Contrasting adaptive responses to cope with drought stress and recovery in Cenchrus ciliaris L. and their implications for tissue lignification
Abstract
Cenchrus ciliaris L. is a widely used species for cattle feed in arid and semi-arid regions due to good forage value and known tolerance to drought conditions. Here, we provide insights to adaptive responses of two contrasting genotypes of C. ciliaris (drought-tolerant “RN51” and drought-sensitive “RN1”) to face drought stress and recovery conditions and the implications for tissue lignification. Drought stress caused a reversible decrease in the leaf water relationship and damage to photosystem II, leading to an increased generation of reactive oxygen species and lipid peroxidation. Plants of RN51 exhibited a pronounced increase of antioxidant enzymatic activities. Unlike the drought-sensitive genotype, RN51 exhibited further development of lignified tissues and bulliform cells and had the greatest thickness of the adaxial epidermis. Drought stress led to the rapid activation of the expression of lignin biosynthesis pathway-related enzymes. The transcript level of the caffeoyl-CoA O-methyltransferase gene decreased in RN1, whereas cinnamoyl-CoA reductase transcripts were increased in RN51. After rewatering, the tolerant genotype recovered more rapidly than RN1. Even though the two genotypes survived when they were exposed to drought stress, RN1 showed the highest reduction in growth parameters, and this reduction was sustained during rewatering. The results indicated that the capacity to regulate lipid peroxidation and mitigate oxidative damage could be one of the mechanisms included in tolerance to drought stress. In addition, the development of foliar characteristics, like thickness of the adaxial epidermis, well-developed bulliform cells, and intensive lignified tissues, are considered anatomical adaptive strategies for drought tolerance in C. ciliaris.
Abbreviations
-
- ADW
-
- aerial dry weight
-
- AFW
-
- aerial fresh weight
-
- APX
-
- ascorbate peroxidase
-
- CAT
-
- catalase
-
- CCoAOMT
-
- caffeoyl-CoA O-methyltransferase
-
- CCR
-
- cinnamoyl-CoA reductase
-
- COMT
-
- caffeic acid-O-methyltransferase
-
- ET
-
- evapotranspiration
-
- FRAP
-
- ferric reducing ability of plasma
-
- Fv/Fm
-
- maximum quantum efficiency
-
- H
-
- height
-
- LRWC
-
- leaf relative water content
-
- MDA
-
- malondialdehyde
-
- P
-
- parenchyma
-
- PCA
-
- principal component analysis
-
- PSII
-
- photosystem II
-
- ROS
-
- reactive oxygen species
-
- S
-
- sclerenchyma
-
- SOD
-
- superoxide dismutase
-
- SWC
-
- soil water content
1 INTRODUCTION
Cenchrus ciliaris L. is an important apomictic forage grass (Kumar, 2017) native to East Africa and Southeast Asia and widely spread in arid and semi-arid regions of the world for feeding livestock (Bowen & Chudleigh, 2018; de Albuquerque et al., 2019). Its demand has increased in recent years because it is a new promising energy source for biofuel (Al-Dakheel & Hussain, 2016; Kumar & Ghosh, 2018). In Argentina, it has been introduced as forage feed for cattle in the northwest of the country due to easy implantation, good forage value for these arid regions (6–9% crude protein and 50% digestibility), and their known tolerance to drought conditions (Arroquy et al., 2014; Guevara et al., 2009). However, the scarcity of rainfall in this area is one of the main abiotic factors that cause severe losses of yield, persistence, and nutritional value (Ruiz & Terenti, 2012). Moreover, future climate scenarios predict more days with higher temperatures and more erratic rainfall, leading to an increase in the frequency of severe drought conditions (Basu et al., 2016).
Water stress caused by drought is a physiological form of water deficit, where there is lower soil water availability than plant demand, resulting in a negative change in the plant's water status away from a reference state (Kumar et al., 2018). In the face of water stress conditions, plants have three main adaptive strategies as recently revised by Volaire (2018): dehydration escape, dehydration avoidance, and dehydration tolerance. These strategies are often combined consecutively or simultaneously and are the most documented strategies across a majority of species (Volaire, 2018). Herbaceous perennials, such as many forage grasses, including C. ciliaris, constitute the biological type with possibly the largest range of strategies. In this sense, Siddiqui et al. (2016) mentioned that this species has the ability to tolerate long dry seasons under varying soil conditions, indicating some degree of dehydration tolerance.
A variety of physiological, biochemical, anatomical, and molecular responses at cellular and organism levels have been described as different types of plant strategies under contrasting environments (Basu et al., 2016; Kumar et al., 2018; Liu et al., 2016). Sensitivity and response time vary depending on the species, genotype, and the duration and intensity of stress (Abid et al., 2018; Basu et al., 2016). It is generally agreed that drought stress generates physiological changes in higher plants, including loss of turgor, osmotic adjustment, and reduced leaf water potential to maintain a well-watered status (Le Gall et al., 2015). The immediate response to water stress is stomatal closure, which results in changes in metabolic pathways, such as photosynthesis and respiration (Basu et al., 2016). Drought stress not only causes important damage to photosynthetic pigments, but also leads to deterioration of thylakoid membranes and reduction in chlorophyll content. Photosynthetic pigments present in photosystems are believed to be damaged by abiotic stress, resulting in reduced light-absorbing efficiency of both photosystems (PSI and PSII) and, hence, a reduced photosynthetic capacity (Asharf & Harris, 2013). Moreover, PSII, with its oxygen-evolving complex (OEC), is considered the component of the photosynthetic apparatus most sensitive to water stress through reduction of maximum quantum efficiency (Fv/Fm; Fracasso et al., 2016).
In the presence of drought stress, the electron transport rate is inhibited and reactive oxygen species (ROS) production is increased (Basu et al., 2016), which generates damage to membrane lipids, proteins, and DNA resulting in cell death (Waszczak et al., 2018). Plants have a complex enzymatic and non-enzymatic antioxidant system to regulate the levels of ROS produced in tissues and, thus, mitigate oxidative damage. Key enzymes, such as superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), and glutathione reductase (GR; You & Chan, 2015, Kumar et al., 2018), are mentioned in the enzymatic system and have been positively correlated in plants with tolerance to abiotic stress (Laxa et al., 2019; Xie et al., 2019). Our previous studies indicated that higher activity levels of the catalase antioxidant enzyme might contribute to better drought tolerance by increasing the protection capacity against oxidative damage (Tommasino et al., 2018).
The rise in ROS is accompanied by an increase in lignin accumulation. Lignin is a polyphenolic polymer constituent of cell walls that is required for reinforcing vascular cells. It displaces the aqueous phase of the cell wall, encasing cellulose and matrix polysaccharides and providing enhanced mechanical strength and a water-impermeable barrier (Chen et al., 2019; Liu, Luo, & Zheng, 2018). This leads to higher lignification of the cell walls of tissues, mainly around the vascular bundles. Besides changes in lignin deposition, plants exposed to water deficit display morphological changes as a result of plant cell wall modifications (Al-Maskri et al., 2014; Hameed et al., 2010, 2012). A decrease in leaf blade thickness, increase in the epidermal layer, reduction in the metaxylem area, and stomata size are mentioned as the most important modifications in tissues of numerous forage species under drought stress (Arias et al., 2018; Hameed et al., 2010, 2012; Mustafa et al., 2019). In C. ciliaris, leaf thickness and cuticle deposition accompanied by a thick epidermal layer were included as modifications when plants were exposed to various regimes of water stress (Mansoor et al., 2019; Nawazish et al., 2006).
The lignification process is regulated by a set of metabolic events that result in the biosynthesis of lignin precursors (monolignols). Some key enzymes of the lignification process include caffeic acid-O-methyltransferase (COMT), 4-coumarate CoA Ligase (4CL), cinnamoyl CoA reductase (CCR), and cinnamon alcohol dehydrogenase (CAD; Moura et al., 2010, Stabile et al., 2012, Baxter & Stewart Jr, 2013). In grasses, expression of genes encoding enzymes from the phenylpropanoid pathway has been shown to be involved in modulating the rate of lignin synthesis (Ralph et al., 2004), and its expression has been negatively associated with cell wall degradability (Fu et al., 2011; Stabile et al., 2012). Several studies mentioned that lignin metabolism was enhanced under various environmental stressors, like drought stress (Liu et al., 2018). In inbred maize lines, expression levels of lignin biosynthesis related genes (CAD and COMT) were significantly positively correlated with drought tolerance (Moura-Sobczak et al., 2011). Moreover, in white clover, higher ROS scavenging systems and more sensitive lignin metabolism could be associated with better drought tolerance during stress and post-drought recovery (Li et al., 2013). However, to our knowledge, the information available regarding the implications of drought stress and post-drought recovery on the expression of genes related to lignin biosynthesis in C. ciliaris is null.
In an environmental context, there is always an interval occurrence of drought and/or rewetting events and even more, their frequencies have been increased under climate change conditions (de la Casa & Ovando, 2014; Nasca et al., 2015). Rapid recovery of damaged plants and regrowth of new tissues following drought stress are important in perennial grasses (Bakhtiari et al., 2019). After withdrawal of drought stress, the availability of even a small amount of rainfall can have a significant effect on plant physiological functions, ranging from whole-plant responses to biochemical and molecular responses (Vandegeer et al., 2020). While there is some evidence for morpho-physiological adaptations of C. ciliaris (Amari et al., 2017; Mansoor et al., 2019; Siddiqui et al., 2016) as a basis for improving production and forage quality under drought stress conditions, a comprehensive study on the physiological, biochemical, anatomical, and molecular responses to drought stress and the rewatering process, as well as information about the relationship between drought tolerance and tissue lignification in this species, is still lacking. Therefore, the aim of this work was to evaluate adaptive responses in contrasting Cenchrus ciliaris L. genotypes under drought stress and recovery and their implications for tissue lignification. We hypothesized that the tolerant C. ciliaris genotype responds differently to drought stress by changing leaf anatomy and affecting the antioxidant system and lignin biosynthesis.
2 MATERIALS AND METHODS
2.1 Plant material
Two Cenchrus ciliaris L. genotypes were used in these experiments, register Number 51 (RN51; cv. originally from Dodoma, Tanganika, Africa) and Register Number 1 (RN1; an introduced sexual source from Texas (EE.UU.)). These genotypes have contrasting responses to different abiotic stresses (tolerant and sensitive genotypes, respectively) as observed in previous studies (Lanza Castelli et al., 2010; Tommasino et al., 2012, 2018).
2.2 General growth conditions and treatment applications
The assay was carried out in a growth chamber under controlled conditions: temperature of 28°C ± 2°C, photoperiod (16/8 h light/dark), humidity (60%) and photosynthetic photon flux density (PAR; 250 μmol m−2 s−1). For all treatments, 0.2 g seeds of individual genotypes were sown in pots (25 cm diameter by 15 cm depth) containing 3 kg sand and soil substrate (1:1) previously dried in a stove at 105°C for 48 h to remove the moisture content. Soil water content was determined via the gravimetric method. After sowing, the pots were irrigated to saturation and once the water drainage was completed, the weight was recorded. This weight was considered as field capacity, the maximum water amount capable of being retained by the substrate and represented 100% soil water content (SWC).
Drought stress assays were applied following the protocol described by Tommasino et al. (2018) with minor modifications. In short, after sowing the plants, the pots (n = 12) were watered daily at 80% SWC. Ten days after sowing, the seedlings emerged and we kept 35 small seedlings in each pot. Thirty days after sowing, when plants had reached 20 cm in height and five leaves were unfolded, pots were divided into two uniform groups: (1) well-watered control plants: the plants were watered daily at 80% SWC throughout the experimental period and (2) drought-stressed plants: the irrigation of pots was interrupted until 20% SWC was reached and kept for 20 days under this condition. Half of the pots of drought-stressed plants were then rewatered at 80% SWC for a total of 15 days for recovery treatment. A randomized complete block design was performed in three independent events to guarantee the reproducibility of the experiments.
Five plants of each pot were collected at 24 and 72 h when reaching 20% SWC to evaluate biochemical and molecular parameters. At the end of the drought stress period, physiological and anatomical parameters and growth performance measurements were evaluated. Regarding recovery treatment, biochemical and molecular parameters were evaluated at 24 and 72 h of rewatering, whereas physiological parameters and growth performance measurements were registered after 15 days of rewatering.
2.3 Evaluation of physiological measurements
2.3.1 Determination of leaf relative water content
Leaf relative water content (LRWC) was evaluated according to Barr and Weatherley (1962) with some modifications. Briefly, the third and fourth leaves of two plants per pot were cut into 7 cm long fragments. The fresh weight (FW) was determined and then the fragments were immersed in distilled water for 24 h and turgor weight was recorded (TW). The samples were dried in a forced air oven at 60°C and dry weight was determined (DW). The LRWC was estimated by the equation proposed by Turner (1986): LRWC = [(FW−DW)/(TW−DW)] × 100.
2.3.2 Evapotranspiration
The water lost by evapotranspiration (ET) was calculated as the amount of water needed to restore the soil water content of the pots to the value in each treatment (80% SWC and 20% SWC). Before and after irrigations, pots were weighed to determine the daily average ET rate (Lo Bianco & Scalisi, 2017).
2.3.3 Chlorophyll fluorescence and JIP test parameters
Measurements of chlorophyll a fluorescence transients were taken using a Pocket PEA (Plant Efficiency Analyzer, Hansatech Instruments Ltd., King's Lynn) between 10:00 and 14:00 h. Before measuring, the leaves were fully dark-adapted for 30 min to achieve the complete oxidation of the primary electron carriers (F0). Then, we proceeded to measure five plants by genotype, treatment, and repetition. Chlorophyll fluorescence induction was prompted by a 3-s pulse of red light (peak wavelength of 637 nm) emitted from an LED lamp filtered by an NIR filter. This pulse was emitted at maximal saturation irradiance of 3500 μmol m−2 s−1. It is possible to label the individual elements of the fluorescence rise. The peaks of the induction curve kinetics are denoted by the letters O, J, I, P; where O is the minimal fluorescence (F0), P is the maximum fluorescence (Fm), and J and I are intermediates inflection points (Strasser et al., 2004). From these two absolute values, the parameter of Fv (variable fluorescence) may be calculated as the difference between them and the Fm value may be displayed as a function of the Fv parameter in order to give the parameter Fv/Fm (maximum quantum efficiency; Maxwell & Johnson, 2000, Ploschuk et al., 2014, Rasouli & Kiani-Pouya, 2015). For further details of terms used by the JIP test, please refer to Table S1.
2.4 Biochemical measurements
2.4.1 Determination of malondialdehyde content (MDA)
Lipid peroxidation in leaves was measured by assessing MDA content, as described by Heath and Packer (1968) with minor modifications for C. ciliaris (Tommasino et al., 2018).
2.4.2 Total reducing power by ferric reducing ability of plasma (FRAP) assay
This parameter was measured using the method described by Benzie and Strain (1996). The FRAP method was used to determine the total reducing power via measuring the reduction of ferric ions to the ferrous form in presence of antioxidant components following the protocol described by Tommasino et al. (2018).
2.4.3 Enzyme activity determination
The samples were processed following the protocol described by Tommasino et al. (2018). SOD (EC 1.15.1.1) activity was estimated according to the method described by Beauchamp and Fridovich (1973). Enzymatic activity was expressed as SOD units (USOD) per mg of protein. Protein content in the enzyme extracts was quantified according to the Bradford (1976) method. CAT activity (EC 1.11.1.6) was measured through the consumption of H2O2 measured by the absorbance at 240 nm (Aebi, 1984). One unit of CAT activity was defined as the amount of enzyme required for catalyzing the conversion of 1 μmol H2O2 into water per minute. Results were expressed as μmol H2O2 extinct per minute per mg of protein.
For measuring APX activity (EC 1.11.1.11), samples were processed following the protocol described by Mizuno et al. (1998). The APX activity was estimated according to the method described by Nakano and Asada (1981). Specific APX activity was expressed as μmol AsA per minute per mg of protein.
2.5 Molecular analysis
2.5.1 Expression of genes coding for enzymes involved in lignin biosynthetic pathways
The used primers were designed with the software Primer3Plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi) and AmplifiX (v.1.7.0 (http://crn2m.univ-mrs.fr/pub/amplifix) on the mRNA sequences deposited in GenBank for Setaria italica (Table 2). The analyzed genes coding for enzymes involved in the biosynthesis of monolignols were: 4-coumarate-CoA ligase (4CL; GenBank: XM_004985609.2), cinnamoyl-CoAreductase (CCR; GenBank: XM_004983487.3), caffeoyl-CoA O-methyltransferase (CCoAOMT; GenBank: XM_004957087.2), cinnamyl alcohol dehydrogenase (CAD; GenBank: XM_004976726.1), phenylalanine ammonia lyase (PAL; GenBank: XM_004953099.2), and Caffeic acid-O-methyltransferase (COMT; GenBank: XM_004972743.3). Three biological replicates for each combination treatment-genotype were used with each primer pair. All primers used (Table 1) were ordered from Ruralex. The amplified product sizes were confirmed by agarose gel electrophoresis and after its purification were sequenced to obtain the real sequences.
| Fluorescence parameters | Control | Drought stress | ||||||
|---|---|---|---|---|---|---|---|---|
| RN51 | RN1 | RN51 | RN1 | |||||
| t for Fm (**) | 185.29 ± 39.11 | c | 193 ± 50.99 | c | 355 ± 46.75 | b | 636 ± 4.72 | a |
| Area (*) | 269124.35 ± 10632.6 | A | 238860.8 ± 13863.2 | A | 171642.33 ± 12655.3 | B | 139995.3 ± 12158.80 | B |
| Fo (**) | 4523.94 ± 152.1 | a | 4557.3 ± 198.3 | a | 4398.25 ± 180.9 | a | 3131.77 ± 173.89 | b |
| Fm (**) | 20713.24 ± 692.87 | a | 20508.1 ± 903.39 | a | 19345.1 ± 824.7 | a | 13179.23 ± 792.33 | b |
| Fv (**) | 16189.29 ± 547.70 | a | 15950.8 ± 714.11 | a | 14946.8 ± 651.9 | a | 10047.5 ± 626.31 | b |
| Fv/Fm (*) | 0.78 ± 0.01 | A | 0.78 ± 0.02 | A | 0.77 ± 0.03 | B | 0.76 ± 0.03 | B |
| ABS/RC (**) | 19.39 ± 0.47 | c | 19.68 ± 0.61 | bc | 20.95 ± 0.61 | b | 24.59 ± 0.54 | a |
| DIo/RC (**) | 0.42 ± 0.02 | c | 0.44 ± 0.02 | bc | 0.48 ± 0.02 | b | 0.6 ± 0.02 | a |
| TRo/RC (**) | 15.15 ± 0.3 | c | 15.31 ± 0.4 | c | 16.14 ± 0.4 | b | 19 ± 0.4 | a |
| ETo/RC (*) | 1 ± 0.02 | A | 0.97 ± 0.03 | A | 0.87 ± 0.03 | B | 0.78 ± 0.03 | B |
| PI total (**) | 16.53 ± 0.92 | a | 7.53 ± 1.85 | b | 0.99 ± 0.7 | c | 0.19 ± 0.03 | c |
- Note: Values represent the means ±se (n = 12). Different lower-case letters indicate significant differences (P ≤ 0.05) for two-way interaction (**) and uppercase letters indicate significant differences between treatments (P ≤ 0.05; *) for variables where interaction was no significant (P > 0.05).
2.5.2 RNA isolation and qPCR
RNA was extracted from leaf tissues harvested at 24 and 72 h after drought treatment. The sampled leaves were frozen immediately in liquid nitrogen and stored at −80°C until processing. High quality total RNA was isolated from 100 mg of frozen tissue using TRIzol™ Reagent, according to the manufacturer's instructions (Invitrogen). Genomic DNA was eliminated after treatment with DNase I for 30 min at room temperature using DNase I (Invitrogen). RNA concentration was measured using a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies). The quality of isolated RNA was determined by measuring the absorbance at 260 and 280 nm, and its integrity was verified by the presence of 18S and 28S rRNA bands after electrophoresis on an agarose gel. First-strand cDNA was synthesized using M-MLV (Promega) according to the manufacturer instructions. In a total reaction volume of 25 μl, 2 μg of total DNase-free RNA was primed with 1 μg d(T)20 (Ruralex). For qPCR, reactions were carried out in a 15-μl reaction mix containing 250 nM of each primer, 1 μl of cDNA sample and iQ SYBR Green Supermix (BioRad). Non-template controls (negative control) were incorporated in each analysis. qPCR reactions were performed using a iQ5 (BioRad) thermocycler. The thermal profile was set to 95°C for 3 min, followed by 40 cycles of 95°C for 30 s, 61°C for 30 s, and 72°C for 30 s. Amplicon specificity was verified by melting curve analysis (55 to 95°C) after 40 PCR cycles.
The qPCR assay was carried out using three biological replicates for each treatment and two technical replicates for each biological replicate. Elongation Factor 1 alpha (EF1α) and Eukaryotic Initiation Factor 4A (ElF4α) genes previously characterized in C. ciliaris (Simon et al., 2013) were selected as reference genes. Amplification efficiencies were determined for each gene following the recommendations of Svec et al. (2015). The efficiency values were between 90% and 100% for all the primers, with exception of PAL, CAD, and 4CL. The relative expression levels of lignin genes were estimated in relation to reference genes according to the 2−ΔΔCt method (Khan-Malek & Wang, 2017; Pfaffl et al., 2002). The analysis was made using InfoStat software (InfoStat versión 2016. Grupo InfoStat. FCA. Universidad Nacional de Córdoba. Argentina. Available at: http://www.infostat.com.ar).
2.5.3 Anatomical observations
Measurements were made according to Carloni et al. (2014) with minor modifications. Segments of 2 cm from the base of the third fully expanded leaf were fixed in a mixture of ethanol:water:formalin:glacial acetic acid (FAA, 5: 3.5: 1: 0.5) for 48 h. They were then placed in 70% ethanol (v/v). Samples were dehydrated in a series of ethanol dilutions (70%, 96%, 100% v/v) and the leaf segments were embedded in paraffin using the conventional technique of D'Ambrogio de Argüeso (1986). Sections (10 μm) obtained with a rotary microtome (Accu-Cut® modelo SRM 200 CW) were stained with Safranin and Fast Green (1%), a simple and differential staining method to identify lignified and unlignified tissues. The lignified sections (stained red) and cellulosic tissues (stained green) were observed using a light microscope (Jia et al., 2015; Wang et al., 2016). Images at magnifications of ×10 and ×40 were analyzed using ImageJ (Image Processing and Analysis in Java; Arias et al., 2018). Measurements were performed in six biological replicates (six cross sections per sample). Two vascular bundles on both sides of the central bundle were taking into account for measurements as described in Arias et al. (2018). In leaves, leaf blade thickness, epidermis thickness, parenchyma area, bulliform cell area, and sclerenchyma area (highly lignified tissue) were recorded. From the parenchyma (P) and sclerenchyma (S) areas, the P:S ratio was calculated.
2.5.4 Evaluation of growth performance
Based on our previous works (Lanza Castelli et al., 2010; Tommasino et al., 2018), the following characteristics were measured: plant height and aerial fresh and dry weights (H, AFW, and ADW, respectively). The plants were dried in a forced air oven at 60°C until constant dry weight was obtained (72 h).
2.6 Statistical analysis
For comparisons between means in LRWC, evapotranspiration, PSII, molecular analysis, anatomical and growth performance parameters, general linear mixed models were used and anova was applied for a two-factor model with interaction between the factors genotype and treatment in a randomized complete block design. For MDA, FRAP and enzymes (CAT, SOD, and APX), two- and three-way interactions were applied between variables (e.g. genotype × treatment × time of measurement). Fisher test at 5% level of significance (P ≤ 0.05) was performed using the InfoStat statistical package (InfoStat versión 2016. Grupo InfoStat. FCA. Universidad Nacional de Córdoba. Argentina. Available at: http://www.infostat.com.ar). The standard error was plotted in all figures.
In all measured parameters where significant differences for two-way interaction were observed, the changes (in terms of percent value) between drought-stressed plants and corresponding control plants were calculated using the following formula: Percent change (%) = [(Xc−Xs)/Xc) × 100]. Where, Xc and Xs denote control and drought-stressed plants.
In order to explore associations between physiological, biochemical, anatomical, and growth performance parameters, a principal component analysis (PCA) was performed. Results of this analysis are visualized through biplot graphs (Gabriel, 1971) constructed from the first and second principal components (PC1 and PC2) derived from the PCA. This technique allows to analyze the whole data set and take into account several factors simultaneously (i.e. the different treatment conditions) and identify associations between observations, between variables, and between variables and observations.
3 RESULTS
3.1 Evaluation of physiological measurements
3.1.1 Leaf relative water content
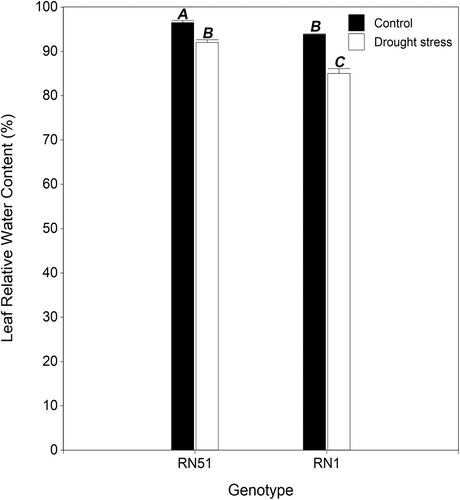
Significant differences for two-way interactions were observed (P ≤ 0.05). Compared with the respective control plants, the LRWC in plants under drought stress was affected. It was decreased by 5.2% and 9.6% in RN51 and RN1, respectively (Figure 1). After recovery from drought stress, no significant differences were found (P ˃ 0.05). Both genotypes recovered the LRWC value to their respective controls.
3.1.2 Evapotranspiration
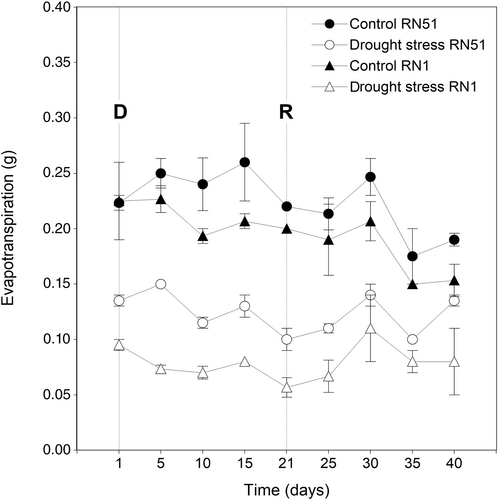
This parameter, measured as the water lost by evapotranspiration (g), during the drought stress period showed no significant differences for two-way interactions (P ˃ 0.05). However, significant differences between genotypes and treatments (P ≤ 0.05) were observed. The average value recorded in RN51 genotype was 0.18 g, whereas for the RN1 genotype, it was 0.14 g. In contrast, the average values for the control and drought stress treatments were 0.22 g, and 0.10 g, respectively. In Figure 2, day 21 denotes the end of the drought stress treatment and the beginning of recovery. After rewatering, the same trend was observed, showing significant differences between genotypes and treatments (P ≤ 0.05). The highest average value was registered in the RN51 genotype (0.16 g), whereas the lowest average value was found in RN1 (0.13 g). Regarding treatment analysis, the average value for the control and rewatered plants was 0.19 g and 0.10 g, respectively.
3.1.3 Chlorophyll fluorescence and JIP test parameters
During drought stress, the average and standard error for each fluorescent parameter are presented in Table 1. Fv/Fm exhibited no significant differences for two-way interactions. However, significant differences were observed between treatments (P ≤ 0.05). The values registered under the control treatment (0.78) were significantly higher than those under drought stress conditions (0.76). A significant increase (P ≤ 0.05) of time to reach Fm (t for Fm) was observed in both genotypes under drought stress, with regards to their respective control plants, and this increase was much higher in RN1 (230%) than in RN51 (92%). The area of the OJIP curve (area) and total performance index (PI total) decreased significantly (P ≤ 0.05) when drought stress was imposed compared to the control treatment but without differences between genotypes. Regarding the specific energy fluxes (per reaction center), the absorption (ABSo/RC) and trapping (TRo/RC) of energy was considerably increased in RN1 (31.6% and 26.6%, respectively) under drought stress compared to control conditions, while in RN51, the increases were smaller (10.5% and 26.6%, respectively). The same trend was observed for the light energy dissipation as latent heat (represented by DIo/RC). RN1 plants under drought stress increased the light energy dissipation by 36% compared to control plants, while RN51 only increased by 12.5%.
After rewatering, most parameters in both genotypes returned to the well-watered level, showing no significant differences for two-way interactions (P ˃ 0.05, Table S2).
3.2 Evaluation of biochemical measurements
3.2.1 Malondialdehyde content (MDA)
During drought stress, the three-way interaction (genotype × treatment × sampling time) was not significant. However, significant differences (P ≤ 0.05) for a two-way interaction (genotypes × treatments) were found in both sampling times. At 24 h, in drought conditions, the highest values were registered. RN1 showed a 133% increase in MDA content compared to its control (17.36 nmol g−1 FW and 7.43 nmol g−1 FW, respectively), followed by the RN51 genotype with a significantly lower increase (84%) between drought stress and corresponding control plants (12.85 nmol g−1 FW and 6.97 nmol g−1 FW, respectively; Figure 3A). Regarding the 72 h of sampling time, RN1 in the drought treatment showed the highest value (12.85 nmol g−1 FW), which represented an increase of 110.65% compared to its control. Conversely, RN51 showed only a 23.5% increase in MDA value between drought and control conditions (9.37 and 7.59 nmol g−1 FW respectively). The last result highlights that the RN51 genotype had lower oxidative damage than RN1 (Figure 3A).
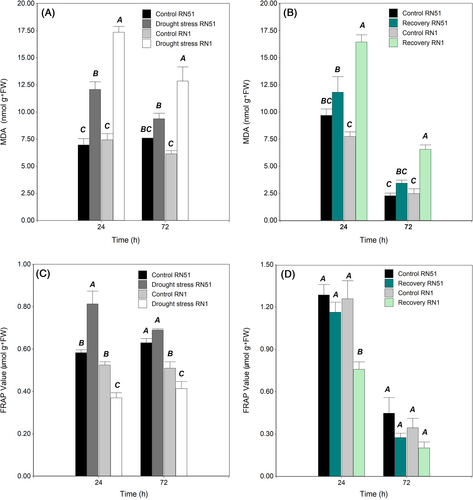
After rewatering, no significant differences were observed for three-way interactions (genotype × treatment × sampling time; Figure 3B). Nevertheless, significant differences (P ≤ 0.05) for a two-way interaction (genotypes × treatments) were found in both sampling times. Lipid peroxidation decreased in recovered plants of both genotypes but did not fully recover to the average MDA value observed in the well-watered treatment. However, recovery of the RN51 genotype (3.4 nmol g−1 FW) was better than that of the RN1 genotype, which almost reached the control level (2.3 nmol g−1 FW), while recovered plants of the RN1 genotype (6.6 nmol g−1 FW) maintained a high difference of 127% regarding their control (2.7 nmol g−1 FW).
3.2.2 Total reducing power by ferric reducing ability of plasma assay (FRAP)
During the drought stress treatment, no significant effect was recorded for the three-way interaction (genotype × treatment × sampling time). As shown in Figure 3C, significant differences (P ≤ 0.05) for a two-way interaction (genotype x treatment) were found at both sampling times. At the 24-h sampling time, when genotypes were exposed to drought stress, RN51 significantly increased the total antioxidant activity (0.81 μmol g−1 FW) by 40%, while RN1 (0.37 μmol g−1 FW) decreased it by 30%, compared to their corresponding control plants (0.58 and 0.53 μmol g−1 FW for RN51 and RN1, respectively). At 72 h of sampling time, RN51 presented the highest FRAP value (0.69 μmol g−1 FW) without differences compared to its control (0.63 μmol g−1 FW), while RN1 (0.41 μmol g−1 FW) showed a value 24% lower than its control (0.51 μmol g−1 FW).
After rewatering, no significant differences were found for three-way or two-way interactions, while significant differences between genotypes, treatments, and sampling time (P ≤ 0.05) were observed (Figure 3D). RN51 showed higher values than RN1, with an average of 0.85 μmol g−1 FW and 0.69 μmol g−1 FW, respectively. In contrast, well-watered plants showed an average value of 0.88 μmol g−1 FW and recovered plants had 0.61 μmol g−1 FW. Regarding sampling time, the FRAP value was 1.13 and 0.33 μmol g−1 FW at 24 and 72 h sampling times, respectively.
3.3 Antioxidant enzyme activity
3.3.1 Superoxide dismutase (SOD) activity
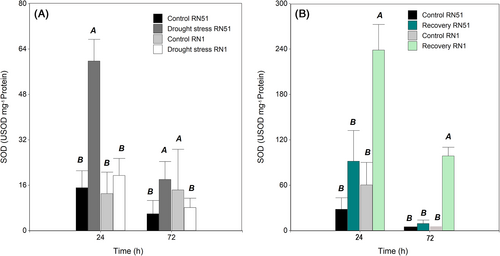
During drought stress treatment, there was no significant difference in the three-way interaction (genotype × treatment × sampling time; P > 0.05). SOD activity showed significant differences for two-way interactions (P ≤ 0.0001) only at the 24–h sampling time. At this time, the highest SOD level under drought treatment was recorded in the RN51 genotype with an average value of 59.79 USOD mg−1 protein, which was 295% higher than its control (15.1 USOD mg−1 protein). Conversely, although RN1 showed a slight increase compared to its control, no significant differences were found between them.
After rewatering, (Figure 4B), significant differences for two-way interactions (P ≤ 0.05) were observed. RN1 showed the highest SOD activity in recovered plants with an average value of 238.58 USOD mg−1 protein after 24 h, 298% higher than its control (60 USOD mg−1 protein). The same trend was observed at 72 h.
3.3.2 Catalase (CAT) activity
During drought stress treatment, the three-way interaction (genotype × treatment × sampling time) was not significant (P > 0.05). At 24 h of drought treatment, CAT activity did not show any significant two-way interactions (P ˃ 0.05), while it exhibited significant differences between treatments (P ≤ 0.05). At this time, higher activity was recorded under drought treatment (with an average value of 0.14 μmol min−1 mg−1 protein) compared to the control treatment (0.07 μmol min−1 mg−1 protein). However, at 72 h, CAT activity showed significant (P ≤ 0.05) two-way interactions. Drought-stressed plants from RN51 genotype showed the highest increase in CAT activity (with an average value of 0.22 μmol min−1 mg−1 protein), which represented an increase of 175% compared to its control (Figure 5A). After rewatering, there were significant differences (P ≤ 0.05) for two-way interaction, with the highest values observed in RN1 genotype at 72 h of sampling time (data no shown).
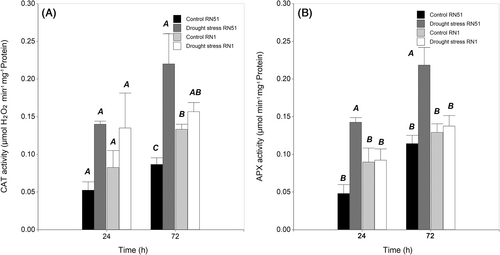
3.3.3 Ascorbate peroxidase (APX) activity
In the drought stress treatment, the three-way interaction (genotype × treatment × sampling time) was not significant (P > 0.05). At the 24-h sampling time, APX activity had a significant two-way interaction, with the highest value recorded in drought-stressed plants from genotype RN51 (0.14 μmol min−1 mg−1 protein), exceeding the value of its control by 180%. The same trend was observed at 72 h of sampling time, with the highest APX activity observed in RN51 when it was exposed to drought stress (0.22 μmol min−1 mg−1 protein), with a value 100% higher than its control (Figure 5B). After rewatering, a significant three-way interaction (P < 0.05) was found. The highest activity was observed in RN51 recovered plants at 72 h (0.05 μmol min−1 mg−1 protein), whereas RN1 only exhibited APX activity values of 0.01 and 0.004 μmol min−1 mg−1 protein at 24 and 72 h, respectively (data not shown).
3.4 Molecular analysis
3.4.1 Expression of genes coding for enzymes involved in lignin biosynthetic pathways
In general, the genes coding for enzymes involved in lignin biosynthetic pathways modified their expression in both genotypes. However, significant differences were observed only at 24 h of drought stress and after rewatering. Regarding drought treatment, expression of the CCoAOMT gene was significantly reduced in the leaves of both genotypes (RN51 and RN1) with respect to the control treatment (log2 ratio = −2.185 and − 9.24, respectively; Table 2). When we compared both genotypes in drought stress conditions, the expression of this gene was reduced with significant differences (log2 ratio = −4.17). Expression of the COMT gene was slightly decreased in both genotypes and no significant differences were observed (P > 0.05). Regarding expression of the CCR gene, drought stress significantly increased its value (log2 ratio = 1.93) in the RN51 genotype with respect to the control treatment, while RN1 did not show significant differences in expression of this gene (Table 2). When we compared both genotypes under drought stress, the expression of this gene was significantly reduced (log2 ratio = −1.2; Table 2).
| Gene name | Primer sequences (5′ - 3′) | Product size (bp) | Genotypes | Treatment | Log2 (ratio) | R2 | P-value |
|---|---|---|---|---|---|---|---|
| Forward /reverse | |||||||
| CCoAOMT | AGCAAAAGCAGTCGTCATAGG CACGGTGGATTCCAGAATC |
138 | RN51 | Drought | −2.185 | 99.6 | 0.0466 |
| RN1 | Drought | −9.24 | 0.0143 | ||||
| RN51:RN1 | Drought | −4.17 | 0.0261 | ||||
| RN51 | Recovery | 0.68 | 0.1174 | ||||
| RN1 | Recovery | 0.41 | 0.1961 | ||||
| RN51:RN1 | Recovery | −4.51 | 0.0289 | ||||
| COMT | TCGAGTTCTACACGGGCTTC AAGTTGACGCCCTTGATCTG |
115 | RN51 | Drought | −0.21 | 99.2 | 0.4273 |
| RN1 | Drought | −1.46 | 0.2275 | ||||
| RN51:RN1 | Drought | −1.11 | 0.2288 | ||||
| RN51 | Recovery | 1.42 | 0.0451 | ||||
| RN1 | Recovery | −4.2 | 0.0442 | ||||
| RN51:RN1 | Recovery | −4.4 | 0.0399 | ||||
| CCR | CCACCAAGTGATGAGCTGAA CCCTAATCCACTCCCCGTAT |
106 | RN51 | Drought | 1.93 | 99.7 | 0.0231 |
| RN1 | Drought | 0.41 | 0.423 | ||||
| RN51:RN1 | Drought | −1.2 | 0.0206 | ||||
| RN51 | Recovery | 0.96 | 0.0376 | ||||
| RN1 | Recovery | −0.85 | 0.2536 | ||||
| RN51:RN1 | Recovery | 1.5 | 0.0415 |
After rewatering, CCR and COMT genes showed significant differences in both genotypes at 24 h, with gene expression increased in the RN51 genotype (log2 ratio = 0.96 and 1.42, respectively), while the RN1 genotype had the opposite behavior (Table 2). The CCoAOMT gene expression was not significantly different (P < 0.05) between genotypes.
3.4.2 Anatomical observations
The average and standard error for anatomical measured parameters (thickness and areas of sclerenchyma, bulliform cells, and parenchyma and epidermis tissues) are presented in Table 3. Two-way interactions were significant (P ≤ 0.05) in most parameters. Under drought stress, RN51 had an increased sclerenchyma area, parenchymal sheath area, and thickness of the adaxial epidermis compared to its control, by 111%, 110%, and 82%, respectively (Figure 6C,D; Table 3). Therefore, RN51 differed significantly from RN1, since the latter decreased regarding its control in these anatomical parameters.
| Anatomical parameters | Control | Drought | ||||||
|---|---|---|---|---|---|---|---|---|
| RN51 | RN1 | RN51 | RN1 | |||||
| Total thickness (μm)** | (2.2 ± 0.3) × 102 | a | (2.17 ± 0.07) × 102 | a | (2 ± 0.16) × 102 | a | (1.22 ± 0.01) × 10 2 | b |
| Sclerenchyma area (S) (μm2)** | (27.37 ± 3.9) × 102 | b | (33.7 ± 3.34) × 102 | b | (57.9 ± 7.5) × 102 | a | (26.8 ± 1.67) × 102 | b |
| Bulliform cell area (μm2)** | - | - | (9.33 ± 0.82) × 103 | - | ||||
| Parenchymal sheath and radiated chlorenchyma (μm2)** | (13.1 ± 1.78) × 103 | b | (12.65 ± 0.88) × 103 | b | (27.53 ± 0.73) × 103 | a | (7.1 ± 0.13) × 103 | c |
| Colorless parenchyma (μm2)** | (95 ± 7.6) × 103 | a | (33.179 ± 6.4) × 103 | b | (18 ± 6.9) × 103 | bc | (10.3 ± 6.9) × 103 | c |
| Parenchyma area (P) (μm2)** | (112.3 ± 8.35) × 103 | a | (47.5 ± 7.6) × 103 | b | (44.94 ± 8.3) × 103 | bc | (17.72 ± 7.05) × 103 | c |
| Total area of the leaf (μm2)* | (123.6 ± 21) × 103 | A | (80.95 ± 5.93) × 103 | B | (72.17 ± 8.51) × 103 | A | (44.46 ± 1.36) × 103 | B |
| P: S** | 41.34 ± 4.32 | a | 14.41 ± 2.26 | b | 8.74 ± 0.59 | c | 6.96 ± 0.46 | d |
| Thickness of abaxial epidermis (μm)* | 25.52 ± 1.83 | A | 20.09 ± 1.83 | B | 25.95 ± 1.83 | A | 19.76 ± 1.83 | B |
| Thickness of adaxial epidermis (μm)** | 16.72 ± 1.4 | c | 21.24 ± 1.38 | b | 31.23 ± 1.38 | a | 19.43 ± 1.83 | bc |
- Note: Two vascular bundles on both sides of the central bundle were measured. Values represent the mean ± se (n = 12). Different lower-case letters indicate significant differences (P ≤ 0.05) for two-way interaction (**). Uppercase letters indicate significant differences between genotypes (P ≤ 0.05; *) within treatments for variables where interaction was no significant (P > 0.05).
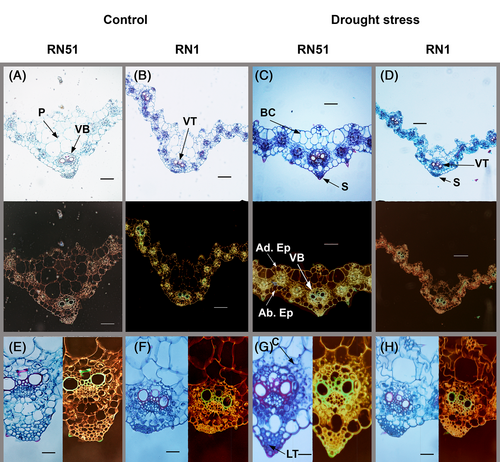
At the intercostal regions in this tissue, groups of four to five colorless epidermal cells (fan shaped), known as bulliform cells, were observed in RN51 under stress conditions, covering an area of 9.33 ± 0.82 * 103 μm2 (Figure 6C). Conversely, the sensitive genotype (RN1) under drought treatment did not show bulliform cells development and decreased significantly the thickness of the leaf cross section by 42% compared to its control (Table 3; Figure 6D). Regarding parenchyma:sclerenchyma ratio (P:S), drought-stressed plants from RN51 showed the highest value (8.74) while RN1 drought-stressed plants the lowest (6.96).
3.4.3 Evaluation of growth performance
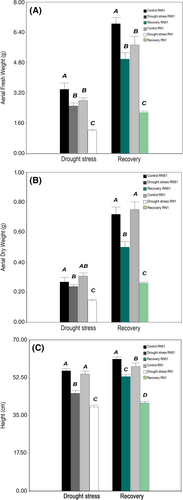
In general, when drought stress treatment was applied, both C. ciliaris genotypes showed lower growth performance than control treatments while RN1 registered the largest reduction in all growth parameters. Under drought stress, aerial fresh weight (AFW) had a significant two-way interaction (P ≤ 0.05). The RN51 genotype exhibited a decrease of 26% with respect to its control, whereas RN1 exhibited a reduction of 56%. The same trend was observed after rewatering, with decreased percentages of 27% and 62%, respectively (Figure 7A). Moreover, aerial dry weight (ADW) had a significant two-way interaction (P ≤ 0.05). RN1 registered the highest decrease for this parameter (46% with respect to its control) when it was exposed to drought stress, while in recovery conditions the reduction was more pronounced (65%; Figure 7B). The same behavior was recorded for plant height (H), with a significant two-way interaction (P ≤ 0.05). Under drought treatment, the reduction observed was 20% and 28% for RN51 and RN1, respectively, and 13% and 29%, respectively, in recovery conditions (Figure 7C).
3.4.4 Associations between physiological-biochemical, molecular, anatomical, and growth performance traits
Under drought stress treatment, the first two principal components (PC1 and PC2) of the PCA explained 92.7% of total variability in the data (cophenetic correlation coefficient = 0.992; Figure 8A). Correlations between the different parameters were explored by the angles between vectors. Acute and obtuse angles indicated positive and negative correlation, respectively, whereas right angles denoted no correlation between traits. PC1 of the biplot (Figure 8A) revealed that the parameters ET (0.31), PI (0.31), and H (0.31), followed by Fv/Fm (0.29), DW (0.29), and LRWC (0.28), were grouped together, being correlated with each other and positively associated with both genotypes in control treatments. The parameters CAT (−0.28) and APX (−0.25) were grouped together and strongly associated to the RN51 genotype under drought stress, while the RN1 genotype under drought stress conditions was strongly associated with MDA (−0.28) content.
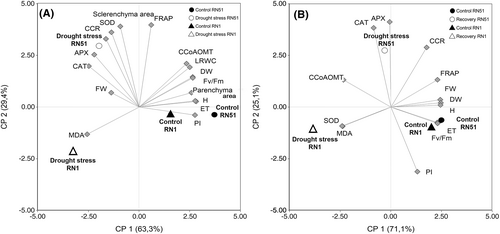
Under recovery conditions, the first two principal components (PC 1 and PC 2) explained 96.2% of total variability in the data (cophenetic correlation coefficient = 0.993; Figure 8B). PC1 of the biplot (Figure 8B) revealed that FW (0.33), DW (0.32), H (0.32), ET (0.31), and Fv/Fm (0.31) remained positively associated among themselves and with both genotypes in control treatments. MDA (−0.32) and SOD (−0.32) vectors were grouped together and oriented to genotype RN1 in recovery conditions. PC2, CAT (0.55), and APX (0.51) were grouped together and correlated, revealing the RN51 genotype under recovery conditions.
4 DISCUSSION
Divergent adaptive mechanisms to cope with drought stress conditions were employed by the contrasting genotypes evaluated in this study and included maintenance of leaf water relations, photosynthetic capacity, ROS detoxification, and changes in leaf anatomy, which enabled the C. ciliaris plants to evade drought-induced damage, thereby allowing tolerant genotypes to more readily recover their physiological functions after rewatering.
Maintaining a well-watered status is crucial to optimal physiological function and growth (Chen et al., 2016; Zhou et al., 2017). In this sense, LRWC can correctly show the balance between water absorbed by the plant and disbursed through transpiration, thereby, it is considered a good indicator of drought tolerance or adaptation in various plant species, including cereals (Rampino et al., 2006; Siddiqui et al., 2016). In our study, drought stress caused a reduction in LRWC of C. ciliaris leaves. Notably, the reduction was higher in the sensitive genotype (RN1) than the tolerant genotype (RN51). It is widely known that plants react to water deficit with a rapid closure of stomata due to decreased leaf turgor and atmospheric vapor pressure (Ashraf & Harris, 2013), thus, avoiding further loss of water through transpiration (Habermann et al., 2019; Huseynova, 2012). Our results suggest that tolerant genotypes could have a better ability to regulate intracellular water relations than susceptible genotypes and, thus, maintain better growth during water restriction. Moreover, it is well documented that reduction in LRWC is related to cell membrane properties and its adaptability to environmental changes (Amari et al., 2017). Chen et al. (2016) and Jian et al. (2012), found similar results and mentioned that return to the state before stress is crucial for recovery, since it involves the rearrangement of metabolic pathways to repair drought-induced damage and resume plant growth.
Damage to PSII is often the first manifestation of stress (Maxwell & Johnson, 2000). Chlorophyll fluorescence measurements have become a widely used method to research photosynthetic apparatus damage and a powerful tool to study plant responses to environmental stress (Ings et al., 2013; Kalaji et al., 2017), including drought stress assays (Lone et al., 2019; Siddiqui et al., 2016; Silva et al., 2010; Zhao et al., 2019). In this study, when plants were exposed to drought stress, alterations in most chlorophyll fluorescence measurements (the OJIP-test parameters) were observed. Fv/Fm showed values lower than 0.83, which suggested that plants were growing under stress and that PSII reaction centers were damaged (Kalaji et al., 2017). Unlike Fv/Fm, which utilizes only extreme values of chlorophyll fluorescence, the performance index (PI) is an indicator of plant vitality that incorporates multiple parameters, including absorption, trapping of excitation energy, electron transport beyond the primary plastoquinone, and dissipation of excitation energy (Ings et al., 2013). In our study, this parameter was affected significantly in the sensitive genotype, suggesting a reduction of energy supply from light-harvesting pigments toward reaction centres (RC). Moreover, the sensitive genotype had the highest energy absorption by reaction centres (ABS/RC) under drought conditions, followed by an increase of heat dissipation per reaction centre (DIo/RC), suggesting that PSII reaction centres were damaged and excessive light energy was accumulated in higher proportions than that in the tolerant genotype. Similar results were found by several authors (Huseynova, 2012; Lepeduš et al., 2012; Luna et al., 2018; Zhao et al., 2019). Regarding all parameters measured, the tolerant genotype showed better ability to keep the integrity of the photosynthetic apparatus when it was exposed to drought stress. Notably, after rewatering, the values of most chlorophyll fluorescence parameters were restored to values similar to those of the control treatment in both genotypes. The latter could suggest reduced drought-associated damage to plant photosynthetic systems is the basis of rapid recovery after rewatering.
MDA content has been considered as an indicator of oxidative damage in various crops, including forage grass species (Bi et al., 2016; Luna et al., 2000; Moller et al., 2007). In this work, a negative association was observed between MDA content and FRAP value, indicating lower lipid peroxidation in leaves and higher total antioxidant power in the tolerant genotype (RN51) in concordance with the observations made by Tommasino et al. (2018) under heat stress. This might be due to the induction of the enzymatic and non-enzymatic antioxidant defense systems. It is well established that antioxidant enzymes are extremely important as a general adaptation strategy used by plants to overcome oxidative stress by detoxifying ROS.
Regarding enzymatic defense systems, our results showed that when genotypes were exposed to drought stress, the tolerant genotype presented the highest SOD activity. Based on current and previous works (Lanza Castelli et al., 2010; López Colomba et al., 2013; Tommasino et al., 2018), the tolerant genotype might have a higher and longer capability to catalyze the dismutation of O2− to H2O2 under drought stress conditions, which is the first step of scavenging ROS. Moreover, it could suggest that the SOD enzyme may play an important role through signaling processes in antioxidant protection to drought damage. Similar comments were mentioned by Berwal and Ram (2018). However, the sensitive genotype (RN1) showed the highest value in this antioxidant enzyme at 24 h after rewatering. This result was also observed in Kentucky bluegrass (Bian & Jiang, 2009) and wheat (Abid et al., 2018) under drought stress. This behavior could suggest that the process of recovery after rewatering did not necessarily limit production of ROS in the sensitive genotype, hence, antioxidant defense was still active. Different results on CAT activities, such as induction, reduction, or stable CAT activities under drought stress and recovery, have been reported (Hossain et al., 2013; Bi et al., 2016; Bian & Jiang, 2009; Boaretto et al., 2014; Gill & Tuteja, 2010; Jin et al., 2016). In our work, during the stress period, the tolerant genotype showed greater CAT activity in leaves compared with the sensitive genotype, suggesting that despite the relatively low affinity for H2O2, the CAT enzyme could efficiently remove photorespiratory H2O2 and maintain the estimated peroxisomal H2O2 concentrations. CAT appeared to be an important enzyme for overcoming drought stress imposed oxidative stress (Waszczak et al., 2018; Xie et al., 2019). Wang et al. (2016) mentioned similar conclusions in wheat. After rewatering, the trend was reversed. Compared to the well-watered control, a greater magnitude of increase in the concentration of the CAT enzyme in the sensitive genotype's leaves relative to those of the tolerant genotype could indicate the potential need for removal of ROS when plants were rehydrated. These cultivar-specific differences in antioxidant enzyme capacity could partially explain differences in drought tolerance, which is in accordance with the previous report on wheat given by Abid et al. (2018). The glutathione-ascorbate cycle (AsA-GSH) involves four enzymes, among them APX is the alternative with a more effective detoxification mechanism against H2O2, operating both in chloroplasts and the cytosol (Pandey et al., 2017). In our study, higher APX activity was observed in the tolerant genotype under drought stress and recovery treatment, while the sensitive genotype did not show activity after rewatering. Sofo et al. (2015) mentioned that genes encoding APXs were particularly important in maintaining the two non-enzymatic antioxidants (ascorbate and glutathione) and were directly or indirectly involved in maintaining high photosynthetic rates in plants under adverse environmental conditions. For instance, the better performance in RN51 to cope with drought stress could be related to greater amounts of these antioxidants. Moreover, ascorbate is involved in other functions, such as plant growth, gene regulation, and modulation of several enzymes. Further studies in gene expression of APX genes will be necessary to establish their role in the tolerance of C. ciliaris.
The modifications in leaf water relations, photosynthetic capacity, and antioxidant defense system observed under drought stress in both tolerant and sensitive genotypes were accompanied by changes in the foliar tissues and its lignification. Several studies have shown that abiotic stress leads to the rapid activation of lignin biosynthesis pathway-related enzymes expression (Chen et al., 2019) and more than a dozen enzymes are involved with CCR, COMT, and CCoAOMT playing an important role in the regulation process of lignification (Christensen & Rasmussen, 2019; Liu, Wu, et al., 2018). In our study, the transcript level of the CCoAOMT gene decreased in the sensitive genotype, whereas CCR transcripts were increased in leaf blade tissues of the tolerant genotype under drought stress conditions. Studies carried out in maize and sugarcane have shown that downregulating the expression of genes coding for CcoAOMT, CCR, and COMT enzymes leads to a decrease in lignin content (Christensen & Rasmussen, 2019). Considering our results, better performance of the tolerant genotype under drought stress and rewatering could be related to increasing tissue lignification through an increase of lignin biosynthesis genes. Several studies indicated lignified tissues as the most relevant factor in forage quality reduction (El Hage et al., 2018; Grabber et al., 2004; Stabile et al., 2012). Nevertheless, further studies would be necessary to determine the effect of drought periods and recovery on forage quality parameters in genotypes evaluated in this study.
Plants adapt to drought stress in the environment through a variety of mechanisms (Basu et al., 2016). In this sense, leaf architecture plays a vital role in resisting a variety of stressors, including drought stress (Hameed et al., 2012). Moreover, structural modifications, like leaf and epidermis thickness and development of bulliform cells, can be directly related to drought tolerance (Arias et al., 2018; Hameed et al., 2010, 2012; Mustafa et al., 2019; Selim et al., 2019). It has been also reported that drought stress causes an increase in sclerenchyma cells and cell wall thickness (Basu et al., 2016; de Paula et al., 2019). In our work, after 21 days of drought stress, the sclerenchyma area of the tolerant genotype increased significantly, and bulliform cells showed great development when plants were subject to drought stress. It could suggest a strategy to increase water storage and strengthen vascular bundles through sclerified tissues and prevent water loss by the development of bulliform cells. Due to the presence of these thin-walled water-containing cells, leaves could roll up during drought stress (Cal et al., 2019; Fang & Xiong, 2015; Xu et al., 2018). Similar conclusions are described for several other species (Basu et al., 2016; Mustafa et al., 2019; Shehzadi et al., 2019) including C. ciliaris (Mansoor et al., 2019; Nawazish et al., 2006).
In forage production, it is important to maintain balance between quality (considered cell wall digestibility) and biomass yield (as dry matter production), which could be altered by abiotic stress and the ability of plants to recover. It has been established that drought stress is a very important limiting constraint in plant growth and crop production (Amari et al., 2017). In the present study and in accordance with the results obtained in C. ciliaris by some authors (Amari et al., 2017; Tommasino et al., 2018), parameters related to growth were notably affected under drought conditions. Even though the two genotypes (sensitive and tolerant) did not die when exposed to drought stress, the sensitive genotype showed the highest reduction in growth parameters, which was sustained during rewatering.
5 CONCLUSIONS
The two genotypes investigated here could tolerate drought and were recovered from the imposed stress condition. However, both genotypes differed significantly in their response. Our findings indicate that the capacity to regulate lipid peroxidation and mitigate oxidative damage in response to soil drying and rewetting, as well as the maintenance of relatively high LRWC and development of foliar characteristics, like thickness of the adaxial epidermis, well-developed bulliform cells, and intensive tissue sclerification, constituting the adaptive mechanisms by which drought-tolerant genotypes cope with water stress.
ACKNOWLEDGMENTS
This work was supported financially by the Instituto Nacional de Tecnología Agropecuaria (INTA). Argentina. Project PNPA-1126072 and the Agencia Nacional de Promoción de Ciencia y Tecnología (ANPCyT project PICT N° 2014–0777).
AUTHOR CONTRIBUTIONS
Iliana M. Carrizo, Eliana López Colomba, and Exequiel Tommasino conducted stress assays in controlled conditions and measurements of physiological, biochemical and molecular parameters in C. ciliaris; Karina Grunberg and Eliana López Colomba were responsible for project design. Edgardo Carloni contributed to evaluating histological analysis. All authors contributed to writing the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




