551,406 | 79,531,820 | 45.345 | 94.755 |
| Treated-Root 3 | 137,220,060 | 76,698,880 | 51.065 | 94.69 |
- Note: control = (0 mM NaCl); treated = (1200 mM NaCl).
3.2 Annotation of unigenes and their functional categorization
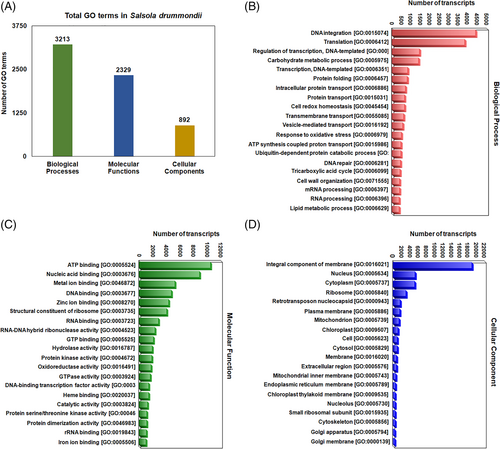
All identified unigenes were searched against nucleotide and protein databases with an e-value cutoff of 10−5 for gene ontology (GO) classification. Of the 272,643 unigenes, 125,918 produced top hits in the BLASTX (nucleotide) sequence database. Based on GO classification with sequences of different species, the majority of 16,907 hits (6.2%) matched Theobroma cacao (cacao), followed by Eucalyptus grandis (flooded gum) with 10,116 hits (3.7%) (Figure S2). GO analysis was performed for all identified unique transcripts to characterize the transcriptome data, in which 6434 unigenes were assigned to different GO terms, including 3213 in the biological processes category, 2329 in the molecular functions category, and 892 in the cellular components category (Figure 2A). GO analysis of the de novo assembly transcripts of S. drummondii revealed the top 20 hits of GO terms among biological processes (Figure 2B), molecular functions (Figure 2C), and cellular components (Figure 2D).
3.3 Identification of salt-responsive transcripts
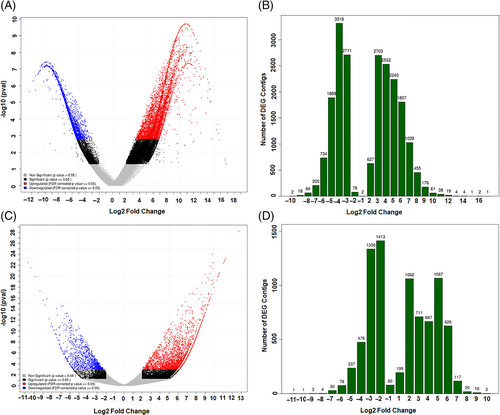
FPKM values of unigenes were calculated to identify salt-responsive transcripts. Comparison of the FPKM expression values between controls and salt-treated plants showed that the expression levels of 17,361 (6.36%) unigenes were altered significantly (>2-fold, FDR <0.05) under salt stress. Salt-responsive genes were identified based on their expression levels using the transcriptome assembly. Transcriptomic comparison between salt-treated and control samples resulted in 12,000 upregulated transcripts (7870 in roots and 4130 in shoots) and 5363 downregulated transcripts (4258 in roots and 1105 in shoots). The numbers of DEGs obtained in roots and shoots under salt treatment are shown in Table 2. In roots of S. drummondii, the total numbers of DEG contigs (each contig is a contiguous length of genomic sequence in which the order of bases is known) obtained are shown as a volcano plot change in Figure 3A and as a histogram depicting the log2(fold change) with annotation (Figure 3B). Similarly, regarding S. drummondii shoots, a volcano plot was produced to visualize the total number of DEG contigs (Figure 3C), and a histogram shows the log2(fold change) obtained through annotation (Figure 3D).
| Plant organ | DEG contigs with adj P value ≤0.05 | DEGs with adj P value ≤0.05 | DEG contigs with P value ≤0.05 | DEGs with P value ≤0.05 | ||||
|---|---|---|---|---|---|---|---|---|
| Down | Up | Down | Up | Down | Up | Down | Up | |
| Root | 4258 | 7870 | 3327 | 6134 | 28,735 | 33,745 | 22,138 | 25,437 |
| Shoot | 1105 | 4130 | 736 | 2944 | 4931 | 15,062 | 3304 | 10,632 |
- Note: Down, downregulated; Up, upregulated.
3.4 Differentially regulated transcripts in S. drummondii under salt stress
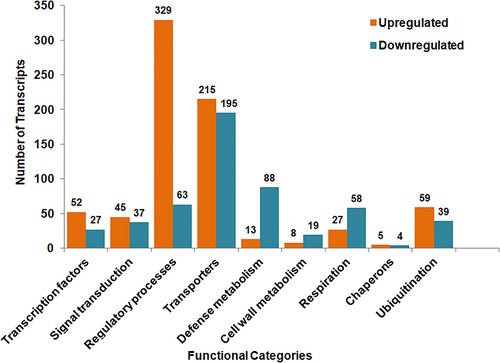
The majority of identified DEGs were involved in transcription regulation (79), signal transduction (82), defense metabolism (101), transportation (410), cell wall metabolism (27), regulatory processes (392), respiration (85), chaperoning (9), and ubiquitination (98) during salt stress (Figure 4).
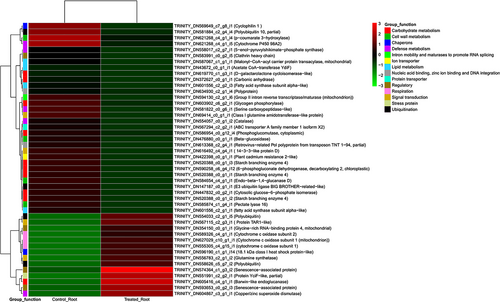
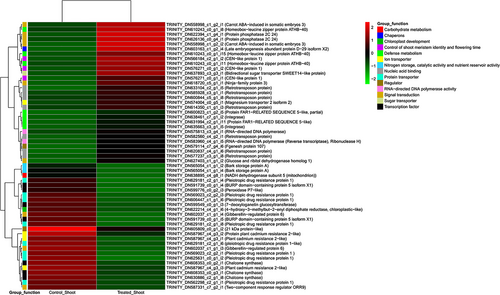
Heatmaps of the top 100 annotated DEGs, which were downregulated or upregulated in S. drummondii roots during salt stress, are shown in Figure S3A,B and those of shoots are shown in Figure S4A,B. Downregulated and upregulated transcripts in treated versus control roots of S. drummondii that were chosen from the top 100 downregulated and top 100 upregulated annotated transcripts are shown in Figure 5 and listed in Table S3. Similarly, downregulated and upregulated transcripts in treated versus control shoots of S. drummondii that were chosen from the top 100 downregulated and top 100 upregulated annotated transcripts are shown in Figure 6 and listed in Table S4.
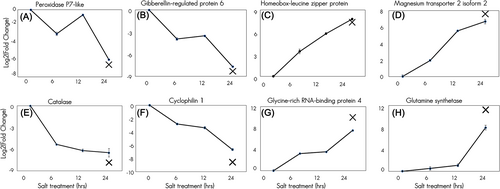
3.5 Validation of DEGs in S. drummondii under salt stress by RT-qPCR
To validate the de novo transcriptome results, RT-qPCR analysis was performed using specific primers for randomly selected DEG candidates. The RT-qPCR results were highly congruent with de novo transcriptome data. The same pattern of differential expression for the shoot and root transcripts was confirmed by RT-qPCR analysis, which revealed comparable levels of up- or downregulation. In the shoots of S. drummondii, PEROXIDASE P7-LIKE and GIBBERELLIN-REGULATED PROTEIN 6 were downregulated (Figure 7A,B). In contrast, the A. thaliana HOMEOBOX-LEUCINE ZIPPER PROTEIN HOMEOBOX 40 (ATHB40) and MAGNESIUM TRANSPORTER 2 (MGT2) were upregulated (Figure 7C,D) in the 1200 mM NaCl treatment, compared to the controls, according to both RT-qPCR and de novo transcriptome analysis. In S. drummondii roots, CATALASE and CYCLOPHILIN 1 were downregulated (Figure 6E,F), whereas GLYCINE-RICH RNA-BINDING PROTEIN 4 and GLUTAMINE SYNTHETASE were upregulated (Figure 6G,H) in the 1200 mM NaCl treatment, compared to the controls, as evidenced by the RT-qPCR and de novo transcriptome results.
4 DISCUSSION
4.1 Identification of DEGs under salt stress
S. drummondii is an excellent model for studying salt stress tolerance mechanisms and identifying potential salinity tolerance-related genes. Transcriptome analysis regarding salt tolerance in S. drummondii identified 17,363 DEGs. Candidate genes among DEGs were assembled into nine functional categories, including transcription factors, signal transduction, defense metabolism, transport, cell wall metabolism, regulatory processes, respiration, chaperoning, and ubiquitination. These candidate genes may have specific biological functions associated with the salt tolerance mechanisms of S. drummondii. The putative functional roles of exemplary candidate genes are discussed below and are summarized in Table 3.
| Transcript ID | Log2(Fold change) | P-value | Gene description | Function | Expression in S. drummondii—salt stress | Expression in other plant species | Stress | References | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | TRINITY_DN610243_c0_g1_i11 andTRINITY_DN610243_c0_g1_i15 |
7.332514952 and 7.259729423 |
1.79E−17 and 6.65E−12 |
Homeobox-leucine zipper protein (ATHB-40) | Transcription factor | ↑Shoots | ↑Prunus persica roots | Drought | (Ksouri, 2016) |
| 2 | TRINITY_DN631994_c2_g1_i11 | 6.949479476 | 8.99E−06 |
FAR1-RELATED SEQUENCE 5-like | Transcription factor | ↑Shoots | ↑Sweet sorghum inbred line root | Salt |
(Yang et al., 2018) |
| 3 | TRINITY_DN558998_c1_g2_i2 | 8.614282772 | 5.83E−15 |
Carrot ABA-induced in somatic embryos 3 | ABA signal transduction | ↑Shoots | ↑Induced in ABA-treated carrot somatic embryos (Daucus carota L.) somatic | Desiccation | (Shiota et al., 2004) |
| 4 | TRINITY_DN622394_c1_g4_i1 andTRINITY_DN626136_c0_g4_i1 | 7.883703289 and 7.770725338 −7.534436 |
1.38E−12 and 2.11E−17 |
Protein phosphatase 2C 24 (PP2Cs) | ABA signal transduction | ↑Shoots | ↑Suaeda fruticosa shoots and roots | Salt | (Diray-Arce et al., 2015) |
| ↑Suaeda glauca shoots | Salt | (Jin et al., 2016) | |||||||
| 5 | TRINITY_DN616492_c4_g4_i1 | 2.18E−06 |
14-3-3-like protein D | ABA signal transduction | ↓Roots | ↑Mangrove leaves |
Salt |
(Wang et al., 2014) |
|
| ↑Brachypodium distachyon leaves | Salt | (Lv et al., 2014) | |||||||
| ↓Brachypodium distachyon roots | H2O2 | (Bian et al., 2015) | |||||||
↓Brachypodium distachyon (seedling roots) |
Drought | (Chen et al., 2018) | |||||||
| 6 | TRINITY_DN602037_c1_g1_i4 and TRINITY_DN602037_c1_g1_i3 | −8.314012419 and −7.474357045 | 6.14E−13 and 7.93E−15 |
Gibberellin-regulated protein 6 | Signal transduction | ↓Shoots | ↓Solanum tuberosum L. plants at tuber bulking stage |
Drought |
(Gong et al., 2015) |
| ↓Hibiscus cannabinus L. seedlings | Salt | (Li et al., 2017) |
|||||||
| ↑Mesembryanthemum crystallinum epidermal bladder cell | Salt | (Oh et al., 2015) |
|||||||
| 7 | TRINITY_DN574004_c0_g5_i1 | 7.607620942 | 3.15E−13 |
Magnesium transporter 2 isoform 2 | Ion transporter | ↑Shoots | ↑Suaeda asparagoides leaves |
Salt |
(Ayarpadikannan et al., 2012) |
| ↓Suaeda fruticosa shoots and roots | Salt | (Diray-Arce et al., 2015) | |||||||
| 8 | TRINITY_ DN587967_ c4_ g3_ i3, TRINITY_DN587967_c4_g3_i2, TRINITY_DN587967_c4_g3_i1, and TRINITY_DN422398_c0_g1_i1 | −5.630730199, −6.068822492, −6.56033105, and −7.402281 | 2.33E−06, 7.88E−09, 6.57E−07, and, 3.31E−06 |
Plant cadmium resistance 2-like | Ion transporter | ↓Shoots ↓Roots | ↑Suaeda fruticosa shoots and roots | Salt | (Diray-Arce et al., 2015) |
| 9 | TRINITY_DN567294_c2_g2_i1 |
−8.623404 | 1.79E−07 |
ABC transporter A family member 1 isoform X2 | ABC transporter | ↓Roots | ↑Salicornia brachiata shoots | Salt and elevated CO2 | (Benjamin et al., 2018) |
| 10 | TRINITY_DN629181_c2_g1_i6, TRINITY_DN625631_c0_g1_i2, TRINITY_DN629181_c2_g1_i8, TRINITY_DN606447_c1_g1_i6, TRINITY_DN569023_c2_g2_i3, TRINITY_DN629181_c2_g1_i4, and TRINITY_DN562298_c2_g1_i1 | −7.135629422, −7.037158789, −6.756601378, −6.400322909, −6.139944146, −5.852165224, and −5.707783672 | 4.83E−09, 1.6E−07, 1.46E−06, 8.87E−12, 3.53E−05, 0.000421, and 1.11E−08 |
Pleiotropic drug resistance 1 | ABC transporter | ↓Shoots | ↑Salt-tolerant rice cultivar FL478 roots |
Salt |
(Senadheera et al., 2009) |
| ↑Hordeum vulgare L., leaves | Salt | (Ueda et al., 2006) | |||||||
| 11 | TRINITY_DN637893_c3_g3_i1 | 7.56815089 | 6.26E−13 |
Bidirectional sugar transporter SWEET14-like | Sugar transporter | ↑Shoot | ↑Suaeda fruticosa shoots and roots | Salt | (Diray-Arce et al., 2015) |
| 12 | TRINITY_DN604867_c3_g1_i1 | 10.92713864 | 3.27E−10 |
Copper/zinc superoxide dismutase (SOD) | Plant defense metabolism | ↑Root | ↑Suaeda maritima seedling | Salt | (Mallik et al., 2011) |
| 13 | TRINITY_DN554057_c0_g1_i2 |
−7.838221 | 8.19E−07 |
Catalase (CAT) | Plant defense metabolism | ↓Root | ↑Cotton roots and leaves | Salt | (Zhang et al., 2016) |
| 14 | TRINITY_DN599776_c0_g2_i3 | −6.533006633 | 7.3E−06 |
Peroxidase P7-like | Plant defense metabolism | ↓Shoot | ↓Peach roots |
Drought |
(Ksouri, 2016) |
| ↑Nictotiana tabacum ecotype K326 seedlings | Alkali and salt stress | (Xu et al., 2019) | |||||||
| 15 | TRINITY_DN630886_c2_g1_i8 and TRINITY_DN608353_c0_g2_i1 | −6.496620982 and −6.860032509 | 8.88E−09 and 3.06E−12 |
Chalcone synthase (CHS) | Plant defense metabolism (flavonoid pathway) | ↓Shoot | ↑Atriplex nummularia Lind. leaves and Atriplex leucoclada Boiss. leaves ↓Soyabean roots and hypocotyls ↓Salix matsudana Koidz roots |
Salt Saline-flooding combined stress Salt |
(Sayed-Hussin, 2007) (Alam et al., 2011) (Qiao et al., 2014) |
| 16 | TRINITY_DN591739_c0_g1_i5 and TRINITY_DN591739_c0_g1_i4 | −7.17077495 and −6.520834472 | 5.99E−10 and 8.79E−06 |
BURP domain-containing protein 5 isoform X1 | Plant defense metabolism | ↓Shoot | ↓Bruguiera gymnorhiza leaves | Salt | (Miyama & Hanagata, 2007) |
| 17 | TRINITY_DN354150_c0_g1_i1 | 10.0007162 | 3.92E−10 |
Glycine-rich RNA-binding protein 4, mitochondrial | Regulatory process | ↑Root | ↑Arabidopsis salt tolerant lines seedlings expressing GRP from Sporobolus virginicus |
Salt | (Tada et al., 2019) |
| 18 | TRINITY_DN583991_c0_g2_i5 | −7.65339 | 1.57E−06 |
Clathrin heavy chain | Regulatory process | ↓Root | ↓Thellungiella halophila seedlings | Salt | (Pang et al., 2010) |
| 19 | TRINITY_DN593653_c0_g2_i3 and TRINITY_DN574364_c1_g3_i2 | 13.17583408 and 11.29722955 | 5.38E−06 and 2.04E−06 |
Senescence associated protein | Regulatory process | ↑Root | ↑Arabidopsis thaliana seedling |
Salt |
(Gong et al., 2005) |
| ↓Mentzelia filifolia leaves | Drought | (Devitt, 2018) |
|||||||
| ↓Suaeda fruticosa shoots and roots | Salt | (Diray-Arce et al., 2015) | |||||||
| 20 | TRINITY_DN621268_c4_g1_i4 | −9.329824 | 1.88E−06 |
p-coumarate 3-hydroxylase | Lignin biosynthesis | ↓Root | Zea mays ↓leaves and ↑roots |
Drought |
(Ludidi & Kolo, 2015) |
| 21 | TRINITY_DN476880_c0_g1_i1 | −8.020764 | 5.57E−07 |
Beta-glucosidase | Cellulose and hemicellulose degradation | ↓Root | ↑Thellungiella halophila seedlings |
Salt |
(Taji et al., 2004, Wong et al., 2006) |
| 22 | TRINITY_DN584654_c4_g1_i1 | −8.12868 | 4.37E−07 |
Probable endo-beta-1,4-glucanase D | Cellulose and hemicellulose degradation | ↓Root | ↑Sorghum bicolor leaves | Salt | (Swami et al., 2011) |
| 23 | TRINITY_DN627403_c1_g1_i2 | 7.48193581 | 1.72E−08 |
Glucose and ribitol dehydrogenase homolog 1 | Carbohydrate metabolism and signaling | ↑Shoot | ↑The mature grain of salinity tolerant barley lines (REC, OWB34, OWB59) | Salt | (Witzel et al., 2010) |
| 24 | TRINITY_DN556783_c2_g1_i2 | 11.24672453 | 2.28E−10 |
Glutamine synthetase | Nitrogen assimilation and signaling | ↑Root | ↑Triticale seedlings | Salt | (Kwinta & Cal, 2005) |
| 25 | TRINITY_DN555305_c4_g15_i1 and TRINITY_DN627029_c10_g1_i1 | 10.34676503 and 10.04959256 | 2.51E−10 and 3.63E−10 |
Cytochrome c oxidase subunit 1 and cytochrome c oxidase subunit 2 | Cellular respiration (electron transport chain) | ↑Root | ↑Triticum aestivum L.) seedling |
Salt |
(Singh et al., 2017) |
| ↑Oryza sativa root | Salt | (Yan et al., 2005) | |||||||
| ↑Tamarix hispida roots | Salt | (Li et al., 2009) | |||||||
| 26 | TRINITY_DN611184_c3_g1_i7 | −8.127123 |
4.8E−07 |
Cytochrome c oxidase subunit 1 | Cellular respiration (electron transport chain) | ↓Root | ↓Medicago truncatula Gaertn. shoots and roots | Drought | (Trindade et al., 2010) |
| 27 | TRINITY_DN603163_c1_g2_i7 and TRINITY_DN603163_c1_g2_i4 | 9.821013361 and 9.815318338 | 3.53E−23 and 1.01E−24 |
Late embryogenesis abundant protein D-29 isoform X2 | Chaperons | ↑Shoot | ↑Suaeda fruticosa shoots and roots | Salt | (Diray-Arce et al., 2015) |
| 28 | TRINITY_DN596190_c1_g1_i14 | 10.32927904 | 2.55E−10 |
18.1 kDa Class 1 heat shock protein-like | Chaperons | ↑Root | ↑Suaeda maritima leaves | Salt | (Sahu and Shaw (2009) |
| 29 | TRINITY_DN607636_c0_g5_i1 | −8.328508 | 3.15E−07 |
Hsp24 | Chaperons | ↓Root | ↓Suaeda fruticose shoots and roots | Salt | (Diray-Arce et al., 2015) |
| 30 | TRINITY_DN558626_c5_g7_i2 and TRINITY_DN554033_c2_g1_i5 | 10.24505127 and 9.858374003 |
2.79E−10 and 5.07E−10 |
Polyubiquitin | Protein degradation | ↑Root | ↑Tamarix hispida roots | Salt | (Li et al., 2009) |
- Note: ↑, upregulated; ↓, downregulated.
4.2 Signal transduction
Signal transduction plays an important role in plant tolerance to abiotic stressors such as salt stress (G.T. Huang, Ma, et al., 2012; Seyfferth & Tsuda, 2014). In the current study, genes involved in signal transduction pathways were differentially expressed under salt stress.
In S. drummondii, CARROT ABA-INDUCED IN SOMATIC EMBRYO 3 (CAISE3) (TRINITY_DN558998_c1_g2_i2) was upregulated in shoots treated with 1200 mM NaCl. CAISE3 expression level is increased under abscisic acid (ABA) treatment and may be involved in desiccation tolerance, as observed in carrot somatic embryos (Shiota et al., 2004). Upregulation of CAISE3 may result from ABA production induced by salt stress in S. drummondii, where the ABA signal transduction pathway contributes to salt tolerance. The present study also highlighted that the gene encoding protein phosphatase 2C 24 (PP2Cs): TRINITY_DN558998_c1_g2_i2 was upregulated in response to salinity in S. drummondii shoots. Plant PP2Cs form a unique class of enzymes, some members of which counteract the mitogen-activated protein kinase pathways, whereas others act as co-receptors for ABA as a phytohormone. Therefore, plant PP2Cs function as regulators of several signal transduction pathways (Fuchs et al., 2013; Rodriguez, 1998; Wang et al., 2007). PP2Cs are also involved in ABA signal transduction and play a notable role in stress signaling (Meyer et al., 1994). Similar results have been reported regarding the upregulation of PP2C under salt stress in other halophytic plants, such as Suaeda fruticosa (Diray-Arce et al., 2015) and Suaeda glauca (Jin et al., 2016). Our transcriptomic results also revealed that salt stress induced ABA signaling similarly to what was previously demonstrated in the two halophytes S. glacua (Jin et al., 2016) and S. fruticosa (Diray-Arce et al., 2015).
Transcriptomic analysis of S. drummondii roots under salt stress showed downregulation of 14-3-3-like protein D (TRINITY_DN616492_c4_g4_i1). A previous study suggested that 14–3-3 proteins regulate plant stress responses (Denison et al., 2011). The 14-3-3-like protein is also increased under salt stress in leaves of a mangrove (Kandelia candel) (Wang et al., 2014) and Brachypodium distachyon seedling leaves (Lv et al., 2014), but is decreased under hydrogen peroxide and osmotic stress in roots of Brachypodium distachyon seedlings (Bian et al., 2015; Chen et al., 2018). ABA affects the expression and protein levels of 14-3-3 isoforms in plants (Schoonheim et al., 2007; Wasilewska et al., 2008), and plants with downregulated 14-3-3 levels show altered expression of ABA-regulated genes (Schoonheim et al., 2007). One effect of ABA signaling is stomatal closure to reduce water loss, which includes a reduction in H+-ATPase (a 14-3-3 client) activity (Merlot et al., 2007). Downregulation of the H+-ATPase pump during salt and drought stress is crucial for membrane depolarization to initiate stomatal closure (Merlot et al., 2007). This process may also be a possible explanation for the downregulation of the 14-3-3 transcript in S. drummondii under salt stress.
The gibberellin-regulated family proteins are associated with plant growth and development. In the present study, salt stress decreased the expression levels of gibberellin-regulated protein 6 (TRINITY_DN602037_c1_g1_i4 and TRINITY_DN602037_c1_g1_i3), a factor involved in gibberellin biosynthesis in plants grown under salt stress (Jithesh et al., 2019). Gibberellin production during abiotic stress also modulates the biosynthesis of antioxidant enzymes and sugar signaling (Fahad et al., 2015). Furthermore, gibberellic acid (GA) signaling regulates stomatal closure (a typical ABA signaling response) (Du et al., 2015), and GA application induces a transient opening of the stomata (Göring et al., 1990). Similar trends have been reported in potatoes in response to drought (Gong et al., 2015) and in kenaf (Hibiscus cannabinus L.) in response to salt stress (Li et al., 2017), where the gibberellin-regulated protein 6 transcript was downregulated. In contrast, transcriptomic analysis of epidermal bladder cells of the halophyte Mesembryanthemum crystallinum exhibited upregulation of the gibberellin-regulated family in response to salt stress (Oh et al., 2015).
TRINITY_DN627403_c1_g1_i2: glucose and ribitol dehydrogenase homolog 1 were upregulated in shoots of S. drummondii under salt stress. Glucose and ribitol dehydrogenase homolog 1 (Glc/RibDH) may function as a short alcohol-polyol-sugar dehydrogenase, probably with carbohydrate metabolism and drought tolerance. Glc/RibDH may also be involved in signal transduction and oxidoreductase activity (Alexander et al., 1994; Kawahara et al., 2013; Shiota et al., 2004). Similar to our results, it has been reported that glucose/ribitol dehydrogenase (Glc/RibDH) is upregulated in mature grains of salt-tolerant barley lines, compared to salt-sensitive lines, under salt stress (Witzel et al., 2010). The protein levels of Glc/RibDH, however, produced contradictory accumulation results in alfalfa (Medicago sativa L. cv. Gabe's) when untreated controls were compared with osmo-primed seeds under salt stress (Yacoubi et al., 2013).
Transcript TRINITY_DN556783_c2_g1_i2, which corresponds to glutamine synthetase (GS), was upregulated in root tissues of S. drummondii seedlings treated with 1200 mM NaCl. Similar results were reported in triticale (Triticosecale Wittm. ex A. Camus.) seedlings, as GS activity was induced by treatment with 100 mM NaCl-enriched medium for 7 days (Kwinta & Cal, 2005). GS is a key enzyme in the assimilation of ammonia into amino acids, where it catalyzes the reaction of ammonium and glutamate to produce glutamine (Bao et al., 2015; Bernard & Habash, 2009).
4.3 Regulatory processes
TRINITY_DN354150_c0_g1_i1 encodes glycine-rich RNA-binding protein (GRP) 4, mitochondrial GRPs were upregulated in S. drummondii roots under salinity treatment. GRPs play a role in the post-transcriptional regulation of genes and are involved in responses to environmental stresses such as salt stress (Kim et al., 2007; Wang et al., 2012). GRPs have been reported among salt tolerance genes in the halophyte Sporobolus virginicus, and they are upregulated in salt-tolerant Arabidopsis thaliana lines expressing GRP from S. virginicus (SvGRP1) in comparison to wild-type controls under salt stress (Tada et al., 2019). In particular, GRP7 is involved in abiotic stress responses by regulating stomatal opening and closure in A. thaliana (Kim et al., 2008). Cao et al. (2006) reported that GRP7 regulates stress responses and ABA signaling in Arabidopsis. Thus, GRPs seem to play a major role in S. drummondii tolerance, possibly by regulating stomatal opening and closure under salinity stress.
The transcript TRINITY_DN583991_c0_g2_i5, which encodes a clathrin heavy chain (CHC), was downregulated in roots of S. drummondii under salt stress. CHCs are a component of clathrin, which is a constitutive element of vesicles of the endomembrane trafficking system (Brodsky et al., 2001; Fotin et al., 2004). Hence, they have a pleiotropic function, including stomatal regulation, coordinate endo-and exocytic traffic gas exchange, vegetative growth, and PIN auxin transporters in Arabidopsis (Kitakura et al., 2011; Larson et al., 2017). CHC2-dependent endocytosis influences the incorporation of activated receptors and is necessary for protection mediated by pattern recognition receptor kinases (Mbengue et al., 2016). In line with our results, CHC is downregulated in the halophyte Thellungiella halophila under salt stress (Pang et al., 2010).
Our analysis revealed that transcripts of senescence-associated proteins (TRINITY_DN593653_c0_g2_i3, TRINITY_DN574364_c1_g3_i2) were upregulated in root tissues. These proteins are also involved in regulatory processes and cellular signal transduction (Gupta & Huang, 2014). During drought stress, senescence-associated proteins are downregulated in leaf tissue of Mentzelia filifolia (Devitt, 2018), and they are also downregulated in the halophyte S. fruticosa under salt stress (Diray-Arce et al., 2015). However, senescence-associated SAG13, which is upregulated in Arabidopsis, is mostly unaffected in Thellungiella halophila under salinity stress (Gong et al., 2005). In salt-stressed Arabidopsis, SAG29 regulates cell viability and cell plasma membrane-localized MtN3 protein (Seo et al., 2011). SAG29 is primarily expressed in senescent plant tissues, and this gene is induced during osmotic stress through an ABA-dependent pathway (Seo et al., 2011). The upregulation of senescence-associated genes indicated that the ABA pathway may regulate salt tolerance mechanisms in S. drummondii.
4.4 Transporters
4.4.1 Ion homeostasis and ion transporters
MGT2 isoform 2 (TRINITY_DN574004_c0_g5_i1) was upregulated in S. drummondii shoots in saline conditions. MGT2 isoform 2 is a magnesium ion transmembrane transporter that can transport other divalent cations. Magnesium transporters play a vital role in salt tolerance in plants (Chen et al., 2017), and this gene has been identified as a salt-responsive gene in halophyte Suaeda asparagoides (Ayarpadikannan et al., 2012). Magnesium transporter-like proteins involved in ionic and osmotic homeostasis are upregulated by salt stress in Populus euphratica (Gu et al., 2004). In contrast, the magnesium transporter NIPA2 is downregulated in the halophyte S. fruticosa during salt stress (Diray-Arce et al., 2015). The observed upregulation of MGT2 isoform 2 in S. drummondii indicates its importance regarding salt tolerance.
Other transcripts related to ion homeostasis were downregulated in salt-treated S. drummondii shoots (TRINITY_DN587967_c4_g3_i3, TRINITY_DN587967_c4_g3_i2, and TRINITY_DN587967_c4_g3_i1) or downregulated in roots (TRINITY_DN422398_c0_g1_i1). One of them (TRINITY_DN587967_c4_g3_i2) corresponds to protein plant cadmium resistance 2-like. Reduced cadmium accumulation has been reported to counteract pathogens and to occur in response to oxidative stress. Similarly, protein plant cadmium resistance 2-like is upregulated in S. fruticosa in response to salinity stress (Diray-Arce et al., 2015).
4.4.2 Protein transporters
The transcript TRINITY_DN567294_c2_g2_i1 (ABC transporter A family member 1 isoform X2) was downregulated in S. drummondii roots under salt stress. Members of the ABC family are multifunctional; they are involved in detoxification processes, plant growth, plant development, plant nutrition, chlorophyll biosynthesis, stomatal movement, and interactions between plants and their environments and responses to biotic and abiotic stressors (Kang et al., 2011; Martinoia et al., 2002). ABC transporter A family member 2-like is involved in N transport in tea plants (Yang et al., 2018). Furthermore, ABC transporters are responsive to salt stress (Alvarado et al., 2004). In addition, ABC transporters improve drought and salt stress tolerance in Arabidopsis (Kim et al., 2010). In contrast to our results, the ABC transporter is upregulated in the halophyte Salicornia brachiata under salt and elevated CO2 conditions (Benjamin et al., 2018).
Our results showed downregulation of the ABC transporter pleiotropic drug resistance PDR1, (TRINITY_DN629181_c2_g1_i4, i6, and i8) in shoots, which is a general defense protein. An opposite result was reported regarding roots of the salt-tolerant rice cultivar FL478 in response to salt stress (Senadheera et al., 2009). PDR5-like ABC was also upregulated in barley leaves during the first 24 h of salt stress (Ueda et al., 2006). As such, the ABC transporter may not play a vital role regarding the salt tolerance mechanisms of S. drummondii.
4.4.3 Sugar transporters
TRINITY_DN637893_c3_g3_i1 corresponding to the bidirectional sugar transporter SWEET14-like protein was upregulated in shoots of S. drummondii. SWEET genes are involved in sugar diffusion across cell membranes and act as key players in sucrose phloem transport (Daba et al., 2019). SWEET family genes exert important functions in response to various environmental stressors (Zhang et al., 2020). SWEET14 is needed for the optimal development of seedlings, seeds, and anthers and for regulating the GA response (Kanno et al., 2016). The bidirectional sugar transporter SWEET3, which is involved in low-affinity influx and efflux of sugars across the cell plasma membrane, is upregulated under salt stress in the halophyte S. fruticosa (Diray-Arce et al., 2015). The bidirectional sugar transporters SWEET12 and SWEET1 are also upregulated, whereas SWEET6 and SWEET4 are downregulated in Cenostigma pyramidale roots after 11 days of salt stress (Frosi et al., 2021).
4.5 Plant defense metabolism
Salt stress increases the production of ROS, which causes oxidative stress in plant cells. Plants counteract oxidative stress by producing enzymatic and nonenzymatic antioxidants to scavenge ROS and maintain redox homeostasis. Antioxidant enzymes include superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX), catalase (CAT), and glutathione reductase (GR). In the present study, the transcript TRINITY_DN604867_c3_g1_i1, which encodes a copper/zinc SOD, was upregulated in roots of salt-stressed S. drummondii. Therefore, SOD may play a critical role in ROS scavenging under salinity stress in S. drummondii as the first line of defense against oxidative stress by catalyzing −O2 molecules to form H2O2 (Alscher et al., 2002). Similar results have been reported previously, with increased Cu/Zn-SOD in seedlings of the halophyte Suaeda maritima under salt stress (Mallik et al., 2011).
In salt-treated S. drummondii, the transcript TRINITY_DN554057_c0_g1_i2, which is a homolog of catalase (CAT), was downregulated in roots, whereas the transcript TRINITY_DN599776_c0_g2_i3, which is a homolog of peroxidase P7-like, was downregulated in shoots. CAT catalyzes the breakdown of H2O2 into water and oxygen (Willekens et al., 1997), and it is upregulated in cotton roots and leaves under salt stress (Zhang et al., 2016). Peroxidase P7-like is a key enzyme that controls plant growth and development and is downregulated in roots of peach plants experiencing drought stress (Ksouri, 2016). In contrast, it has been shown that peroxidase P7-like is upregulated under alkali and salt stress in tobacco, reflecting its involvement in the tolerance process (Xu et al., 2019). Based on our results, we hypothesize that ROS production due to high salt concentrations may be mainly regulated by SOD.
Flavonoids are involved in the defense against many stressors, including salt stress, wounding, and UV-B (Mechri et al., 2015; Murai et al., 2015). In the present study, TRINITY_DN630886_c2_g1_i8 and TRINITY_DN608353_c0_g2_i1, which correspond to chalcone synthase (CHS), were downregulated in shoots of salt-stressed S. drummondii. CHS was suggested as a salt-responsive candidate gene in the halophytes Atriplex nummularia and Atriplex leucoclada (Sayed-Hussin, 2007), and it is the first key enzyme in the flavonoid/isoflavonoid biosynthesis pathway. In the present study, the expression level of the CHS gene was downregulated in shoots under salt stress, which suggests that salt stress affects the expression of the chalcone synthase gene in S. drummondii. Additionally, treatment with 1200 mM NaCl for 24 h did not induce the production of flavonoids in S. drummondii. Similar results have been reported in the roots of the medicinal halophyte Limonium bicolor, whereas an opposite trend was observed in the leaves of the same plant (Wang et al., 2016). In accordance with our results, CHS was downregulated during combined high salinity and flooding stress in soybean (Glycine max L. Merr. cv. Taegwang) roots and hypocotyls (Alam et al., 2011) and in roots of Salix matsudana Koidz under salt stress (Qiao et al., 2014).
The transcripts TRINITY_DN591739_c0_g1_i5 and TRINITY_DN591739_c0_g1_i4 (which encodes BURP domain-containing protein 5 isoform X1) were downregulated in S. drummondii shoots subjected to salt stress. BURP domain-containing proteins play a vital role in plant development, metabolism, and defense against environmental stressors (Shunwu et al., 2004). The plant-specific BURP domain family plays a role in A. thaliana drought tolerance (Harshavardhan et al., 2014). Similar results have been reported by Miyama and Hanagata (2007), showing that the expression of BURP domain-containing protein was strongly downregulated in leaves of salt-stressed Bruguiera gymnorhiza (L.) (Burma mangrove family).
4.6 Cell wall metabolism
The plant cell wall is the first to be affected by and respond to abiotic stressors (Wimmer & Eichert, 2013). Stress signals induce cell wall remodeling to preserve flexibility and protect plant cells against ionic disturbances (Hofmann, 2016). Examples of different transcripts associated with cell wall metabolism that have been identified in S. drummondii under salt stress are discussed below.
4.6.1 Lignin biosynthesis pathway
TRINITY_DN621268_c4_g1_i4: p-coumarate 3-hydroxylase (C3H) was downregulated in the roots of S. drummondii under salt stress. C3H is involved in lignin biosynthesis, and lignin is one of the most important compounds in vascular plants and is required for structural support and water transport (Xu et al., 2010). Lignin composition and content in plants may be altered due to environmental stress (Moura et al., 2010). ZmC3H expression is reduced in leaves but increased in roots of Zea mays subjected to drought stress (Ludidi & Kolo, 2015). Downregulation of C3H in S. drummondii roots may reduce root lignification (where root growth diminishes), which is considered an adaptive mechanism to harsh conditions, as observed in roots of Amaranthus hypochondriacus L. under drought stress (Huerta-Ocampo et al., 2011).
4.6.2 Cellulose and hemicellulose degradation
TRINITY_DN476880_c0_g1_i1 and TRINITY_DN476880_c0_g1_i1, which encodes beta-glucosidase were downregulated in roots of S. drummondii under salt stress. Beta-glucosidases play a role regarding abiotic stress, and downregulation of beta-glucosidase was suggested to be linked to the inhibition of cell wall elongation under stress, which reduces root growth (Kong et al., 2010). The beta-glucosidase gene is also upregulated in Thellungiella halophila under salt stress (Taji et al., 2004; Wong et al., 2006), and endo-beta-1,4-glucanase D (TRINITY_DN584654_c4_g1_i1), involved in cellulose degradation, was downregulated in salt-stressed S. drummondii roots. Endo-beta-1,4-glucanase D is involved in carbohydrate metabolism, cellulose degradation, and polysaccharide degradation (Le Gall et al., 2015). Endo-b-1,4-glucanases affect plant cell wall development by affecting cellulose crystallization (Glass et al., 2015). Several studies suggested that beta-1,3-glucanase-related proteins are involved in salinity responses in salt-tolerant tomato lines (Sadder et al., 2014), Sorghum bicolor leaves (Swami et al., 2011), and grapes (Daldoul et al., 2008). Moreover, downregulation of B eta-1,4-glucanase may lead to changes in cell wall structure and root growth as part of an adaptive mechanism to reduce the root surface area exposed to stressors (Le Gall et al., 2015; Nanjo et al., 2013).
In S. drummondii roots, transcripts involved in lignin biosynthesis (p-coumarate 3-hydroxylase), cellulose, and hemicellulose degradation (beta-glucosidase and endo-beta-1,4-glucanase D) were downregulated under salinity stress. These results suggest that changes in cell wall metabolism may be one mechanism of salt tolerance in S. drummondii. In this case, decreasing lignification and cell wall degradation may reduce root growth, which would then reduce the root surface area exposed to saline conditions.
4.7 Respiration
In the present study, the transcripts of cytochrome c oxidase (COX) subunit 1 (TRINITY_DN555305_c4_g15_i, and TRINITY_DN627029_c10_g1_i1), and COX subunit 2 (TRINITY_DN589326_c4_g1_i1), which are involved in the respiratory pathway and sucrose metabolism (Pang et al., 2010), were upregulated in S. drummondii roots under salt stress. COX is involved in the reduction of O2 to H2O and is the last electron acceptor in the mitochondrial respiratory chain (Mansilla et al., 2018). Similar to our results, COX subunit 1 was upregulated in wheat (Triticum aestivum L.) under salt stress (Singh et al., 2017). COX subunit 6b-1 expression is also induced in salt-treated rice roots (Yan et al., 2005) as well as in roots of the halophyte Tamarix hispida under salt stress (Li et al., 2009). COX subunit 1 is also upregulated in many other plants under salt stress, including Arabidopsis and rice (Barkla et al., 2013; Pang et al., 2010; Yan et al., 2005).
4.8 Chaperons
TRINITY_DN603163_c1_g2_i7 and TRINITY_DN603163_c1_g2_i4 are two transcripts which encode the late embryogenesis abundant protein D-29 isoform X2. These two transcripts were highly upregulated in shoots of S. drummondii in the salt treatment. The respective genes are strongly induced by ABA, which is produced under salt stress to protect the plant against dehydration (Hundertmark & Hincha, 2008). Late embryogenesis abundant protein D-29 isoform X2 is a putative stress protein that reflects tolerance to salt stress in the halophyte Thellungiella halophila (Zhang et al., 2008). Similar results have been reported regarding the halophyte S. fruticosa (Diray-Arce et al., 2015).
TRINITY_DN596190_c1_g1_i14, which encodes an 18.1-kDa class 1 heat shock protein (HSP)-like, was upregulated in the roots of S. drummondii after salt stress. HSPs are involved in protein metabolism and can correct protein folding and inhibit toxic aggregate formation (Domżalska et al., 2017; Teyssier et al., 2014). HSP70 is a molecular chaperone that has been associated with resistance to salt stress in the halophyte S. maritima L. (Sahu & Shaw, 2009). A different transcript, TRINITY_DN607636_c0_g5_i1: Hsp24 protein, was downregulated in the roots of salt-stressed S. drummondii. Similar results were reported in S. fruticosa where the HSP20-like chaperone protein superfamily was downregulated under salt stress (Diray-Arce et al., 2015).
4.9 Ubiquitination
Ubiquitination is a process that degrades harmful cellular proteins and modulates the involved regulatory elements, and it is one of the key mechanisms underlying abiotic stress tolerance in plants (Cheng et al., 2012). E3 ligases serve as main determinants of substrate specificity and may be involved in the regulation of signal transduction under salt and drought stress in A. thaliana (Lee & Kim, 2011). Transcriptomic analysis of S. drummondii roots showed upregulation of polyubiquitin TRINITY_DN558626_c5_g7_i2 and TRINITY_DN554033_c2_g1_i5 under salt stress. Polyubiquitin genes are involved in the regulation of a wide array of biological processes, including cell cycle control, gene silencing, DNA repair, transcription, protein processing, endocytosis, apoptosis, signal transduction, and stress responses (Kommaddi & Shenoy, 2013; Lyzenga & Stone, 2012). Similar results demonstrated that polyubiquitin was upregulated under salt stress in roots of the woody halophyte Tamarix hispida, suggesting its involvement in salt tolerance mechanisms (Li et al., 2009). Gharat and Shaw (2015) proposed that ubiquitination genes may be associated with the responses of halophytes to salinity.
4.10 Transcription factors
TRINITY_DN610243_c0_g1_i11 and TRINITY_DN610243_c0_g1_i15: homeobox-leucine zipper protein (ATHB-40) were upregulated in the shoots of S. drummondii under salt stress. Transcription factors such as homeobox 6 (ATHB-6), ATHB-12, and HD-Zip are involved in many physiological functions, including the mediation of growth responses under water deficit (Gharat et al., 2016; Tan & Irish, 2006), the regulation of floral development (Olsson et al., 2004), and cell cycle regulation (Hur et al., 2015). The observed upregulation of HD-Zip transcription factors in S. maritima L. in response to salt treatment indicates their essential role in abiotic stress tolerance (Gharat et al., 2016). ATHB-12 regulates leaf growth (specifically adaxial leaf fates) by stimulating cell expansion and endo-reduplication (Hur et al., 2015). In the current study, S. drummondii transcript ATHB-40, which is involved in stress tolerance (van Muijen et al., 2013), was also upregulated under salt stress. Similar to our findings, the ATHB-40-like was upregulated in the roots of Prunus persica under drought stress (Ksouri, 2016).
A different transcription factor that was upregulated in shoots under salt stress was TRINITY_DN631994_c2_g1_i11, which encodes the protein FAR1-RELATED SEQUENCE 5-like. The protein FAR1-RELATED SEQUENCE 5-like regulates numerous functions, including light control of development, zinc ion binding, and transcription. Yang et al. (2018) reported that Sb09g006170, encoding FAR1 and suberin synthesis, was significantly upregulated in two genotypes of sweet sorghum inbred lines differing in salinity tolerance (salt-sensitive and salt-tolerant, respectively). Moreover, FAR1-related Sb04g022400 was downregulated only in the roots of the salt-sensitive genotypes. Hence, the FAR1-RELATED SEQUENCE 5-like transcription factor may play a role in the salt tolerance mechanisms of S. drummondii.
5 CONCLUSION
S. drummondii is a habitat-indifferent halophyte with currently no genome data available. Hence, we performed comprehensive transcriptomic analyses of S. drummondii shoots and roots in response to salt stress using Illumina Hiseq 2500 sequencing as a first step for investigating S. drummondii responses to salinity at a molecular level. We assessed the expression of candidate genes involved in salt stress regulation through de novo transcriptome assembly. Our results revealed many functionally annotated DEGs and unigenes In shoot tissues, salinity tolerance of S. drummondii may depend on the induction of genes involved in the ABA pathway, ion transporters, sucrose transport, carbohydrate metabolism, and transcription factors. In addition, salt tolerance of S. drummondii may depend on the induction of some genes in root tissues, which play a role in nitrogen assimilation, redox homeostasis, chaperones, respiratory pathway, sucrose metabolism, and polysaccharide metabolism and transport. Thus, our study provides valuable information on salt tolerance-related genes and key metabolic pathways related to salt stress tolerance in habitat-indifferent halophytes. This information may help develop salt-tolerant crops, enhance plant tolerance to salinity stress and desalination, and restore saline soils.
ACKNOWLEDGMENTS
This research was supported by the Office of VC for Research and Graduate Studies at the University of Sharjah, Project No. 1602145041-P. The authors would like to thank Ms. Masarra Elgabra and Ms. Muna Alhammadi for their assistance.
AUTHOR CONTRIBUTIONS
Conceptualization: Kareem A. Mosa, Ali El-Keblawy; Experimental design: Kareem A. Mosa, Ali El-Keblawy; Software: Attiat Elnaggar, Kareem A. Mosa, Kalidoss Ramamoorthy, Sameh S.M. Soliman; Validation: Kareem A. Mosa, Attiat Elnaggar, Kalidoss Ramamoorthy; Formal analysis: Kareem A. Mosa, Attiat Elnaggar, Kalidoss Ramamoorthy; Investigation: Kareem A. Mosa, Attiat Elnaggar, Kalidoss Ramamoorthy; Resources: Kareem A. Mosa, Ali El-Keblawy; Data curation: Kareem A. Mosa, Attiat Elnaggar, Kalidoss Ramamoorthy, Ali El-Keblawy, Sameh S.M. Soliman; Writing—original draft preparation: Kareem A. Mosa, Attiat Elnaggar; Writing—review and editing: Kareem A. Mosa, Attiat Elnaggar, Ali El-Keblawy, Teresa Navarro, Sameh S.M. Soliman; Supervision: Ali El-Keblawy, Teresa Navarro, Kareem A. Mosa; Funding acquisition: Kareem A. Mosa, Ali El-Keblawy. All authors have read and agreed to the published version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the Supporting Information of this article.




