Negative impact of long-term exposure of salinity and drought stress on native Tetraena mandavillei L.
Funding information: Researchers supporting project number, Grant/Award Number: RSP-2020/193; King Saud University
Abstract
Tetraena mandavillei L. is a perennial shrub native to the Middle Eastern countries of Asia, which is extensively regarded as a drought-tolerant plant. However, the plant reduces growth and biomass when grown in high concentrations of sodium chloride in the soil. We conducted a pot experiment to influence the negative impact of different levels of salinity (0, 10, and 20 dSm−1) and drought stress (100, 80, 60, and 40% water field capacity), to study different growth-related parameters, physiological alterations and ion uptake by T. mandavillei. Both salinity and drought stress caused a negative impact by affecting several attributes of T. mandavillei, but the plants showed some resistance against drought stress conditions in terms of growth and biomass. In addition to that, we noticed that a combinatorial and individual impact of drought and salinity stress decreased photosynthetic pigments and gas exchange parameters in T. mandavillei. Results also depicted that the combination of the abiotic stress conditions drought and salinity induced reactive oxygen species (ROS), indicating that the plants undergo oxidative damaged. However, due to the active plant defense system, the plant enhanced its performance under abiotic stress conditions, but due to the severe drought condition (40% water field capacity), a significant (P < 0.05) decrease in the activities of antioxidant compounds was caused. Furthermore, osmolytes also increased under both salinity and drought stress conditions in this study. Our results also showed that increased salinity and drought stress in the soil caused a significant increase in sodium (Na+) and chloride (Cl−) ions in roots and shoots of T. mandavillei. In contrast to that, the contents of Calcium (Ca2+) and potassium (K+) were decreased in all organs of the plants with increasing levels of salinity and drought stress. Taken together, T. mandavillei can be classified as a facultative halophyte with the ability to tolerate drought stress and using salt accumulation mechanisms to tolerate salinity stress.
Abbreviations
-
- APX
-
- ascorbate peroxidase
-
- AsA
-
- ascorbic acid
-
- PCA
-
- principal component analysis
-
- ROS
-
- reactive oxygen species
-
- TBA
-
- thiobarbituric acid
-
- TCA
-
- trichloroacetic acid
-
- WFC
-
- water field capacity
1 INTRODUCTION
Plants are typically exposed to a broad myriad of biotic and abiotic stresses, including feeding from wild animals and insects, weed infestation, hail, mechanical injury, diseases, low soil fertility, drought, salinity and others that can diminish the plant photosynthetic area, and thus, the attained total plant biomass or grain yield (Adeyemi et al., 2020; Adnan et al., 2020; Battaglia et al., 2018; Battaglia, Lee, Thomason, Fike, et al., 2019; Battaglia, Lee, Thomason, & Van Mullekom, 2019; Diatta, Fike, et al., 2020; Diatta, Thomason, et al., 2020; Farsad et al., 2019; Thomason & Battaglia, 2020) In the case of plants grown in a hot, arid or semi-arid climate two of the main yield and biomass-limiting stresses are salinity and drought (Saleem, Ali, Hussain, et al., 2020; Saleem, Ali, Rehman, Hasanuzzaman, et al., 2020; Zamin et al., 2019). In this context, the use of native and perennial C4 plant species, that can remain highly productive in environments with saline soils and recurrent drought periods, is an important strategy to cope against these predominant abiotic stresses in arid and semi-arid climates (Battaglia, Fike, et al.,2019; Khan, Zhang, Luo, Liu, Rizwan, et al., 2019b; Kumar, Lai, Battaglia, et al., 2019; Kumar, Lai, Kumar, et al., 2019; Mohamed et al., 2020). Recent research has shown that native plant species may exhibit stronger tolerance or better and faster mechanisms to adjust or withstand abiotic stress conditions such as drought and salinity stress compared to their cultivated relatives (Saleem, Ali, et al., 2019; Saleem, Rehman, et al.,2020; Yaseen et al., 2020). With the cultivation of indigenous crops, landscape architects not only produced vernacular ecosystems, but also provided solutions to the increased air temperatures experienced at a global scale in the last 50 years and the wasteful use of water channels (Parker & Hay, 2005; Valliere et al., 2019). The selection of plants in urban landscaping scenarios is important in order to avoid future environmental and financial losses (Saleem, Ali, Hussain, et al., 2020; Saleem, Ali, Rehman, Hasanuzzaman, et al., 2020). According to Fernández-García et al. (2004), several taxa of native plants (both arid and semi-arid) are listed as resistant to drought, mostly based on anecdotal observations of plant performance in countryside planting. However, the eventual success of these species in more protected, anthropogenic managed environments is uncertain to date. Moreover, for several species, especially native species, specific information on their tolerance to salt and drought stresses is lacking or scarce.
Soil salinity in agricultural soil is responsible for decreasing plant growth and yield in numerous cereal crops worldwide (Adhikari et al., 2020; Kamran et al., 2019; Safdar et al., 2019), in a global scenario where food production will need to be increased by more than 70% by 2050 to feed the growing human population (Saleem, Ali, Hussain, et al., 2020; Saleem, Ali, Rehman, Hasanuzzaman, et al., 2020). Soil salinity means a high sodium (Na+) and chloride (Cl−) concentration in the soil, and this has an adverse effect on plant growth and yield (Munns & Tester, 2008; Safdar et al., 2019). According to the UN Environment Program (UNEP), approximately 50% of the total soils under agriculture are now characterized as saline soils throughout the world (Zafar et al., 2019). Salinity affects plant growth through osmotic stress, where high levels of soluble salts in the soil decrease the uptake of important plant nutrients such as Ca, K, and N and the plant water content (Chantre Nongpiur et al., 2016; Kang et al., 2014; Ketterings & Czymmek, 2007). Plants can also build up highly soluble salt contents in their structures, contributing to plant toxicity and eventually altering plant physiology and biochemical processes under saline conditions (Nizam, 2011; Zafar et al., 2015). However, the negative impact of salinity depends upon the number of climatic conditions, light intensity, plant species and soil conditions (Kamran et al., 2019; Parida & Das, 2005). In addition, a reduction in the plant growth rate and changes in structural composition as a result of high saline environments can lead to an alteration in the photosynthetic machinery, exclusion of ions, osmotic adjustment and nutrient imbalances (Hussain et al., 2016; Shafiq et al., 2020).
Drought conditions can lead to a number of changes in plants, including reduced growth, lower fresh and dry biomass, lower rates of photosynthesis, and reduced absorption of essential nutrients from the soil (Ahmad et al., 2017; Akram et al., 2018; Khan, Zhang, Luo, Liu, Rizwan, et al., 2019b). Reductions in chlorophyll content and oxidation of membrane lipids and proteins are the crucial reasons for plant damage caused by the production of drought-induced highly reactive oxygen species (ROS), leading to modification of the cellular redox status (Sakya & Prahasto, 2020; Wu et al., 2018). However, the negative impacts of drought depend on the total length of the plant growth cycle, the degree of drought stress and frequency of drought conditions, growth conditions and plant species (Khan, Zhang, Luo, Liu, Rizwan, et al., 2019b, Hesham & Fahad, 2020). Under environmental stress, imbalance in ROS accumulation and generation can occur, which results in the formation of hydrogen peroxide (H2O2), superoxide radicles (O−) and hydroxide ions (OH), all of which are known as stress indicators at cellular levels (Saleem, Ali, Rehman, Rana, et al., 2020; Saleem, Fahad, Khan, et al.,2020; Saleem, Kamran, et al., 2020). These ROS are toxic to plants and scavenged by various types of antioxidants like SOD (superoxide dismutase), POD (peroxidase), catalase, etc. to maintain cellular homeostasis (Rehman et al., 2019; Zaheer, Ali, Saleem, Imran, et al., 2020). Previously, many researchers concluded that varieties of antioxidants have increased their activities under salt stressed environments in Vigna radiata (Islam et al., 2016), Triticum aestivum (Shafiq et al., 2020), and Plantago ovata (Kala, 2015). Additionally, drought conditions reduce the photosynthetic rates, expansion of leaves, increase stomatal closure, the levels of ROS and early leaf catabiotic, and decrease the translocation within the plant, resulting in overall decreased crop yields (Ahmad et al., 2017; Anjum et al., 2017).
Tetraena mandavillei L. (Hadidi) Beier & Thulin [Syn. Zygophyllum mandavillei (Moq.)] is a common perennial shrub found in sand plains between dunes areas (Alzahrani & Albokhari, 2017). T. mandavillei and related species are considered to be tolerant toward salt and water stress and are therefore recommended for planting in urban greening programs (Alam et al., 2020). Along with the required proper management techniques, the maintenance of T. mandavillei in landscape plantings and its water requirements are still unknown. We conducted a field experiment which will give us new insights into T. mandavillei tolerance and resistance and help to use this plant species for landscaping purposes. Findings from this study will add to our knowledge about (1) the effect of individual or combinatorial stress of drought and salinity on plant growth and biomass, (2) content of photosynthetic pigments and gas exchange parameters, (3) oxidative stress and antioxidant response, and (4) ion uptake into different organs of the plants. Moreover, these findings will provide new insights about the tolerance and resistance mechanisms to abiotic stresses in arid and semi-arid environments that will be helpful to develop appropriate maintenance in arid and semi-arid urban landscapes with incorporation of native plants.
2 MATERIALS AND METHODS
2.1 Site area and plant material
To study the eco-physiological response of T. mandavillei to different salt and water stresses, the plants were planted in pots and exposed to various stress conditions. The experiments were carried out at the AL-Foa Research Farm, United Arab Emirates University, Al Ain, Abu Dhabi, UAE, during the years 2015–2016. The seeds of T. mandavillei were sown in germinating trays with potting soil and sand in a ratio of 1:1 v/v. At the same time, external fertilization (N, P, K) was applied, as recommended by Islam and Rahman (2008). Approximately 21 days after emergence, the plants were shifted to 20 cm pots that were filled with desert sand. All cultural practices, i.e., fertilization, weeding, etc., were the same for all plants during the experiment. The pots were placed in a controlled greenhouse environment, where the plants received natural light and humidity and were protected from the occurrence of rains and storms during the length of the study.
2.2 Stress treatments
In this study, we applied different treatments of salt and water stress to T. mandavillei. The salinity levels for T. mandavillei included three treatments, as follows: no NaCl of 0 dSm−1 (control), standard salinity levels of 10 dSm−1 and high salinity levels with 20 dSm−1 (Zamin et al., 2019; Zamin & Khattak, 2017). These treatments were applied by using sodium chloride (NaCl, purity 99.5%). NaCl was mixed with tap water in 500-gallon water tanks to get the solutions of desired electrical conductivity (EC) such as 0, 10 and 20 dSm−1. All these solutions were used as irrigation water with different quantities (100, 80, 60, and 40% of field capacity) to the plants. All planted pots were irrigated with tap water after 60 days from seed emergence. After that time, the pots were categorized into four different levels of water stress, including 100, 80, 60, and 40% of water field capacity (WFC). As a result of this, we evaluated the individual and combined effects of drought and salinity stress in form of 12 treatments, which are as follow: (1) 100% WFC + 0 dSm−1, (2) 100% WFC + 10 dSm−1, (3) 100% WFC + 20 dSm−1, (4) 80% WFC + 0 dSm−1, (5) 80% WFC + 10 dSm−1, (6) 80% WFC + 20 dSm−1, (7) 60% WFC + 0 dSm−1, (8) 60% WFC + 10 dSm−1, (9) 60% WFC + 20 dSm−1, (10) 40% WFC + 0 dSm−1, (11) 40% WFC + 10 dSm−1, and (12) 40% WFC + 20 dSm−1. Each treatment combination was repeated three times (i.e. total of 36 pots or experimental units, each one with one plant) and the experiment was executed as a complete randomized design.
2.3 Sample harvesting
Whole plants were harvested to study different biological traits and harvested after 120 days from seed germination, which completed 60 full day of water and salinity stress treatment application. For the quantitative chemical analysis, representative specimens of each plant (each from a treatment) were frozen and stored at – 80°C. To study agronomic traits of the plants, root and shoot lengths were measured using measuring scale and dry plant weight was determined after drying the plants in an oven at 60°C for 72 h for all three replications.
2.4 Chlorophyll content determination
The total chlorophyll content was measured by following the method of Lichtenthaler (1987) for each treatment and then means were calculated. Gaseous exchange parameters were measured in a full sunny day between the 9:00 AM and 11:00 AM using a portable IRGA before harvesting the plants. Total plant leaves were detached from plants in each treatment and stored at −80°C for the physiological and biochemical analysis.
2.5 Measurement of MDA content
MDA content was determined following the method of Hesham and Fahad (2020) by spectrophotometrically using the thiobarbituric acid (TBA) technique. An aliquot solution having 2 ml of leaf extract was added to a test tube comprising 1 ml of 20% trichloroacetic acid (TCA) and 0.5% TBA. The solution was heated for 30 min at 95 °C in a water bath and left for cooling at room temperature. Afterwards it was centrifuged at 14000 g for 10 min and then the absorbance and non-specific absorbance were measured at 532 and 600 nm, respectively.
2.6 H2O2 content determination
H2O2 was measured according to the method of Hesham and Fahad (2020) with a slight modification from the method of Jana and Choudhuri (1981). Samples were prepared from 5 g of fresh leaf samples. Then 50 μl of 5% titanyl sulfate (w/v) and concentrated NH4OH solution were added to the leaf samples. Afterwards 15 ml of 2 M H2SO2 were added and the column was topped up with cold water to 20 ml. The absorbance was measured at 415 nm.
2.7 Electrolyte leakage (EC)
Membrane permeability was measured by determining the electrolyte leakage following the procedure of Dionisio-Sese and Tobita (1998). The EC was measured as EC = (EC1/EC2) × 100.
2.8 Measurements of enzymatic antioxidants
The activities of antioxidant enzymes such SOD, POD, and CAT measured by following the method of Hesham and Fahad (2020). One unit of SOD activity was defined as the amount of enzyme needed for 1-mg tissue proteins in 1 ml of a reaction mixture to increase SOD inhibition rates to 50% at 550 nm. For POD activity measurements the 3 ml reaction solution consisted of 50 mM phosphate buffer (pH 7.8), 25 mM guaiacol, 200 mM H2O2 and 0.5 ml leaf extract. The 3 ml CAT activity measurement solution consisted of 50 mM phosphate buffer (pH 7.0), 200 mM H2O2, and 50 μl leaf extract. By adding the leaf extract, the enzymatic reaction was initiated. Changes in the absorbance were determined at 240 nm. Ascorbate peroxidase (APX) activity was measured according to the method of Nakano and Asada (1981). The assay depends on the decrease in absorbance at 290 nm when ascorbate is oxidized. The reaction mixture contained 50 mM K phosphate buffer (pH 7.0), 0.5 mM ascorbate, 0.1 mM EDTA Na2, 0.1 mM H2O2 and 0.1 ml of leaf extract in a final assay volume of 1 ml. The concentration of oxidized ascorbate was calculated by using the extinction coefficient of 2.8 mM−1 cm−1. One unit of APX was defined as 1 mmol ml−1 ascorbate oxidized min−1.
2.9 Metabolite measurements
Free proline, soluble sugars, ascorbic acid, and glutathione contents were determined by following the method of Bates et al. (1973), Irigoyen et al. (1992), Mukherjee and Choudhuri (1983), and Griffith (1980), respectively. For the proline determination, frozen leave samples at room temperature were homogenized in 3% (w/v) sulfosalicylic acid (1 ml) and then stored overnight at 4°C. After the addition of 2 ml acid ninhydrin and 2 ml of glacial acetic acid, the sample was heated in a water bath for 45 min at 100°C. The change in absorbance was measured at 519 nm. Soluble sugars were determined by taking frozen leaf sample (1 g) which was homogenized in 5 ml 80% ethanol. Using the Whatman No. 42 (Whatman plc) the mixture was filtered and diluted with distilled water to a total volume of 50 ml. 1 ml taken from the 50 ml solution was added in the test tube that already contained 5% p-phenol solution (1 ml), followed by the addition of 5 ml 95% sulfuric acid. Then the change in absorbance was measured at 490 nm. Ascorbic acid was measured by mixing frozen leaf samples (∼0.2 g FW) in a solution containing 1.5 ml 2% (v/v) metaphosphoric acid containing 1 mM ethylenediaminetetraacetate. Samples were centrifuged for 12 min at 13,000 g and at 4°C. The filtered supernatant was utilized to determine antioxidants by capillary electrophoresis using a buffer containing 60 mM NAH2PO4, 60 mM NaCl (pH 7) and 0.0001% hexadimethrine bromide under the following conditions: −15 kV potential, 50 μm-internal diameter and 30/40.2 cm-long capillary tube with indirect UV detection at 256 nm. To obtain total AsA pools, samples were treated with dithiothreitol. The reaction mixture for the determination of glutathione content contained 0.1 M K-P buffer (pH 7.0), 1 mM EDTA, 1 mM glutathione disulfide (GSSG), 0.2 mM NADPH and leaf extract (final volume 1 ml). The reaction was initiated with GSSG and the decrease in absorbance was measured at 340 nm.
Soluble protein content was quantified according to the method of Bradford (1976). First, frozen leaf samples (0.5 g) were ground and proteins were extracted via a sodium phosphate buffer. Then centrifugation of the mixtures was performed at 4000 g, 4 °C for 10 min. The supernatants were standardized using bovine serum albumin solution and then absorbance was measured at 595 nm.
2.10 Determination of essential nutrients
The amounts of several essential nutrients (Na, Cl, Ca, and K) from different parts of the plants (roots and shoots, previously ground to pass a 2-mm screen, as suggested by Weidhuner et al., 2019) were determined by the method presented by Kruse et al. (2007) and expressed in mg kg−1. The samples were run against the standard of measured nutrients through using an AA 800 Atomic Absorption Spectrophotometer. Total contents of essential nutrients were estimated by using the following formula, where “r” represents roots and “s” represents shoots of the plants: Nutrients uptake = (Total nutrients)r + (Total nutrients)s.
2.11 Statistical analysis
All the data were analyzed with the SPSS software through multivariate post hoc test, pursued by Duncan's test in order to determine the interaction among significant values. Considerable variation among different observed values were calculated at P < 0.05 and displayed with various letters. Graphical representation was carried out using Origin 2017 and R Studio.
3 RESULTS
3.1 Survival rate and plant growth
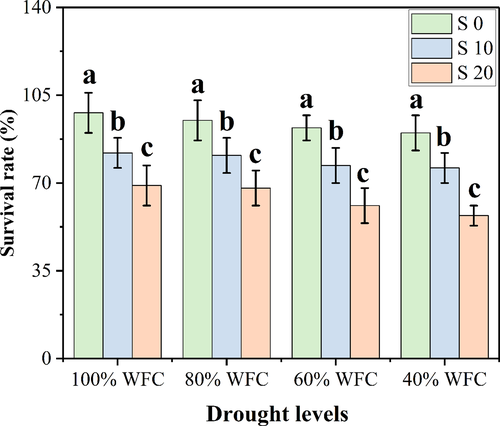
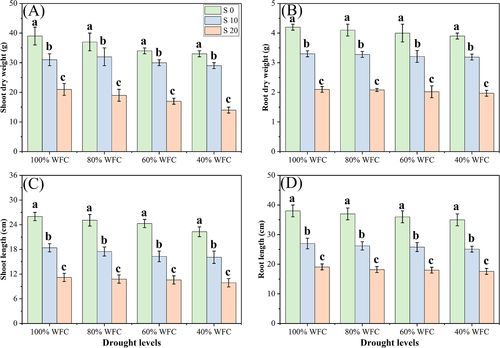
In the current study, we studied the survival rate of plants as well as the plant growth and biomass yields under various levels of drought and salinity stresses (Figures 1 and 2). Our results show that T. mandavillei has considerable potential to favorably cope with water deficient conditions, but excess amount of NaCl in the soil affected both survival rate and plant growth and biomass. According to these results, drought stress (40% WFC) decreased survival rate of the plant by 8.3% compared to control plants (100% WFC), while salinity stress decreased survival rate of the plant by 29.7% in the plants grown in 20 dSm−1 with 40% WFC, compared to those grown in 0 dSm−1 and 40% WFC. Shoot length, shoot dry weight, root length, and root dry weight were not affected by different water stress levels. However, salinity stress reduced these parameters at all levels of drought stress, with maximum decreases observed in 20 dSm−1 with 40% WFC by 37.5, 51.6, 62.9, and 32.2%, respectively, compared to plants, which were grown in 0 dSm−1 with 40% WFC.
3.2 Photosynthetic pigments and gas exchange characteristics
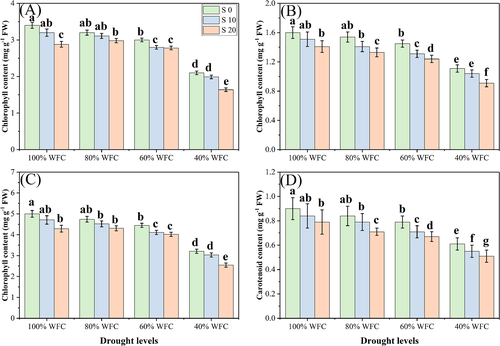
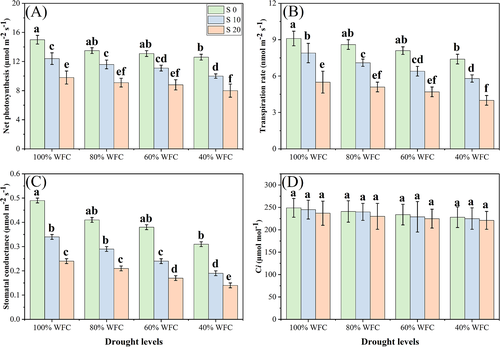
Both salinity and drought stresses had detrimental impacts on photosynthetic pigments and gas exchange parameters, compared to the control plants without stress. The Ci was not significantly different across different levels of salinity and drought stress. Results regarding chlorophyll contents are presented in Fig. 3 and results regarding various gas exchange characteristics are presented in Fig. 4. When plants were grown in 40% WFC without addition of NaCl in the soil, total chl contents, carotenoid contents, Pn, Tr, and Gs decreased by 47.6, 31.4, 13.0, 16.8, and 34.6%, respectively, compared to plants grown in 100% WFC. Moreover, addition of NaCl in the soil decreased these parameters at all levels, with maximum reductions in the chlorophyll, carotenoid, Pn, Tr and Gs contents of up to 9.4, 19.4, 14.3, 27.6, and 34.5%, respectively, observed in the 40% WFC with 20 dSm−1 treatment when compared to 40% WFC with 0 dSm−1 treatment.
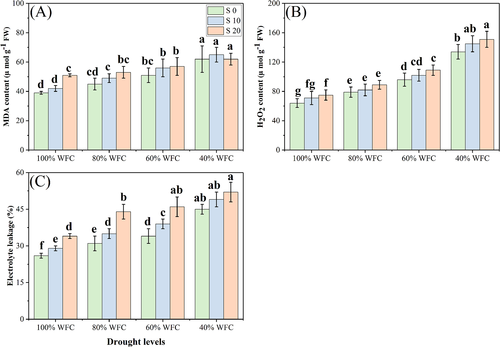
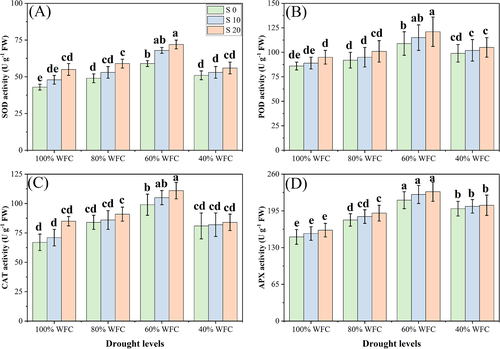
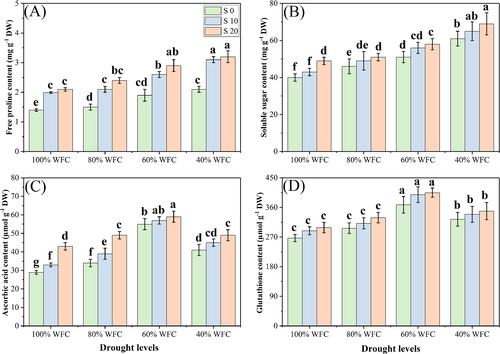
3.3 Oxidative stress, enzymatic, and non-enzymatic antioxidants and osmolytes
In our study, both the individual and the combined effect of drought and salinity stress increased the oxidative stress, enzymatic, and non-enzymatic antioxidants, and the osmolyte concentration in T. mandavillei, compared to control plants grown without stress. The results regarding oxidative stress indicators, enzymatic and non-enzymatic antioxidants, and osmolytes are presented in Figures 5-7, respectively. Results show that the oxidative stress indicators, non-enzymatic antioxidants, and osmolytes increased with increasing salinity and drought stress. However, enzymatic antioxidant activities were increased up to a drought level of 60% WFC, then decreased (P < 0.05) with a drought level of 40% WFC, compared to plants grown in 100% WFC. Although, absolute values for enzymatic antioxidant activities numerically increased at all levels of salinity, these increases were not statistically significant. Our results show that the contents of MDA, H2O2, EL (%), proline, soluble sugar, ascorbic acid (AsA) and GSH were increased by 43.5, 89.7, 28.0, 166.6, 189.8, 276.5, and 180.2%, respectively, in the plants grown in the 40% compared to plants grown under 100% WFC without the addition of NaCl in the soil. The activities of the enzymes SOD, POD, CAT, and APX were increased by 13.4, 24.6, 14.9, and 18.7%, respectively in the plants grown in 60% WFC, compared to 100% WFC without the addition of NaCl in the soil. These parameters were also increased at all levels of salinity in drought stress conditions.
3.4 Ion uptake
In this study, we determined different ions from the roots and shoots of T. mandavillei grown at different salinity and drought stress levels. The data related to Na+, Cl−, Ca2+, and K+ uptake from roots and shoots of T. mandavillei are given in Table 1. The Na+ and Cl− contents in plant organs increased with the addition of NaCl and with the occurrence of drought stress, while the contents of Ca2+ and K+ in the roots and shoots of T. mandavillei decreased at all levels of salinity and drought stress. Maximum increase of Na+ and Cl− was observed in plants grown in 40% WFC with 20 dSm−1 of salinity stress, compared to plants grown in 100% WFC and 0 dSm−1. The contents of Na+ and Cl− were increased by 312.5% and 241.4% in the roots and also increased by 118.1% and 164.6%, respectively, in the shoots in the plants grown in 40% WFC with 20 dSm−1, compared to plants grown in 100% WFC and 0 dSm−1. The Ca2+ and K+ decreased by 76.4% and 86.6% in the roots, and by 84.6% and 71.9% in the shoots when plants were grown in 40% WFC with 20 dSm−1, compared to 100% WFC and 0 dSm−1.
| Drought levels (%) | Salinity levels | Na+ (roots) | Na+ (shoots) | Cl− (roots) | Cl− (shoots) | Ca2+ (roots) | Ca2+ (shoots) | K+ (roots) | K+ (shoots) |
|---|---|---|---|---|---|---|---|---|---|
| 100 | |||||||||
| S 0 | 0.24 ± 0.01 h | 0.61 ± 0.01 g | 0.79 ± 0.01 e | 1.21 ± 0.03 g | 0.45 ± 0.01 a | 0.43 ± 0.01 a | 0.59 ± 0.03 a | 0.79 ± 0.04 a | |
| S 10 | 0.28 ± 0.01 g | 0.71 ± 0.01 ef | 0.82 ± 0.01 e | 1.26 ± 0.02 g | 0.42 ± 0.01 a | 0.43 ± 0.02 a | 0.58 ± 0.02 a | 0.77 ± 0.03 a | |
| S 20 | 0.34 ± 0.01 f | 0.77 ± 0.01 e | 0.86 ± 0.02 e | 1.27 ± 0.02 f | 0.43 ± 0.02 a | 0.41 ± 0.01 a | 0.57 ± 0.04 a | 0.75 ± 0.03 a | |
| 80 | |||||||||
| S 0 | 0.45 ± 0.01 e | 0.83 ± 0.01 d | 1.12 ± 0.03 d | 1.42 ± 0.04 e | 0.34 ± 0.01 b | 0.36 ± 0.01 b | 0.47 ± 0.03 b | 0.68 ± 0.02 b | |
| S 10 | 0.49 ± 0.01 e | 0.86 ± 0.01 d | 1.16 ± 0.02 d | 1.46 ± 0.03 e | 0.33 ± 0.01 b | 0.35 ± 0.02 b | 0.46 ± 0.02 b | 0.65 ± 0.04 b | |
| S 20 | 0.52 ± 0.01 e | 0.91 ± 0.01 d | 1.18 ± 0.03 d | 1.51 ± 0.01 e | 0.32 ± 0.01 b | 0.34 ± 0.02 b | 0.47 ± 0.03 b | 0.61 ± 0.04 c | |
| 60 | |||||||||
| S 0 | 0.62 ± 0.01 d | 1.12 ± 0.01 c | 1.39 ± 0.05 c | 1.67 ± 0.06 d | 0.27 ± 0.01 c | 0.29 ± 0.01 c | 0.38 ± 0.02 c | 0.54 ± 0.02 d | |
| S 10 | 0.65 ± 0.01 cd | 1.15 ± 0.02 c | 1.45 ± 0.06 bc | 1.71 ± 0.07 d | 0.26 ± 0.01 c | 0.29 ± 0.02 c | 0.37 ± 0.02 c | 0.52 ± 0.02 d | |
| S 20 | 0.69 ± 0.01 c | 1.16 ± 0.01 c | 1.51 ± 0.05 b | 1.81 ± 0.06 c | 0.26 ± 0.01 c | 0.28 ± 0.01 c | 0.36 ± 0.04 c | 0.51 ± 0.02 d | |
| 40 | |||||||||
| S 0 | 0.91 ± 0.01 b | 1.24 ± 0.02 bc | 1.81 ± 0.04 a | 2.22 ± 0.06 b | 0.19 ± 0.01 d | 0.24 ± 0.01 d | 0.24 ± 0.02 d | 0.41 ± 0.02 e | |
| S 10 | 0.92 ± 0.02 b | 1.29 ± 0.01 b | 1.86 ± 0.06 a | 2.29 ± 0.1 a | 0.19 ± 0.02 d | 0.23 ± 0.01 d | 0.21 ± 0.01 d | 0.39 ± 0.02 e | |
| S 20 | 0.99 ± 0.02 a | 1.33 ± 0.01 a | 1.91 ± 0.05 a | 2.35 ± 0.1 a | 0.18 ± 0.01 d | 0.23 ± 0.02 d | 0.19 ± 0.01 d | 0.37 ± 0.01 e |
- Note: Values are demonstrated as means of three replicates along with standard deviation (sd; n = 3). Two-way anova was performed and means differences were tested by HSD (P < 0.05).
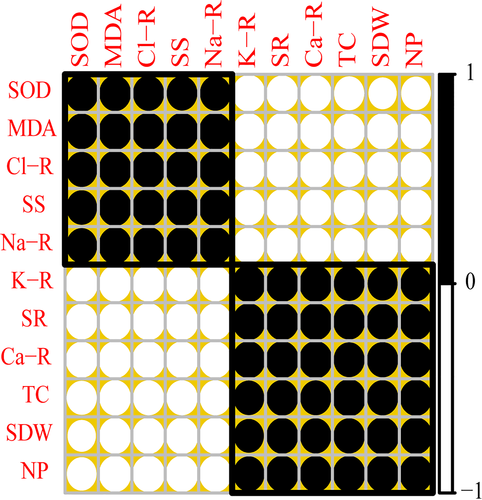
3.5 Correlation analysis
Pearson's correlation analysis among the different parameters presented in this study is shown in Figure 8. According to this correlation analysis, Na+ contents in the roots were positively correlated with Cl− contents in the roots, soluble sugar, MDA contents, and SOD activity, but negatively correlated with Ca2+ and K+ contents in the roots, survival rate, total chlorophyll contents, shoot dry weight, and net photosynthesis. In contrast, Ca2+ contents in the roots were positively correlated with K+ contents in the roots survival rate, total chlorophyll contents, shoot dry weight, and net photosynthesis, but negatively correlated with Na+ and Cl− contents in the roots, soluble sugar, MDA contents, and SOD activity. This correlation depicts a close connection between ion uptake and different morpho-physiological traits of T. mandavillei.
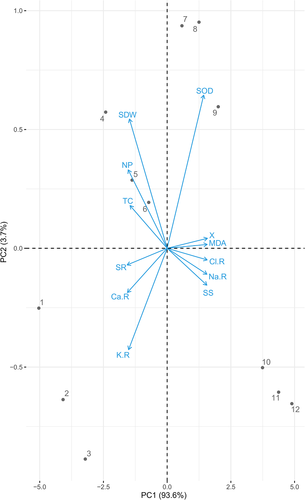
3.6 Principal component analysis of morphological traits and ion uptake
The score and loading plots of principal component analysis (PCA) to evaluate different morpho-physiological traits and ions uptake by T. mandavillei plants are presented in Fig. 9. Among all the components, the first two components, i.e., PC1 (Dim1) and PC2 (Dim2), exhibited maximum contribution and accounted for 93.6% and 3.7% of the total variance in the dataset. The 12 salinity-drought treatments imposed in this study were successfully dispersed across the whole dataset. This distribution of treatments across the entire data base gives a clear indication that drought and salinity stress induce a negative impact on the growth and composition of T. mandavillei. The treatment 1 was most displaced from other treatments of this study, which is also suggesting that different levels of drought and salinity stress imposed negative impact on growth and composition of T. mandavillei plants. The first group of variables positively correlated with PCA1 includes variables such as Na+ and Cl− contents in the roots, soluble sugar, MDA contents and SOD activity, while Ca2+ and K+ contents in the roots, survival rate, total chlorophyll contents, shoot dry weight, and net photosynthesis were negatively correlated in the dataset.
4 DISCUSSION
Studies of the effects of salt and water stresses are frequently treated separately, (Adhikari et al., 2020; Ahmad et al., 2017; Akram et al., 2018; Kamran et al., 2019; Khan, Zhang, Luo, Liu, Rizwan, et al., 2019b; Safdar et al., 2019), even though they are closely interrelated, resulting in detrimental impacts in overall plant growth and productivity as a plant responses to biotic stresses (Kamran et al., 2019; Parida & Das, 2005; Rana, Hu, et al., 2020; Zaheer, Ali, Saleem, Noor, et al., 2020). However, some halophytes show resistance to water-deficient conditions, and do not reduce their plant growth and biomass productivity when grown under drought conditions (Saad et al., 2010) and can survive in very saline environments (Parihar et al., 2015; Safdar et al., 2019). In this study, we found that high amount of NaCl in the soil reduced the plants survival rate, its growth and biomass, but water deficient stress treatments did not affect plant growth and yield (Figures 1 and 2). This is because salinity stress affects halophytes considerably more than water-deficient conditions or the combined effect of salinity and drought stress (Goharrizi et al., 2020; Li et al., 2020; Trabelsi et al., 2019). These findings are in agreement with findings from Tsamaidi et al. (2017) in Anethum graveolens and Miranda-Apodaca et al. (2018) in Chenopodium quinoa. Previously, many studies showed that soil salinity causes a drastic effect on plant growth and development due to a reduction in the biosynthesis of proteins, which is essential for plant growth and composition (Adhikari et al., 2020; Chantre et al., 2016).
Photosynthetic pigments (Figure 3) and gas exchange characteristics (Figure 4) decreased under individual or the combined effect of salinity and drought stress in our study. Soil salinity induces hazardous impact on plant photosynthesis and causes stomatal closure, which is linked to the biosynthesis of chlorophyll and also causes ultra-structural alteration in chloroplast and stomatal aperture (Chantre Nongpiur et al., 2016; Dolatabadian et al., 2011; Jabeen & Ahmad, 2017). In addition to that, a reduction in plant growth was correlated with decreased photosynthetic pigments, gas exchange characteristics and stomatal properties, similar to findings reported from other researchers (Dolatabadian et al., 2011; Parihar et al., 2015; Sadak & Abdelhamid, 2015). However, in water limited environments, photosynthesis rates typically decrease, alongside with a reduction in leaf distension, stomatal narrowing and block, early leaf catabiosis, less acquisition of nutrient translocation and overall reduced crop yield (Khan, Zhang, Luo, Liu, Ni, et al., 2019a; Nikolaeva et al., 2010). In addition, drought also negatively affects the water-use efficiency, leaf water capacity, stomatal opening, transpiration rate, and plant canopy temperature (Kosar et al., 2015; Saud et al., 2017). The reduction of photosynthetic pigments associated with many cellular mechanisms, including stomatal limitations and also salinity stress, causes stomata to be closed in water stressed environments (Iqbal et al., 2015; Munns & Tester, 2008; Zafar et al., 2015). When plants close their stomata, the exchange of gasses between chloroplasts and the atmosphere ceases, thus reducing photosynthetic pigments, and ultimately overall plant growth (Dolatabadian & Jouneghani, 2009; Hassini et al., 2017; Kamran et al., 2019). Moreover, the decrease in photosynthetic rate is not only associated with stomatal closure but also with the decrease in chlorophyll contents (Hussain et al., 2016). Net photosynthesis is reduced by regulation mechanisms, which include decreases in stomatal behavior and photosynthetic pigments when plants are exposed to drought. Previously, it was observed that optimum supplementation of water showed significantly higher photosynthetic pigments, gaseous exchange parameters, and stomatal aperture, compared to the plants grown in water limited environments (Bashir et al., 2020; Khan, Zhang, Luo, Liu, Rizwan, et al., 2019b; Sharma et al., 2020). In addition, when the salt concentration in the soil is high, ROS is formed in the cells and tissues, which further contributes to chlorophyll degradation (Hasanuzzaman et al., 2013; Kamran et al., 2019).
Enzymatic and non-enzymatic antioxidants mitigate and repair ROS-generated damage and allow plants to evolve complex defense systems to elevate cell protection strategies against stress-induced oxidative stress (Saleem et al., 2019; Saleem, Fahad, Rehman, et al., 2020; Saleem, Rehman, et al., 2019). As reported by Hesham and Fahad (2020), with antioxidants such as AsA and GSH, additional ROS may be scavenged within the plant, therefore increasing the plants tolerance against environmental stressors. In the case of plants with oxidative stress resistance, ascorbate is a redox active molecule that interacts with H2O2, OH, and lipid hydroperoxides and is involved in various physiological activities (Imran et al., 2019; Kamran et al., 2019). Furthermore, osmo-protectants also have redox active properties, which overcome the production of ROS as well as participate in the ascorbate-glutathione cycle (Rana, Bhantana, et al., 2020; Rehman et al., 2019). However, better and more efficient antioxidant systems in plants can help to tolerate salinity stress, while surviving when soil salinity is very high (Dolatabadian & Jouneghani, 2009; Kang et al., 2014; Munns & Tester, 2008). In this study, oxidative stress indicators were increased in the plants grown in 40% WFC (Figure 5) and induced oxidative stress in T. mandavillei. Oxidative stress was overcome by the actions of antioxidants, which were also increased in drought stressed plants compared to the control up to a certain level of 60% WFC (Figure 6). The increase in the content of proline and soluble sugars in drought-stressed plants (Figure 7) showed that improved cell osmotic adjustments may help to maintain higher water and salt contents in plant cells and lower electrolyte leakage (Anjum et al., 2017; Hasanuzzaman et al., 2018; Khan, Zhang, Luo, Liu, Ni, et al., 2019a). So accumulation of different compatible solutes and their involvement in osmotic adjustment might lead to a noteworthy improvement in water stress tolerance in T. mandavillei plants. The decrease in antioxidant activities in T. mandavillei plants at a drought level of 40 WFC is directly associated with the relative gene expression and also to the function of some proteins in different plant tissues (Kamran et al., 2019).
It is well-known that high contents of Na+ and Cl− are negative for plant development. However, some water stress tolerant crops such as T. mandavillei have developed a mechanism to take up high contents of Na+ and Cl− under elevated levels of salt stress in the soil (Munns & Tester, 2008; Safdar et al., 2019). In the present study, we found that increasing levels of a combined effect of salinity and drought stress in the soil caused a significant increase in Na+ and Cl− uptake by the roots and shoots in T. mandavillei (Table 1), resulting in reduced plant growth, similar to results from Liu et al. (2020) in H. vulgare. If plants are exposed to a low level of water in the soil, they cannot absorb enough nutrients like Ca2+ and K+ that are important for their growth (Waraich et al., 2011, Dimkpa et al., 2020). Plant roots take up most essential nutrients in the form of ions, and water plays an important role as a medium in this process. Nevertheless, the movement of these nutrients ceases when there is a water shortage in the soil. Hence, the plants are less able to take up and accumulate nutrients under drought conditions (Raza et al., 2013). Therefore, drought conditions result in low absorption rates of essential nutrients, as less water is available for the transport of ions, and decreased root development hinders the process of ion absorption.
5 CONCLUSIONS
According to the results of this study, individual and combinatorial effects of drought and NaCl stress are harmful for plants, as they affect plant growth and development. However, halophytes like T. mandavillei have considerable potential to cope with water shortage conditions and did not reduce growth, although the combined effect of salinity and drought decreased photosynthetic pigments and gas exchange characteristics. Furthermore, drought and NaCl in the soil increased the amount of ROS which induce the oxidative damage in plant tissues. T. mandavillei can tolerate water stress and is a better option to cultivate in the Arabian deserts. Further studies need to be conducted to find the possible molecular mechanisms that are responsible for the enhanced drought and salinity tolerance observed in T. mandavillei.
ACKNOWLEDGMENTS
The findings of this study are a part of the Doctorate studies of Hasnain Alam. The authors extend their appreciation to the Researchers supporting project number (RSP-2020/193) King Saud University, Riyadh, Saudi Arabia.
AUTHOR CONTRIBUTIONS
Hasnain Alam, Jabar Zaman Khan Khattak, and Shah Fahad initiated and designed the research, Hasnain Alam performed the experiments, Taoufik Saleh Ksiksi, Muhammad Hamzah Saleem, Hamza Sohail, and Qasim Ali analyzed the data while Muhammad Zamin, Mohamed A. El-Esawi, Xue Jiang, and Mona S Alwahibi wrote the manuscript. Shah Fahad, Shah Saud, Jawaher Alkahtani, and Jabar Zaman Khan Khattak revised and edited the manuscript and also provided advices on the experiments.
Open Research
DATA AVAILABILITY STATEMENT
N/A




