Seed inoculation of desert-plant growth-promoting rhizobacteria induce biochemical alterations and develop resistance against water stress in wheat
Rabisa Zia and Muhammad Shoib Nawaz contributed equally to this study.
Edited by: M. Ahanger
Abstract
Water shortage limits agricultural productivity, so strategies to get higher yields in dry agricultural systems is vital to circumvent the effect of climate change and land-shortage. The plant rhizosphere harbors beneficial bacteria able to confer biotic/abiotic tolerance along with a positive impact on plant growth. Herein, three bacterial strains, Proteus mirabilis R2, Pseudomonas balearica RF-2 and Cronobacter sakazakii RF-4 (accessions: LS975374, LS975373, LS975370, respectively) isolated from native desert-weeds were investigated for their response to improve wheat growth under drought stress. The bacteria showed drought tolerance up to 20% polyethylene glycol (PEG; −0.6 MPa), and salt (65–97 g l−1), 1-aminocyclopropane-1-carboxylate (ACC)-deaminase activity, P/Zn/K-solubilization, calcite degradation, IAA, and siderophore production. The plant growth-promoting rhizobacteria (PGPR) were evaluated on wheat under water stress. The P. balearica strain RF-2 primed seeds showed a maximum promptness index and germination index under PEG-stress, that is, 68% and 100%, respectively. Inoculation significantly improved plant growth, leaf area, and biomass under water stress. P. mirabilis R2 inoculated plant leaves showed the highest water contents as compared to the plants inoculated with other strains. C. sakazakii RF-4 inoculated plants showed minimum cell injury, electrolyte leakage, and maximum cell membrane stability at PEG stress. After 13 days exposure to drought, C. sakazakii RF-4 treated plants showed an overall higher expression of cytosolic ascorbate peroxidase (cAPX) and ribulose-bisphosphate carboxylase (rbcL) genes. The activity of stress-induced catalase and polyphenol oxidase was reduced, while that of peroxidase and superoxide dismutase increased after inoculation but the response was temporal. Taken together, this data explains that different PGPR (especially C. sakazakii RF-4) modulate differential responses in wheat that eventually leads towards drought tolerance, hence, it has the potential to enhance crop production in arid regions.
1 INTRODUCTION
The last few decades have shown alarming evidence of climate change (Fischer & Knutti, 2015) on the entire planet. The world population is projected to hit 9.8 billion by 2050 (UN World Population Prospects), half of which will be concentrated in the countries which are most vulnerable to climate change. Climate experts forecast an increase of 3–4°C in temperature in Pakistan and other South Asian countries by the end of this century (Defra, 2005). An increase in temperature leads to the development of transient or stable, mild-to-extreme drought conditions leaving soils from really-wet to really-dry, very fast. Both heat and drought are the key challenges for plant growth and development, severely affecting yield (Yordanov et al., 2000). Climate change is a global problem that has aggravated the challenges confronting the agriculture sector and a decline of 25% in yield of staple crops is anticipated by 2050 in more than half of arable land (Kasim et al., 2013).
Wheat is a globally important food and cash crop which is highly sensitive to drought and heat stress, particularly during flowering and grain filling stage. Recurrent drought associated with climate change is among principal constraints to global wheat productivity. Global wheat production was 755 million metric tons in 2016 compared to 578 million metric tons in 1996, but per acre production has decreased over time (Ray et al., 2012) and is expected to be reduced by 30% by the mid-century (Ortiz et al., 2008). With an expected increase of 1.6% in wheat demand, the severity of drought in wheat-growing areas, and the upcoming shortage of water and land (Trenberth et al., 2014) serious efforts are required for improvements in water-use-efficiency and technologies for providing effective use of available water. The strategies that can maximize wheat growth and yield per unit volume of water and land area without compromising ecosystem sustainability (Shen et al., 2016) while feeding a rapidly growing population (Gregersen et al., 2013) are of immediate concern.
Breeding and genetic engineering strategies for tolerance to drought (Eisenstein, 2013; Philippot et al., 2013) have largely ignored the potentials of soil ecology (Morrissey et al., 2004). Millions of rhizosphere microbes form complex ecological communities, directly, or indirectly influence the growth and productivity of crops. Plant growth-promoting rhizobacteria (PGPR)-induced drought tolerance is known in plants (Timmusk and Wagner 1999). PGPR enhances plant stress tolerance through 1-aminocyclopropane-1-carboxylate deaminase (ACCd). Under abiotic stress, especially drought, plants endogenously produce ethylene to maintain or enhance homeostasis from the precursor 1-aminocyclopropane-1-carboxylate (ACC; Hardoim et al., 2008). ACC break down and the inhibition of ethylene synthesis by ACCd decreases the damage and maintains the homeostasis in and around the plant root, especially at early stages of stress exposure. Hence, inoculation of plants with ACC-deaminase producing bacteria improves plant growth (Glick, 2005), productivity and fruit ripening (Arshad et al., 2008) under drought stress. Apart from stress management, these bacteria exhibit other plant beneficial functions, for example, the production of indole acetic acid (IAA), phosphate solubilization, siderophore production, and nitrogen fixation. In this way, they promote plant growth, hence are termed as PGPR (Bashan & Holguin, 1997).
PGPR-inoculation technology is a well-known, economical, and environment-friendly strategy for crop fertilization (Majeed et al., 2015; Naqqash et al., 2016; Rajput et al., 2013). However, little is known about its effectiveness in stressed environments, especially the interaction with wheat under drought stress. The addition or inoculation of drought-tolerant bacterial communities can make plants cope with drought stress and improve their health (Cherif et al., 2015). We hypothesized that due to severe drought conditions at the Cholistan desert, the indigenous bacterial community would contain certain bacteria to help plants to take up more nutrients, induce root development and enable them to survive under extreme conditions.
The present study was designed to screen drought-tolerant bacteria from desert soil and rhizosphere, to evaluate the plant growth-promoting potential of these bacteria in vitro as well as in vivo and finally to evaluate this technology for wheat growth under drought stress. We have identified 21 potentially beneficial bacteria and three of them have been tested on wheat and reported in this study. The present study describes the potential of these microbes in solving food security issues related to drought stress.
2 MATERIALS AND METHODS
2.1 Site description, soil and plant sampling, enrichment, and bacterial isolation
The native weeds (Aerva tomentosa, Penicum turgidum) and soil samples were collected from the Cholistan Desert (28.5°N70°E). Cholistan is a hot hyper-arid sandy desert with a mean annual rainfall of less than 100 mm (Figure S1). The soils are generally saline (pH 8.2–8.4), alkaline and gypsiferous composed of granites, schists, gneiss, slates, and a complex blend of river alluvium and eolian sandy soil. Underground water is at a depth of 30–50 m, generally brackish containing 9000–24 000 mg l−1 salts (Akbar et al., 1996; Chaudhry & Nasim, 1995).
The soil tightly adhering to the roots was separated and 1 g of each soil sample was used for enrichment. Similarly, roots were washed with sterile water and then 1 g root was crushed in 1 ml saline and used for enrichment. Enrichment was performed in test tubes containing 5 ml of each media: (1) LB broth supplemented with 1% polyethylene glycol-6000 (PEG-6000) and (2) semi-solid Nitrogen Free Medium (NFM) with malate as carbon source (Okon & Kapulnik, 1986). The test tubes were incubated at 28 ± 2°C until the appearance of visible growth. Samples of 1 ml were taken out of the test tube and serially diluted in saline as described by Somasegaran and Hoben (1994). The dilutions 10−4 and 10−5 were spread onto LB agar plates and incubated at 28 ± 2°C until the appearance of colonies. These cultures were enriched from LB medium containing 5, 10, and 20% PEG-6000. Enriched cultures were also spread and streaked on LB agar repeatedly to attain purity.
2.2 Screening for drought tolerance
Drought stress screening was carried out on different concentrations of PEG-6000 as an osmotic agent to induce water stress in vitro. Nonstressed media contained 0% PEG (0 MPa) while drought stress of −0.15, −0.3, −0.45, and − 0.6 MPa was created by dissolving 5, 10, 15, and 20 g PEG-6000 in 100 ml of water or respective media. The osmotic potential of the media was confirmed by a Vapor Pressure Osmometer (Wescor 5520) at 25°C as described by Michel and Kaufmann (1973).
The pre-grown bacteria (108 CFU ml−1) were inoculated in broth media containing different PEG concentrations and incubated on a shaker for 2–3 days at 28 ± 2°C. Optical density was measured using a spectrophotometer at 600 nm after 3 days as an indication of the tolerance to specific drought levels created with PEG-6000.
2.3 Salt and temperature tolerance
Temperature tolerance was tested by inoculating pre-grown bacterial culture into 100 ml LB broth and incubation at 28°C, 37°C, 40°C, and 45°C for 48 h. Salt tolerance was checked following the protocols mentioned in Rajput et al. (2013) in 100 ml halophilic medium containing a concentration of 32, 65, and 97 g l−1 NaCl. The cultures were incubated on a shaker for 2–3 days. Optical density was measured using a spectrophotometer at 600 nm after 3 days as an indication of the tolerance to a specific level of temperature or salt.
2.4 Identification of bacteria and detailed functional characterization
The colony morphology and growth characteristics of bacterial isolates were examined after overnight growth on LB at 28 ± 2°C (Somasegaran & Hoben, 1994). Cell size, shape, and motility were observed under a Light microscope (Leica DM 750). Acid/alkali production was checked on LB agar plates containing 0.025% (w/v) bromothymol blue (pH indicator). Gram staining was performed as described by Vincent and Humphrey (1970). The catalase activity was detected by mixing H2O2 onto a glass slide with bacterial culture and observed for bubble formation which indicates the positive activity for catalase enzyme. Antibiotic sensitivity testing was performed by the Kirby-Bauer method (Valverde et al., 2005) on antibiotic sulphonamide sensitivity test agar plates (Merck) using commercially available discs (Bioanalyse). The utilization of different carbon sources and enzymatic reactions were performed using the QTS-24 kit (DESTO, Karachi) following the manufacturer's protocol.
Bacteria were identified by sequence analysis of 16S rRNA genes. An over-night grown colony was dissolved in 100 μl of de-ionized distilled water and 3 μl of this suspension was used for PCR amplification of the 16S rRNA gene with universal primers P1and P6 (Tan et al., 1997). The amplification was carried out as described by Imran et al. (2010). The PCR product was purified using the QI quick gel extraction kit (QIAGEN) and sequenced commercially by Macrogen. NCBІ BlastN was used to compare the gene sequences with the sequences in the GenBank database. Multiple sequence alignments were performed by Clustal X and phylogeny was determined by the neighbor-joining method using the MEGA6 software. The sequences were submitted to the NCBI database.
2.5 Indole-3-acetic acid production
For the detection of indole-3-acetic acid (IAA), the bacteria were grown for 2–3 days in LB-broth containing 100 mg l−1 tryptophan in media with PEG-6000 (0, 5, 10, 15, and 20%). The development of pink color by the culture supernatant and Salkowski reagent was considered as positive for IAA production as described by Gordon and Weber (1951). IAA was extracted from the culture supernatant using ethyl acetate as described by Tien et al. (1979). The extract was evaporated to dry and re-collected in methanol. The extract was analyzed by an HPLC (Perkin Elmer) equipped with the Turbochrom software using a C-18 column at a flow rate of 0.5 ml min−1 following the protocol of Rasul et al. (1998). IAA production was quantified by comparing the retention time and peak area of a 1000 ppm synthetic IAA standard.
2.6 Phosphate, zinc, calcite, and potassium solubilization
The solubilization potential of bacteria was tested in different media containing PEG-6000 (0, 5, 10, 15, and 20%). Phosphate and zinc solubilization were checked by spot inoculation of pre-grown bacterial cultures onto Pikoviskaya's agar plates containing tri-calcium phosphate (Pikovskaya, 1948) and LGI medium containing zinc oxide/sulfate (Bunt & Rovira, 1955), respectively. Calcite degradation was tested on calcium carbonate solubilization medium (CCS) containing 5 g l−1 insoluble calcium carbonate as described by Rana et al. (2015). Potassium solubilization was checked on Aleksandrov agar medium containing potassium aluminate silicate as an insoluble K source (Sugumaran & Janarthanam, 2007).
The inoculated plates were incubated at 28 ± 2°C for 10–14 days and examined daily for the formation of a clear zone around the bacterial growth spot. The appearance of a halo zone around the inoculated spot was considered as an indication of P/Zn/calcite/K solubilization ability of the bacterium. Solubilization indices were calculated by dividing the clearance zone with the colony diameter as described by Shanware et al. (2014).
Available phosphorous in the inoculated media was quantified by the phosphomolybdate blue color method using a spectrophotometer (λ = 882 nm) as described by Murphy and Riley (1962).
2.7 ACC-deaminase activity
The ability of bacteria to breakdown ACC was tested in Dworkin and Foster (DF) salt minimal medium (Penrose & Glick, 2003) containing 0.5 M ACC and PEG-6000 (0, 5, 10, 15, and 20%). The bacteria were grown for 24–48 h with constant shaking at 28 ± 2°C. The appearance of turbidity indicated the ACC-deaminase activity.
2.8 Siderophore production
Siderophore production by bacteria was determined through the method described by Schwyn and Neilands (1987), without PEG. Briefly, the pure bacterial colonies were spotted on the Petri plates containing chrome azurol S media (Sigma) and incubated at 28 ± 2°C for three days. The appearance of a purple or an orange zone around the bacterial colonies was considered as siderophore production.
2.9 Plant inoculation experiments
2.9.1 Plant material
Wheat seeds (Var. Punjab 2008) were obtained from NIBGE, Faisalabad. Apparently, healthy seeds were used for plant inoculation studies. The seeds were surface sterilized by dipping in 2.5% sodium hypochlorite solution for 15 min. The seeds were then washed with a 70% ethanol solution for 30 s and then with sterile distilled water 3–4 times.
2.9.2 Seed priming
Bacterial cultures were grown individually overnight in LB broth at 28 ± 2°C. Bacterial cultures were centrifuged and pellets were washed with saline and resuspended in normal saline at an approximate bacterial cell concentration of approximately 1.0 × 109 (OD 600 nm = 1.0). Seed priming was performed by soaking the sterilized seeds in 100 ml of bacterial cell suspension for 15–20 min. In some experiments, a mixture of bacteria was used which was prepared by mixing equal volumes of bacterial suspensions (1:1:1) and designated as the mix.
2.10 Effect of PGPR priming on the germination stress index
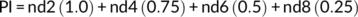
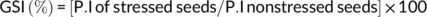
2.11 Effect of PGPR inoculation on early growth of wheat at different water stress levels in sand
A completely randomized design (CRD) based experiment was set up in small pots filled with 200 g sterilized sand. PGPR-primed seeds (n = 5) were sown in each pot. For each of the strains R2, RF-2, and RF-4 four different levels of water stress were used: 50, 37.5, 25, and 12.5%, each with three replicates. The water stress levels were maintained by determining the sand water holding capacity. One hundred grams of sand were saturated with water and weighed (W1). The sand was allowed to dry for 3–4 days and weighed again (W2). The difference in weight (W1–W2) gives the water holding capacity (WHC). The water stress levels were maintained as WHC = 100%, ½ WHC = 50%, 3/8 WHC = 37.5%, ¼ WHC = 25%, and 1/8 WHC = 12.5%. The noninoculated controls were set up at the same stress levels. The plants were watered alternately with full-strength Hoagland solution maintaining the same stress levels throughout the experiment for up to 50 days.
Flag leaves from each replicate were cut in the morning at day 30 post-germination and placed on a white sheet to take pictures with the help of a digital camera. The pictures were processed in Image J for measurement of leaf area as described in detail by Ahmad et al. (2015). Plants were uprooted carefully from the pots at day 50. Roots were carefully washed to remove sand particles and placed in an oven (65°C) for 72 h to dry out completely. The biomass was then calculated from all the treatments and the average was calculated.
2.12 Effect of PGPR inoculation on physiological parameters, enzymes, and gene expression of wheat grown at different water stress levels in the soil
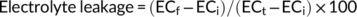

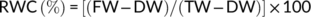
RNA was extracted from 100 mg each of three independent replicate leaves as described in Amin et al. (2011). cDNA was synthesized using the first-strand cDNA synthesis kit (Thermo Fisher Scientific) using the recommended protocol. Analysis of the expression pattern of cAPX and RUBISCO genes (rbcL and rbcS) was analyzed by RT-PCR using wheat specific primers designed in this study (Table 3). The gene amplification was performed at: denaturation at 95°C for 5 min, 35 cycles of denaturation at 95°C for 30 s, annealing at 59°C for 30 s, extension at 72°C for 45 s. and final extension at 72°C for 10 min. Semi-quantitative analysis of PCR amplicons and relative expression of stress-related genes was carried out using the Image J software as described by Antiabong et al. (2016).
For enzyme assays of polyphenol oxidase (PPO), catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD) 0.1 g of fresh leaf samples were ground in 2 ml of 0.1 M sodium phosphate buffer (pH 6). Leaf samples were collected at 2nd, 5th, 10th, 12th, and 16th-day post-induction of water stress (dpi). The homogenate was centrifuged at 12 000g for 15 min at 4°C, the supernatant was transferred to separate microfuge tubes and stored at −80°C for further analysis.
The CAT activity was assayed according to Worthington (1988) and Weisany et al. (2012). The activity of PPO was analyzed as described in Worthington Enzyme Manual (Shanti et al., 2014; Worthington, 1988). The POD activity was evaluated by following the protocols from Hammerschmidt et al. (1982) and Liang et al. (2011). The activity of superoxide dismutase (SOD) enzyme was assayed as described by Paoletti et al. (1986), McCord and Fridovich (1969), and calculated as mentioned by Zhang et al. (2016).
2.13 Statistical analysis
The correlation analysis and plant inoculation data were statistically analyzed by one-way and/or two-way ANOVA (Steel et al., 1997) using the software Statistix 8.1. The comparison among treatment means was done at 5% probability level using Tukey's HSD or Fischer's LSD. The categorical principal component analysis (CATPCA) was performed using IBM SPSS software Package version 20 (SPSS, Inc.).
3 RESULTS
3.1 Isolation, morphological, and biochemical characterization of bacteria
Three bacterial isolates (R2, RF-2, and RF-4) were obtained from different media inoculated with soil/root samples collected from the desert. Bacterial isolate R2 was purified from soil enriched in PEG-LB, while RF-2 and RF-4 were obtained from root samples inoculated to nitrogen free medium (NFM) after enrichment. The details of morphological and biochemical characteristics of bacterial strains are mentioned in Table 1. Growth of RF-2 and RF-4 in nitrogen-free semi-solid NFM medium with Malate as the only source of carbon indicates that the bacteria are putative diazotrophs and may contain nitrogen fixation ability. Both isolates showed tolerance to 20% PEG and the OD of cultures was similar to that grown without PEG supplementation (OD: 0.45 λ600).
| Characteristics | P. mirabilis sp. strain R2 | P. balearica sp. strain RF-2 | C. sakazakii sp. strain RF-4 | Characteristics | P. mirabilis sp. strain R2 | P. balearica sp. strain RF-2 | C. sakazakii sp. strain RF-4 |
|---|---|---|---|---|---|---|---|
| 16S rRNA accession | LS975374 | LS975373 | LS975370 | Catalase test | ++ | ++ | + |
| Colony morphology | Concentric | Round | Round | Cytochrome oxidase test | − | + | + |
| Colony color | Light Yellow | White | Pale Yellow | Drought tolerance 10% PEG 20% PEG |
++ | ++ | ++ |
| Isolated from/medium | Soil/ LB Agar | Root/NFM | Root/NFM | Siderophore production | − | − | + |
| Shape/motility | Rods/highly motile | Rods/Motile | Rods/very slow motility | ACC-deaminase activity | + | + | + |
| Salt tolerance up to | |||||||
| 32 g ml−1 | +++ | +++ | +++ | Temp. tolerance | + | + | + |
| 65 g ml−1 | +++ | +++ | +++ | 37°C | − | − | − |
| 97 g ml−1 | +++ | − | − | 45°C | |||
| Resistance to antibiotics | |||||||
| Gentamicin | S | S | S | Ofloxacin | S | S | S |
| Ciprofloxacin | S | S | S | Rifampicin | S | S | S |
| Aztreonam | R | R | S | Cefepime | R | S | S |
| Amikacin | R | S | S | Nalidixic acid | R | R | S |
| Biochemical characteristics | |||||||
| ONPG | + | + | + | H2S production | + | − | − |
| Sodium citrate | + | + | + | Urea hydrolysis | + | +/− | + |
| Sodium malonate | + | + | + | Tryptophan deaminase | + | − | − |
| Ornithine decarboxylase | + | − | + | Nitrate reduction | + | + | + |
| Indole | − | − | − | Lysine decarboxylase | NI | NI | NI |
| Voges-Proskauer (Acetion) | − | − | + | Arginine dihydrolase | NI | − | NI |
| Gelatin hydrolysis | + | − | + | Acid from glucose | + | + | + |
| Acid from maltose | − | + | + | Acid from ARAB | − | − | − |
| Acid from SUC | − | − | − | Acid from RHAM | − | − | + |
| Acid from Mann | − | − | + | Acid from SOR | − | NI | + |
| Acid from INOS | − | − | − | Acid from MEL | − | − | + |
| Acid from ADO | − | − | − | Acid from RAF | − | − | + |
- +, presence of any activity/growth, − , absence of any activity/growth, ++, moderate growth/activity, +++, high growth or activity.
- Abbreviations: ADO, adonitol; ARAB, arabinose; INOS, inositol; mann, mannose; MEL, malate; NFM, nitrogen-free medium; NI, test cannot be categorized as positive or negative; R, resistant; RAF, raffinose; RHAM, rhamnose; S, sensitive; SOR, sorbitol; SUC, sucrose.
All three bacteria contained multifarious plant growth-promoting characteristics showing a combination of stress-tolerance and PGP activities (Tables 1 and 2). The R2 is a rod-shaped, highly motile bacterium with light yellow-colored colonies on LB, catalase-positive, and cytochrome oxidase negative strain identified as Proteus mirabilis based on 16S rRNA gene sequence analysis (accession no. LS975374). The bacterium was drought tolerant to up to PEG 20%, extremely halophilic (tolerant up to 97 g l−1 NaCl) and could grow at 37°C. The bacterium was resistant to aztreonam, amikacin, cefepime, and nalidixic acid antibiotics and was metabolically not very diverse (Table 1).
| Strain | Phosphate solubilization | Zn solubilization | K solubilization | Calcite degradation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone diameter (cm) | Sol. index | Solubilized P (ppm) | Zone diameter (cm) | Sol. Index | Zone diameter (cm) | Sol. Index | Zone diameter (cm) | Sol. index | ||||||||||
| DPI | 3 | 6 | 3 | 6 | 3 | 6 | 3 | 6 | 3 | 6 | 3 | 6 | 3 | 6 | 3 | 6 | 3 | 6 |
| R2 | 0.62 | 1.92 | 1.12 | 1.54 | 3.15 | 3.24 | 1.03 | 1.73 | 1.32 | 1.38 | — | — | — | — | 0.53 | 1.21 | 1.25 | 2.00 |
| RF-2 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.81 | 0.83 | 1.66 | 1.33 |
| RF4 | 1.61 | 2.00 | 4.23 | 5.00 | 19.51 | 19.89 | 2.50 | 3.32 | 4.83 | 4.72 | 2.52 | 2.94 | 7.01 | 7.25 | — | — | — | — |
- DPI, activity of solubilization/degradation at certain days post-inoculation on specific media.
| Gene | Primer | Primer sequence (5′–3′) | Product size (bp) |
|---|---|---|---|
Cytosolic ascorbate peroxidase |
cAPX-F cAPX-R |
TGGTGGACGCCGAGTACCTGCGCCAG GGGTGTACTCTTCCTCGTATCTAATTG |
183 |
| RUBISCO-larger subunit | rbcL-F rbcL-R |
CCGAGTAAGTCCTCAGCCTGGGGT GTAACGGAACCCTCTTCAAATAGGTC |
222 |
RUBISCO-smaller subunit |
rbcS-F rbcS-R |
GATCCGCTCCAAGTGGGTGCCCTGCC CGATGACGCGGACATAGGCGTCAGGG |
207 |
| Actin | act-F act-R |
ATGGCTGACGGTGAGGACATCCAGCC AGGTGAGGATACCCCTCTTGGATTGG |
205 |
RF-2 is a rod-shaped, motile bacterium with round, white colonies on LB plates, while RF-4 is a slow-motile, rod-shaped bacterium with pale-yellow colored colonies on LB plates. Both are catalase- and cytochrome oxidase-positive strains. RF-2 was identified as Pseudomonas balearica based on 16S rRNA gene sequence analysis (accession no. LS975373) while RF-4 was identified as Cronobacter sakazakii (accession no. LS975370). The bacteria were drought-tolerant at up to PEG 20%, halophilic with up to 65 g l−1 NaCl, but could not grow above 37°C. Both were sensitive to most of the antibiotics tested and metabolically active (Table 1).
3.2 Bioassay for plant growth-promoting traits
PGPR traits of some of the bacteria are shown in Figure 1. PGPR traits of P. mirabilis R2 included: ACC-deaminase activity, production of IAA and widespread nutrient mobilization/solubilization ability for insoluble phosphorus, zinc, and calcite (Figure 1; Table 2). The solubilization indices were 1.5 for phosphorus, 1.38 for zinc, and 2 for calcite. The amount of P solubilized was 3.24 ppm within 6 days.
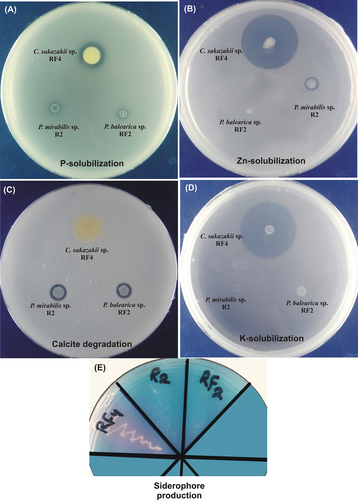
P. balearica RF-2 exhibited ACC-deaminase activity, putative nitrogen-fixation ability, and degradation of calcite (Figure 1; Table 2) with a solubilization index of 1.33 after 6 days of inoculation.
C. Sakazakii RF-4 was a putative nitrogen-fixing bacterium with ACC-deaminase activity. P-solubilization index was 5 and it solubilized 19.89 ppm phosphorus. It also contained Zn-solubilization with an index of 4.7 and K-solubilization with an index of 7.25 after 6 days. The P-/Zn-solubilization activity of RF-4 was highest among all bacteria in this study. RF-4 also produced a catechol type of siderophore as indicated by the development of the purple color in the chromazoral S medium (Figure 1).
Analysis of PGP traits under PEG-stress media showed that P. mirabilis R2 produced a small amount of IAA at 5% PEG stress. Similarly, the solubilization of P/Zn/K was severely affected at 5% PEG stress while no strain exhibited PGP activity above 5% stress level.
3.3 Effect of PGPR priming on seed germination under PEG-induced stress
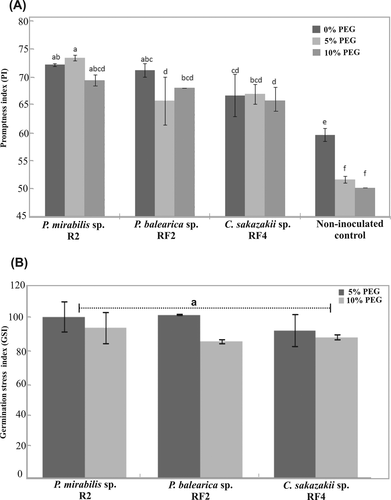
The promptness index (PI) ranged from 72.25–63 at 0%, 57.5–74 at 5% and 63–70.5 at 10% PEG in different inoculated treatments. RF-2 primed seeds showed significantly higher PI at 0 and 5% PEG (72.3–73.5) compared with other inoculated treatments (Figure 2A). The germination stress tolerance index (GSI) ranged from 92.1 to 101 at 5% and 85.5–93% at 10% PEG. Although, the P. mirabilis strain R2 and P. balearica strain RF-2 showed higher GSI at 5% PEG compared to all other treatments (Figure 2B). GSI data were statistically nonsignificant among inoculated treatments at both stress levels.
3.4 Early growth of wheat under induced water stress in sand
Visually the growth of noninoculated plants was negatively affected by water stress as compared to inoculated plants (Figure 3A). Furthermore, the shoot development, leaf length, and area were visually better in the inoculated treatments compared to respective noninoculated treatments under the same water stress level (Figure 3A, B). The leaves of inoculated plants remained green and mainly unaffected up to 25% WHC stress (Figure 3B) compared to a significant reduction in noninoculated plants.
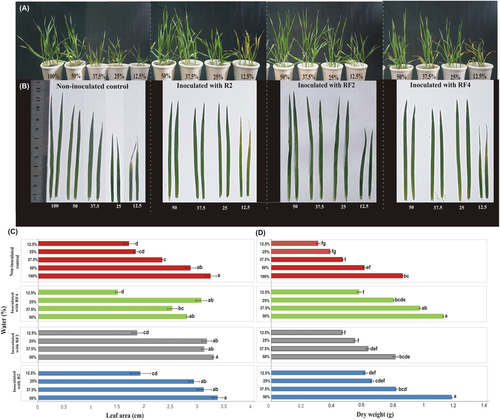
The analysis showed that drought stress significantly reduced the leaf area at different stress levels compared to nonstressed plants (Figure 3C). Maximum leaf area was observed in plants inoculated with P. mirabilis strain R2 (3.7 cm) followed by plants inoculated with P. balearica strain RF-2 (3.61 cm) at 50% WHC and noninoculated plants at 100% WHC (3.56 cm). The inoculated plants showed a slight decrease in leaf area with increasing stress level from 50% to 25% WHC, but a sharp decline at 12.5% WHC which was statistically significant. Furthermore, the leaf area of noninoculated plants was generally lower than the inoculated plants at all stress levels except RF-4 inoculated plants at 12.5% WHC.
A similar trend was observed for plant biomass which was considerably higher in the P. mirabilis R2 and C. sakazakii strain RF-4-inoculated plants at 50% WHC as compared to noninoculated control plants even at 100% WHC (Figure 3D). Furthermore, the plants inoculated with the C. sakazakii strain RF-4 at 37.5% WHC also showed significantly higher biomass then both controls.
3.5 Effect of PGPR inoculation on physiological parameters of wheat grown at different water stress levels in the soil
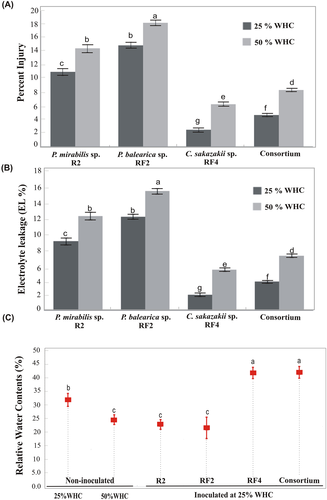
Water stress enforced injury and electrolyte leakage in plant leaves was more significant at 50% WHC than at 25% WHC (Figure 4A). However, inoculation with the C. sakazakii strain RF4 and a mix-inoculation of bacteria significantly reduced the percentage of injury and electrolyte leakage (Figure 4A, B) with corresponding higher cell membrane stability. Overall, the plants inoculated with the P. balearica bacterial strain RF-2 showed maximum injury and electrolyte leakage at 50% WHC stress. Under induced water deficit stress, relative water contents of plants inoculated with the P. balearica strain RF-2 were the lowest (Figure 4C ), whereas the RWC were maximum for the plants inoculated with RF-4 and mixed bacterial inoculation.
3.6 Effect of PGPR inoculation on gene expression analysis of wheat grown at different water stress levels in the soil
The temporal expression pattern of the antioxidant genes cAPX involved in the plant defense system, plant physiology and photosynthesis (rbcL and rbcS) was assessed by RT-PCR in inoculated and noninoculated plants grown under stress (Figure 5). The gel photographs (Figure 5A) depict that different genes showed a temporal increase or decrease in expression levels. The expression of cAPX (Figure 5A, B) showed a differential expression among different inoculated and noninoculated treatments under stress. Its expression was higher under noninoculated stressed plants (Figure 5B), but a clear downregulation of this gene was observed in R2-inoculated plants. In RF-2 inoculated plants a gradual increase in the expression was observed with time. However, in RF-4 inoculated plants the expression was maximum and remained almost constant throughout the experiment. While in plants inoculated with a mix-bacterial treatment, a gradual decrease in the expression of cAPX was observed.
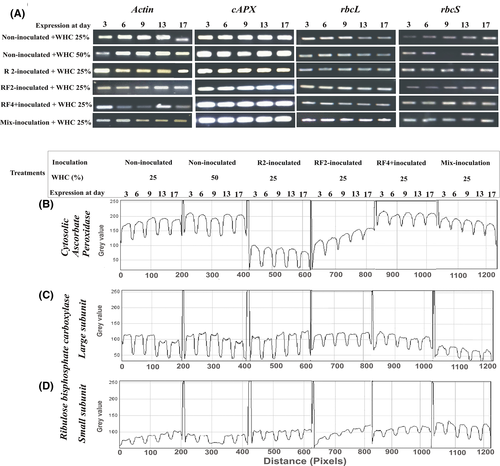
To observe the impact of the inoculation on the photosynthetic efficiency of wheat exposed to drought stress, the transcript level of the biofunctional enzyme Rubisco and its large and small subunits (rbcL and rbcS, respectively) were analyzed. The expression of rbcL in the noninoculated plants under stress (Figure 5A, C) during early days of stress, that is, day 3–9 remains almost constant but then decreased gradually until day 17. A similar trend was observed for plants inoculated with RF-4. On the contrary, in R2- and RF-2 -inoculated plants, a gradual increase in expression of the rbcL gene was observed with time as the stress progressed until day 17. However, the plants inoculated with bacterial consortium showed minimum levels of rbcL gene expression which further decreased as the drought stress prolonged.
The expression of the rbcS gene was very low in noninoculated plants under stress (Figure 5A, D). In plants inoculated with different bacteria, a gradual change in expression pattern of the rbcL gene was observed. Where the expression was increased in RF-2 and RF-4 inoculated plants, remained constant in R2 inoculated plants, and decreased in mix-bacterial inoculation treatment as the water stress progressed from day 3 until day 17.
The gel data were normalized with the actin gene and quantification was done using the Image J software. The statistical analysis of expression data as a function of PGPR inoculation averaged over time (Table S1) showing that the highest expression of rbcL was observed in RF-2 inoculated plants while it occurred in inoculated plants for rbcS and cAPX in RF-4.
CATPCA analysis showed that the RF-4 inoculated and mix-bacterial inoculation treatments loaded on the positive quadrant while noninoculated control treatments loaded onto the negative quadrant (Figure S2A). The treatments were clearly separated on the graph and loading clearly shows an effect of bacterial inoculation on gene expression. Higher values of variance (86.8%) explained by both components/dimensions validates the statistical design and analysis.
3.7 Effect of PGPR inoculation on enzyme expression analysis of wheat grown at different water stress levels in the soil
The antioxidant enzyme activities were measured from the same plants processed for gene expression analysis. To investigate the impact of bacterial inoculation on the analogous enzymatic system of wheat plants in response to drought stress, the leaf CAT, PPO, POD, and SOD activities were measured at different times after drought induction.
Catalase activity (CAT) remained low throughout the drought period in most of the inoculated plants except a significant activity in the mix-consortium inoculated plants at 2 dpi and noninoculated plants at 5, 10, and 16 dpi (Figure 6A). From day 2 until the end of the experiment at 16 dpi, all the inoculated plants exhibited very low catalase activity except a significantly higher value observed in mix-bacterial inoculation treatments at day 2.

Polyphenol oxidase (PPO) activity was higher during the start of drought induction (2 dpi) in all plants and significantly decreased afterward in all inoculated/noinoculated treatments. A slight induction of PPO activity was observed in plants (except RF-2) at 10 and 16 dpi (Figure 6B). Maximum PPO activity was observed in mix-bacterial inoculation treatments at day 2 and 5 after drought induction.
Peroxidase activity (POD) remained high during the early period of drought induction, that is, day 2–5. In noninoculated plants, it was at maximum at day 5 after drought induction. In RF-4 inoculated plants POD activity was highest during the early period of drought induction, that is,, day 2–5 but decreased at day 10 and again showed a gradual increase until day 16 (Figure 6C). In mix-bacterial inoculation treatments, the activity of POD gradually increased over drought induction and was at its maximum at later stages of drought induction, that is, 12–16 dpi.
Superoxide dismutase activity (SOD) remained higher in most of the inoculated plants, showing a gradual increase with time (Figure 6D). R2 and RF-2 inoculated plants showed a stimulation of SOD activity during early days of drought which declined as drought progressed but again increased at later stages. On the other hand, RF-4 inoculated plants showed increased SOD at later stages of drought, that is, day 12–16 dpi which show a relationship of SOD with RF-4.
CATPCA analysis (Figure S2B) showed that the mix-bacterial inoculation and plants treated with R2 loaded on the positive quadrant. Noninoculated control treatments were scattered on the graph but were mostly loaded onto the negative quadrant. The treatments were not very clearly separated on the graph but loading shows an effect of bacterial inoculation on enzyme expression. Higher values of variance (>70%) validates the statistical design and analysis.
4 DISCUSSION
Agricultural productivity is directly related to water availability. To increase the yield of global staple crops like rice and wheat under water deficit conditions, a practically profitable, economically feasible, and ecologically safe strategy is to exploit the potential of PGPR native to drought-prone soils. The present study has revealed the potential of three multi-stress-tolerant PGPR for improving wheat growth under water stress. The strains were obtained from a tropical desert which is one of the driest and hottest areas of Pakistan and receives very scanty rainfall, with drought periods sometimes extending from 1 to 3 years.
A polyphasic characterization was carried out to screen putative stress-tolerant PGPR from the desert weeds using classical tests. The bacteria showed low metabolic potential due to innate desert-background where nutrients are scarce but as wheat is rich in root exudates, it may support the rhizosphere colonization by these bacteria. The bacteria identified by 16S rRNA sequencing as P. mirabilis sp. R2, P. balearica sp. RF-2. and C. sakazakii sp. RF-4 and showed tolerance to 20% PEG-induced drought (−0.6 MPa), salt (65–97 g l−1), temperature (37°C) and exhibited multiple plant growth-promoting traits. These stress-tolerant bacteria (P. mirabilis, P. balearica, C. sakazakii) are part of the normal desert microbes (Bhatnagar & Bhatnagar, 2005) and have well-characterized taxonomic relatives for conferring salinity and zinc tolerance in plants (Babalola, 2003), but we have demonstrated the drought tolerance potential of these bacteria with the capacity to promote wheat growth under water-limited conditions which was not studied earlier.
Functional characterization of these putative multi-stress-tolerant bacteria for plant growth-promoting (PGP) traits showed IAA production, solubilization of different minerals, production of siderophore, ACC-deaminase activity, and nitrogen fixation ability along with a multi-stress tolerance. It has been already reported that various bacteria use this multiple stress-tolerance and PGP-abilities to decipher plant growth promotion under normal as well as stressed conditions (Glick, 1995). Usually, the bacteria having more than one PGP trait are preferred candidates for the development of crop-inocula (Ali et al., 2020), hence, these bacteria were selected for detailed analysis.
The analysis of PGP traits demonstrated that only the P. mirabilis strain R2 showed IAA production up to 5% PEG stress. IAA is the most significant plant growth enhancer which stimulates root development, subsequently improving nutrient uptake under various conditions including drought (Egamberdieva et al., 2017, Nabti et al., 2010). This trait helps plants to explore larger soil areas to extract water and nutrients under stressed conditions, hence, supports plants under stressed soils. Moreover, larger roots offer the extra home to microbial communities (including inoculated bacteria) to dwell and ultimately facilitate in nutrient recycling and therefore more availability of nutrients to plants. This fact was further validated in the plant inoculation experiment where inoculation with the P. mirabilis strain R2 produced maximum dry weight at 50% WHC (Figure 3D).
All three bacteria showed ACC deaminase activity as well as catalase enzyme activity. ACC-deaminase regulates the ethylene levels during stress (Singh et al., 2015) while catalase helps in maintaining reactive oxygen species (ROS; Ali et al., 2015; Sharma et al., 2012) and therefore enhances plant survival under stress (e.g., biotic and abiotic stress, hydrocarbons, heavy metals, salts, water, temperature, light). These characteristics of the bacteria further support their potential for use as inocula under stress conditions.
The three PGPR (R2, RF-2, RF-4) showed the potential of multiple mineral (P, Zn, K, Ca) solubilizations, hence, have great potential for facilitating in the uptake of macro/micronutrient in plants under stressed conditions. It is reported that in vegetated soils, many nutrients are readily converted to insoluble forms after field application (Khan et al., 2007) and are not available for plant uptake subsequently leading to drastically affected plant growth. The mineral-solubilizing bacteria screened in this study are able to solubilize a range of bound minerals releasing various nutrients, for example,, P, Zn, Ca, K, etc. in the rhizosphere for plant uptake with subsequent plant growth promotion. Mineral solubilizing bacteria also enhance the microbial community (Arzanesh et al., 2011) with pronounced positive effects on plant growth (Hanif et al., 2020; Martínez-Rodríguez et al., 2014). It has been reported that mineral solubilizing bacteria also alleviate stress, for example, in stress microbial calcite degradation releases Ca2+ in the rhizosphere and subsequent intracellular Ca2+ level elevation leads to transcriptional activation of stress-responsive genes (Kudla et al., 2010; Reddy et al., 2011). Similarly, nitrogen is an essential macroelement required for plant growth and development under normal as well as stressed conditions and bacterial (biological) nitrogen fixation (BNF) significantly contributes to plant growth, but BNF activity usually decreased under stress. The P. balearica strain RF-2 and the C. Sakazaki strain RF-4 showed growth in nitrogen-free medium demonstrating their ability to fix atmospheric nitrogen and improve plant nitrogen contents. However, an acetylene reduction assay is required before the final validation of these putative diazotrophs.
We hypothesized that inoculation with stress-tolerant PGPR may provide protection to seeds and help in seed germination because seed germination in many crops is severely affected under drought (Chandra et al., 2004). PGPR-inoculation showed increased seed germination and seed vigor (Gholami et al., 2009). The results of the present study demonstrate that R2-inoculated plants at 5% PEG showed maximum Promptness index (PI) while noninoculated plants at 10% PEG stress showed minimum PI, which validates that PGPR-inoculation improves seed germination under normal as well as water stress conditions (Figure 2A). PI and GSI indicate the drought-tolerance and increase in these indices supporting the concept that bacterial inoculation helped wheat seeds to withstand and germinate under stress. In wheat and triticale, seeds with higher GSI and PI show higher drought tolerance (Mohammadi et al., 2003; Zarei et al., 2007) as both indices are quantitative measurements to characterize drought tolerance (Razzaq et al., 2017).
The analysis of the effect of varying water stress levels maintained in sand shows that the growth of the inoculated plants even at 50% and 37.5% WHC is significantly better compared to noninoculated plants with or without water stress. Visual plant growth is an indication of plant health and the inoculated plants showed better growth than noninoculated plants (Figure 3). Dry weight and leaf area of R2 inoculated plants were at their maximum at 50% WHC compared to other bacterial inoculation treatments and even 100% watered plants. This can be attributed to the IAA-production ability of R2 as described earlier. The data shows the potential of these bacteria even at very low water content to adjust plant growth.
The analysis of soil-grown inoculated wheat plants under stress shows that RF-4 inoculated plants exhibited minimum injury and electrolyte leakage, consequently, higher cell membrane stability (Figure 4) and leaf water contents. Cell membrane stability and electrolyte leakage (EL) are indications of plant stress tolerance and a measure of stress-induced injury (Lee & Zhu, 2010). EL is always accompanied by the production of ROS caused by oxidative damage to plants (Chen et al., 2008) but the catalase, polyphenol oxidase, or the cytosolic ascorbate peroxidase activity of RF-4 might be helped to destroy ROS damage and retain higher water content. It is already reported that PGPR play a significant role to counteract the oxidative and osmotic stresses due to drought stress and help in the physiological adjustment of the plant under these conditions (Gou et al., 2015; Grover et al., 2014; Sandhya et al., 2010), ultimately leading to enhanced crop productivity. Although R2 and RF-2 inoculated plants did not show physiological adjustments in other experiments, but their inoculation showed significantly higher leaf area and dry weight which can be attributed to their multiple PGP traits and stress-tolerance. Together, the results of our in vivo and in vitro plant inoculation experiments validated our hypothesis that inoculation with stress-tolerant PGPR may provide protection to plants under stress.
The effect of PGPR on physiological markers of drought stress was examined by the expression of genes encoding cAPX and the Rubisco large and small subunits (rbcL and rbcS). In the C. sakazakii strain RF4 treated plants the expression of cAPX and rbcL was significantly higher, showing the physiological adjustment during the drought stress because cAPX acts as a quencher for ROS produced in stress (Ren et al., 2016). In the P. mirabilis strain R2-treated plants the level of cAPX expression was significantly lower as compared to the control. On the other hand, the expression of rbcS was maximum in RF-2 inoculated plants. Both rbcL and rbcS genes in noninoculated plants decreased gradually as the drought prolonged, showing that the photosynthetic activity is being affected due to water stress as down-regulation of Rubisco is usually observed under stressed conditions (Bagherikia et al., 2019).
ROS in plants are neutralized by enzymatic and nonenzymatic scavengers like catalase (CAT), peroxidase (POD) and polyphenol oxidase (PPO), superoxide dismutase (SOD), ascorbate peroxidase (APX), and their activities are altered due to drought (Agarwal & Pandey, 2004). We found a significant decrease in the activity of the CAT enzyme by bacterial inoculation (except in mix bacterial inoculation at 2 dpi), a gradual decrease in the activity of PPO from day 2 until 16 in the inoculated treatments (except in R2 inoculated plants at 10 dpi and RF-4 inoculated plants at 12 dpi), and marked variations in the activities of POD and SOD. These decreased activities may be attributed to the inherent genetic variability, multiple PGP, and stress tolerance traits of inoculated strains. Contrary to our results, it was reported that the activities of these enzymes are increased due to ROS scavenging during most of the drought stress. Although it is known that CAT activity in leaf tissues may decrease in some stress conditions (Azevedo et al., 1998; Hertwig et al., 1992) because the CAT protein is prone to photo-inactivation upon exposure to intense light (Streb et al., 1999). Furthermore, PGPR strains have been shown to significantly enhance APX, SOD, and CAT, POD enzyme activities under drought stress (Gururani et al., 2013; Kohler et al., 2008) with a significant relation between drought stress and antioxidant enzyme activity (Han & Lee, 2005; Ullah et al., 2019). The data sets show high variation in leaf confronting stresses, so it is too complex and difficult to elucidate any relationship of these enzymes with the bacterial inoculation treatments. A clearer picture of enzyme activities can be obtained by the analysis of activities just after the induction of drought during the early hours and analysis of more enzymes as well as including roots in the assays which are the first organ of the plant confronting drought stress.
The study concludes that the utilization of PGPR is a user-friendly and cheaper technology as compared to available systems (e.g., variety development, gene transformation, or genome editing tools) for increasing stress tolerance in plants. The results of the present study demonstrate that the multi-stress tolerant PGPR have multiple plant beneficial traits and the potential to support wheat growth individually or in the form of a consortium and alleviate water stress by adjusting the physiological and enzymatic systems of plants. Besides, these bacteria could make plants healthy and strong by providing enough phosphate, zinc, calcium, nitrogen, and protection against various pathogens. These microbes or their consortium exhibit substantial potential for wheat growth in lab studies and among all the effects, those by the C. sakazakii strain RF-4 were found maximum. However, further research is required for field validation under different stresses (e.g., salt and the combined effect of drought and salt), biosafety and ecological safety (especially for C. sakazakii) before recommending them for commercial inoculum production. The same strategy may be adapted to screen bacteria for growing other crops that require high amounts of water or growing crops under salts.
ACKNOWLEDGMENTS
The work mentioned in the manuscript is part of M.Phil Research work of the 1st and 2nd Authors and was not financially supported by any funding agency. The funds already available at MEL, National Institute for Biotechnology and Genetic Engineering (NIBGE) were utilized to carry out this research work.
AUTHOR CONTRIBUTIONS
Rabisa Zia and Muhammad Shoib Nawaz carried out the basic experimentation and wrote the first draft, Sumaira Yousaf helped in enzyme analysis, Imran Amin helped in RNA work, Muhammad Shoib Nawaz helped in drafting and bacterial isolation, Asma Imran1 designed, supervised the studies and finalized the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




