Peroxidase activity and operation of photo-protective component of NPQ play key roles in drought tolerance of mung bean [Vigna radiata (L.) Wilcziek]
Edited by: P. Ahmad
Abstract
Developing drought-tolerant cultivars is mainly restricted due to poor knowledge of the mechanism behind drought tolerance. In the present work, available germplasm of Vigna radiata (mung bean) was screened for drought tolerance using multiple agronomic and physiological parameters and used to selected one drought-tolerant (NM-13-1) and one drought-sensitive (NM-54) cultivar for further studies. Plant water status and PSII activity were found to be potential physiological discriminating traits. Changes in PSII and PSI activity, accumulation of proline, oxidative damage, and antioxidants were further assessed in selected drought-sensitive and drought-tolerant cultivars. Drought stress reduced PSII efficiency and electron transport in both mung bean cultivars. Drought increased NPQ and Y(NPQ), a greater increase in NPQ and Y(NPQ) was found in the drought-tolerant cv NM-13-1, indicating that the drought-tolerant cultivar managed over-excitation of PSII by safe heat dissipation via photo-protective component of NPQ. A decrease in PSI efficiency with an increase in donor end limitation of PSI in both mung bean cultivars further confirmed that the electron transport through PSII became down-regulated. However, the drought-sensitive cv. NM-54 had poor ability to manage over-excitation of PSII through buildup of Y(NPQ) thereby causing greater oxidative stress. Mung bean cultivars counteracted oxidative stress by accumulation of proline and increasing POD activities. Drought-tolerant cv. NM-13-1 had higher proline accumulation and antioxidant potential than in the drought-sensitive cultivar. Overall, drought tolerance in the mung bean cultivars can be related to plant water status, PSII activity, Y(NPQ), and POD activity, which can be effectively used for selecting mung bean cultivars for drought tolerance.
1 INTRODUCTION
Crop yield is severely affected by drought worldwide. Crop improvement for drought tolerance is of great importance to meet the food demand of the future. However, limited progress has been made in this area during past 50 years due to limited knowledge about the mechanism of drought tolerance in plants and effective methods for selection (Blum, 2014; Blum & Tuberosa, 2018). Drought-tolerant plants are able to survive desiccation either by maintaining plant water status or by maintaining the metabolic activity even at low plant water status (Blum, 2017; Morgan, 1984). Plants produce specific stress proteins to protect the sub-cellular structures or membranes (Blum, 2011). However, sometimes components of these mechanisms (physiological indicators) play a role in drought tolerance such as photosynthetic activity, antioxidant potential, etc. (Berger et al., 2016). For example, some plants lowers the oxidative stress by lowering the reactive oxygen species (ROS) through regulating electron transport and safe dissipation of heat to protect the thylakoidal membrane protein complexes in both photosystems (Foyer, 2018). Some plants increase the amount and activity of antioxidant enzymes to scavenge the ROS rather than down-regulating electron transport (Foyer, 2018; Foyer & Shigeoka, 2011). However, the later approach requires metabolic energy for the biosynthesis of antioxidant enzymes.
Reduction in photosynthesis due to drought stress is generally attributed to destruction of photosynthetic pigments, changes in activity of PSII and PSI, reduction in stomatal conduction, influx of CO2 through stomata and its fixation by Calvin Cycle enzymes (Ashraf & Harris, 2013; Athar & Ashraf, 2005; Lawlor & Tezara, 2009). To monitor the activity of PSII, electron transport, and safe dissipation of heat (photo-protective component of nonphotochemical quenching, NPQ), the use of pulse amplitude chlorophyll fluorescence meters in response to drought stress has become a powerful and popular physiological tool (Bresson et al., 2015; Brestic et al., 2016; Kauser et al., 2006). In view of this information, it was hypothesized that crop cultivars differing in drought tolerance may also differ in their regulation of photosynthetic efficiency.
Mung bean [Vigna radiata (L.) Wilcziek] belong to the family Leguminosae, and is a rich source of protein, carbohydrate, minerals, and vitamins and is specially a good meat replacer protein for vegetarian. It is highly sensitive to drought stress. Thus, the purpose of the current study was to assess to what extent down-regulation of electron transport through PSII and PSI play a role in lowering in the generation of ROS and mediating drought tolerance in mung bean cultivars at the vegetative stage. Moreover, whether such photosynthetic attributes measured through slow chlorophyll a fluorescence kinetic analysis at various intensities of photosynthetically active radiation (PAR) could be used as potential physiological indicators for drought tolerance.
2 MATERIALS AND METHODS
A pot experiment was conducted at the Botanic Gardens of Bahauddin Zakariya University Multan, Pakistan (30°15 N and 71°30 E) during the spring season from March to May, 2018. The average photoperiod during whole experiment was 12–13.7 h with average high-temperature 28.5–40.4°C. Seeds of 10 mung bean cultivars were obtained from the Nuclear Institute of Agriculture and Biology (NIAB) Faisalabad. Seeds were surface sterilized by using 5% sodium hypochlorite solution for 5 min and then rinsed with distilled water thrice before sowing. Ten seeds of each cultivar were sown in plastic pots (30 × 30 cm) filled with 9 kg garden soil. Ten days after sowing, germinated plants were thinned to four plants per pot keeping in mind that plants were equidistantly placed and were of uniform size. The experiment was arranged in completely randomized design with 10 cultivars, two drought treatments (Control and Drought), and three replicates. The seedlings were irrigated with tap water. Two weeks after germination, half the set of plants in pots were subjected to drought stress by withholding water, while the control set was irrigated continuously at regular intervals with tap water till the end of the experiment. Various physiological and biochemical parameters were determined/quantified under drought stress following standard laboratory standard protocols. Based on growth and physiological attributes, one drought-tolerant, and one drought-sensitive mung bean cultivar were chosen for the next experiment. The second experiment was conducted in the same way as mentioned above, except only two cultivars (one drought-tolerant and one drought-sensitive) were used. However, in the second experiment, rapid light curves for PSII and PSI efficiencies, oxidative stress as H2O2, oxidative damage as malondialdehyde (MDA) content, and antioxidant potential were also measured in addition to general physio-biochemical attributes.
2.1 Leaf relative water contents
Fully mature 3rd leaf was taken from the top of the plants of each cultivar, and these leaves were used for all analysis. After measuring fresh weight the samples were dipped in distilled water for 4 h and then leaf turgid weight (TW) was recorded. The leaves of each cultivar were oven-dried at 70°C for 48 h and the dry weight of each leaf was measured. relative water contents (LRWC) was calculated as RWC (%) = [(leaf fresh wt. – leaf dry wt.)/(leaf turgid wt. – leaf dry wt.) × 100].
2.2 Quantum yield
To measure quantum yield (QY) of PSII, fully mature but young leaf usually third leaf from the top was used. Measurements were taken by using FlourPen FP-100 MX-LM (Photon System Instruments). QY of PSII was measured on third day of drought and then continued to measure on daily basis till the termination of the experiment. The intensity of saturation pulse was 3000 μmol m−2 s−1 with 0.8 s width.
2.3 Photosynthetic pigments
Measurement of total chlorophyll contents was recorded using SPAD-502 (Minolta) a pocket-size instrument. Measurements were taken from fully expanded matured leaves of 5-week-old plants. Moreover, chlorophyll contents were also determined after extraction with acetone following the method of Arnon (1949). Leaf sample (0.2 g) from each cultivar was ground in 10 ml of 80% acetone in pestle and mortar and then filtered. The absorbance of the filtrate was recorded at 645, 663, and 480 nm on a double beam spectrophotometer.
2.4 Quantification of total soluble proteins and proline
Total soluble proteins were extracted in phosphate buffer and then measured by following Bradford (1976). However, proline contents in the leaves were estimated by following Bates et al. (1973).
2.5 Measurement of PSII and PSI efficiency, electron transport through PSII and PSI, and nonphotochemical quenching
Young but mature leaf from each plant was used for the simultaneous assessment of PSII and PSI efficiency. Before taking measurements, the leaf from each cultivar was dark adapted for 10 min by covering it with an aluminum foil. After 10 min dark adaptation, the aluminum cover was carefully removed and immediately the leaves were mounted to the measuring head of the DUAL-PAM-100 (Walz). Leaf absorbance for PSI at 830 and 870 nm was balanced first. A weak red light was used to measure Fo and then a saturated pulse of 8000 μmol m−2 s−1 with 0.8 s width was applied to measure Fm and maximum QY (Fv/Fm) of PSII photochemistry. Various intensities of red actinic light was used which increased stepwise from 0, 11, 18, 27, 58, 100, 131, 221, 344, 536, and 830 in 20 s intervals over 5 min. Quantum efficiency of PSII, electron transport through PSII, and nonphotochemical quenching (NPQ) were calculated. Photo-protective component of NPQ as Y(NPQ) and nonregulated heat dissipation as Y(NO) were also calculated. Similarly, changes in redox status of PSI were measured in analogy to Fo and Fm as Po, and Pm. The QY of PSI, electron transport through PSI, acceptor end limitation for QY of PSI and donor end limitation were also calculated following Klughammer and Schreiber (2008).
2.6 Hydrogen peroxide determination
Hydrogen peroxide (H2O2) was measured following Velikova et al. (2000).
2.7 MDA determination
Drought-induced oxidative damage was assessed by determining the amount of MDA in the leaf tissue, following the method of Cakmak and Horst (1991).
2.8 Catalase and peroxidase
Activities of catalase (CAT) and peroxidase (POD) were determined by following protocol of Chance and Maehly (1955).
2.9 Fresh and dry biomass
Plants of each cultivar were uprooted and separated into shoots and roots. Fresh biomass of shoots and roots of the plants were recorded. Harvested plant parts were dried at 70°C in oven for 1 week and their dry weights were recorded.
2.10 Statistical analysis
All mung bean cultivars examined in the present study were ranked in three classes (drought tolerant, moderate, and sensitive) according to the ranges of observations and general trends of data. Class intervals were determined as the difference between high and low values of data, scores were assigned to cultivars (1, 2, and 3) from highest to lowest in all attributes. Means and standard errors were calculated using MS Excel and the data were presented as bar graphs or XY scatter line graphs. The collected data were subjected to a two-way analysis of variance (ANOVA) using the CoSTAT (v. 6.5) computer package (Cohort). Means of each treatment were compared with LSD calculated at 0.05 probability level following Snedecor and Cochran (1980).
3 RESULTS
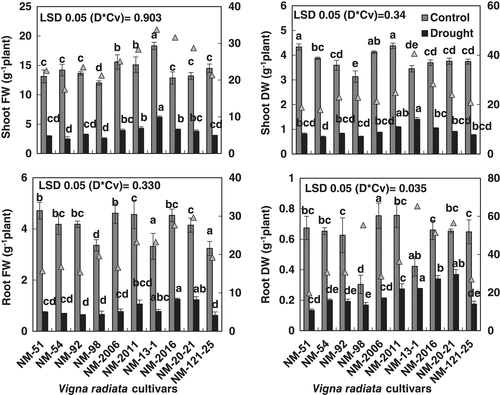
A pot experiment was carried out to select a drought-tolerant and a drought-sensitive cultivar of mung bean using growth and physio-biochemical attributes. Data presented in Figure 1; Table 1 showed that shoot fresh and dry weights of all cultivars decreased significantly (P ≤ 0.001) due to drought stress. However, reduction in shoot fresh and dry weights due to drought stress varied in all cultivars. Moreover, maximum reduction in shoot fresh and dry weights due to drought stress was found in cultivar NM-54, whereas the least reduction in biomass was observed in cultivar NM-13-1 (Figure 1). Likewise, drought-stressed plants of cv. NM-13-1 had the highest root fresh and dry weights, whereas NM-54 scored lowest with regards to these growth attributes (Figure 1).
| Source of variance | Df | Shoot fresh weight | Shoot dry weight | Root-fresh weight | Root-dry weight |
|---|---|---|---|---|---|
| Drought | 1 | 1808.500*** | 125.4848*** | 174.982*** | 2.139*** |
| Cultivars | 9 | 13.552*** | 0.2929*** | 0.573*** | 0.025*** |
| Drought × cultivars | 9 | 1.8594*** | 0.2965*** | 0.305*** | 0.011*** |
| Error | 40 | 0.2992 | 0.042 | 0.040 | 4.583 |
| Source of variance | Df | Chlorophyll a | Chlorophyll b | Chlorophyll a/b | Total chlorophyll | Chlorophyll (SPAD) |
|---|---|---|---|---|---|---|
| Drought | 1 | 1.2068*** | 0.1579*** | 0.397*** | 1.095*** | 118.962 ns |
| Cultivars | 9 | 0.0309 ns | 0.0057 ns | 0.0031 ns | 0.0518*** | 35.420*** |
| Drought × cultivars | 9 | 0.0313 ns | 0.0057 ns | 0.004 ns | 0.0402*** | 39.402* |
| Error | 60 | 0.0336 | 0.0067 | 0.0026 | 0.0102 | 18.882 |
| Source of variance | Df | Quantum yield of PSII | Relative water content | Total soluble proteins | Proline content |
|---|---|---|---|---|---|
| Drought | 1 | 0.0343*** | 4154.743*** | 32.854*** | 37093.2170*** |
| Cultivars | 9 | 0.2100*** | 1090.347*** | 1.395 ns | 1505.3612*** |
| Drought × cultivars | 9 | 0.0204*** | 1062.530*** | 1.698* | 1582.0287*** |
| Error | 60 | 0.002 | 4.489 | 0.719 | 214.0116 |
- Note: ns, nonsignificant; *, **, ***significant at 0.05, 0.01 and 0.001 probability levels, respectively.
Photosynthetic pigments (Figure 2; Table 1) reduced in all mung bean cultivars when grown under drought stress condition. Cultivars did not differ significantly in chlorophyll a, b, and a/b ratio under normal or water stress conditions. However, mung bean cultivars differed significantly (P ≤ 0.001) in total chlorophyll contents when exposed to drought stress (Figure 2; Table 1). In addition, drought-stressed plants of cv. NM-13-1 had greater total chlorophyll content than those in other mung bean cultivars.
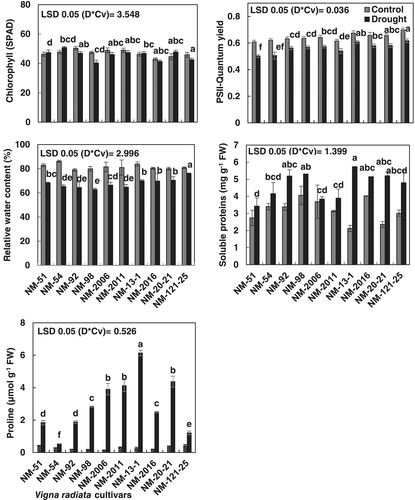
Imposition of drought stress caused a significant reduction in leaf relative water content (RWC) in all studied mung bean cultivars (Figure 2; Table 1). Mung bean cultivars differed significantly in RWC under drought stress conditions. Cultivar NM-13-1 had maximal RWC in their leaves under drought stress conditions, while cv. NM-54 was the lowest in this plant water status attribute. Drought stress significantly enhanced (P ≤ 0.001) the proline contents and total soluble proteins in the leaves of all cultivars of mung bean (Table 1). Cultivar NM-13-1 had higher proline and total soluble proteins content compared to all other cultivars under drought stress (Figure 3).

Correlation analysis presented in Table 2, indicated that growth attributes (fresh and dry weights of shoots and roots) were positively correlated with the RWC content, QY of PSII and total soluble proteins under drought stress. Whereas negative correlation was found for each of growth attributes with accumulation of proline. Significantly positive correlation was also found between QY of PSII and RWC and proline.
| SFWT | RFWT | SDWT | RDWT | RWC | TSP | Proline content | QY PS(II) | Chl. (SPAD) | |
|---|---|---|---|---|---|---|---|---|---|
| SFWT | 1 | ||||||||
| RFWT | 0.977 | 1 | |||||||
| SDWT | 0.964 | 0.976 | 1 | ||||||
| RDWT | 0.957 | 0.967 | 0.961 | 1 | |||||
| RWC | 0.803 | 0.790 | 0.808 | 0.751 | 1 | ||||
| TSP | 0.430 | 0.399 | 0.395 | 0.275 | 0.427 | 1 | |||
| Proline content | −0.065 | −0.010 | −0.028 | 0.071 | −0.205 | 0.061 | 1 | ||
| QY PS (II) | 0.816 | 0.758 | 0.755 | 0.783 | 0.743 | 0.560 | 0.269 | 1 | |
| Chl. (SPAD) | 0.248 | 0.224 | 0.271 | 0.316 | 0.464 | −0.276 | 0.136 | 0.324 | 1 |
- Abbreviations: Chl. (SPAD), chlorophyll (SPAD); QY of PS(II), quantum yield of photosystem II; RDWT, root dry weight; RFWT, root fresh weight; RWC, relative water content; SDWT, shoot dry weight; SFWT, shoot fresh weight; TSP, total soluble proteins.
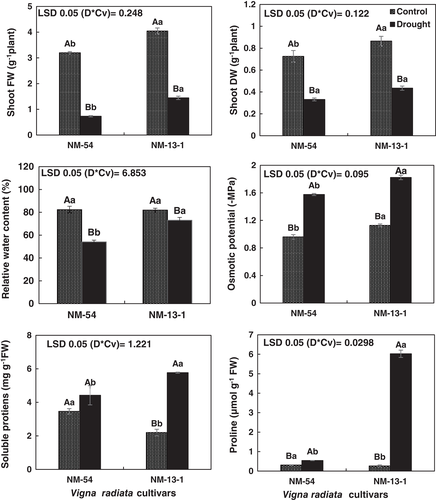
Based on growth, photosynthetic pigments, RWC, proline, and total soluble proteins the NM-13-1 cultivar was considered the most drought tolerant, six of the other cultivars were ranked as moderately drought tolerant while the three remaining cultivars were ranked as drought sensitive out of the 10 tested mung bean cultivars. It is important to mention here that if cultivars ranked based on only growth attributes two cultivars (NM-13-1, NM-2016) ranked as drought tolerant. Cultivar NM-2016 exhibited lower increase in total soluble proteins, proline, and intermediate cultivar in having RWC, QY of PSII, and photosynthetic pigments. Similarly, seven cultivars were ranked as drought sensitive based on growth attributes, but only two cultivars ranked the lowest for all physiological and biochemical attributes. Thus, it is preferred to select drought tolerant and sensitive cultivars based on combined and multiple physiological parameters which will help better in assessing physiological mechanism of drought tolerance. Since the drought-tolerant cultivar had more photosynthetic pigments and higher QY of PSII than in the drought-sensitive cultivar (Figure 3; Figure 4), detailed analysis of PSII and PSI activity in relation to generation of ROS, antioxidant activity were further assessed.
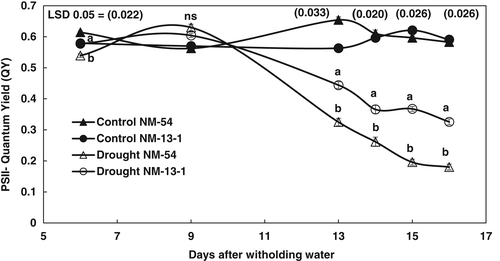
Photosynthetic efficiency of both mung bean cultivars was measured as QY of PSII over progressive drought stress. Reduction in QY of PSII was noted in both cultivars due to drought stress, and reduction in this attribute was more obvious on the 13th day after withholding water (Figure 5). In addition, QY of PSII was further decreased with increasing number of days of drought stress and cultivars also become different on 13th day of water stress) Drought-tolerant cv. NM-13-1 had higher quantum yield of PSII from 13–16th day of water stress than in drought-sensitive cv. NM-54 (Figure 5).
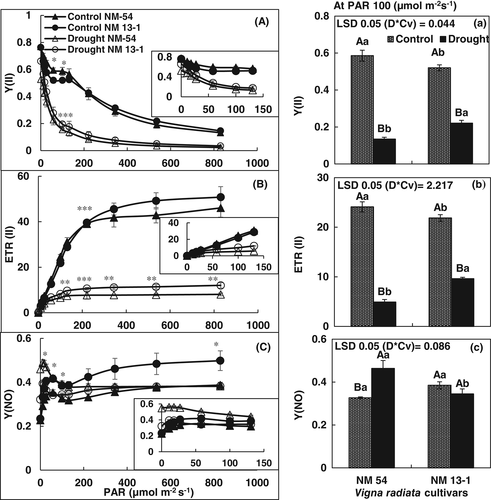
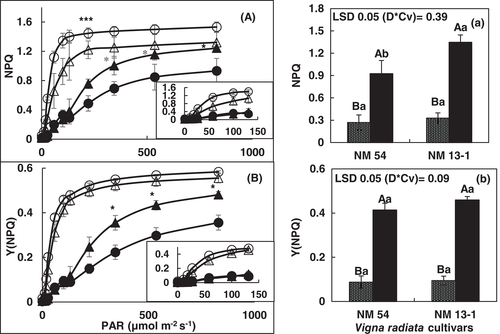
Rapid light response curve revealed changes in efficiency of PSII (YII), electron transport (ETRII), and heat dissipation as NPQ in both bean cultivars under normal and water stress conditions. QY of PSII decreased in both cultivars of mung bean with increase in light intensity under both normal and water stress conditions. However, up to 200 μmol m−2 s−1 PAR, there was a lesser decrease in Y(II) in both cultivars under normal conditions. At lower PAR, drought-sensitive cv. NM-54 had greater Y(II) than in drought-tolerant NM-13-1 under normal conditions. In contrast, under stress condition, Y(II) was significantly greater in drought-tolerant NM-13-1 than in drought-sensitive cv. NM-54 (Figure 6A). Moreover, cultivar differences in Y(II) at higher light intensity were not clear under normal or water stress conditions. ETR(II) determines relative activity of linear electron transport through PSII and drought stress decreased ETR(II) in both cultivars. However, greater decrease in ETR(II) was found in drought-stressed plants of cv. NM-54 as compared to cv. NM-13-1. Nonphotochemical quenching (NPQ) and Y(NPQ) increased due to drought stress in both cultivars. Development of NPQ at 200–800 μmol m−2 s−1 PAR is lower in drought-tolerant cultivar NM-13-1. In contrast, under water stress conditions values of NPQ were higher in drought-tolerant cultivar NM-13-1. However, NPQ in NM-13-1 was significantly higher at 221 μmol m−2 s−1 (Figure 7). QY of nonregulated energy loss as heat in PSII or Y(NO) increased at 200–800 μmol m−2 s−1 PAR intensity in drought-sensitive cultivar NM-54 under normal conditions. However, at 100 PAR, it was greater in drought-sensitive cultivar than in drought-tolerant mung bean cultivar under drought conditions (Figure 6). In mung bean cultivars, the acceptor-side limitation Y(NA) was generally low, becoming negligible at moderate-to-high light intensities, while donor-side limitation Y(ND) become increased (Figure 8).

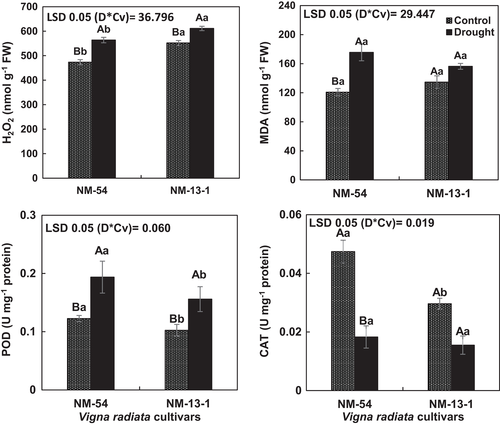
Drought stress increased the ROS (H2O2) and membrane damage (MDA) in both mung bean cultivars. Although membrane damage due to drought stress was greater in drought-sensitive cultivar, both mung bean cultivars were similar in ROS generation (Figure 9). Drought stress enhanced (P ≤ 0.005) the activities of peroxidase (POD) in both cultivars of mung bean. However, cultivar NM-54 had greater POD activity than in cv. NM-13-1 under drought stress. Drought stress reduced the CAT activity in both cultivars and the cultivars did not differ in CAT activity (Figure 9).
4 DISCUSSION
In the present study, considerable genetic variation for drought tolerance was observed in mung bean cultivars. However, based on growth attributes only two cv. NM-13-1 and NM-2016 were found as drought tolerant while seven cultivars ranked as drought sensitive. Since it is presumed that such genetic variability in drought tolerance in mung bean cultivars might have been due to variation in number of physiological and biochemical attributes, ranking of cultivars or screening for abiotic stress tolerance should be carried out on multiple physiological parameters as suggested earlier (El-Hendawy et al., 2005; Oyiga et al., 2016; Ulfat et al., 2007). Based on some of physiological attributes, such as RWC, proline, total soluble proteins, photosynthetic pigments, and QY of PSII, NM-13-1 ranked as drought tolerant and NM-54 as drought sensitive. Thus, discrimination between the mung bean cultivars for drought tolerance based on multiple physiological parameters along with growth attributes seems to be better than only on growth attributes. Although drought tolerance in 10 mung bean cultivars is positively associated with RWC, total soluble proteins, photosynthetic pigments, and QY of PSII, two attributes namely RWC and QY had greater contribution in drought tolerance (Table 2).
Drought tolerance is linked with maintenance of plant water status through osmotic adjustment or maintaining balance between water-uptake and loss of water through transpiration (Blum, 2017; Kauser et al., 2006). In the present study, drought-tolerant cultivar has greater ability to maintain water status, which is positively associated with accumulation of proline, and total soluble proteins. These results are similar to several studies in which it has been demonstrated that accumulation of osmoprotective substances such as soluble sugars, soluble proteins, and proline in leaves helped the plants in maintaining plant water status and drought tolerance in different plant species, for example, tomato (Shamim et al., 2013), canola (Kauser et al., 2006), and mung bean (Batra et al., 2014).
Photosynthetic capacity is very important as it directly contributes to plant growth and productivity under drought stress (Lawlor, 2002). Photosynthetic capacity of plants is represented by the amount of photosynthetic tissue, photosynthetic pigments, influx of CO2 through stomata, and its fixation efficiency and efficiency of solar energy conversion into biochemical energy (Ashraf & Harris, 2013; Dąbrowski et al., 2019). In this study, drought decreased the amount of photosynthetic pigments in mung bean. This can be explained by drought inhibited pigment biosynthesis or increased pigment degradation in mung bean cultivars as observed previously in mung bean (Batra et al., 2014). Decline in photosynthetic pigments may affect the efficiency of photosystem (PSII), which plays a key role in photosynthetic response to drought stress (Lawlor & Tezara, 2009). This study showed that QY of PSII decreased under drought stress which is indicative of photo-inhibitory damage (Athar & Ashraf, 2005; Golding & Johnson, 2003; Lawlor & Tezara, 2009). Drought stress caused a rapid disassembly of the light-harvesting complex II (LHCII). PSII dimers kept stable under the short-term drought stress but it decreased only after 15 days of drought stress in Arabidopsis (Chen et al., 2016). This suggested that PSII-LHCII super-complexes and LHCII assembly play a role in preventing photo-damages to PSII under drought stress (Chen et al., 2016). However, Foyer (2018) were of the view that continuous decrease in QY of PSII (Fv/Fm ratio) under stress conditions are more probably related to PSII downregulation via sustained components of protective NPQ and hence should not be used as a measure of PSII damage.
The maintenance of photosynthetic efficiency constitutes an important mechanisms of drought tolerance in plants. Efficiency of PSII and PSI measurements through rapid light curve or induction and relaxation analysis can provide detailed information about extent of PSII damage, electron transport, and contribution of photo-protective mechanism in stress tolerance of plants (Nath et al., 2017). Light response curve from our results showed that decrease in efficiency of PSII due to increase in light intensity (0–800 μmol m−2 s−1) is positively correlated with increase in electron transport rate through PSII and increase in nonphotochemical quenching under normal or drought stress in both mung bean cultivars. Drought stress reduced the ETR(II) and increased NPQ in both mung bean cultivars indicated that drought stress caused the accumulation of more electrons at the PSII acceptor side that leads to increase in nonphotochemical quenching. This increase in NPQ could be due either to an increase in the photo-protective component of NPQ or nonregulated photo-inhibition. In this study, photo-protective component or Y(NPQ) increased substantially due to drought stress in both mung bean cultivars and it is greater in drought-tolerant cultivar cv. NM-13-1. Although Y(NO) or photo-inhibitory component remained unchanged at higher PAR level in both mung bean cultivars due to drought stress, it was higher in drought-sensitive cv. NM-54 at lower irradiance level, that is, 11–100 μmol m−2 s−1. These results suggested that 16 days drought stress did not cause PSII photo-damage in the drought-tolerant cv. NM-13-1 cultivars and decrease in photochemistry was possibly linked with rapid buildup of photo-protective component of NPQ through xanthophyll cycle. Reduction in PSII efficiency and ETR(II) can also be reasoned to decrease the antenna size by degrading light harvesting complex proteins. This argument is supported by the fact that drought stress reduced several light harvesting complex proteins but increased the PsbS protein (a potential xanthophyll cycle mediator protein of PSII) in Arabidopsis after the long-term drought stress (Chen et al., 2016). Recently, Redekop et al. (2020) proposed that PsbS is transiently required during high light acclimation for the reorganization of thylakoid membranes and/or antenna proteins along with the activation of NPQ and adjustment of electron transfer characteristics, and that degradation of PsbS is essential in the fully high light acclimated state.
The buildup of NPQ is directly or indirectly related to the processes of light harvesting by the photosynthetic antenna complexes, their structure, captured energy transfer to reaction centers, electron transport, proton translocation across the membrane, ATPase activity, and carbon assimilation (Demmig-Adams et al., 2014). Golding and Johnson (2003) studied the effects of drought on the regulation of electron transport through photosystems I and II (PSI and PSII) in Hordeum vulgare L. cv. Chariot, they demonstrated that electron flow through PSII decreased in response to drought. This was due to regulation, as opposed to photo-inhibition. At the same time, nonphotochemical quenching (NPQ) increases, alleviating the excitation pressure placed on PSII. An increase in the proportion of PSI centers that are “active” (i.e., can be oxidized with a saturating flash and then rapidly re-reduced) under drought when NPQ is increased. Golding and Johnson (2003) suggested that these additional centers are primarily involved in cyclic electron transport, which generates the pH gradient to support NPQ and protect PSII (Golding & Johnson, 2003).
It has been proposed that the role of PSII damage is actually protection of PSI (Sonoike, 2011). In the present study, decrease in electron transport due to drought stress induce PSI nonphotochemical energy dissipation owing to the donor-side limitation and regulated energy dissipation in PSII, and maintained higher NPQ at PSII. In mung bean cultivars, the acceptor-side limitation was generally low, becoming negligible at moderate-to-high light intensities, while donor-side limitation become increased. The nonregulated dissipation Y(ND) represents the fraction of total P700 which is nonphotochemical energy dissipation in PSI (Klughammer & Schreiber, 1994) which is oxidized in given state (P700+/P700). Hence, it is a measure of the PSI donor-side limitation causing nonphotochemical energy dissipation in PSI (Klughammer & Schreiber, 1994). Furthermore, there was a fraction of P700 that was not reduced by saturation flash in a given state, that is, PSI acceptors Y(NA) that are not fully oxidized.
Oxidative stress in plants is closely linked to photosynthetic activity, as the transfer of electrons to oxygen can lead to ROS generation and oxidative stress, which cause photo-damage of PSII and PSI (Foyer, 2018; Foyer & Shigeoka, 2011). Imbalance between the donor and acceptor sides of photosystem I (PSI) can lead to inactivation of PSI (Lima-Melo et al., 2019). Several studies have revealed the importance of chloroplastic ATP synthase for PSI and PSII photo-protection through suppressing ROS production in PSI and adjusting the redox state of reaction center chlorophyll in PSI during photosynthesis (Foyer & Shigeoka, 2011; Lawlor & Tezara, 2009; Sonoike, 2011). Recent findings reported by Lima-Melo et al. (2019) that a partially inhibited PSI pool can support normal CO2 metabolism, inactivation of PSI may be more affordable than commonly thought.
The activities of antioxidant enzyme generally increase to scavenge ROS such as H2O2 and O2•−. In this study, activities of CAT decreased in mung bean cultivars under drought stress. These results are similar to those of Sharma and Dubey (2005) who reported a decrease in CAT activity in rice seedlings due to drought stress. Similarly, combined salt and drought stress decreased the CAT activity in Glycyrrhiza uralensis seedlings (Pan et al., 2006). In this study, activities of POD increased in drought-stressed plants of both mung bean cultivars. Moreover, activity of POD was greater in the drought-tolerant NM-13-1 than in the drought-sensitive mung bean cultivar NM-54. The drought-tolerant cultivar also had lower MDA content than the drought-sensitive mung bean cultivar. These findings suggest that the POD activity in the drought-tolerant cultivar NM-13-1 had a prominent role in scavenging ROS and drought tolerance. Peroxidase activity played a key role in decreasing H2O2 content and differential drought tolerance in wheat cultivars (Csiszár et al., 2012), pea (Mittler & Zilinskas, 1994) and in transgenic tobacco (Faize et al., 2011).
5 CONCLUSIONS
Drought stress reduced the growth of mung bean cultivars by reducing plant water status, oxidative damage, and photosynthetic activity. Greater drought tolerance in cv. NM-13-1 was mainly due to its better ability to maintain RWC and proline accumulation, manage over-excitation of PSII by heat dissipation safely through photo-protective component of NPQ, regulate the electron transport through PSII and PSI, as well as enhanced peroxides activity.
ACKNOWLEDGEMENT
The research work presented is a part of PhD thesis work of Mrs. Hussan Bano (PhD scholar).
AUTHOR CONTRIBUTION
Habib-ur-Rehman Athar, Zafar Ullah Zafar, and Muhammad Ashraf contributed to experimentation, Hussan Bano and Zafar Ullah Zafar contributed to data analysis, Hussan Bano, Habib-ur-Rehman Athar and Zafar Ullah Zafar contributed to data validation, Chukwuma C. Ogbaga, Habib-ur-Rehman Athar, and Muhammad Ashraf contributed to first draft preparation and funding acquisition, Hussan Bano and Habib-ur-Rehman Athar contributed to final draft preparation, review, and editing. HRA, ZUZ and MA concieved the idea and experimental design, HB and ZUZ performed the experimentation, and did the data analysis, HB, HRA, and ZUZ validated the data, HB and CCO prepared the first draft, HB, HRA, and MA revised the manuscript. HB, HRA and MA edited the final version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




