Stacking for future: Pyramiding genes to improve drought and salinity tolerance in rice
Funding information: International Atomic Energy Agency (Vienna); Indo-US Advanced Bioenergy Consortium; Indo-US Science and Technology Forum; University Grants Commission, Government of India
Abstract
Abiotic stresses, such as drought and salinity, adversely affect rice production and cause a severe threat to food security. Conventional crop breeding techniques alone are inadequate for achieving drought stress tolerance in crop plants. Using transgenic technology, incremental improvements in tolerance to drought and salinity have been successfully attained via manipulation of gene(s) in several crop species. However, achieving the goal via pyramiding multiple genes from the same or different tolerance mechanisms has received little attention. Pyramiding of multiple genes can be achieved either through breeding, by using marker-assisted selection, or by genetic engineering through molecular stacking co-transformation or re-transformation. Transgene stacking into a single locus has added advantages over breeding or re-transformation since the former assures co-inheritance of genes, contributing to more effective tolerance in transgenic plants for generations. Drought, being a polygenic trait, the potential candidate genes for gene stacking are those contributing to cellular detoxification, osmolyte accumulation, antioxidant machinery, and signaling pathways. Since cellular dehydration is inbuilt in salinity stress, manipulation of these genes results in improving tolerance to salinity along with drought in most of the cases. In this review, attempts have been made to provide a critical assessment of transgenic plants developed through transgene stacking and approaches to achieve the same. Identification and functional validation of more such candidate genes is needed for research programs targeting the gene stacking for developing crop plants with high precision in the shortest possible time to ensure sustainable crop productivity under marginal lands.
Abbreviations
-
- Act1
-
- actin promoter
-
- AHA
-
- H+-ATPase
-
- APX
-
- ascorbate peroxidase
-
- BtCry3A
-
- B. thuringiensis crystal protein
-
- CaMV
-
- 35 S cauliflower mosaic virus
-
- DMT2
-
- dimethylglycine methyltransferase gene
-
- EPSPs
-
- 5-enolpyruvylshikimate-3-phosphate synthase
-
- ER
-
- ethylene receptor
-
- GDP
-
- Guanosine diphosphate
-
- GGP
-
- GDP-L-Gal phosphorylase
-
- GLY I/II
-
- glyoxalase І/II
-
- GME
-
- GDP-Mannose 30,50-epimerase
-
- GMP
-
- GDP-D-mannose pyrophosphorylase
-
- GPP
-
- L-Gal-1-P phosphatase
-
- GS
-
- glutamine synthetase
-
- GSMT2
-
- glycine sarcosine methyltransferase gene
-
- HK
-
- histidine kinase
-
- HPT
-
- histidine phosphotransferase
-
- HVA1
-
- Hordeum vulgare gene
-
- JERF36
-
- jasmonic acid ethylene-responsive element
-
- MAS
-
- marker-assisted selection
-
- MDA
-
- malondialdehyde
-
- MG
-
- methylglyoxal
-
- mtlD
-
- mannitol-1-phosphate dehydrogenase
-
- NER
-
- nonethylene receptor
-
- NHX
-
- H+-ATPases vacuolar Na+/H+ exchanger
-
- NO
-
- nitric oxide
-
- Nos
-
- nopaline synthase
-
- OC-I
-
- oryzacystatin I
-
- Pin II
-
- protease inhibitor II
-
- ROS
-
- reactive oxygen species
-
- RR
-
- response regulator
-
- SacB
-
- levansucrase gene
-
- SOD
-
- superoxide dismutase
-
- SOS
-
- salt overly sensitive
-
- SRP3-1
-
- salt responsive protein 3–1
-
- SWPA2
-
- sweet potato peroxidase anionic 2
-
- TBARS
-
- thiobarbituric acid reactive substance
-
- TCS
-
- two-component system
-
- Vgb
-
- vitreoscilla globin gene
-
- VHAc1
-
- vacuolar H+-ATPase subunit c1
-
- WT
-
- wild type
1 INTRODUCTION
Rice is a staple food crop consumed by nearly half of the human population. Asia alone contributes to 90% of the rice produced worldwide(Maclean et al., 2002). However, changing climatic conditions, biotic stress (such as fungi, bacteria, insects, viruses), and abiotic stress (including salinity, drought, submergence, extreme temperature, wounding, mineral deficiency, and oxidative stress) affect rice plants in many ways, ultimately contributing to reduction in crop yield (Akos et al., 2019; Kaya et al., 2020). Among these abiotic stresses, drought alone affects more than 50% of the rice production (~23 million ha of rain-fed rice globally) (Bouman et al., 2005; Serraj et al., 2011). In South Asia, besides drought, salinity also drastically affects the cultivation of rice (amounting to ~90 million hectares of uncultivated land), resulting in its low production (Poonamperuma & Bandyopadhya, 1980). In rice, the seedling stage is reported to be the most sensitive to salinity where even mild stress (5–6 dSm−1 of soil electrical conductivity) can reduce shoot and root growth (Akbar & Yabuno, 1974; Pearson et al., 1966). The first symptom of salinity stress in rice at the vegetative stage is “white leaf tip,” whereas “leaf rolling” occurs in case of drought stress, followed by “tip burning,” which expands toward the base. Extreme drought and/or high salinity eventually lead to plant death (Gazal et al., 2018).
Rice, like any other plant, adapts to these abiotic stresses and copes by using several signaling cascades at the cellular level (Vinocur & Altman, 2005). The basic units of life are cells and efficient cell signaling is required for functioning and development (Li, 2012). The cell signaling, critical for survival under stress via control and regulation of the current biochemical information from the external environment to inside of the cells, is achieved by the diverse signaling cascades operating at cellular and tissue levels in a plant. Usually, the plasma membrane is the location where a stimulus is detected by a diverse set of receptors, further resulting in the activation of complex signaling machineries such as covalent modification cycles (Catozzi et al., 2016). Kinase cascades are a progression of such sequences and are conserved in nature. In these kinase cascades, one protein is activated in one tier and assists the next protein's activation on the next level (Bluthgen et al., 2006). Like animal cells, plant cells also communicate and coordinate with each other. Plant cells perceive signals like light and chemicals from the external environment and respond on a physiological and chemical level via signaling pathways which contribute to the fine regulation of processes such as seed germination, photosynthesis, flowering, fruiting, and development (Fordham-Skelton & Lindsey, 2001).
Tolerance against abiotic stresses, including drought and salinity in rice, is multigenic and complex in nature and therefore hard to control and engineer. Exploiting genetic engineering can help to build tolerance based on manipulation of key genes participating in signaling and regulatory pathways (Vinocur & Altman, 2005). However, gene pyramiding presents an attractive option to achieve enhanced and durable stress tolerance. In this review, we present various approaches employed to perform the gene stacking in plants. We also present a critical account of various experiments where gene stacking has been performed for improved stress tolerance, primarily in rice but examples from other plants have also been mentioned deliberately to present a holistic picture. Since salinity causes both ionic as well as osmotic stress in a cell, appropriate examples have been picked up to reflect upon this commonality between drought and salinity stress response in plants. For brevity sake, only representative examples have been discussed.
1.1 Approaches for achieving “gene stacking” in plants
In plants, responses to abiotic stresses such as drought, require alteration in numerous genes and their interconnected pathways. Several strategies have been in practice to stack or pyramid the desired transgenes in different crops. Broadly speaking, two types of gene stacking approaches are used: the conventional approach which is the marker-assisted selection (MAS), and the advanced molecular stacking. However, in the conventional approach, nonlinking and segregation of transgenes as well as development of homozygous lines is still a challenge (Que et al., 2010). A recent system for flexible and in vivo stacking of multiple genes within a T-DNA of Agrobacterium, termed as GAANTRY, has also been reported (Collier et al., 2018). Figure 1 presents a bird's eye view of these approaches being used for gene pyramiding in crops and the following text briefly describes the same.
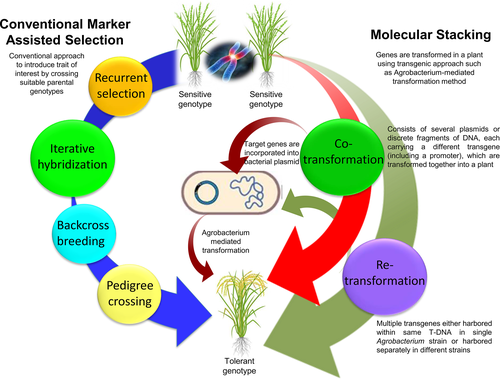
1.2 Gene pyramiding via MAS
Pyramiding of key genes into a single line for enhanced stress tolerance is a powerful strategy in crop breeding. To develop multi-stack hybrids, gene stacking is a strategy to successfully introduce one or more transgenes by crossing the desired parents having different transgenes. The conventional crop breeding strategies utilize traditional techniques and routine natural processes that require extensive field trials (Dormatey et al., 2020; Janila et al., 2016). These strategies include recurrent selection, iterative hybridization, backcross breeding, and pedigree crossing (Figure 1). However, it is difficult to trace the presence of targeted genes, limiting the selection of desired progeny (Malav et al., 2016). Pyramiding of genes/Quantitative Trait Loci (QTLs) to confer tolerance to drought stress is now feasible due to advancements in molecular markers (Das & Rao, 2015). Plant breeders use MAS to transfer genes from pyramided lines into the required crop to enhance the proportion of the desired population of improved genotypes (Das & Rao, 2015; Magar et al., 2014; Pradhan et al., 2015; Shamsudin et al., 2016). By using MAS, plant scientists successfully utilize genes/QTL allele-linked markers to improve qualitative as well as quantitative traits against abiotic stress such as drought, besides monitoring these polygenic traits at a time (Dormatey et al., 2020). There are several recent reviews published on this approach (for reference see Gouda et al., 2020) and therefore, presenting the details of this approach is beyond the scope of this review.
1.3 Gene pyramiding via molecular stacking
Molecular gene stacking or simultaneous gene pyramiding is another strategy where a few genes are introduced at the same time in a plant using transgenic approaches such as Agrobacterium-mediated transformation or by biolistic methods (Keshavareddy & Kumar, 2018; Srivastava et al., 2017). The molecular gene stacking can be achieved in plants via two routes: co-transformation or re-transformation, for which the details are presented below.
1.4 Co-transformation mediated gene pyramiding
Co-transformation is one of the most efficient strategies for the introduction of multi genes in plants. It can be done either by multiple plasmid co-transformation of unlinked transgenes consisting of various plasmids, (or discrete DNA fragments) or by single plasmid co-transformation of the linked transgene, where each transgene is linked with its own promoter and they are transformed together into the plant via Agrobacterium-mediated transformation or the biolistic method (Gupta et al., 2018, Segolela et al., 2019; Figure 1). A major advantage of this method is that the co-introduced transgenes tend to co-integrate at the same chromosomal position in a high proportion of transgenics. In contrast, it is difficult to assemble complex plasmids with multiple gene cassettes using similar promoters. Moreover, the integration of the transgene in high copy number and undesirable incorporation of T-DNA segments has also been reported in many cases (Douglas & Halpin, 2010; Salim et al., 2020).
Recent advancements in molecular biology have enabled the development of more useful and highly refined tools such as GAANTRY (Gene Assembly in Agrobacterium by Nucleic acid Transfer using Recombinase technologY) which can overcome some of the above-mentioned limitations of gene pyramiding. GAANTRY is an excellent platform for genetic engineering of plants by stably stacking multiple genes in the T-DNA of Agrobacterium plasmids (Collier et al., 2018). This system was designed to efficiently utilize cloning vectors and sequential site-specific recombination events to insert transgenes of interest in controlled orientation within a T-DNA and swapping of selection markers carried on an Agrobacterium virulence plasmid, eliminating the need for a binary vector. GAANTRY is a simple, efficient, inexpensive, high-fidelity procedure, and demonstrated to assemble and stably maintain extremely large T-DNAs by sequentially stacking genes to produce high-quality transgenic events of low copy number and free of backbone contaminations. Using this system, researchers have generated high-quality transgenic events by stacking 10 cargo sequences together (Collier et al., 2018).
1.5 Re-transformation mediated gene pyramiding
Re-transformation is a multi-trait or combined trait event, where a plant harboring a transgene is transformed several times with multiple genes. In this method of gene pyramiding, there are fair chances for the transgenes to get inserted at multiple loci and each event critically requires a separate selectable marker (Figure 1). This method is primarily used in crops that are uneasy to propagate through sexual cultivation. However, the induction of transgenes and the requirement of a range of selectable markers limits its utilization. Further, it is difficult to retain transgenes in the offspring after genetic segregation. In this regard, marker-free transformation provides an alternative approach which is also considered to be more environment-friendly (Joshi et al., 2020).
1.6 Gene pyramiding for enhanced tolerance to drought
In terms of plant responses to abiotic stresses, salinity and drought are strongly interconnected as similar cellular destruction and related signaling pathways have been reported to be activated (Urao et al., 2000; Zhu, 2001; Zhu, 2002). Plants face critical osmotic and mechanical injuries during drought stress, leading to the production of reactive oxygen species (ROS) that denature cellular proteins (Ahmad et al., 2010; Begum et al., 2019; Kosar et al., 2020; Thomashow, 1999; Tripathy et al., 2012). Although single gene engineering has been found to be useful towards improving drought tolerance in crops, future improvements of complex traits will likely require and benefit from the introduction of multiple genes. The transformation of the desired crops with the genes involved in drought and salt stress in a predictable and precise manner has been achieved by genetic engineering (Lawlor, 2013). A set of stress-responsive genes have been reported getting activated in response to drought and salinity stress which in turn leads to the activation of several stress-related proteins and the accumulation of protective metabolites. These stress-responsive genes are the primary targets for genetic manipulation to enhance drought tolerance in crops. Regarding this, initial studies focused on the single gene transgenics, including the manipulation of genes that targeted only metabolite accumulation to confer tolerance against salinity or drought stress (Bajaj et al., 1999; Blumwald, 2003). However, attempts have also been made to target pyramiding of multi-genes that can enhance endurance several folds. Some of the representative success stories of attaining improved tolerance against drought and salinity stress by using the gene pyramiding approach are listed in Table 1.
| Target plant for gene pyramiding | Cellular pathways targeted | Genes used for pyramiding | Transformation method | Characteristics of the transgenic plants | Phenotype assessment for stress tolerance | References |
| Nicotiana tabaccum, Solanum lycopersicum, Oryza sativa | Glyoxalase pathway | Gly I + Gly II | Agrobacterium-mediated transformation (pCAMBIA1304 vector under the 35 S CaMV promoter) | Lower lipid peroxidation, lower ROS production, low accumulation of Methylglyoxal, less chloroplast damage and more chlorophyll retention, high shoot and root biomass | Salinity and drought | Singla-Pareek et al., 2003, Viveros et al., 2013, Gupta et al., 2018 |
| Oryza sativa | Glutamine synthesis pathway | OsGS1 + OsGS2 | Agrobacterium-mediated transformation (pMDC99 Vector under the Actin promoter) | High proline levels, low malondialdehyde (MDA) content, and electrolyte leakage, enhanced nitrogen metabolism | Salinity and drought | James et al., 2018 |
| Solanum lycopersicum | Ascorbate biosynthesis pathway | GME + GMP + GGP + GPP | Agrobacterium-mediated transformation (pMV vector under the 35 S CaMV promoter) | Enhanced ascorbic acid content | Oxidative stress | Li et al., 2019 |
| Arabidopsis thaliana | Salt overly sensitive pathway | AtNHX1 + SOS1, AtNHX1 + SOS3, SOS2 + SOS3, SOS1 + SOS2 + SOS3, SOS1 + AHA1 | Agrobacterium-mediated transformation (pCAMBIA3301 vector under the 35 S CaMV promoter) | Lower accumulation of Na+, less growth retardation, fewer yellow leaves with decreased death rate | Salinity | Yang et al., 2009, Ma et al., 2014, Pehlivan et al., 2016, Fan et al., 2019 |
| Festuca arundinacea | Salt overly sensitive pathway | SOS1 + SOS2 + SOS3 | Agrobacterium-mediated transformation (pSOS vector under the rd29A promoter) |
|||
| Oryza sativa | Ion sequestration pathway | PgNHX1 + AtAVP1 | Enhanced root growth, lower percent reduction in chlorophyll content, higher cell viability, higher cell membrane stability, and stabilized higher K+/Na+ ratio | Salinity and drought | Sushma et al., 2014 | |
| Nicotiana tabacum | Betaine synthesis pathway | BADH + SeNHX1, betA + AtNHX1 | Agrobacterium-mediated transformation (pBinBS vector under the 35 S CaMV promoter) | Higher accumulation of Na+ and betaine in leaves and vacuoles | Salinity | Zhou et al., 2008, Duan et al., 2009 |
| Zea mays | Betaine synthesis pathway | betA + TsVP | Agrobacterium-mediated transformation (pCAMBIA1300 vector under the maize ubiquitin promoter) | High amounts of soluble sugar, proline and Glycinebetaine | Drought | Wei et al., 2011 |
| Oryza sativa | Ion sequestration pathway | SaSRP3-1 + SaVHAc1 | Agrobacterium-mediated transformation (pCAMBIA1305 vector under the 35 S CaMV promoter) | Increased K+/Na+, chlorophyll content, RWC, shoot-root growth | Salinity | Biradar et al., 2018 |
| Populus | Fructan biosynthesis pathway | vgb + SacB + JERF36 + BtCry3A + OC-I | Particle bombardment | Higher chlorophyll content, higher total biomass, higher proline content | Salinity and drought | Su et al., 2011 |
| Zea mays | Mannitol biosynthesis pathway | mtlD + HVA1 | Particle bombardment | Improved RWC, plant survival, increase in mannitol and fructose, reduction in glucose and sucrose content | Salinity and drought | Nguyen et al., 2013 |
| Nicotiana tabacum, Zea mays | glycinebetaine synthesis pathway | ApGSMT2 + ApDMT2 | Agrobacterium-mediated transformation (pCUE-GSD vector under the 35 S CaMV promoter) | Lower electrolyte leakage, lower Malondialdehyde content, higher glycinebetaine content and chlorophyll content | Drought | He et al., 2011, He et al., 2013 |
1.7 Gene pyramiding for engineering metabolic pathways
Methylglyoxal (MG) is a cytotoxic metabolite produced in response to abiotic stress in plants, including salinity and drought (Kaur et al., 2014). As a byproduct of the glycolysis pathway (Sousa et al., 2013), MG leads to ROS generation in cells (Singla-Pareek et al., 2003). The glyoxalase pathway involves two enzymes, that is, glyoxalase І (GLY I) and glyoxalase II (GLY II) that detoxify MG (Singla-Pareek et al., 2003, Mustafiz et al., 2011; Figure 1). Initial attempts for gene pyramiding for the two key genes of this metabolic pathway were carried out in the model plant tobacco followed by rice (Gupta et al., 2018; Singla-Pareek et al., 2003). The GlyI gene was cloned from Brassica juncea and the GlyII gene was cloned from Oryza sativa into plant transformation vectors under the control of the 35S cauliflower mosaic virus (CaMV) promoter. The construct was transformed into tobacco through Agrobacterium-mediated transformation. In the process, both single and double-transgenic plants were generated. The transgenic plants carrying overexpression of both the Gly genes were found to show a synergistic effect for enhanced stress tolerance than the plants carrying overexpression for either of the single genes of the pathway (Singla-Pareek et al., 2003). This proof of concept in tobacco led to the translation of this technology to rice. Attempts were made to engineer the glyoxalase pathway in a high yielding rice IR64 (Gupta et al., 2018). The GlyI gene from Brassica juncea and the GlyII gene from Oryza sativa under the 35S CaMV promoter were cloned together into the pCAMBIA1304 vector (Gupta et al., 2018). The construct was transformed into rice through Agrobacterium-mediated transformation, and overexpression lines were generated. Higher amounts of GlyI and GlyII proteins and low accumulation of MG in the transgenic plants were observed compared to wild-type (WT) plants, even under nonstress conditions, thus confirming that both the transgenes were stable and functionally active (Gupta et al., 2018). Surprisingly, 80% of transgenic seeds showed germination in the presence of 200 mM NaCl and also were able to maintain their greenness at the vegetative stage. The double transgenic plants, overexpressing both GlyI and GlyII genes together, exhibited less chloroplast damage and 70% chlorophyll retention under salinity stress. ROS accumulation was significantly lower in transgenic plants as compared to WT under salinity conditions. At the same time, the glyoxalase-overexpressing transgenic plants showed high shoot and root biomass as compared to WT plants under water-deprived conditions. However, typical drought stress symptoms like leaf rolling and chlorophyll bleaching were observed under 25 days of drought conditions in the transgenic lines. Upon rewatering, these symptoms disappeared and healthy transgenic plants were observed (Gupta et al., 2018). A similar attempt was made in tomato where lower lipid peroxidation and ROS production were observed, representing enhanced salt tolerance in the transgenic plants (Viveros et al., 2013). These reports clearly demonstrated the advantage of gene pyramiding in tackling drought and salinity stress as compared to single gene manipulation. Another important finding of the study was the fact that even though the two transgenes were regulated under the control of a constitutive 35S promoter, it did not result in any yield penalty or silencing of the transgenes. In contrast, the use of the same promoter to derive the transgene constitutively has been shown to have a yield penalty in rice (Xiao et al., 2007) and/or gene silencing, when present in more copies in several plants species (Matsunaga et al., 2019). Further investigations need to be done in this area to conclude the usefulness of the same promoter for gene pyramiding programs.
In another set of experiments, the glutamine synthetase (GS) enzymes which catalyze critical nitrogen metabolic reactions in plants, were attempted for gene pyramiding. GS has two isoforms, the cytosolic GS1 which is responsible for the primary assimilation of inorganic nitrogen available from the soil in the form of nitrate or ammonia, and the re-assimilation of NH4+ released by protein degradation (Bernard & Habash, 2009). On the other hand, the chloroplastic GS2 is responsible for re-assimilation of NH4+ released during photorespiration and nitrate reduction (Lam et al., 1996; Leegood et al., 1995). In a recent report by James et al. (2018), the cytosolic OsGS1 and chloroplastic OsGS2 genes were co-expressed in rice (Figure 2). The genes were cloned in the pMDC99 vector next to the actin promoter and then transformed into rice using agrobacterium. The dual-overexpression lines showed higher chlorophyll content, fresh weight, and relative water content compared to WT under drought stress. The transgenic plants showed very high proline levels while the malondialdehyde (MDA) content and electrolyte leakage were reported to be lower in pyramid lines as compared to the single transgenic plants (James et al., 2018).
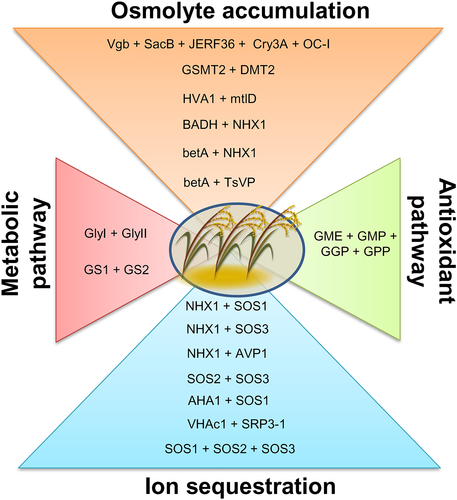
1.8 Gene pyramiding for engineering antioxidant pathways
Ascorbic acid (AsA), also known as vitamin C, is an antioxidant involved in ROS scavenging, programmed cell death, premature senescence, cell division, and elongation, and oxidative stress tolerance mechanisms (Akram et al., 2017). Li et al. (2019) engineered the ascorbic acid metabolic pathway by pyramiding guanosine diphosphate (GDP)-mannose 3,5-epimerase (GME), GDP-D-mannose pyrophosphorylase (GMP), GDP-L-Gal phosphorylase (GGP), and L-Gal-1-P phosphatase (GPP) genes into tomato plants. These genes were cloned under the control of CaMV35S into the pMV vector and transformed into Agrobacterium tumefaciens by electroporation. They observed that multi-gene overexpression lines had increase in ascorbic acid content compared to the single-gene transgenic lines. Furthermore, they discovered that with an increase in AsA, the tolerance to oxidative stress was also enhanced in pyramided lines (Li et al., 2019). Recently, it has also been demonstrated that exogenously applied ascorbic acid has a positive effect on the growth of safflower plants under drought stress (Farooq et al., 2020).
Superoxide dismutase (SOD), a ROS scavenging enzyme detoxifies hydrogen peroxide (H2O2) into water and oxygen with the help of the ascorbate peroxidase (APX; Asada, 1999). Shafi, Gill, et al. (2019a) developed transgenic Arabidopsis lines overexpressing cytosolic Cu/Zn-SOD and APX genes isolated from Potentilla atrosanguinea and Rheum australe, respectively. The genes were individually overexpressed in Arabidopsis thaliana and later crossed to obtain dual transgenic lines. The transgenic lines showed enhanced antioxidant enzyme activity, callus induction, and shoot regeneration (Shafi, Gill, et al., 2019a) besides higher cellulose biosynthesis under salinity stress (Shafi et al., 2015; Shafi, Zahoor, et al., 2019b). Similarly, potato plants overexpressing PaSOD and RaAPX showed higher activities of SOD and APX, increased starch accumulation, improved growth, enhanced expression of genes and transcription factors directly involved in lignin biosynthesis, and reduced accumulation of ROS under salt stress (Shafi et al., 2017). Further, transgenic Festuca arundinacea (Lee et al., 2007) and potato plants (Tang et al., 2004) overexpressing Cu/Zn-SOD and APX in chloroplasts under the control of the oxidative stress-inducible promoter SWPA2 showed reduced thiobarbituric acid reactive substances (TBARS), ion leakage, and chlorophyll degradation under methyl viologen, H2O2, and heavy metal stress. In addition, enhanced root growth under salt stress was observed in sweet potato plants overexpressing Cu/Zn-SOD and APX under the control of the SWPA2 promoter (Yan et al., 2016).
1.9 Gene pyramiding for engineering ion sequestration in cells
One of the most important components of high salinity is the ionic stress which is due to accumulation of excess of Na+ ions in the cell. Excessive Na+ disrupts the K+ homeostasis and interferes with numerous metabolic processes that affect plant growth and development (Assaha et al., 2017; Kronzucker et al., 2013; Nieves-Cordones et al., 2016). Therefore, maintaining a balanced cytosolic Na+/K+ ratio has become a crucial target to improve salinity tolerance. One way to achieve this target is to overexpress the genes involved in ion homeostasis, for example, salt overly sensitive (SOS) genes (Shi et al., 2003), AVP1 (Gaxiola et al., 2001), and H+-ATPases and vacuolar Na+/H+ exchanger (AtNHX1) genes (Apse et al., 1999; Zhang et al., 2001). The ion homeostasis pathway includes SOS1, SOS2, and SOS3 genes in Arabidopsis (Zhu, 2001). It has been reported that the transformation of SOS1, SOS2, and SOS3 genes together in yeast results in higher tolerance against salt stress in contrast to single SOS gene transformation (Quintero et al., 2002). AtNHX1 is a vacuolar protein involved in Na+ transport into vacuoles (Apse et al., 1999), and its activity is controlled by SOS genes (Qiu et al., 2004). As SOS1 and AtNHX1 activity depend on SOS2 and SOS3 expression, scientists developed overexpression lines of AtNHX1 + SOS3, SOS2 + SOS3, and SOS1 + SOS2 + SOS3 in A. thaliana and observed lower accumulation of Na+ in the transgenic plants under salinity conditions (Yang et al., 2009; Figure 2). Unexpectedly, the AtNHX1 + SOS3 and SOS1 + SOS2 + SOS3 lines did not show greatly improved salt tolerance compared to single transgenic plants. Furthermore, the same attempt to generate transgenic lines co-expressing SOS1 + SOS2 + SOS3 was made in tall fescue (Ma et al., 2014). The three SOS genes, under the control of the stress-inducible rd29A promoter were cloned together into a single vector. Enhanced tolerance against salt stress was observed in the transgenic plants with pyramided genes. Additionally, SOS1 + SOS2 + SOS3 overexpression lines showed less growth retardation, fewer yellow leaves with decreased death rate, indicating a healthy phenotype under salinity conditions as compared to the WT plants (Ma et al., 2014). Likewise, Pehlivan et al. (2016) developed transgenic lines co-expressing AtNHX1 + SOS1 and observed a highly enhanced salinity tolerance (250 mM NaCl) in Arabidopsis. A similar approach was used by Fan et al. (2019) where they co-expressed SOS1 and H+-ATPase (AHA1) genes from Sesuvium portulacastrum into Arabidopsis to enhance salinity tolerance (Fan et al., 2019).
Sushma et al. (2014) co-expressed PgNHX1 and AtAVP1 in rice where enhanced salt and drought stress tolerance was observed. The PgNHX1-AtAVP1 overexpressing plants showed root growth enhancement, a lower percent reduction in chlorophyll content, higher cell viability, higher cell membrane stability, and stabilized higher K+/Na+ ratios under NaCl and PEG treatment. These results clearly state that the PgNHX1-AtAVP1 overexpressing lines had higher tolerance against salinity and drought stress as compared to the WT plants (Sushma et al., 2014). Recently, Biradar et al. (2018) attempted pyramiding of the salt responsive protein 3–1 (SaSRP3-1) gene and the vacuolar H+-ATPase subunit c1 (SaVHAc1) from Spartina alterniflora (halophyte grass) into rice. SaSRP3-1 and SaVHAc1 were independently transformed into pCAMBIA 1305.2 vectors and later used to develop a single transgenic plant. After that, a double transgenic plant harboring SaSRP3-1 and SaVHAc1 genes was developed by crossing individual transgenic lines of SaSRP3-1 and SaVHAc. They observed improved salinity tolerance in the double transgenic plants at the seedling, vegetative, and reproductive stages. Increased K+/Na+, chlorophyll content, RWC, and shoot-root growth was also reported in double transgenic plants as compared to the WT under salt stress (Biradar et al., 2018).
1.10 Gene pyramiding for engineering osmolyte accumulation
Betaine is an osmolyte known to have a role in salinity tolerance. Zhou et al. (2008) co-expressed the betaine synthesis gene (BADH) from the halophyte Atriplex hortensis with the NHX1 gene from the halophyte Salicornia europaea in tobacco. Tobacco transformation was carried out using the agrobacterium vector pBinBS having different cassettes of BADH and SeNHX1 under the CaMV35S promoter. They observed higher accumulation of Na+ and betaine in leaves and vacuoles in the double transgenic plant as compared to single transgenics under salinity stress. Also, the osmotic pressure and biomass were high in co-transformed plants (Zhou et al., 2008). Similarly, Duan et al. (2009) generated overexpression lines of tobacco to enhance their salinity tolerance, harboring the betA (betaine) gene from Escherichia coli encoding choline dehydrogenase and the AtNHX1 gene from A. thaliana. Firstly, single transgenic lines were generated using Agrobacterium-mediated transformation, followed by the double transgenic plant through the cross-breeding of the betA and AtNHX1 transgenic plants. They observed highly improved tolerance in the double transgenic plants since the two genes belong to different salt tolerance pathways (Duan et al., 2009). Similarly, a transgenic maize plant co-expressing betA from Escherichia coli and TsVP from Thellungiella halophila was also developed and tested for its improved drought tolerance (Wei et al., 2011). The authors observed higher glycine betaine content in pyramided lines as compared to the WT and single transgenic lines under drought stress conditions. Moreover, the solute potential of pyramided lines was found to be more negative than the WT and single transgenic lines after drought treatment, indicating that pyramided lines had great potential for osmotic adjustment to facilitate water uptake (Wei et al., 2011). Another group attempted the pyramiding of five genes, including the Vitreoscilla globin gene (vgb), the levansucrase gene (SacB), jasmonic acid ethylene-responsive element (JERF36), the Bacillus thuringiensis crystal protein (BtCry3A), and oryzacystatin I (OC-I) into a hybrid poplar plant and observed enhanced tolerance against drought and salinity conditions (Su et al., 2011). Populus × euramericana “Guariento” hybrid obtained from crossing Populus nigra and Populus deltoids was used in the study. All the five genes were regulated by the CaMV promoter and were transformed into hybrid poplar using particle bombardment. The authors reported that the combination of five genes in hybrid poplar conferred tolerance to waterlogging, drought, and salinity conditions and also was resistant to insects (Su et al., 2011).
Nguyen et al. (2013) cloned the abscisic acid-inducible protein PHV A1-Hordeum vulgare gene (HVA1) and the mannitol-1-phosphate dehydrogenase gene (mtlD) in maize and reported enhanced drought and salt stress tolerance. The HVA1 and mtlD genes were cloned in pBY520 and JS101 vectors, respectively, under the control of the actin promoter of rice (Act1) and the potato protease inhibitor II terminator (pin II). These constructs also contained a selectable marker under 35S CaMV promoter and nopaline synthase (Nos) terminator. Transformation into maize calli was done by bombardment technique using tungsten particles coated with plasmids containing the desired genes. WT plants and single/double transformants were subjected to drought (15 days no watering) and salinity stress (upto 300 mM NaCl). They observed 67% survival for the double transgenic plants, that is highest compared to single transgenics (52 and 45% of HVA1 and mtlD) and WT (35%) under drought stress. Also, the relative water content (RWC) was 85% in HVA1 + mtlD, 81% in HVA1, 77.6% in mtlD transgenic plants in contrast to the RWC (57.1%) reported in the WT plants. The percentage of reduction in shoot fresh weight and shoot and root dry weights of the HVA1 + mtlD plants were 13, 18.4, and 21.4%, respectively. These reductions were 32.1, 14.8, and 25.0% for HVA1 transgenic plants and 25.9, 27.0, and 21.9% for the mtlD transgenic plants, respectively. This study showed that the double transgenic plants were surviving better than the WT and accumulated higher mannitol under drought conditions as compared to single transgenics (Nguyen et al., 2013). He et al. (2011) enhanced glycinebetaine synthesis in transgenic tobacco by co-overexpressing two bacterial (Aphanothece halophytica) genes, that is glycine sarcosine methyltransferase gene (ApGSMT2) and dimethylglycine methyltransferase gene (ApDMT2). Both lower the genes were cloned into the pCUE-GSD vector under the control of the CaMV35S promoter, and the selection marker was for 5-enolpyruvylshikimate-3-phosphate synthase (EPSPs) gene, which provides tolerance to the herbicide glyphosate. Agrobacterium-mediated transformation was used to generate double-transgenic lines. The dual-overexpression lines showed lower electrolyte leakage and lower MDA content. Higher glycine betaine content and chlorophyll content under drought stress in transgenic plants indicated enhanced osmoprotection as compared to the WT plants (He et al., 2011). Later, the same team performed the pyramiding of these genes in maize which again resulted in enhanced drought tolerance (He et al., 2013).
2 CONCLUSIONS AND FUTURE PERSPECTIVES
Various abiotic stresses cause devastating effects on cultivation and productivity of crops across the globe. To fulfill the needs of the world's growing population, developing multiple stress tolerances in crop plants is a foremost challenge. Since the first approval of dual hybrid cotton stacking Cry1A(b) and esps gene in 1995, several conventional breeding and genetic engineering techniques of gene stacking into a single genotype are currently available. In case of molecular gene stacking, the choice of the pathways to be engineered to improve the stress tolerance in crop plants is the most critical decision to be made beforehand. Eventually, the target genes also need to be picked very carefully for the pyramiding purpose to ensure the synergistic advantage of the genes being together at the same loci in the genome. It is also critical to use the right combination of promoters for the genes in hand. Nonetheless, the recent reports where enhanced stress tolerance has been reported based on multi gene pyramiding have raised a strong hope that the multigenic traits such as drought and salinity can be tackled in the years to come. Owing to the impressive progress made in recent years toward sequencing of plant genomes and more efficient transformation methods available for recalcitrant species, have given us a strong confidence that soon the scientific community will be able to raise drought and salinity-tolerant plants and thus contribute toward food security worldwide.
ACKNOWLEDGMENTS
Anjali Shailani acknowledges CSIR-UGC (University Grants Commission, Government of India) for the Junior Research Fellowship (JRF). This research was supported by the Indo-US Science and Technology Forum (IUSSTF) through Indo-US Advanced Bioenergy Consortium (IUABC), International Atomic Energy Agency (Vienna), and Institutional Umbrella support over the years under DST-FIST and -PURSE; UGC-UPEII, -DRS, and -Networking.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest among them.
AUTHOR CONTRIBUTIONS
The study was conceptualized by Ashwani Pareek and compiled by Anjali Shailani. Ashwani Pareek, Rohit Joshi, Sneh Lata Singla-Pareek, and Ashwani Pareek contributed to the writing and editing of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing does not apply to this article, as no new data were created or analyzed in this study.




