Overexpression of Os-microRNA408 enhances drought tolerance in perennial ryegrass
Nan Hang and Tianran Shi contributed equally to this study.
Funding information: National Natural Science Foundation of China, Grant/Award Number: 31472140; Natural Science Foundation of Beijing Municipality, Grant/Award Number: 6182025
Abstract
As a conserved microRNA (miRNA) family in plants, miR408 is known to be involved in different abiotic stress responses, including drought. Interestingly, some studies indicated a species- and/or cultivar-specific drought-responsive characteristic of miR408 in plant drought stress. Moreover, the functions of miR408 in perennial grass species are unknown. In this study, we investigated the role of miR408 in perennial ryegrass (Lolium perenne L.) by withholding water for 10 days for both wild type and transgenic plants with heterologous expression of rice (Oryza sativa L.) miR408 gene, Os-miR408. The results showed that transgenic perennial ryegrass plants displayed morphological changes under normal growth conditions, such as curl leaves and sunken stomata, which could be related to decreased leaf water loss. Moreover, transgenic perennial ryegrass exhibited improved drought tolerance, as demonstrated by maintaining higher leaf relative water content (RWC), lower electrolyte leakage (EL), and less lipid peroxidation compared to WT plants under drought stress. Furthermore, the transgenic plants showed higher antioxidative capacity under drought. These results showed that the improved drought tolerance in Os-miR408 transgenic plants could be due to leaf morphological changes favoring the maintenance of water status and to increased antioxidative capacity protecting against the reactive oxygen species damages under stress. These findings implied that miR408 could serve as a potential target for genetic manipulations to engineer perennial grass plants for improved water stress tolerance.
Abbreviations
-
- ABA
-
- abscisic acid
-
- APX
-
- ascorbate peroxidase
-
- CAT
-
- catalase
-
- EL
-
- electrolyte leakage
-
- MDA
-
- malondialdehyde
-
- miRNA408
-
- microRNA408
-
- POD
-
- guaiacol peroxidase
-
- ROS
-
- reactive oxygen species
-
- RWC
-
- relative water content
-
- SOD
-
- superoxide dismutase
-
- TG
-
- transgenic
-
- WT
-
- wild type
1 INTRODUCTION
As sessile organisms living in constantly changing environment, plants are often exposed to abiotic stress, such as drought, salt, and temperature stresses (Zhu, 2016), which leads to more than 50% yield loss in major crops worldwide each year (Boyer, 1982; Lobell et al., 2011). Meanwhile, due to global climate change and world population growth, water scarcity is already a critical concern in many parts of the world, and drought has become one of the most adverse factors of plant growth and productivity (Shao et al., 2009). Therefore, it is critical to breed drought-tolerant crops and study plants' mechanism in response to drought stress.
Plants can perceive abiotic stress signals and elicit appropriate responses by altering metabolism, growth, and development (Bartels & Sunkar, 2005). The primary signal caused by drought is hyperosmotic stress, which causes the accumulation of reactive oxygen species (ROS), damage to cellular components such as membrane lipids, proteins, nucleic acids, and metabolic dysfunction (Zhu, 2016). The hyperosmotic signal also increases the phytohormone abscisic acid (ABA) content in plants, which in turn elicits many adaptive responses including stomatal closure and regulation of dehydration-responsive genes (Fujita et al., 2011; Shinozaki & Yamaguchi-Shinozaki, 2007; Zhu, 2016). Many drought-inducible genes with various functions have been identified by molecular and genomic analyses, including genes encoding water channels transporters, detoxification enzymes, LEA (Late embryogenesis abundant) proteins, key enzymes for osmolyte biosynthesis, and so on, as well as a number of transcription factors and microRNAs that regulate stress-inducible gene expression (Shinozaki & Yamaguchi-Shinozaki, 2007).
MicroRNA (miRNAs) are a class of endogenous small RNAs of 19–25 nucleotide that regulate the expression of genes encoding transcription factors, stress response proteins, and other proteins that impact the development, growth, and physiology of plants at post-transcriptional levels in a range of organisms (Axtell, 2013; Rogers & Chen, 2013). Recent findings have demonstrated that some miRNAs play a role in drought stress response, such as participating in morphological changes, ABA response, auxin signaling, osmoprotection, and antioxidant defense, which can be used for crop genetic engineering, such as miR169, miR319 and miR408 (Ding et al., 2013; Ferdous et al., 2015; Golldack et al., 2011; Zhou et al., 2013). Here, we focused on miRNA408, a conserved miRNA firstly identified to target genes encoding copper-containing proteins in plants (Abdel-Ghany & Pilon, 2008). Later, it was also recognized as an important component of the HY5-SPL7 gene network with a role in plant copper homeostasis and response to light (Alaba et al., 2015; Yamasaki et al., 2009; Zhang et al., 2014). Previous studies also suggest that miRNA408 responds to multiple kinds of stresses and might somehow have different role in different species for drought stress. For example, overexpression of At-miR408 in Arabidopsis (Arabidopsis thaliana [L.] Heynh.) leads to improved tolerance to salinity, cold and oxidative stress by enhancing cellular antioxidant, but enhanced sensitivity to drought/osmotic stress possibly due to altered redox state (Ma et al., 2015). Similarly, in rice (Oryza sativa), Os-miR408 plays opposite roles in cold and drought, with an enhanced cold tolerance but a decreased drought tolerance (Sun et al., 2018). However, in chickpea (Cicer arietinum L.), it has been demonstrated that the higher expression of At-miR408 could enhance drought tolerance (Hajyzadeh et al., 2015). Moreover, heterologous expression of Anthurium andraeanum Aa-miR408 in Arabidopsis improved plant tolerance to various abiotic stresses, including salt, oxidative stress, and osmotic/water stress (Jiang et al., 2018). In addition, the expression of miR408 varies among plant species under drought/dehydration stress. For instance, drought up-regulates miR408 in Arabidopsis (Liu et al., 2008), barley (Hordeum vulgare L.; Kantar et al., 2010), barrel clover (Medicago truncatula Gaertn.; Trindade et al., 2010), rice (Balyan et al., 2017; Mutum et al., 2013), chickpea (Hajyzadeh et al., 2015), wheat (Triticum aestivum L.; Akdogan et al., 2016), Ipomoea campanulata (Ghorecha et al., 2017), peach (Prunus persica L. Batsch; Esmaeili et al., 2017) and A. andraeanum (Jiang et al., 2018), but down-regulates it in rice (Zhou et al., 2010), populus (Populus tomentosa Carrière; Ren et al., 2012), pea (Pisum sativum L.; Jovanović et al., 2014), cotton (Gossypium hirsutum L.; Xie et al., 2014), Arabidopsis (Ma et al., 2015), tomato (Lycopersicon esculentum Mill.; Candar-Cakir et al., 2016), wild rice (O. rufipogon Griff.; Zhang et al., 2016), and almond (Prunus dulcis Mill. D.A. Webb; Esmaeili et al., 2017).
Other than the inconsistent role of miR408 in drought/osmotic stress tolerance and/or response among different plant species, how miR408 functions in perennial grass species are not known yet, especially perennial forage grass or/and turfgrass. Here, we used perennial ryegrass, one of the most widely used perennial forage and turfgrass species in cool climate, to investigate the function of miRNA408 in plants' response to drought stress. And we sought to answer whether miR408 could improve drought stress performance in ryegrass and how it might function at morphological and physiological levels.
2 MATERIALS AND METHODS
2.1 Multiple sequence alignment and phylogenetic analysis
Multiple sequence alignment of the stem-loop sequence of selected miR408 genes was performed using the DNAMAN version 8.0 software under default parameters (Lynnon Biosoft). All the sequences were downloaded from miRBase (http://www.mirbase.org/; Griffiths-Jones et al., 2006, Kozomara et al., 2019), except the one of the perennial ryegrass lpe-miR408, which was retrieved from the deposited perennial ryegrass draft genome database at NCBI (National Center for Biotechnology Information; https://www.ncbi.nlm.nih.gov/genome/484?genome_assembly_id=283917) using BLAST search, with the rice, Arabidopsis, and Brachypodium distachyon miR408 genes as queries. A phylogenetic tree was subsequently constructed using the Neighbor-joining method with MEGA-X software with the following parameters: bootstrap test method (1000 replicates), maximum composite likelihood, and pairwise deletion (Kumar et al., 2018). All the RNA sequences from miRBase were converted into the DNA sequences before analysis.
2.2 Cloning of Os-miR408 and overexpression in perennial ryegrass
The genomic DNA fragment corresponding to pre-miR408 was amplified from genomic DNA of rice (O. sativa L. japonica. cv. Nipponbare) extracted from leaves using a modified CTAB (cetyl trimethylammonium bromide) method (Allen et al., 2006). The gene fragment was amplified by PCR using KOD Plus Neo (TOYOBO) and the forward & reverse primer set (Table 1) (Sun et al., 2018). The full length of precursor sequence of rice Os-miR408 was cloned into the binary vector pZH01 (Zhou et al., 2013) containing one CaMV 35S promoter driving Os-miR408 (35S::Os-miR408) and another CaMV 35S promoter driving the hyg gene (35S::HYG) for hygromycin resistance as a selectable marker (Figure 1). The plasmid was transferred into the Agrobacterium tumefaciens strain LBA4404 for plant transformation. The transformed Agrobacterium containing the overexpression construct of Os-miR408 was used to infect the embryonic callus induced from the mature seeds of perennial ryegrass “Citation Fore” (PureSeed). Transgenic plants overexpressing Os-miR408 were produced and maintained as described previously (Zhang et al., 2013).
| Primer name | Primer sequence (5′–3′) | Purpose |
|---|---|---|
| miR408-F | GGATGGCGTTGGCCTAACCGGATT | Gene isolation |
| miR408-R | CAACAGCAGAAAATGAGTGAACTT | Gene isolation |
| 408_XbaI-F | TCTAGA GGATGGCGTTGGCCTAACCGGATT | Vector construction |
| 408_SalI-R | GTCGACCAACAGCAGAAAATGAGTGAACTT | Vector construction |
| Hyg-F | TACTTCTACACAAGCATCGGTCCAG | PCR analysis |
| Hyg-R | CTTGACATTGGGGAGTTTAGCGA GA | PCR analysis |
| 408_RT-F | GGCTGTTCCCGTGCTTGTT | RT-PCR analysis |
| 408_RT-R | TCCTCGCCGTTGTTGCTG | RT-PCR analysis |
| EF1A-F | CCGTTTTGTCGAGTTTGGT | RT-PCR reference |
| EF1A-R | AGCAACTGTAACCGAACATAGC | RT-PCR reference |
| 408_StRT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGCCAGGGA | Stem-loop RT |
| 408_StRT-F | GGATGCACTGCCTCT | Stem-loop RT-PCR analysis |
| miR-Universal primer | GTGCAGGGTCCGAGGT | Stem-loop RT-PCR analysis |
| ACTIN-F | ATAAGAGAATCCGTGAGATCCCG | Stem-loop RT-PCR reference |
| ACTIN-R | GCTGTTTTCCCTAGCATTGTTGG | Stem-loop RT-PCR reference |
2.3 Identification of transgenic plants, isolation of plant RNA, and gene expression analysis
Genomic DNA was isolated from the putative transgenic and wild-type plants by the CTAB method. The PCR was used to screen for putative transgenic plants with the primers of the HYG gene (Table 1), using the following program: 2 min denaturation at 94°C, followed by 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s and completed with an extension step of 10 min at 72°C. Total RNA was extracted from plant leaves with the Trizol reagent and treated with RNase-free DNaseI (Invitrogen). First-strand cDNA was synthesized using SuperScript III Reverse Transcriptase Kit (Invitrogen) following the manufacturer's protocol. EF1A (ELONGATION FACTOR-1a) and ACTIN genes were used as internal references for assessing gene expression level in perennial ryegrass with the specific primers (Table 1). Semi-quantitative reverse transcription PCR (RT–PCR) was conducted using the following program with the primers (Table 1): 2 min denaturation at 94°C, followed by 35 cycles of 94°C for 30 s, 53°C for 30 s, and 72°C for 30 s and completed with an extension step of 10 min at 72°C. Stem-loop RT-PCR of mature miR408 was conducted using the following program with the primers (Table 1): 1 min denaturation at 95°C, followed by 30 cycles of 95°C for 30 s, 65°C for 15 s, and 72°C for 5 s and completed with an extension step of 5 min at 72°C.
2.4 Plant maintenance and drought stress treatment
Transgenic perennial ryegrass plants (TG) and wild-type (WT) controls grew in plastic pots (10-cm diameter, 8.5-cm depth) filled with a soil mixture of soil:peat:vermiculite (1:1:1) placed in greenhouse (25 ± 3 /15 ± 2°C, day [d]/night [n]) under normal water condition for 6 months. To study plant responses to drought stress, 24 pots (third pots/replications per treatment) of the clonally-propagated plants from tillers of three transgenic lines (TG1-1, TG5-2, TG7-1) and WT controls at the same growth status were transferred to growth chambers (22/16°C, 14/10 h, d/n, PAR [photosynthetically active radiation] at 450 μmol s−1 m−2) and adapted for 2 weeks before being subjected to drought stress treatment. Drought was imposed by water withholding for 10 days. After the stress, the plants recovered with sufficient watering for 14 days. To reduce the microenvironment effects, plants in the growth chamber were rotated daily. The grass plants were clipped and fertilized weekly to achieve uniform plant growth during the whole project except during the adaptation and drought stress periods. The leaf samples were collected at 0, 5, and 10 days after drought treatment. Samples were immediately frozen with liquid nitrogen and stored at −80°C until analysis except those for leaf relative water content (RWC), electrolyte leakage (EL), and malondialdehyde content (MDA).
2.5 Phenotypic analysis of Os-miR408 transgenic perennial ryegrass
Phenotypic measurements were performed in 6-month-old transgenic plants (TG1-1, TG3-2, TG5-2, and TG7-1) and wild-type plants grown under normal condition. We determined the length of the plant leaves by taking the first fully developed leaf of each tiller (counting from the top to the bottom of the plant) and measuring the length of the leaves from leaf collar to the leaf tip. The width of the leaves of the plants was also determined by taking the first mature leaf of each tiller and measuring the width of the widest part of the leaves. Twenty values were determined for each plant line.
2.6 Plant microscopic analysis and electron microscopy
For histological analysis, leaf samples were fixed in FAA (formaldehyde:glacial acetic acid:ethanol [1:1:18]) for 24 h at 4°C and then dehydrated in a graded ethanol series. Dehydrated samples in absolute ethanol were replaced with xylene, embedded in paraffin wax, and then sectioned at a thickness of 9 μm using a rotary microtome. The microtome sections were observed with a light microscope (SMZ25, Nikon). For scanning electron microscopy (SEM) analysis, the leaf samples at the same relative positions from the longest tiller of WT and TG plants were fixed in 2.5% (v/v) glutaraldehyde. Samples were sputter-coated with gold particles and the coated surfaces were viewed using a scanning electron microscope (SU8010, Hitachi).
2.7 Measurement of physiological parameters and ABA content
Plant chlorophyll content (represented by the measured SPAD index value) was recorded using a chlorophyll meter according to the user guide (SPAD-502Plus, Konica Minolta). Stomatal conductance was measured with a leaf porometer following the user manual (SC-1, METER Group). Leaf RWC was determined using 10 fully expanded leaves per plant per pot, according to Barrs and Weatherley (1962) with modification (DaCosta & Huang, 2006). EL was measured according to the method of Blum and Ebercon (1981) with some modifications (Wang & Jiang, 2007). Water loss rate (WLR) in detached leaves was monitored using the technique described by Ristic and Jenks (2002) with modification (Shi et al., 2012). Leaf proline was extracted and determined spectrophotometrically at 520 nm using the method of Bates et al. (1973). Lipid peroxidation of the leaf tissue was measured in terms of MDA content with an extinction coefficient of 155 mm−1 cm−1, and the activities of superoxide dismutase (SOD; EC 1.15.1.1), guaiacol peroxidase (POD; EC 1.11.1.7), and catalase (CAT; EC 1.11.1.6) were measured following previous protocols (Dhindsa et al., 1981; Wang et al., 2012). Leaf hydrogen peroxide (H2O2) was detected by the DAB (3,3′-diaminobenzidine) staining method (Ouyang et al., 2010), and the staining intensity was further quantified using Bio-rad Quantity One software. The method for extraction and purification of ABA was modified from previously described by He (1993) and Zhang et al. (2008). The content of ABA was determined by an enzyme-linked immunosorbent assay (ELISA) method following previous protocols (Yang et al., 2001; Zhang et al., 2009).
2.8 Experimental design and statistical analysis
The experiment was a randomized complete block design with three replicates (three pots of plants for WT and each line of TG perennial ryegrass). In the study herein, drought was not considered a treatment for statistical analysis, but more an abiotic stress to which all grasses were uniformly exposed. All measurements were analyzed using the samples collected at the sampling time mentioned above (0, 5, and 10 days after water withholding), unless otherwise stated. Data were analyzed using PROC GLM (SAS Institute, version 9.1). Treatment means were separated using Fisher's-protected least significant difference (LSD) test at a 0.05 significance level.
3 RESULTS
3.1 Phylogenetic relationship and conservation of miR408 family genes
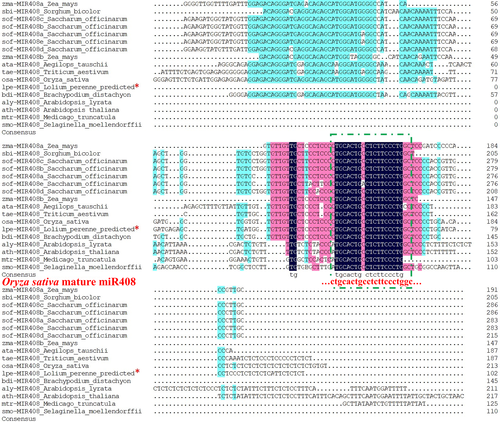
Fifty-three precursor miR408 (pre-miR408) sequences of different land plant species were used for phylogenetic analysis, and 17 of the 53 sequences were selected for sequence alignments to identify conserved regions/sequences. According to our analysis, the mature miR408 sequences were nearly identical among species, especially within plant families (Figure 1). Perennial ryegrass has the same mature miR408 sequence as the one of rice (…CUGCACUGCCUCUUCCCUGGC…), which is only one base different (out of the 21 nucleotides) from that of Arabidopsis (…AUGCACUGCCUCUUCCCUGGC…). MiR408 genes from the same lineage tended to cluster together in the phylogenetic tree, and the predicted perennial ryegrass miR408 was more similar to the rice miR408 gene. Based on the topology and clade support values, the 53 miR408 genes could be generally classified into four groups: (1) Angiospermae/Monocotyledoneae, (2) Angiospermae/Dicotyledoneae, (3) Gymnospermae, and (4) Pteridophyta & Bryophyta, which suggested that bryophytes might be the evolutionary ancestors of the miR408 family (Figure 2).

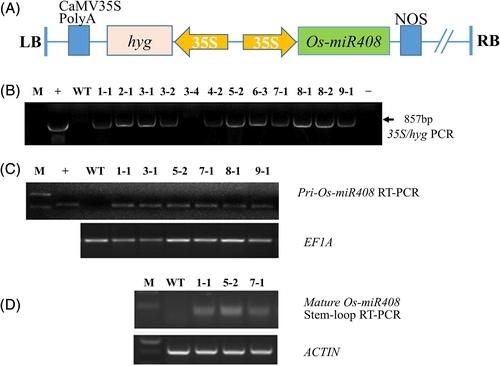
3.2 Generation of transgenic perennial ryegrass overexpressing the rice miR408 gene
To investigate the function of miR408 gene in perennial ryegrass, an Os-miR408 overexpression construct (35S::Os-miR408/35S::HYG, Figure 3A) was constructed and introduced into perennial ryegrass by Agrobacterium-mediated plant transformation. A total of 12 transgenic (TG) lines were identified using PCR for an 875-bp HYG fragment (Figure 3B). And six independent lines were further confirmed by semi-quantitative RT-PCR of the primary Os-miR408 transcripts (Figure 3C). All the six independent TG lines were morphologically similar and performed similarly when grown in the greenhouse. We selected three (TG1-1, TG5-2, and TG7-1) out of the six TG lines for further detection of the mature miR408, and its expression was observed in all the three lines, but not in the WT plants (Figure 3D).
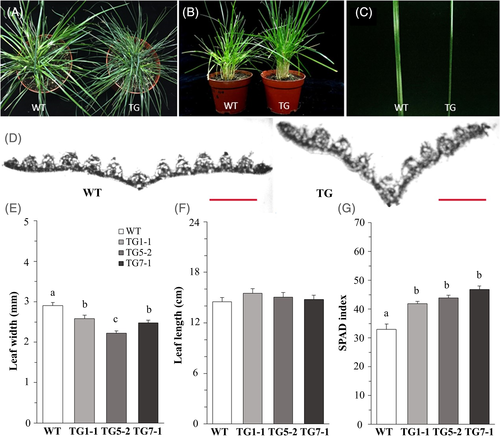
3.3 Phenotypic characterization of transgenic plants
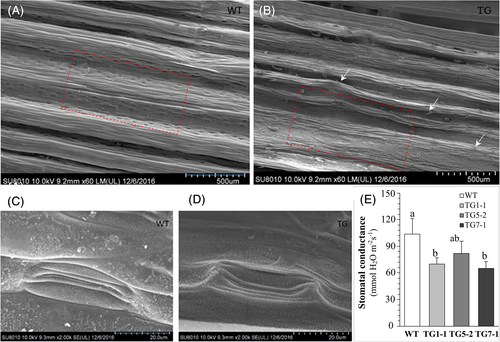
To determine the phenotypic impact of the ectopic expression of the rice miR408 in perennial ryegrass, we compared the leaf traits of 6-month-old TG and WT plants. We observed that the TG plants had significantly narrower leaves of similar length (Figure 4A–F). Microscopic analysis of the plant leaf samples indicated that, compared with the WT plants, TG plants exhibited less vein number and more closely folded leaves (Figure 4D). In addition, we found the TG plants were greener than the WT, and further examination of the chlorophyll contents (represented by the measured SPAD value) showed that the three TG lines had 27–42% higher leaf SPAD values than the WT (Figure 4A,B,G). Scanning electron microscope analysis found that there were more bristle-like trichomes on the leaf surface of the TG plants in comparison with WT controls. And, under low magnification, the stomata could be seen clearly on the leaf surface of the WT plants, but not the TG plants (Figure 5A,B). More interestingly, magnified pictures showed that the stomata of TG plants were relatively smaller and more sunken than those of WT plants (Figure 5C,D). Meanwhile, we measured the stomatal conductance and found that the stomatal conductance of all the three lines of TG plants was significantly lower than that of the WT plants, except TG5-2 (Figure 5E).
3.4 Overexpression of Os-microRNA408 enhances drought tolerance in transgenic perennial ryegrass plants
To understand the role of miRNA408 in plant drought tolerance, WT and three independent TG lines grown under optimal conditions were subjected to water withholding for 10 days (Figure 6A–D). The leaves showed a more severe wilting phenotype after 5 days of drought treatment in WT controls than in TG plants (Figure 6B). After water withholding for 10 days, serious tissue damage was observed in WT controls, while most TG plants remained turgid and showed less tissue damage (Figure 6C). Then, all plants were re-watered and allowed for a recovery of 14 days (Figure 6D). Transgenic plants all recovered from the drought-elicited damage, whereas nearly all the WT controls died or recovered less, suggesting an improved drought tolerance in Os-miR408 transgenic plants.
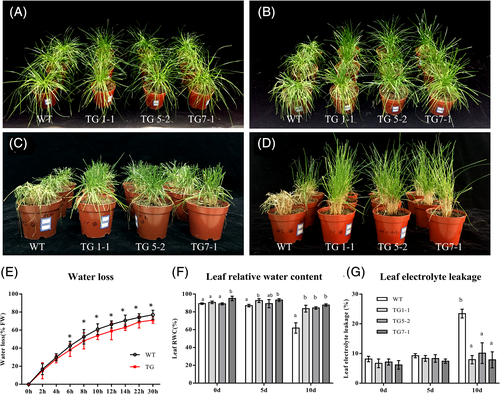
To further investigate the physiological mechanism of enhanced drought tolerance in Os-miR408 perennial ryegrass plants, we measured some physiological parameters in comparison with WT controls. Water deficit caused a reduction in relative water content of plants. The result revealed that the leaf RWC in TG1-1, TG5-2, and TG7-1 plants was 35.1, 36.3, and 41.5%, respectively, higher than that in WT controls after 10-day water withholding (Figure 6F). Moreover, detached TG plant leaves displayed an overall 5–10% lower WLR than WT controls during the 30-h drying period, supporting a correlation with enhanced drought stress resistance in TG lines (Figure 6E). While the leaf electrolyte leakage (EL) was similar in WT controls and three TG lines under normal growth conditions and 5 days after water withholding, it was significantly higher in WT plants than that in TG plants after a 10-day drought stress (Figure 6G). These results indicate that drought-elicited cell membrane damage in TG plants was significantly less than that in WT controls.
3.5 MiRNA408 increased plant antioxidant capacity, but does not stimulate ABA and proline accumulation under drought stress
ABA is a very important signaling molecule, which can be induced by many adverse environmental stresses, especially drought. Therefore, we detected the ABA content in TG and WT leaves before and after drought stress. The results showed that there was no significant difference in ABA content between WT controls and TG5-2, which was slightly higher than that of TG1-1 and TG7-1 under normal conditions. After drought stress, the ABA content of WT increased significantly,but not that of TG plants, except TG1-1. And the ABA content of WT was about onefold higher than the average across all three lines of TG plants after 10-day water withholding (Figure 7A). Similarly, we found low levels of proline in both WT and TG plants under normal growth conditions, while more proline accumulated in the WT than TG after 10 days of drought treatment, suggesting that WT plants might be more sensitive to drought stress and need to accumulate more proline for water potential regulation (Figure 7B).
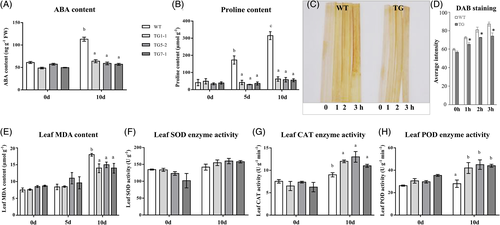
For MDA, the contents in WT and TG plants were similar under normal growth conditions. Although it was induced upon water deficit in both TG and WT plants, the accumulation of MDA in TG lines was significantly less than that in WT control, indicating less oxidative damage to the cell membranes in TG plants under drought treatment (Figure 7E). DAB staining was applied on the detached leaves of TG plants and WT plants before and after water deficit treatment to visualize the leaf H2O2 content. We found that the leaves of TG plants were stained lighter than WT after treatment, suggesting that the TG plants accumulated less H2O2 under drought condition and were less affected by oxidative stress caused by drought stress (Figure 7C,D).
Meanwhile, we measured the activities of three major antioxidant enzymes in TG plants and WT plants before and 10 d after water withholding. The activities of CAT and POD in TG and WT control plants were at the same level under normal growth conditions, while the two enzymes in TG plants accumulated significantly more than that in WT plants after 10 days of drought, with an average of 36.6 and 53.1% higher activities for CAT and POD, respectively, in TG plants across the three lines, compared to wild-type (Figure 7G,H). For SOD, a similar accumulation trend was observed, but it was not significantly different between TG and WT plants (Figure 7F). The results indicated that the TG plants had better ability to scavenge ROS under drought conditions.
4 DISCUSSION
Drought stress is one of the main limiting factors of global agricultural production due to the complexity of water-restricted environments and climate change (Boyer, 1982; Farooq et al., 2009). Thus developing crop plants with improved drought stress tolerance using traditional breeding or transgenic approaches is of increasing importance. MiRNAs is a type of key gene regulators at the post-transcriptional level, recent exciting research implicated an important role for some of them in drought stress responses (Ferdous et al., 2015; Khraiwesh et al., 2012). Over-expression of these microRNAs have been demonstrated to improve drought stress resistance in different transgenic plant species, including miR164, miR169, miR319, miR393a, miR394a, miR408, and miR827 (Ferdous et al., 2015; Ferdous et al., 2017; Hajyzadeh et al., 2015; Zhao et al., 2019). For example, ectopic expression of Arabidopsis miR408 can enhance drought tolerance of chickpea (Hajyzadeh et al., 2015), and over-expression of A. andraeanum miR408 improved Arabidopsis tolerance to osmotic stress (Jiang et al., 2018). In accordance with the studies above, we found that the miR408-overexpressing perennial ryegrass plants showed better growth under drought stress treatment than the control plants struggling with more severe symptoms of stress (Figure 6A–D). The enhanced water stress tolerance of transgenic perennial ryegrass plants was further demonstrated by higher leaf relative water content and lower leaf cell damage (lower leaf EL) than those in WT control plants under drought stress (Figure 4A,G). However, a study of transgenic Arabidopsis plants with altered miR408 expression revealed that higher miR408 expression led to enhanced sensitivity to drought and osmotic stress (Ma et al., 2015). In rice, the OsmiR408 transgenic lines also exhibited decreased drought tolerance, along with greater water loss (Sun et al., 2018). Interestingly, two other recent studies on rice observed that the expression level of miR408 responded differently in different varieties, with reduced expression in drought susceptible varieties, but significantly elevated level in resistant genotypes under drought stress, which strongly suggest higher miR408 could be important for better drought resistance (Balyan et al., 2017; Mutum et al., 2013). These contrasting observations of the role of miR408 in plant drought stress might indicate a species- and/or cultivar-specific drought-responsive characteristic for miR408, although the miR408 family is known to be conserved and has been annotated in over 30 plant species (Kozomara & Griffiths-Jones, 2011; Liu et al., 2017). Another possible reason could be the variation between the drought stress treatment (intensity, duration, etc.) and/or plant growth stage (seedling, vegetative growth, etc.) among all the studies reported. Further interrogation is needed to address the questions above.
MiR408 also functions in plant development. Transgenic plants overexpressing miR408 exhibit a wide range of morphological changes, such as leaf area, leaf pigment accumulation, petiole length, plant height, flower size, grain number, and grain size, etc. (Hajyzadeh et al., 2015; Pan et al., 2018; Song et al., 2017; Zhang et al., 2011; Zhang et al., 2018; Zhang & Li, 2013). However, findings among studies are somehow paradoxical, particularly for plant height, leaf area, and pigment accumulations. In Arabidopsis, the accumulation of miR408 in transgenic plants promoted vegetative growth along with larger leaves and bigger plant, and increased leaf chlorophyll and anthocyanin contents in young seedlings (Zhang et al., 2011). Similarly, another study on miR408 in Arabidopsis showed that plant and leaf sizes were higher in transgenic lines overexpressing miR408 than in control, but no accumulation of leaf chlorophyll was observed in transgenic lines (Ma et al., 2015). In the same study, a decrease of chlorophyll content in transgenic plants was even found when the plants were grown on MS (Murashige and Skoog) agar plates at high plant density, instead of being grown in soil under optimal conditions (Ma et al., 2015). Interestingly, heterologous expression of an Arabidopsis miR408 gene in tobacco decreased the chlorophyll content of transgenic plants (Feng et al., 2010), while Arabidopsis plants over-expressing an A. andraeanum miR408 gene showed no significant difference in chlorophyll accumulation compared to wild-type control (Jiang et al., 2018).
In contrast to the promotive role of miR408 in growth and leaf size reported in Arabidopsis, overexpression of Arabidopsis miR408 in chickpea clearly showed an inhibitory effect on vegetative growth with a shorter plant height in transgenic plants, but no morphological difference in the leaf area was observed between transgenic and control plants (Hajyzadeh et al., 2015). In our study, no significant change was found for plant size between transgenic plants and control plants (data not shown), but the leaves of transgenic plants were slightly longer (not significantly), finer, and more closely folded (Figure 4A–F). In addition, the transgenic plants had higher leaf chlorophyll content, as indicated by the SPAD index (Figure 4G). Interestingly, the transgenic plants also had smaller and more deeply sunken stomata (Figure 5). Several studies on stomatal development have implicated miRNAs in stomatal lineage determination (Jover-Gil et al., 2012; Kutter et al., 2007), and a recent one identified more than 200 differentially expressed (DE) miRNAs (including two miR408s) among different stomatal development stages (Zhu et al., 2020), which further suggested that miRNA dynamics and function may be crucial for stomatal development. Moreover, several miRNAs (miR829, miR3932, and miR861) were further proved to affect stomatal development, including number and patterning, indicating that the interaction between environmental changes and miRNA expression can modulate stomatal development to better adapt to the environmental changes, such as heat, salt, and drought conditions (Gan et al., 2010; Lau et al., 2018; Zhu et al., 2020). However, how miR408 might function in stomatal development remains unclear. Further verification and characterization of the role of miR408 in the regulation of stomatal development would help to know how miR408 interacts with the environment. At the morphological level, leaves play a critical role in plant adaptation to drought stresses (Larcher, 2003; Tsukaya, 2005), as they are the plant organ responsible for converting solar energy through photosythesis for plant growth and development. In general, thicker leaves, higher weight-area ratio, more total wax coverage, and narrower leaf width are thought to be positively correlated with plant drought stress tolerance (Begg, 1980; Deak et al., 2011; Zhang et al., 2005). And leaf folding/rolling is known to be a typical water-saving response to water deficit by decreasing the transpiration area (Deak et al., 2011; Lakso, 1983). Meanwhile, lower stomata density, less stomata aperture, and sunken stomata can result in less water loss and increase water content in plants, and are thought to be an adaptation to water deficit (Basu et al., 2016; Huang et al., 2009; Larcher, 2003). Here, the leaf morphological changes (Figures 4 and 5) helped to reduce water loss and made the transgenic plants maintain a better water status under water-holding condition (Figure 6).
Other than the morphological adaptation mechanism, plants have also evolved a mechanism at the physiological and biochemical levels to overcome water deficit conditions (Bartels & Sunkar, 2005; Gupta et al., 2020; Zhu, 2016). ABA is one of the most important plant hormones that confer abiotic stress tolerance in plants (Zhu, 2016). Many studies have shown that ABA plays a crucial role in plant resistance to drought stress (Li et al., 2015; Yoshida et al., 2010). Here, we measured the ABA content and wanted to determine whether miR408 regulates plant drought tolerance through the ABA pathway. Overall, the ABA contents increased both in WT and transgenic ryegrass under drought condition, but to a much higher extent in WT (Figure 7A). In two studies comparing ABA accumulation for Kentucky bluegrass cultivars differing in drought tolerance, sensitive cultivars had higher ABA accumulation along with lower RWC and higher EL than drought-tolerant ones under drought stress (Wang et al., 2004; Wang & Huang, 2003). Drought induced the accumulation of ABA, and the rise of ABA content is largely controlled by leaf water status and depends on the severity of water stress (DaCosta et al., 2004; Quarrie, 1989). In response to drought stress, plants synthesize ABA, which triggers stomatal closure, thus reducing water loss during the early phase of stress (Fujita et al., 2011; Schroeder et al., 2001). Therefore, the rate of ABA accumulation in leaves is thought to be an indication of the severity of physiological injuries at certain conditions (Wang et al., 2004), which is consistent with the physiological indicators of lower RLW, higher EL and MDA contents in WT plants (Figures 6E–G and 7E). Thus, our results implied that miR408 might not regulate plant resistance to drought through ABA signaling, at least not the major way. Similarly, the proline accumulation showed a similar pattern as ABA with a higher amount in WT than transgenic plants under stress (Figure 7B). Proline is one of the most common osmolytes accumulated in drought-stressed plants, and many studies supported a correlation between proline accumulation and increased stress resistance (Bartels & Sunkar, 2005). However, such a correlation is not always the case in all studies (Krasavina et al., 2014). Some studies imply that proline synthesis is not always required for plant drought resistance (Schafleitner et al., 2007; Vasquez-Robinet et al., 2008), but is rather related to their response to stress (Deyholos, 2010; Legay et al., 2011). Together with the data of ABA content and other physiological indicators (RLW, EL, and MDA) in WT plants (Figures 6E–G and 7A,E), the higher proline accumulation in WT plants is rather a marker of more severe stress in the leaves than a component of drought resistance. In a comparison of proline accumulation between two cassava (Manihot esculeta L.) cultivars with contrasting drought tolerance, Sundaresan and Sudhakaran (1995) reported that the susceptible cultivar (M-4) accumulated higher proline than the tolerant cultivar (S-1215) under water stress.
Drought stress commonly induces the generation of ROS, such as O2, H2O2, O2−, and OH−, which may disturb the spatial configurations of various membrane proteins/enzymes and causes membrane lipid peroxidation, leading to increased membrane permeability and ion leakage, pigment destruction, metabolism perturbations, and even severe injury or plant death (Gill & Tuteja, 2010; Schneider et al., 2019). Plants have developed defense mechanisms against oxidative damage, including non-enzymatic and enzymatic antioxidant components (Mittler, 2017; Mittler et al., 2011). Major enzymes of the enzymatic antioxidant defense system such as SOD, CAT, and POD play an important role in scavenging ROS and resisting oxidative stress (Gill & Tuteja, 2010; Mittler, 2017). In the present study, plants with enhanced miR408 expression had higher activities of SOD, CAT, and POD under drought stress compared with the wild-type controls. Meanwhile, all the plants accumulated higher levels of H2O2 and MDA under stress, while the transgenic plants exhibited lower accumulation of H2O2 and MDA than WT (Figure 7C,D,E). As an indicator of oxidative damage, MDA is usually accumulated in plants under stress (Pauls & Thompson, 1980). These results indicate that elevated levels of miR408 in the transgenic plants lead to better antioxidative capacity, which may in turn improve the plants' ability to cope with the drought stress. In Arabidopsis, the ROS levels in the miR408-overexpressing lines were lower under stress when compared to WT control, along with a higher expression of genes involved in the antioxidative response to abiotic stresses, including two SOD genes (Ma et al., 2015). More recently, Guo et al. (2018) reported transgenic tobacco overexpressing Sm-MIR408 reduced the ROS accumulation under salt stress, which is in consistence with both the increased transcript level of antioxidative genes (SOD, POD, CAT) and their enzyme activity. Taken together, we inferred that miR408 might increase the drought resistance of transgenic plants by improving the antioxidant capacity.
In summary, our results showed that overexpression of Os-miR408 in perennial ryegrass improved plant drought tolerance. MiR408 caused leaf morphological changes associated with a reduced water loss. It also improved the antioxdiative response by increasing the antioxdiative capacity beneficial to plants responding to drought condition. MiR408 might serve as a potential target for genetic manipulations to engineer perennial grass plants for improved water stress tolerance. Considering the possible species-specific role of miR408 in plant stress tolerance, further studies in more perennial grass species would be worthwhile.
ACKNOWLEDGMENTS
This research was supported by the Beijing Municipal Natural Science Foundation (6182025) and National Natural Science Foundation of China (31472140). We thank Yunwei Zhang and Bin Xu for useful discussions and insightful comments on the manuscript.
AUTHOR CONTRIBUTIONS
Yan Sun, Wanjun Zhang, Kehua Wang: Conceived and designed the experiments. Nan Hang, Tianran Shi, Yanrong Liu, Geli Taier, Wenxin Ye: Performed the experiments. Nan Hang, Tianran Shi, Geli Taier: Analyzed the data. Nan Hang, Tianran Shi, Wanjun Zhang, Kehua Wang: Contributed to the writing of the manuscript. All authors approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as all new created data is already contained within this article.




