Chemical composition and reproductive functionality of contrasting faba bean genotypes in response to water deficit
Abstract
Water deficit (WD), a major contributor to yield reductions in faba bean (Vicia faba), is a complex phenomenon that varies across daily to seasonal cycles. Several studies have identified various morphological and physiological indicators of WD tolerance, which generally show limited water use during WD. Limited information is available on the impact of WD on nutrient content and reproductive biology of the faba bean. We studied carbohydrates, amino acids, mineral nutrients and the abundance of naturally occurring carbon isotopes (δ13C) in leaf and grain tissues of faba bean genotypes grown under well-watered (WW) and WD conditions. δ13C of leaf tissues were found to indicate changes in water use due to WD but this was not reflected in grain tissues. Nutrient concentrations with regard to amino acids and minerals were not influenced by WD. However, carbohydrate accumulation was found to be significant for WD, specifically through the presence of a higher concentration of myo-inositol in WD leaf tissues. Alternatively, sucrose concentration in grain tissues was reduced under WD treatment. WD hampered reproductive functionality by reducing pollen viability and germination with the severity and duration of stress and this reduction was less prominent in the drought-tolerant genotype (AC0805#4912) compared to the sensitive one (11NF010c-4). It was also demonstrated that WD caused developmental impairment in the stamen and pistil, where the pistil appeared more sensitive than stamen. These findings suggest that WD impairs pollen viability and pistil function reducing yield volume, but the nutrient content of the resulting yield is not significantly affected.
Abbreviations
-
- ANOVA
-
- analysis of variance
-
- GC-QQQ
-
- gas chromatography-triple quadrupole
-
- WD
-
- water deficit
-
- WUE
-
- water use efficiency
-
- WW
-
- well watered
1 INTRODUCTION
Faba bean is one of the important cultivated grain legumes grown over a wide range of environments. The yield of faba bean has doubled over the past 50 years whereas the planted area declined by almost half (Foyer et al., 2016). The most common factor for this decline was irregular rainfall over the growing season. Faba bean is sensitive to the effects of WD with yield losses recorded as much as 50% (Mwanamwenge et al., 1999). Consequently, drought tolerance in faba bean has been investigated via physiological characterisations and biological markers (Annicchiarico & Iannucci, 2008; Khan et al., 2007; Khazaei et al., 2013), with great potential for successful drought tolerance screening for multiple faba bean genotypes (Ammar et al., 2015; Kabbadj et al., 2017).
Water deficit (WD) is a primary factor among a range of environmental conditions associated with the predicted effects of climate change that will negatively impact global crop production (Hussain et al., 2018). Yield reduction due to WD is influenced by the interactive effects of crop growth stage and stress severity. Plants deploy an array of physiological and biochemical strategies to cope with WD (Anjum et al., 2017; Anjum et al., 2017). Physiologically, stomata play an important role in regulating the gaseous diffusion of carbon dioxide and water. To investigate the impact of WD, tools that can quantify the physiological and biochemical changes in different plant organs (leaf, grain and root) need to be developed to promote and predict crop production under limited water environments. One common response of plants against stress conditions is to accumulate minerals and synthesize osmoprotectants in the form of stress metabolites (Samarah et al., 2004). Among many functions, the accumulation of such compounds assists in the maintenance of cell turgor pressure during WD conditions (Hare et al., 1998). The identity of stress metabolites varies among plant genera, although they commonly belong to groups with similar physiochemical properties. Rapid accumulation of metabolites in plant tissues may indicate stress conditions, either through the accumulation in response to the stress or as a consequence of it, while also influencing nutritional and/or energetic content. Identification of these metabolites may hold importance for the development of chemically based tools for the detection and/or mitigation of stress conditions.
In flowering plants, flower production followed by seed formation determines yield but is constrained among many cropping systems by the effects of abiotic stresses on seed development. WD hampers the productivity of all growing stages, although the incidence during reproductive and grain filling stage is most sensitive for legumes, causing significant yield loss (Fang et al., 2010; Kong et al., 2015). Considering the importance of faba bean as a food source, as feed and its importance in conservation agriculture, this study was undertaken to define how WD changes the concentration of metabolites in different plant tissues that underpin yield quality and quantity. As the response of leaves and seeds to abiotic stresses may follow opposite response mechanisms, enhanced or inhibited development patterns, the development of physiochemical tools for the evaluation of stress response mechanisms must consider the identity of tissues used in the performed analyses. To this end, we investigated the chemical and carbon isotopic composition of leaves and grains in contrasting faba bean genotypes during WD. These data may provide insights into plant physiological performance through established modelling techniques and nutritional status that may help to develop suitable management packages against drought stress and/or to indicate superior genotypes for selection in plant breeding programmes. We also focused on physiological changes of reproductive organs like pistils and pollen at anthesis in response to WD to explain how WD affects fertilization. Our objectives were (1) to identify biochemical indicators from leaf and grain tissues of contrasting faba bean genotypes; (2) to test whether WD can impact on nutrient concentration of grains; and (3) to examine the impact of WD on reproductive biology, that is, pollen viability, pollen germination and pistil function towards seed development. Combined, characterizing these properties will identify potential biochemical markers by assessing the impact of WD on the nutritional content of the overall yield of faba bean.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
This experiment was completed at the Centre for Carbon, Water and Food, University of Sydney. Faba bean seeds of three contrasting genotypes (AC0805#4912, 11NF010c-4 and PBA Warda) were chosen for their diverse leaf δ13C value in this experiment, obtained from breeding trails performed under field conditions at the University of Sydney's I. A. Watson Grains Research Centre, Narrabri (30°27′S, 149°80′E).
The temperature of the growth chamber was maintained at 25 ± 2°C for 14 h, and 15 ± 2°C for 10 h for light and dark period, respectively. Light intensity and relative humidity were fixed at 400 μmol m−2 s−1 and 60% ± 5%, respectively. Plants were grown in 9 L plastic pots filled with Osmocote potting mix (Scotts). No additional fertilizer was added. Plants were placed into a commercial potting mix that was embedded (5 g l−1) with a slow-release fertilizer consisting of a N, P, K mix (17.3, 3.5 and 6.8% by weight) and a comprehensive supply of micronutrients. All plants received the same nutrient supply. Seeds were germinated in trays with the same potting mix and were transplanted into individual pots after three weeks. At planting, the potting mix in each pot was inoculated with Nodule N Strain WSM 1455, a traditional sterile peat formulation for seed coating prior to planting.
Evaporation and evapotranspiration were calculated to measure the amount of water to be applied in the well-watered (WW) and WD treatments. Pots filled with potting mix were flooded with water, then allowed to leach the excess water and weighed. The following day, approximately 24 h later, the pots were weighed again. The average difference in weights was used as an estimation of evaporation from the surface. Average evapotranspiration of a faba bean plant was calculated following the same process with the pots having faba bean plants. Transpiration was calculated from the difference between evapotranspiration and evaporation. This process was completed for five pots for each genotype. AC0805#4912 transpired 170 ml whereas PBA Warda and 11NF010c-4 transpired 210 ml and 233 ml, respectively. For treatments, eight pots were used for each genotype—four each for WW and WD treatments and each pot was considered as a replicate having a single plant. Thus, there were six treatment combinations (three genotypes and two water regimes) and each treatment and genotype had four replicates. Control plants were watered on a daily basis to full water holding capacity until final harvest. WD plants received full irrigation until the three-pod stage after which the irrigation was restricted for two weeks or until wilting was observed. WD plants then received 20% of transpiration until maturity to harvest seeds from the treated plants.
2.2 Tissue collection and carbon isotope analysis
Leaf samples were collected between 4:00 and 5:00 PM. From each pot, leaves were collected with a sharp razor and immediately put into a 2 ml Eppendorf tube and transferred into an icebox to reduce metabolism. Within 2 h, leaf samples were placed in an oven at 65°C for 48 h for complete drying. An oscillating matrix mill was used to grind the dried leaf samples. Approximately 3 mg of ground leaf was then placed into silver capsules (IVA Analysentechnik) and then analysed to determine the Δ and %N of samples according to the protocol outlined in (Smith, Fuentes, & Merchant, 2019). Grain samples were harvested at maturity and stored at −20°C for further analysis. Whole grain was initially ground with a traditional coffee grinder and further ground using an oscillating matrix mill. Subsequently, the same protocol was used for both N and C isotope analysis as described for leaf samples.
2.3 Quantification of soluble sugars, amino acids and nutrients
Soluble sugar and amino acid analysis were performed according to the protocol outlined in Smith, Fuentes, and Merchant (2019). For the extraction process, 40 mg of milled leaf/grain samples were poured into a screw cap microtube. The methanol, chloroform and water extraction was performed according to the protocol outlined by Merchant, Richter, et al. (2006). Samples were stored at −80°C for further gas chromatography triple quadrupole (GC-QQQ) mass spectrometer analysis, which was performed according to Merchant, Tausz, et al. (2006). Soluble sugar and amino acid determination from extracted leaf samples was completed on an Agilent 7890A GC-QQQ mass spectrometer with a HP5 column (Agilent Technologies).
Determination of soluble nutrients in extracted samples was completed using an inductively coupled plasma optical emission spectrometer (Varian Vista, Agilent Technologies; Smith et al., 2019). Samples were prepared with a dilution of 400 μl of the supernatant in 10 ml of ultra-pure Milli-Q water. The nutrients calcium (Ca), iron (Fe), potassium (K), magnesium (Mg), phosphorus (P), sodium (Na), sulphur (S) and zinc (Zn) were chosen for analysis. Any results lower than the detection limits of the instrument were not considered for analysis.
2.4 Pollen viability and pollen germination
In vitro pollen viability and pollen germination was measured at 0, 3, 6, 9, 12 and 15 days after the WD treatments. In general, viable pollen under stress conditions is an indication of stress tolerance. Five unfertilized flowers (not open) from each genotype and treatment were collected just before anthesis and placed in a petri dish with a cover and carried over to the laboratory for pollen viability analysis. Pollen grains were spread by a soft pointed paintbrush on microscope slides having a drop of 2% w/v carmine stain (Sigma C1022) in 45% acetic acid and covered with a coverslip to observe under the microscope (Ahmad & Martin, 2017). On each slide, pollen grains were counted and scored in three fields of view per slide (100 pollen grains per field) based on their stain retention. Unstained pollen grains were scored as non-viable. Similarly, five more flowers were collected from each genotype and treatment to carry out in vitro pollen germination assays. Pollen germination medium was prepared according to the recipe outlined in Bishop et al. (2016). Viability and germination were tested around 10:00 AM to 12:00 PM on each sampling day.
A reciprocal in vivo cross-pollination assay was also completed to determine the influence of WD on the pistil/pollen function. Twenty flowers from each genotype and treatment were emasculated at 4:00 PM to 6:00 PM on the previous day of pollination. The following morning approximately 12 h later, fresh pollen samples from fully opened flowers were used to pollinate those emasculated flowers according to genotype and treatment. Treatment combinations were as follows: WW + WW = stigmas of WW plants pollinated with pollen from WW plants, WW + WD = stigmas of WW plants pollinated with pollen from WD plants, WD + WW = stigmas of WD pollinated with pollen from WW plants and WD + WD = stigmas of WD pollinated with pollen from WD plants. Following pollination, each flower was tagged and the number of pollinated flowers and the total number of formed pods having seeds were recorded.
2.5 Statistical analysis
The effects of treatments on amino acids, soluble sugars, nutrient concentrations and δ13C were examined by a restricted maximum likelihood analysis using GenStat 18th Edition (VSN International, Hemel Hempstead). Analyses of variance (ANOVA) were calculated for the biochemical parameters under WW and WD treatments using a completely randomized design considering genotypes and water treatment as source of variance. Fisher's unprotected least significant difference test was used for post-hoc test (Fisher, 1960).
3 RESULTS
3.1 WD induced changes in isotopic abundance in leaves but not in grains
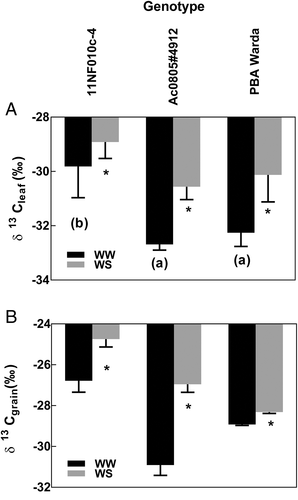
Significant genotypic differences were observed for both plant tissues, that is, leaves and grains. Among the plant organs, the leaf was enriched with δ13C significantly by WD for all genotypes (P < 0.05) at the three-pod stage (Figure 1A). Besides significant genotypic differences, a significant treatment effect was also observed for grain δ13C content among the tested genotypes (Figure 1B).
3.2 WD impact on nutrient composition in leaves and grains
Among the tested genotypes, we found significant (P < 0.05) differences for some of the detected nutrients in the leaf samples. ANOVA revealed that K, Na, P, S, Zn and %N significantly differed among genotypes (Table S1). All identified nutrients from WW and WD plants were non-significant except P, Mg and Ca, which was statistically significant for the genotypes 11NF010c-4 and AC0805#4912 (Figure 2A,B), and P and %N for PBA Warda (Figure 2C). Among the nutrients, K was found at the highest concentration followed by Ca and Mg. Fe was absent in leaf samples but detected at trace levels in grains.
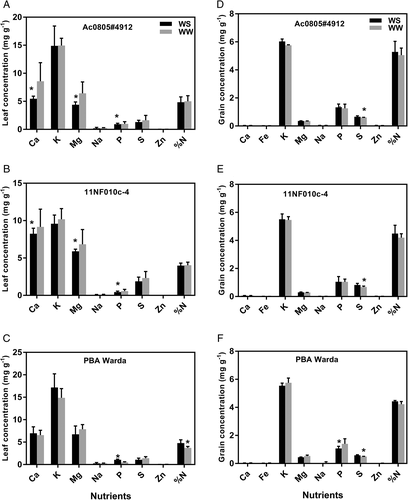
For grain nutrient abundance, Ca, Fe, K, Mg, S, Zn and %N were found to be statistically significant (P < 0.05) among tested genotypes (Table S1). Treatments significantly influenced S concentrations in grains for all tested genotypes with the presence of lower concentrations in WD treatments (Figure 2D–F). Exceptionally, PBA Warda showed a significant treatment effect for P (Figure 2F). For grain samples, K was found to be the most abundant nutrient followed by P and S. Percentage of N exhibited a higher value in grains than in the leaf but had no statistically significant difference between treatments.
3.3 WD impact on sugar abundance in leaves and grains
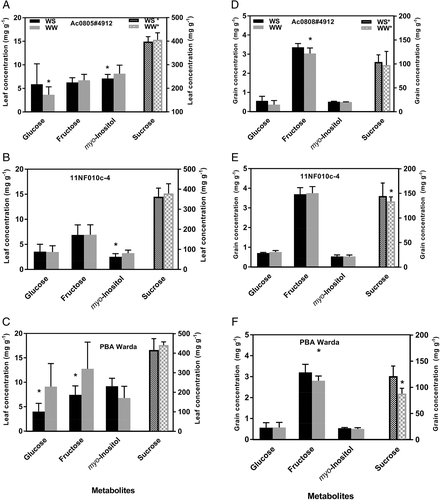
Individual sugar and polyol concentrations were similar for all tested genotypes in leaves and grains, among which there were few treatments or genotypic differences (Figure 3; Table S2). Sugar concentrations in the leaf soluble fraction were almost double that of the soluble grain tissues (Figure 3). Generally, leaf tissues from WD plants contained a higher concentration of sugars than grain tissues. In leaf tissues all the studied sugars were statistically significant except sucrose (P < 0.05). In grain tissues, sugar concentration was significantly higher in WW plants. Overall, sucrose concentration was found to be the most abundant, both in leaf and in grain tissues. In leaf tissues, myo-inositol responded against treatment for genotypes 11NF010c-4 and AC0805#4912 but not for PBA Warda (Figures 3A–C). In grain tissues, sucrose had a significant treatment effect (P < 0.05), that is WW plants had a higher concentration for all tested genotypes (Figures 3E,F) except of AC0805#4912. Glucose concentration was non-significant for all tested genotypes.
3.4 WD impact on amino acid abundance in leaves and grains
The concentration of amino acids varied between leaf and grain tissues (Figure 4). Leaf tissues contained almost 10 times higher amounts of amino acids compared to grain tissues. Thirteen amino acids were detected in leaf tissues: alanine, asparagine, aspartic acid, glutamine, glutamic acid, isoleucine, leucine, methionine, phenylalanine, proline, serine, threonine and valine. Proline was found to be the most abundant in leaf tissues for all tested genotypes (Figures 4A–C). Alanine and serine were found higher in WD plants for all tested genotypes (P < 0.05). In addition, asparagine, methionine, proline, serine, threonine and valine differed among treatments. Unlike in leaf tissues, only 10 amino acids were detected in grain tissues: alanine, asparagine, aspartic acid, glutamine, glutamic acid, isoleucine, leucine, phenylalanine, proline and valine. Among them, glutamine and phenylalanine were found to be the most abundant (Figures 4D–F). No statistically significant differences among treatments were observed in grain tissues for any amino acids. The concentrations of amino acids did not follow a regular pattern, neither among genotypes nor between treatments. Alanine, asparagine, methionine, proline and serine showed significant genotypic variations among three genotypes (Table S3). In addition, methionine, serine and threonine were completely absent in grain tissues of tested genotypes.

3.5 WD impact on pollen viability and pollen germination of faba bean genotypes
In vitro pollen viability was tested using an acetocarmine stain and counted as a percentage (Ahmad & Martin, 2017). Pollen viability of tested faba bean genotypes reduced with increasing severity of stress (Figure 5A–C). During the 2 weeks of stress imposition, pollen viability was more or less similar in WW and WD plants for the first 6 days. Pollen viability dropped sharply to almost 60% after 9 days of the stress treatment. Pollen viability for the tolerant genotype AC0805#4912 recorded 95% ± 2.16 and 49% ± 4.08 in WW and WD plants, respectively. The drought sensitive genotype 11NF010c-4 had the lowest pollen viability with 42% ± 1.70 after the stress period.

Pollen germination rates in WW and WD plants were similar for the pollen collected from the first 6 days of the treatment period (Figure 5D–F). Germination in WD plants had a sharp decline from Day 9 and was reduced by almost 33% at the end of the treatment period. After 2 weeks of the stress period, pollen germination for the drought-tolerant genotype AC0805#4912 was 83 ± 1.29% and 37 ± 2.38% in WW and WD plants, respectively. The drought-sensitive genotype 11NF010c-4 had similar germination rates in WW plants (82 ± 3.86%) but they were reduced in WD plants (30 ± 2.21%) compared to the drought-tolerant genotype AC0805#4912.
3.6 WD impact on the pod formation of faba bean genotypes
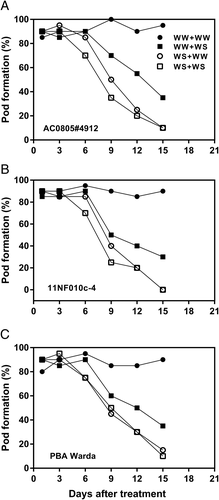
In vivo reciprocal cross-pollination was compared among combinations of treatments. Pollen from WW and WD plants were used to pollinate the stigma of WW and WD plants. A similar pattern of pollen viability and germination were also observed in pod formation. During the first 6 days of the treatment period, pod formation was more or less similar (80–85%) in WD plants irrespective of the genotypes. A slight reduction was observed among WD plants from Day 6. In the drought-tolerant genotype (AC0805#4912) 35% of pod formation was obtained when using pollen from WW plants to pollinate the stigma from WD plants but it was reduced to 10% when both (pollen and stigma) involved WD plants (Figure 6A). No pod formation occurred in the WD sensitive genotype (11NF010c-4) when WD flowers were pollinated with WD pollen (Figure 6B). However, when stigmas of WW plants were pollinated with the pollen from WD plants almost 30% pods were developed (Figure 6B).
4 DISCUSSION
Under the conditions experienced in this study, WD impacted plant nutrient and carbohydrate allocation as well as the viability of reproductive organ development (pistil and stamen). Within this response, genotypic variation in stress responses was observed among tested faba bean genotypes on physiological, biochemical and reproductive properties which may assist to identify superior genotypes and thus improve faba bean production in sub-optimal environments.
4.1 Impact of WD on isotopes, nutrients, carbohydrate and amino acid contents in leaves and grains
WD significantly increased leaf level δ13C of contrasting faba bean genotypes between WW and WD treatments. However, similar difference was also observed on carbon isotope compositions in grains (Figure 1A,B). The relatively small shift in isotopic abundance suggested carbon isotope fractionation did not significantly alter between leaves and grains; therefore, it can be used as a robust character to select plant materials for increased water use efficiency (WUE) irrespective of the source material. Our findings closely align both in magnitude and direction with previous studies in faba bean (Khan et al., 2007) and common bean (Smith, Fuentes, & Merchant, 2019). Availability of soil water and acquisition through roots are major determinants of nutrient accumulation and mobilization within and among plant tissues. One of the objectives of this study was to examine the biochemical changes that occur among three contrasting faba bean genotypes under WD conditions and to uncover robust biological indicators linked to improved plant growth under WD conditions. In general, nutrient concentrations in leaves and grains among different genotypes changed significantly in the current study. But the changes due to treatment effect were not significant. One possible explanation may be the prevalence of the isohydric response limiting water uptake, hence restricting the bulk flow of solutes from the soil. Typically, an isohydric response would restrict our ability to detect differences in WUE through the use of isotopes as significantly reduced carbon uptake would impede the transfer of an altered isotope composition into the carbohydrate pool. These results suggest that the severity of our WD treatment was sufficient to allow continued nutrient uptake but not exceed the threshold for plant mortality.
Overall, nutrient concentration did not change significantly in both leaf and grain tissues in response to treatments in the leaves (Figure 2A,B) and grains (Figure 2D,E). Such minor changes in nutrient concentrations demonstrated that faba beans could adjust under WD conditions by the maintenance of leaf water content. In our experiment, we found increased P in WW leaf tissues for the genotypes AC0805#4912 and 11NF010c-4. This may be due to the function of the stomatal apparatus and/or the genetic control of specific genotypes. Our study also agrees with previous results (Daoui et al., 2012) that P acquisition and consumption is controlled genetically among faba bean genotypes. Grain mineral content also had no significant treatment effect, except sulphur (S) accumulation and P for PBA Warda. WW plants of all studied genotypes had a higher concentration of S compared to their counterpart. Sulphur is crucial to plant function as it regulates the metabolism of S-rich amino acids like methionine and cysteine (Scherer, 2001). In our study, reduction in S due to WD conditions may lead to down-regulated metabolism of S containing amino acids which are also in agreement with Hawkesford and De Kok (2006). Accumulation of higher P content in grain of PBA Warda make the genotype stable against drought stress. The significant genetic variation for some of the nutrients in leaves and grains can be a good option for future nutrient enriched breeding programmes.
Generally, increases or decreases of a given carbohydrate depend on the species as well as the water content of the tissue under WD conditions (Amede et al., 2003). Increases in the concentration of a carbohydrate can occur due to enhanced synthesis, decreased metabolism or changes in the transport and allocation of carbohydrates to different tissues. Increased myo-inositol in WD plants observed here may be an osmoregulatory response. Myo-inositol is a significant osmoticum that was found in water stressed common bean leaf tissues (Lockhart et al., 2016) and faba beans (Amede et al., 2003). In our study, sucrose was the most abundant metabolite in both leaves and grains. Significant changes in sucrose concentration were only detected in response to WD in grain tissues. The synthesis and degradation of sucrose in faba bean is impacted by water status (Weber et al., 1996) and difficult to interpret on a background of inhibited growth and resource allocation. Nevertheless, our study aligns with the findings of Abid et al. (2017) where sucrose accumulates in stressed faba bean leaf tissues, presumably for osmotic adjustments. Differences in the impact of WD on sucrose concentrations in grains agree with previous observations by Cuellar-Ortiz et al. (2008) that carbohydrate partitioning is impacted by WD.
Amino acid composition of studied genotypes indicated the presence of 13 amino acids in leaf tissues and 10 amino acids in grain tissues, while no significant differences within treatments were observed for grain tissues. For leaves, two non-essential amino acids (alanine and serine) for human nutrition were found to be significant among genotypes (11NF010c-4 and PBA Warda) but they did not follow any specific pattern. For example, genotype 11NF010c-4 had a higher concentration in WW samples whereas PBA Warda had lower concentrations. Among detected amino acids, proline was the most abundant and its accumulation in response to WD in faba bean has been observed previously (Abid et al., 2017; Siddiqui et al., 2015). The present study did not detect any treatment effect for any amino acids in our study. A possible explanation may have been insufficient treatment severity to elicit significant changes in nutrient availability or simply that the nutritional concentration for each seed is genetically predisposed to achieve seed viability. Once a set nutrient content is achieved, priority is then given to other seeds.
4.2 Impact of WD on the reproductive biology of contrasting faba bean genotypes
The plants developmental stage under stress conditions has a critical influence on the yield. Faba bean plants are mostly prone to drought at the pod set and early pod filling stage, mainly as a consequence of plant size (Khan et al., 2010). Our study demonstrated that WD reduced in vitro pollen viability as well as germination with the progression of WD. Though pollen viability is mostly regulated by environmental factors (Johri & Vasil, 1961), our study provides evidence for genetic influence, demonstrated by higher pollen viability and germination in genotype AC0805#4912. A similar result was observed in chickpea plants for heat stress (Devasirvatham et al., 2012). In vegetative tissues, plants are able to adjust WD by reducing water loss or increasing water uptake, but no such mechanism was involved in reproductive tissues. Another reason for reduced pollen viability may be the lower water potential of flowers than that of leaves, which was also proven in soybean by Kokubun et al. (2001). The critical period for faba bean pollen viability and germination starts from approximately Day 6 after the WD treatment. Microsporogenesis is likely to be impacted by WD leading to reduced pollen viability and germination which is also observed in common bean (Shen & Webster, 1986). A substantial decrease in pollen viability and germination would lead to less seed formation and subsequently yield reduction. For example, in common bean (Shen & Webster, 1986) and chickpea (Fang et al., 2010) WD affects pollen viability, germination and pod development.
We also demonstrated that WD caused impairment in stamen and pistil viability where pistil was more sensitive than stamen. A reciprocal cross confirmed unsuccessful fertilization and the incapability of ovaries grown under WD to form pods irrespective of whether the pollen was from WD or WW treatments (Figure 6). Genotypic differences were observed and the drought-tolerant genotype AC0805#4912 was able to develop seeds (10%) when pistils of WD plants were pollinated with WD plants, but no pod formation was observed in sensitive genotypes in the same condition, that is, WD + WD (Figure 6A,B). This impairment suggested that stigma receptivity and functionality is more critical than pollen for pod development in faba bean. This has been observed by Fang et al. (2010) and Nayyar et al. (2005) in chickpea plants. This sensitivity is probably due to the presence of higher levels of abscisic acid in the female parts of the flower. Combined, these results illustrate the importance of reproductive biology and the critical stages of pollen, stamen and pistil viability which appear to be under significant genetic control.
5 CONCLUSIONS
Understanding the nutritional status of crops during stress conditions has significant consequences for plant adaptation strategies under changing climatic conditions. As a rainfed crop that is sensitive to WD, faba bean production is at risk due to changes in climate. Overall, the effect of WD on nutrient acquisition was likely a result of an isohydric response to reduced water availability, namely that severe reductions in water transport impeded nutrient uptake. With treatment-specific changes in their carbon isotope abundance, faba bean plants did not exhibit significant treatment differences in nutrient concentrations. The abundance of selected metabolites in leaves responded to WD treatments, while this was not observed in grains. Most critically for yield loss, WD significantly reduced the formation of viable pods due to reduced pollen viability and stigma receptivity. Genotypic differences exist for these traits, indicating selection is possible. While WD conditions are likely to reduce the volume of faba bean production, the nutrient content of that production appears resilient to its effects.
AUTHOR CONTRIBUTIONS
Md Abdul Muktadir conceived the ideas, designed the research, conducted the experiments, analysed the data and wrote the first draft. Kedar N. Adhikari and Andrew Merchant supervised the experiments, provided available resources and critically reviewed the manuscript. Nabil Ahmad assisted in designing a part of the research, provided resources to conduct experiments and critically reviewed the manuscript. All authors contributed to the subsequent development and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




