Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses
Funding information: Innovative Team Program of Hainan Natural Science Foundation, Grant/Award Number: 2018CXTD334; National Natural Science Foundation of China, Grant/Award Number: 41871041
Abstract
Recently, melatonin has gained significant importance in plant research. The presence of melatonin in the plant kingdom has been known since 1995. It is a molecule that is conserved in a wide array of evolutionary distant organisms. Its functions and characteristics have been found to be similar in both plants and animals. The review focuses on the role of melatonin pertaining to physiological functions in higher plants. Melatonin regulates physiological functions regarding auxin activity, root, shoot, and explant growth, activates germination of seeds, promotes rhizogenesis (growth of adventitious and lateral roots), and holds up impelled leaf senescence. Melatonin is a natural bio-stimulant that creates resistance in field crops against various abiotic stress, including heat, chemical pollutants, cold, drought, salinity, and harmful ultra-violet radiation. The full potential of melatonin in regulating physiological functions in higher plants still needs to be explored by further research.
Abbreviations
-
- ABA
-
- abscisic acid
-
- SA
-
- salicylic acid
-
- PAO
-
- pheophorbide an oxygenase
-
- ROS
-
- reactive oxygen species
-
- APX
-
- ascorbate peroxidase
-
- POX
-
- peroxidase
-
- CAT
-
- catalase
-
- ET
-
- ethylene
-
- IAA
-
- indole-3-acetic acid
-
- PSII
-
- photosystem II
-
- JA
-
- jasmonic acid
-
- RNS
-
- reactive nitrogen species
-
- SDG
-
- secoisolariciresinol DiGlucoside
-
- SOD
-
- superoxide dismutase
1 INTRODUCTION
When plants are exposed to specific stimuli, they produce nutrients and organic compounds, such as phytohormones (plant originated hormones). These phytohormones, at very low doses, regulate various physiological functions at all developmental stages of plants, such as germination, rooting, flowering, dormancy, and so on. Therefore, as endogenous signals, such compounds act both temporally and spatially (Demir & Celikel, 2019; Gundesli et al., 2019; Sajjad et al., 2017). Melatonin is a bio-molecule which is widely studied, with the molecular name of N-acetyl-5-methoxytryptamine. The functions of this bio-molecule have been investigated in plants, bacteria, fish, reptiles, amphibians, birds, and mammals. In 1958 melatonin was found in pineal glands of bovine species (Lerner et al., 1958). In 1959, it was identified as N-acetyl-5-methoxytryptamine, and soon after this discovery, the biosynthetic pathway of melatonin from tryptophan was discovered (Axelrod & Weissbach, 1960; Lerner, Case, et al., 1959; Weissbach et al., 1960). Its presence in humans was described in 1959 (Lerner, Case, Mori, et al., 1959). In higher plants, it was discovered in 1995. Also, in most edible plant species, the presence of melatonin was demonstrated explicitly in the same year (Dubbels et al., 1995; Hattori et al., 1995). A wide variation in the amount of melatonin was found, which was quantified between species of plant samples, developmental stages, tissue type, and sometimes within a single experiment (Erland et al., 2015). Melatonin has been implicated in many physiological processes, which are important in plants responses to different environmental cues like developing tissues protection from stress responses, damage and redirecting growth and development of plants (Arnao & Hernández-Ruiz, 2020; Erland et al., 2015, 2017; Murch et al., 2009; Reiter et al., 2015; Tan et al., 2009). Currently, the number of publications associated with plant melatonin in the last decade has shown a rapid uprise because plant melatonin related research is in a growth phase, and also in many plants, the function of this compound has been uncovered (Arnao & Hernández-Ruiz, 2015; Reiter et al., 2015; Shi et al., 2016), with main focus on primary root architecture, seed germination, lateral root architecture, photoprotection, fruit ripening, flowering time, circadian rhythm, leaf senescence, and biomass production (Arnao & Hernández-Ruiz, 2018; Shi et al., 2017).
According to Reiter (1991), melatonin lies amidst animal hormones responsible for regulating photoperiodism, sleep, reproduction, aging, and circadian rhythm like physiological processes; thus cells could be informed by the daily profile of melatonin levels about the time and season (Manchester et al., 2015; Singh & Jadhav, 2014). Melatonin is a remarkable molecule in animals, as it not only serves as a time cue, but also promotes cytoprotective and immunomodulatory properties. Moreover, in animal physiology, melatonin has been declared as a neurotransmitter and hormone (Brenner et al., 2006). Being inexpensive and safe for animals it act as a cost-effective approach to remove environmental contaminants. Moreover, it also serves as an antioxidant and radical scavenger in animals (Tan et al., 2003), just like in plants. Protection of organisms against reactive nitrogen and oxygen species (RNS and ROS) is exhibited in the literature (Manchester et al., 2000, 2015; Reiter et al., 2000, 2003; Rodriguez et al., 2004; Tan et al., 2002, 2015; Tan, Manchester, Terron, et al., 2007).
Since the presence of melatonin in higher plants was discovered by Hattori et al. (1995) and Dubbels et al. (1995), a pool of information has accumulated through several studies regarding the presence of melatonin in plants. At first research into plant melatonin was slow with only 146 articles published up to the year 2010. Since then a exponential rise in the number of scientific publications has been recorded, reaching a maximum in 2019 with 183 articles published, thus uptil 2019 a total of 870 articles on the topic have been published (Figure S1). Journal of Pineal Research and Melatonin Research are the top publishing journals of melatonin related work. Although great interest has been shown by other journals in recent years, for sharing work-related to melatonin, including Frontiers in Plant Sciences, Molecules, Biomolecules, International Journal of Molecular Science, Plants, Agronomy, Plant Signaling Behavior, and Food Chemistry, among others.
In the current review, the information available in literature related to melatonin biosynthesis, its role and function in plants is summarized. The objective is to enlighten the readers of the notable role of melatonin in plant resistance against abiotic stress and encourage scientists to investigate the further potential role of both exogenous and endogenous in creating resistance mechanisms in engineered plants.
2 OCCURRENCE OF MELATONIN IN PLANTS
Other than in the animal kingdom, the first report of melatonin was in the dinoflagellate Gonyaulax polyhedra, and subsequently, it was identified in plants, algae, fungi, and bacteria (Arnao & Hernández-Ruiz, 2019a; Tan et al., 2012). There has been an increasing number of research, since melatonin was first discovered in plants, reporting the occurrence in various plants and plant tissues, including cereals, fruits, medicinal herbs, vegetables, and seeds (Reiter et al., 2007).
In the majority of plant species, the presence of melatonin is determined (Table 1). The seeds, roots, bulbs, flowers, and leaves were found enriched in melatonin in most of the examined plants. An especially rich source of melatonin (7.11 μg g−1) is the roots of Scutellaria biacalensis (Huang-qin), belonging to the family Lamiaceae (Reiter & Tan, 2002). The majority of plants that contain melatonin are from the following families Vitaceae, Rosaceae, Brassicaceae, Apiaceae, and Poaceae. However, some other plant families also have large quantities of melatonin. It is possible that concentrations of melatonin may be higher in higher plants, which are yet unstudied. The concentration of melatonin in different plant species is affected by factors like the development stage, genotype, and environmental factors such as photoperiod and temperature (Byeon & Back, 2014a; Zhao, Sun, et al., 2012). The concentration may also vary between different cultivars of the same species. Wang et al. (2009) quantified melatonin levels in 25 different varieties of rice and 58 altered corn varieties that were grown at the same geographical location, and found huge variations in concentrations. The values of melatonin were in the range of 11–234 ng g−1 in rice and 11–2034 ng g−1 in corn. These huge differences in melatonin concentrations suggest that the method of determination is the source for the seen variation between varieties. When rice seedlings are maintained under dark conditions or exposed to high temperatures, the biosynthesis of melatonin is enhanced. When seedlings of rice were exposed to darkness, at a temperature of 55°C, the concentration of melatonin increased from 2.95 to 4.9 ng g−1. This upsurge in melatonin was linked with greater activity of hydroxyindole-O-methyltransferase/acetyl serotonin methyl transferase (HIOMT/ASMT) and serotonin-N-acetyltransferase (SNAT), which are involved in the biosynthetic pathway of melatonin (Byeon & Back, 2014a). Similarly, Riga et al. (2014) reported that levels of melatonin were increased (135%) in tomato plants when they were exposed to shade. Two obvious peaks in endogenous melatonin levels were recorded in sweet cherry, the first one at 5:00 am hand the other one at 2:00 pm (Zhao, Tan, et al., 2012); the first one was linked with darkness, and the other one was related to high light intensity or high temperature (stress conditions).
| Plant organ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Specie name | Fruit | Shoot | Seed | Leaf | Root | Blub | Whole plant | Coleoptiles | Skin | References |
| Actinidiaceae family | ||||||||||
| Kiwifruit | Hattori et al., 1995 | |||||||||
| Arecaceae family | ||||||||||
| Date palm | Zohar et al., 2011 | |||||||||
| Asteraceae family | ||||||||||
| Butterbur | Hattori et al., 1995 | |||||||||
| Fever few, gold | Murch & Saxena, 2006 | |||||||||
| Milk thistle | Reiter & Tan, 2002 | |||||||||
| Fever few, gold | Hattori et al., 1995 | |||||||||
| Sunflower | Reiter & Tan, 2002 | |||||||||
| Fever few, green | Chen et al., 2003 | |||||||||
| Fever few, green | Murch & Saxena, 2006 | |||||||||
| Apiaceae (Umbelliferae) family | ||||||||||
| Coriander | Reiter & Tan, 2002 | |||||||||
| Carrot | Badria, 2002 | |||||||||
| Anise | Reiter & Tan, 2002 | |||||||||
| Carrot | Hattori et al., 1995 | |||||||||
| Fennel | Reiter & Tan, 2002 | |||||||||
| Celery | Reiter & Tan, 2002 | |||||||||
| Amaranthaceae family | ||||||||||
| Beet Roots | Dubbels et al., 1995 | |||||||||
| Malabar spinach | Hattori et al., 1995 | |||||||||
| Amaryllidaceae family | ||||||||||
| Onion | Badria, 2002 | |||||||||
| Onion | Aguilera et al., 2015 | |||||||||
| Garlic | Badria, 2002 | |||||||||
| Asparagaceae family | ||||||||||
| Asparagus | Hattori et al., 1995 | |||||||||
| Asparagus | Chen et al., 2003 | |||||||||
| Anacardiaceae family | ||||||||||
| Mango | Johns et al., 2013 | |||||||||
| Brassicaceae family | ||||||||||
| Red cabbage | Aguilera et al., 2015 | |||||||||
| White mustard | Reiter & Tan, 2002 | |||||||||
| Red radish | Chen et al., 2003 | |||||||||
| Black mustard | Reiter & Tan, 2002 | |||||||||
| Radish | Aguilera et al., 2015 | |||||||||
| Chinese cabbage | Hattori et al., 1995 | |||||||||
| Broccoli | Aguilera et al., 2015 | |||||||||
| Cabbage | Hattori et al., 1995 | |||||||||
| Turnip | Badria, 2002 | |||||||||
| Red radish | Hattori et al., 1995 | |||||||||
| Radish | Badria, 2002 | |||||||||
| Radish | Gomez et al., 2015 | |||||||||
| Red radish | Hattori et al., 1995 | |||||||||
| Bromeliaceae family | ||||||||||
| Pineapple | Badria, 2002 | |||||||||
| Cucurbitaceae family | ||||||||||
| Cucumber | Posmyk et al., 2009 | |||||||||
| Cucumber | Hattori et al., 1995 | |||||||||
| Caricaceae family | ||||||||||
| Papaya | Johns et al., 2013 | |||||||||
| Chenopodiaceae family | ||||||||||
| Red pigweed | Kolár et al., 1997 | |||||||||
| Convolvulaceae family | ||||||||||
| Morning glory | Van-Tassel et al., 2001 | |||||||||
| Morning glory | Van-Tassel et al., 2001 | |||||||||
| Fabaceae family | ||||||||||
| Mung bean | Aguilera et al., 2015 | |||||||||
| Lupin | Arnao & Hernández-Ruiz, 2013 | |||||||||
| Alfalfa | Aguilera et al., 2015 | |||||||||
| Alfalfa | Reiter & Tan, 2002 | |||||||||
| Lentil | Aguilera et al., 2015 | |||||||||
| Fenugreek | Reiter & Tan, 2002 | |||||||||
| Lupin | Arnao & Hernández-Ruiz, 2013 | |||||||||
| Hypericaceae family | ||||||||||
| St. John's wort | Murch & Saxena, 2006 | |||||||||
| Juglandaceae family | ||||||||||
| Walnut | Reiter et al., 2005 | |||||||||
| Lamiaceae family | ||||||||||
| Huang-qin | Manchester et al., 2000 | |||||||||
| Huang-qin | Chen et al., 2003 | |||||||||
| Huang-qin | Reiter & Tan, 2002 | |||||||||
| Lythraceae family | ||||||||||
| Pomegranate | Badria, 2002 | |||||||||
| Linaceae family | ||||||||||
| Flax | Reiter & Tan, 2002 | |||||||||
| Moraceae family | ||||||||||
| Fig | Zohar et al., 2011 | |||||||||
| While Mulberry | Zohar et al., 2011 | |||||||||
| While Mulberry | Chen et al., 2003 | |||||||||
| Musaceae family | ||||||||||
| Banana | Badria, 2002 | |||||||||
| Banana | Dubbels et al., 1995 | |||||||||
| Meliaceae family | ||||||||||
| China berry tree | Zohar et al., 2011 | |||||||||
| Oleracea family | ||||||||||
| European olive | Zohar et al., 2011 | |||||||||
| Green olive tree | Zohar et al., 2011 | |||||||||
| Poaceae family | ||||||||||
| Wheat | Hernández-Ruiz et al., 2005 | |||||||||
| Oat | Hattori et al., 1995 | |||||||||
| Oat | Hernández-Ruiz et al., 2005 | |||||||||
| Rice | Wang et al., 2009 | |||||||||
| Barley | Badria, 2002 | |||||||||
| Corn | Wang et al., 2009 | |||||||||
| Canary grass | Hernández-Ruiz et al., 2005 | |||||||||
| Barley | Hernández-Ruiz et al., 2005 | |||||||||
| Tall fescue | Hattori et al., 1995 | |||||||||
| Papaveraceae family | ||||||||||
| Poppy | Reiter & Tan, 2002 | |||||||||
| Rosaceae family | ||||||||||
| Pico Negro cherry | Gonzalez-Gomez et al., 2009 | |||||||||
| Hongdeng cherry | Badria, 2002 | |||||||||
| Burlat cherry | Gonzalez-Gomez et al., 2009 | |||||||||
| Navalinda cherry | Gonzalez-Gomez et al., 2009 | |||||||||
| Rainier cherry | Badria, 2002 | |||||||||
| Van cherry | Gonzalez-Gomez et al., 2009 | |||||||||
| Sweetheart cherry | Gonzalez-Gomez et al., 2009 | |||||||||
| Pico Colorado cherry | Gonzalez-Gomez et al., 2009 | |||||||||
| Apple | Hattori et al., 1995 | |||||||||
| Almond | Manchester et al., 2000 | |||||||||
| Camarosa strawberry | Sturtz et al., 2011 | |||||||||
| Primoris strawberry | Sturtz et al., 2011 | |||||||||
| Festival strawberry | Sturtz et al., 2011 | |||||||||
| Candonga strawberry | Sturtz et al., 2011 | |||||||||
| Wild strawberry | Hattori et al., 1995 | |||||||||
| Cherry | Burkhardt et al., 2001 | |||||||||
| Tart cherry (Balaton) | Kirakosyan et al., 2009 | |||||||||
| Raspberry | Chen et al., 2003 | |||||||||
| Rubus | Zohar et al., 2011 | |||||||||
| Rutaceae family | ||||||||||
| Orange | Johns et al., 2013 | |||||||||
| Santalaceae family | ||||||||||
| Osyris | Zohar et al., 2011 | |||||||||
| Meliaceae family | ||||||||||
| China berry tree | Zohar et al., 2011 | |||||||||
| Oleracea family | ||||||||||
| European olive | Zohar et al., 2011 | |||||||||
| Green olive tree | Zohar et al., 2011 | |||||||||
| Poaceae family | ||||||||||
| Wheat | Hernández-Ruiz et al., 2005 | |||||||||
| Oat | Hattori et al., 1995 | |||||||||
| Oat | Hernández-Ruiz et al., 2005 | |||||||||
| Barley | Badria, 2002 | |||||||||
| Rice | Wang et al., 2009 | |||||||||
| Canary grass | Hernández-Ruiz et al., 2005 | |||||||||
| Corn | Wang et al., 2009 | |||||||||
| Tall fescue | Hattori et al., 1995 | |||||||||
| Barley | Hernández-Ruiz et al., 2005 | |||||||||
| Papaveraceae family | ||||||||||
| Poppy | Reiter & Tan, 2002 | |||||||||
| Rosaceae family | ||||||||||
| Pico Negro cherry | Gonzalez-Gomez et al., 2009 | |||||||||
| Burlat cherry | Gonzalez-Gomez et al., 2009 | |||||||||
| Hongdeng cherry | Badria, 2002 | |||||||||
| Navalinda cherry | Gonzalez-Gomez et al., 2009 | |||||||||
| Rainier cherry | Badria, 2002 | |||||||||
| Van cherry | Gonzalez-Gomez et al., 2009 | |||||||||
| Sweetheart cherry | Gonzalez-Gomez et al., 2009 | |||||||||
| Pico Colorado cherry | Gonzalez-Gomez et al., 2009 | |||||||||
| Apple | Hattori et al., 1995 | |||||||||
| Almond | Manchester et al., 2000 | |||||||||
| Camarosa strawberry | Sturtz et al., 2011 | |||||||||
| Primoris strawberry | Sturtz et al., 2011 | |||||||||
| Festival strawberry | Sturtz et al., 2011 | |||||||||
| Candonga strawberry | Sturtz et al., 2011 | |||||||||
| Wild strawberry | Hattori et al., 1995 | |||||||||
| Cherry | Burkhardt et al., 2001 | |||||||||
| Tart cherry (Balaton) | Kirakosyan et al., 2009 | |||||||||
| Raspberry | Chen et al., 2003 | |||||||||
| Rubus | Zohar et al., 2011 | |||||||||
| Rutaceae family | ||||||||||
| Orange | Johns et al., 2013 | |||||||||
| Santalaceae family | ||||||||||
| Osyris | Zohar et al., 2011 | |||||||||
| Solanaceae family | ||||||||||
| Tomato | Hattori et al., 1995 | |||||||||
| Tomato | Riga et al., 2014 | |||||||||
| Tomato | Pape & Luning, 2006 | |||||||||
| Tomato | Dubbels et al., 1995 | |||||||||
| Tomato | Sturtz et al., 2011 | |||||||||
| Solanaceae family | ||||||||||
| Tomato | Wang et al., 2014 | |||||||||
| Tomato | Okazaki & Ezura, 2009 | |||||||||
| Wolfberry | Manchester et al., 2000 | |||||||||
| Currant tomato | Dubbels et al., 1995 | |||||||||
| Chilies | Riga et al., 2014 | |||||||||
| Wolfberry | Chen et al., 2003 | |||||||||
| Bell pepper | Huang & Mazza, 2011 | |||||||||
| Wolfberry | Reiter & Tan, 2002 | |||||||||
| Theaceae family | ||||||||||
| Tea plant | Gomez et al., 2015 | |||||||||
| Vitaceae family | ||||||||||
| Albana grape | Gonzalez-Gomez et al., 2009 | |||||||||
| Sangiovese grape | Gonzalez-Gomez et al., 2009 | |||||||||
| Merlot grape | Iriti et al., 2006 | |||||||||
| Nebbiolo grape | Iriti et al., 2006 | |||||||||
| Sangiovese grape | Iriti et al., 2006 | |||||||||
| Croatina grape | Iriti et al., 2006 | |||||||||
| Marzemino grape | Iriti et al., 2006 | |||||||||
| Barbera grape | Iriti et al., 2006 | |||||||||
| Cabernet | Iriti et al., 2006 | |||||||||
| sauvignon grape | Iriti et al., 2006 | |||||||||
| Cabernet franc grape | Iriti et al., 2006 | |||||||||
| Verbenaceae family | ||||||||||
| Lantana | Zohar et al., 2011 | |||||||||
| Asphodelaceae family | ||||||||||
| Aloe vera | Chen et al., 2003 | |||||||||
| Zingiberaceae family | ||||||||||
| Ginger | Pape & Luning, 2006 | |||||||||
| Ginger | Badria, 2002 | |||||||||
| Green cardamom | Manchester et al., 2000 | |||||||||
The melatonin contents of tomato lines overexpressing sheep AANAT and oHIOMT genes were higher than their wild type. This ultimately indicates that animal genes could also be used to enhance melatonin synthesis in plants (Wang et al., 2014). Similarly, Park and Back (2012) found that levels of melatonin were higher in transgenic rice plants, overexpressing a sheep SNAT as compared to wild type plants. Introducing genes from vertebrates into plants can alter the biosynthesis of melatonin; such genetically altered plants can be useful since they have induced resistance against diseases, but also due to increased yield and enhanced nutritional value.
Moreover, besides the plants mentioned in Table 1, melatonin levels were also measured in 64 commonly used medicinal herbs (Chen et al., 2003); the concentration of melatonin ranged from 0.012 to 3.771 μg g−1. It is of great interest that herbs used to treat free radical-associated diseases (e.g., neurological disorders) and retard aging exhibited the highest melatonin concentration. Extra virgin olive oil, which is beneficial for human health, was also noted to be with melatonin (De la Puerta et al., 2007). Colored grains from purple wheat crops were also found enriched in melatonin and SDG (Secoisolariciresinol DiGlucoside), besides having anthocyanins (Hosseinian et al., 2008). Thus, it is proposed that additive health benefits of these cereals may be due to the presence of phytochemicals. Moreover, four altered varieties from Pistachio (Pistacia vera L.) were found to have the highest values of melatonin (227–233 μg g−1) reported for any plant organ (Oladi et al., 2014). The reproductive organs, mostly seeds, and flowers, had the highest levels, and it may be connected with their higher sensitivity toward environmental stresses like UV radiations (Afreen et al., 2006).
In 2003, for the determination of melatonin content, 108 Chinese medical herbs were selected. In all herbs, melatonin was found per gram of tissue, with a range of levels between limited nanograms and numerous thousand nanograms (Chen et al., 2003). Such huge variances demonstrated between different species propose that there must be a variance between the function of melatonin in plants. Following this, the presence of melatonin in different plants and even within different parts of the same plant was confirmed by several studies (Table 1).
There are several methods for melatonin detection in plants like ELISA (enzyme-linked immunosorbent assay), RIA (radioimmunoassay), GS-MS (gas chromatographic-mass spectrometric), HPLC (high-performance liquid chromatography), HPLC-FD (high-performance liquid chromatography with fluorescence detection), HPLC-ECD (high-performance liquid chromatography coupled to electrochemical detection), or HPLC–MS (high-performance liquid chromatography-mass spectrometer). Kolár and Macháčková (2005) suggested that RIA is not a reliable method for melatonin detection in plant samples, since the measurements have not been validated by other methods. Various extraction solvents have also been used for the different methods. Oladi et al. (2014) used GC–MS for measuring melatonin, while an ultrasound-assisted solid–liquid extraction method was employed for melatonin extraction. They explored the effect of solvent volume, choice of solvent, pH, sonication time, and temperature on extraction efficiency.
2.1 Melatonin biosynthesis
Looking at the work by Reiter (1991) and Falcón et al. (2009) in vertebrates, the biosynthesis pathway of melatonin, which takes place in the pineal gland, is quite known. Falcón et al. (2009) discovered that the amino acid tryptophan is transformed by the enzyme tryptophan 5-hydroxylase (T5H) to 5-hydroxytryptophan, which is then transformed into serotonin by the enzyme tryptophan decarboxylase. This pathway is also involved in the production of serotonin in the Hypericum perforatum L. plants (Murch et al., 2000; Murch & Saxena, 2006). However, Park and Back (2012) revealed that in rice, the synthesis is more effectively caused by the tryptamine pathway (tryptophan-tryptamine-serotonin). This pathway has been found in many plant species. In both animals and plants, serotonin is transformed to N-acetylserotonin via the enzyme SNAT. After that, HIOMT (hydroxyIndole-O-methyl transferase), also known as ASMT (acetyl serotonin methyl transferase), methylates N-acetyl serotonin to form melatonin. Tryptamine produces N-acetylserotonin in plants, especially rice, with the facilitation of N-acetyltryptamine, where SNAT and tryptophan 5-hydroxylase catalyzes the pathway. Serotonin synthesizes melatonin with the intermediation of 5-methoxytryptamine, SNAT, and HIOMT/ASMT acting as catalysts (Arnao & Hernandez-Ruiz, 2014). Tryptamine also produces an important auxin named indole acetic acid (IAA) with the intermediation of indole-3-acetyl aldehyde (Arnao & Hernandez-Ruiz, 2014) (Figure 1).
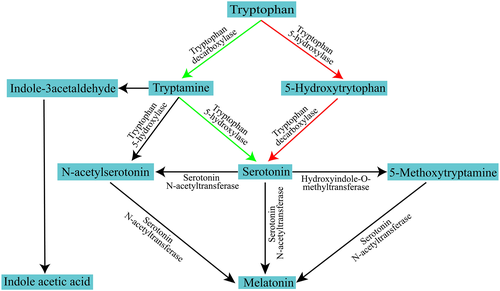
Source: Modification from Arnao & Hernandez-Ruiz, 2014
As described by Arnao and Hernandez-Ruiz (2014), melatonin in plants is synthesized in higher concentrations, and its biosynthesis is more intricate in plants compared to animals. Since the information pertaining to melatonin biosynthesis is very limited in plants; its distinctive pathway is yet to be finalized (Byeon, Tan, et al., 2015). A recent study revealed that the genes for all the enzymes involved in melatonin biosynthesis are present in transgenic rice (Byeon et al., 2013), which may contribute to the description of the precise details of the biosynthetic pathway of melatonin in plants. Contrary to the biosynthesis, melatonin also undergoes the process of degradation. N1-acetyl-N2-formyl-5methoxykynuramine (AFMK) is a secondary metabolite present in vascular plants that degrades melatonin either through an enzymatic or non-enzymatic reaction (Tan, Manchester, Mascio, et al., 2007). Among metabolites of melatonin, 2-hydroxymelatonin (99%) and 4-hydroxymelatonin (0.05%), to a much lesser extent, are being documented in rice (Byeon, Tan, et al., 2015).
2.2 Effects of melatonin on plant growth and development
Murch et al. (1997) described the regulatory role of melatonin in Hypericum perforatum L. They detected induced root growth with increased concentration of melatonin and promoted shoot growth with serotonin (melatonin precursor) accumulation in vitro cultures. However, inhibitors of melatonin and serotonin transport were shown to block auxin-promoted root formation. On the other hand, substances inhibiting the conversion of serotonin into melatonin stimulate the formation of the cytokinin-induced shoot growth, as shown by Murch and Saxena (2002). So, the morphogenetic abilities of cultures of Hypericum perforatum L. seem to be dependent on a proper ratio of serotonin/melatonin, similarly like changes in the ratio of phytohormones like cytokinins to auxins cause modification in plant growth (Jones et al., 2007; Murch & Saxena, 2002).
Many studies have explained the regulation of the biological function of melatonin in plants, like the growth of explants, shoots, roots, and seed germination is generally improved by melatonin (Arnao & Hernández-Ruiz, 2019b; Murch et al., 2001). In 2005, Hernández-Ruiz et al. reported a direct melatonin involvement in plant growth stimulation; they observed the extension of coleoptiles (10–55%) due to melatonin in wheat, oat, barley, and canary grass (monocots). Later, Park and Back (2012) explained that enhancement in the length of initial seminal roots, root biomass, and growth of transgenic varieties of rice was observed by 0.5–1 μM application of melatonin. It is now known that melatonin is involved in altering many plant characteristics, including grain yields, variation in time of flowering, senescence, and seedling growth (Wang, Sun, Chang, et al., 2013) and germination (Zhang et al., 2014). Overexpressing sheep SNAT in transgenic rice, induced early seedling growth by melatonin, but flowering was delayed, and grain yield was reduced, as found by Byeon and Back (2014b). Wang, Sun, Chang, et al. (2013) showed that the metabolic status was altered and protein degradation was delayed in apple plants (Malus hupehensis Rehd.) due to the long term application of melatonin (100 μM) in soil. They also found an increase in photosynthetic end products (sorbitol, sucrose, and starch), photosynthetic rates, and chlorophyll contents compared with control plants. All such changes are linked with improved preservation capacity of proteins. In line with these findings, it was reported by Wang, Sun, Li, et al. (2013) that drought-induced leaf senescence was delayed in Hanfu apple (Malus domestica Borkh) by long term application of melatonin (100 μM), by suppressing upregulation of SAG-12 (senescence-associated gene 12) and PAO (pheophorbide an oxygenase) and reducing oxidative stress. Also, in Arabidopsis, natural leaf senescence showed a delay by exogenous application of melatonin (Shi, Reiter, et al., 2015).
As stated by the FAO (Food and Agricultural Organization of the United Nation), the fruit and vegetable post-harvest losses are very high (20–40%). In 2009 out of all food produced, 32% was lost or wasted, as shown by Lipinski et al. (2013). Keeping in view the interval of senescence and aging owing to melatonin pre as well as post-application, there exists a possibility that in an extension of fruits and vegetable shelf life, melatonin may play a significant role and prove useful in fruits' on-tree storage, both of which may decrease post-harvest losses and thus, increase production in fresh horticultural produce. In sweet cherry, Zhao, Tan, et al. (2012) detected that melatonin concentration during the second stage of fruit development was much higher. At the same time, it was comparatively much lower during the first and third stages. Seed and embryo development, cell elongation, and cell expansion occur during the second stage.
2.3 Action of melatonin as a bio stimulator and plant growth regulator
Melatonin is an influential antioxidant that abates the concentrations of ROS (reactive oxygen species) and RNS (reactive nitrogen species) and detoxifies a wide array of chemical pollutants. Melatonin stabilizes the membranes, facilitates its fluidity, and regulates gene expression. Melatonin also acts as a bio-stimulator by modifying the redox network gene expression responsible for triggering an anti-stress response in plants by ameliorating the photosynthetic activity and inhibiting genes responsible for senescence. The endogenous levels of melatonin enhance the activation of anti-stress genes caused by abiotic stress. Melatonin, as a growth regulator in plants, regulates enzymes' expression, promoters, and receptors of hormones pertaining to growth response such as branching and rooting (Arnao & Hernandez-Ruiz, 2014; Debnath et al., 2019). Moreover, melatonin may enhance the IAA levels in plants by changing their phenotypic response. Despite the thorough scrutiny of the role of melatonin in higher plants, limited studies are available on its overall role (Table 2). Figures 2 and 3 show the action of melatonin in plants as a plant growth regulator and bio-stimulator, based on the available information.
| Function of melatonin in plants | |
|---|---|
| Enhanced seed germination rates under different stresses | Tan, Manchester, 2007; Posmyk et al., 2008, 2009 |
| Enhanced photosynthesis system and mineral accumulation | Dawood & El-Awadi, 2015 |
| Improved root architecture system in different plant species | Altaf et al., 2020; Nawaz et al., 2018 |
| Activation and inhibition of primary roots growth | Chen et al., 2009; Hernández-Ruiz et al., 2004 |
| Improved plant physiology, yield and quality under different abiotic stress | Nawaz et al., 2016 |
| Balancing growth pattern and branching of leaves and stems | Okazaki et al., 2010; Wang et al., 2014 |
| Functions as a chronoregulator | Tan, Manchester, Mascio, et al., 2007; Tal et al., 2011 |
| Act as a growth promoter | Hernandez-Ruiz et al., 2004 |
| Act as a protective agent against environmental stress such as environmental pollution, salinity, heat stress, UV exposure, drought | Arnao & Hernández-Ruiz, 2018, 2019a |
| Delays chlorophylls lost during induced leaf senescence | Arnao, 2014 |
| Auxin activity and is an excellent antioxidant | Arnao,2014 |
| Stimulating the growth of different seedlings under environmental stress | Hernández-Ruiz & Arnao, 2008 |
| Encouraging lateral and adventitious rooting in different plants species | Park & Back, 2012; Sarropoulou et al., 2012 |
| Stimulating rhizogenesis and caulogenesis in explant cultures | Jones et al., 2007; Murch & Saxena, 2002 |
| Increased ascorbic acid content proline level | Kabiri et al., 2018; Sarropoulou et al., 2012 |
| Effects on flowering | Murch et al., 2009 |
| Various concentrations found at seed formation and fruit development | Okazaki & Ezura, 2009; Murch et al., 2010 |
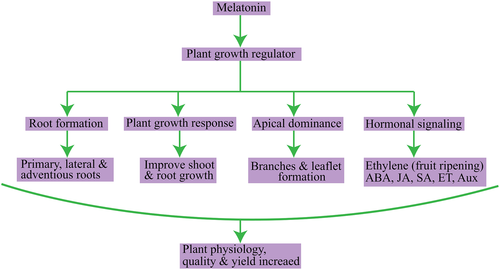
Source: Modification from Arnao & Hernandez-Ruiz, 2014
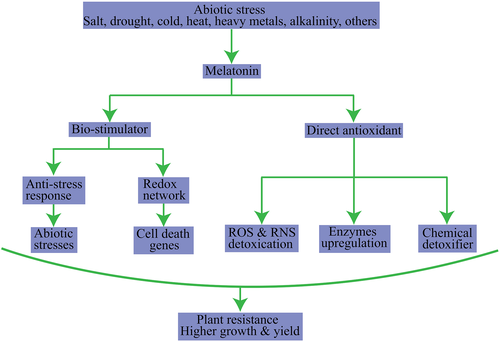
Source: Modification from Arnao & Hernandez-Ruiz, 2014
2.4 Exogenous application of melatonin to plants
After describing melatonin in animals and its detection in plants, studies concerning the effect of exogenous treatment of melatonin in different abiotic stresses were soon conducted. In this regard, both recent, as well as pioneer studies, are discussed in this section.
From the initial studies where the cold-induced apoptosis in culture cells of carrot (Daucus carota) was attenuated by the presence of exogenous melatonin (Lei et al., 2004), the protective role of melatonin against biotic and abiotic stresses in plants was postulated (Arnao & Hernández-Ruiz, 2019c, 2019d; Nawaz et al., 2016). Thus, the analysis of many stress situations and the effect of melatonin has been done. Most studies are on pea plant (Pisum sativum), where the survival and tolerance of plants in copper contaminated soil were enhanced with the application of exogenous melatonin (Tan, Manchester, & Helton, 2007). Also, in seedlings of red cabbage (Brassica oleracea rubrum), copper toxicity was reduced after the pretreatment of seeds with melatonin (Posmyk et al., 2008). During chilling stress, the germination rate was improved by pretreatment of cucumber seeds with melatonin compared to untreated seed (Posmyk et al., 2009). Similarly, an apparent increase in root growth and seed germination was shown in melatonin-treated cucumber plants under water stress indicated the minimization of water stress due to melatonin application (Zhang et al., 2013).
In apple species (Malus hupehensis) under salt stress, pretreatment of seedlings with melatonin showed that plants were less affected than untreated plants. Moreover, saline-induced inhibition would be reduced by induction of ROS-metabolizing enzymes (peroxidase, ascorbate peroxidase, and catalase activities), upregulation of K+, and Na+ transporters (AKT1 and NHX1), and halved hydrogen peroxide levels. (Szafranska et al., 2013). In meristem cells of mung bean (Vigna radiata), the protective role of melatonin applied exogenously after chilling was also observed (Szafranska et al., 2013). In plants, for long term storage of germplasm of cell culture, the usefulness of melatonin was demonstrated by the enhancement of cold shoot explants growth in Ulmus americana (American elm) due to the presence of both pre-culture and regrowth melatonin media (Uchendu et al., 2013). In a recent study of Arabidopsis grown at 4°C and treated with melatonin, fresh weight, shoot height, and primary root length was significantly greater in treated than untreated plants; the effect was dependent on both concentration and time (Bajwa et al., 2014).
Another study obtained similar data for soybean plants (Glycine max). Many parameters were optimized in seeds imbibed with melatonin, such as leaf size, seedling growth, biomass, pod number, seed number, and plant height. Also, in this species, both drought and salt tolerance were improved by melatonin treatment, which demonstrates the potential for field crops' improvement (Wei et al., 2015). Bermuda grass (Cynodon dactylon), a widely used turfgrass, showed similar results, as cold, drought, and salt stress situations were actively protected with melatonin applied exogenously as compared to untreated grass. A lower electrolyte leakage, cell damage and ROS burst and higher height and weight of plants, high levels of organic acids, amino acids, sugar alcohols, and sugars were shown in stressed melatonin treated plants than untreated plants, thus clearly affecting nitrogen and carbohydrates metabolism which are the main solutes having involvement in osmotic-stress response (Shi, Jiang, et al., 2015).
Another recent studies obtained similar results under salt stress in seedlings of Citrus aurantium (Kostopoulou et al., 2015); in cell cultures of Chara australis (Beilby et al., 2015); in cucumber seeds (Zhang et al., 2014) and seedlings, cotyledons and roots of sunflower (Mukherjee et al., 2014). Also, underwater stress in Vitis vinifera cuttings, under alkaline stress in tomato plants (Liu, Jin, et al., 2015), and under high-temperature stress in cucumber seedlings (Zhao, Sun, et al., 2012a), the importance of melatonin was revealed.
Special consideration is needed concerning the effects of melatonin on the photosynthetic process. The pioneering work of Arnao and Hernández-Ruiz (2009a) showed a delay in the loss of chlorophylls and retarding of induced senescence in leaves of barley as compared to untreated leaves, by exogenous melatonin. This effect of melatonin was contrasted with the retardant effect of kinetin and inductive effect of ABA (a synthetic cytokinin and abscisic acid, respectively) on foliar senescence. Later studies also confirmed this in other species, including Arabidopsis (Weeda et al., 2014), cucumber (Zhang et al., 2013), rice (Byeon et al., 2012, 2013), apple (Wang et al., 2012; Wang, Sun, Li, et al., 2013) and cherry (Sarropoulou et al., 2012). In apple leaves, through the development of some ROS activities, dark-induced senescence was delayed by exogenous melatonin, which contributed to the maintenance of higher glutathione and ascorbic acid contents and elimination of the excess of H2O2 generated in stressed leaves as compared to control leaves (Wang et al., 2012).
In apple trees, better efficiency of photosystem II was proved under both dark and light conditions. Photosynthetic efficiency may also be improved by melatonin in plants, besides its protective role against leaf senescence. Under drought stress, melatonin alleviates the inhibition in photosynthesis and allows leaves to maintain capacity for stomatal conductance and CO2 assimilation (Wang, Sun, Li, et al., 2013). Similarly, data were obtained in cucumber seedlings under water stress. Adverse effects of water stress were reversed by melatonin treatment, as activities of ROS scavenging enzymes and photosynthetic rates were increased, and chlorophyll degradation was reduced (Zhang et al., 2013). Also, in cherry, the role of melatonin in plant stress metabolism has been indicated, as the content of the photosynthetic pigment, total carbohydrates, and total biomass was enhanced. Proline contents of roots were reduced by the exogenous application of less concentrated melatonin in shoot tip explants of the rootstock of cherry (Sarropoulou et al., 2012).
Several previous studies have also described preservative effects of melatonin on chlorophyll content, in freshwater C. australis (Lazar et al., 2013) and macroalga Ulva sp. (Tal et al., 2011), where the efficiency of the reaction centers of photosystem II was increased. Recently, similar research has been done regarding the protective role of melatonin on photosynthetic pigments under alkaline stress in tomato plants; in sunflower under salt-stress (Mukherjee et al., 2014); in wheat under cold-stress (Turk et al., 2014); and in citrus under salt-stress (Kostopoulou et al., 2015).
A clear relationship has been described in some cases between the beneficial effects of exogenous melatonin and leaf anatomical and morphological changes. Thus, underwater deficiency stress, oxidative damage was alleviated by melatonin in grape cuttings, by increasing antioxidative enzymes' activity (SOD, CAT, POX) and decreasing malondialdehyde content and ROS burst. Moreover, there was an increase in levels of antioxidant metabolites like ascorbic acid and glutathione, as well as proline content.
Direct evidence has been provided by a recent study of Wei et al. (2015), where melatonin coated seeds of soybean plants showed a significant increase in plant height, seeds, and pods per plant, fatty acid substances, and leaf area. New paths for enhancing the plant yield and revolutionizing the seed industry has been suggested by this study, as for a considerable number of essential horticultural and agronomic plants, melatonin-coated seeds could be used commercially. As described in Table 3, the application of melatonin resulted in substantial alleviation in growth reduction, photosynthetic inhibition, chlorophyll loss, and enhance antioxidant activities in response to biotic and abiotic stresses in many plants is shown in several studies.
| Plant name | Stress types | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salinity | Drought | Heavy metal | Heat | Cold | Light | Chemical | Pathogen | UV-B | Alkalinity | Acid | ||
| Asteraceae family | ||||||||||||
| Sunflower | Arora & Bhatla, 2017 | |||||||||||
| safflower | Namdjoyan et al., 2020 | |||||||||||
| Sunflower | Mukherjee et al., 2014 | |||||||||||
| Sunflower | Kaur & Bhatla, 2016 | |||||||||||
| Actinidiaceae family | ||||||||||||
| Kiwifruit | Liang, Gao, et al., 2018 | |||||||||||
| Kiwifruit | Liu et al., 2019 | |||||||||||
| Kiwifruit | Xia et al., 2017, 2020 | |||||||||||
| Kiwifruit | Liang et al., 2019 | |||||||||||
| Amaranthaceae family | ||||||||||||
| Spinach | Moussa & Algamal, 2017 | |||||||||||
| Apocynaceae family | ||||||||||||
| Catharanthus | Nabaei & Amooaghaie, 2019 | |||||||||||
| Betulaceae family | ||||||||||||
| White birch | Li, Pei, et al., 2019 | |||||||||||
| Boraginaceae | ||||||||||||
| Lacy phacelia | Tiryaki & Keles, 2012 | |||||||||||
| Brassicaceae family | ||||||||||||
| Arabidopsis | Zheng et al., 2017 | |||||||||||
| Arabidopsis | Zhang, Cui, et al., 2019 | |||||||||||
| Arabidopsis | Chen et al., 2017 | |||||||||||
| Arabidopsis | Yang et al., 2019 | |||||||||||
| Arabidopsis | Zuo et al., 2014 | |||||||||||
| Arabidopsis | Shi, Tan, et al., 2015 | |||||||||||
| Arabidopsis | Zhou, Liu, et al., 2016 | |||||||||||
| Arabidopsis | Shi, Qian, et al., 2015 | |||||||||||
| Arabidopsis | Bajwa et al., 2014 | |||||||||||
| Arabidopsis | Zhang, Li, et al., 2019 | |||||||||||
| Arabidopsis | Shi & Chan, 2014 | |||||||||||
| Arabidopsis | Rodriguez-Naranjo et al., 2012 | |||||||||||
| Rapeseed | Li, Zeng, et al., 2018 | |||||||||||
| Rapeseed | Khan et al., 2019, 2020 | |||||||||||
| Cabbage | Posmyk et al., 2008 | |||||||||||
| Rapeseed | Zhao et al., 2018 | |||||||||||
| Radish | Jia et al., 2019 | |||||||||||
| Radish | Yao et al., 2017 | |||||||||||
| Radish | Jiang et al., 2017 | |||||||||||
| Characeae family | ||||||||||||
| Chara | Beilby et al., 2015 | |||||||||||
| Cucurbitaceae family | ||||||||||||
| Cucumber | Wang et al., 2016 | |||||||||||
| Cucumber | Zhang et al., 2014 | |||||||||||
| Cucumber | Zhang, Sun, et al., 2017 | |||||||||||
| Cucumber | Zhang et al., 2013 | |||||||||||
| Cucumber | Cao et al., 2018 | |||||||||||
| Cucumber | Zhao et al., 2017 | |||||||||||
| Cucurbitaceae family | ||||||||||||
| Cucumber | Ahammed et al., 2020 | |||||||||||
| Cucumber | Zhang et al., 2020 | |||||||||||
| Cucumber | Li, Li, et al., 2017 | |||||||||||
| Cucumber | Posmyk et al., 2009 | |||||||||||
| Cucumber | Shah et al., 2020 | |||||||||||
| Watermelon | Li, Chang, Chen, et al., 2017; Li, Chang, Zheng, et al., 2017 | |||||||||||
| Watermelon | Nawaz et al., 2018 | |||||||||||
| Melon | Castañares & Bouzo, 2019 | |||||||||||
| Melon | Wu et al., 2019 | |||||||||||
| Euphorbiaceae family | ||||||||||||
| Cassava | Ding et al., 2019 | |||||||||||
| Fabaceae family | ||||||||||||
| Alfalfa | Antoniou et al., 2017 | |||||||||||
| Fenugreek | Zamani et al., 2019 | |||||||||||
| Alfalfa | Gu et al., 2017 | |||||||||||
| Soybean | Cao, Jin, et al., 2019 | |||||||||||
| Soybean | Wei et al., 2015 | |||||||||||
| Soybean | Zhang, Zeng, et al., 2017 | |||||||||||
| Soybean | Zou et al., 2019 | |||||||||||
| Pea | Tan, Manchester, & Helton 2007 | |||||||||||
| Fava bean | Siddiqui et al., 2020 | |||||||||||
| Pea | Szafranska et al., 2017 | |||||||||||
| Pigeon pea | Yadu et al., 2018 | |||||||||||
| Chinese liquorice | Afreen et al., 2006 | |||||||||||
| Juglandaceae family | ||||||||||||
| Chinese hickory | Sharma et al., 2020 | |||||||||||
| Lamiaceae family | ||||||||||||
| Perilla | Xiang et al., 2019 | |||||||||||
| Lemon balm | Hodzic et al., 2018 | |||||||||||
| Moldavian balm | Kabiri et al., 2018 | |||||||||||
| Basel | Bahcesular et al., 2020 | |||||||||||
| Moringaceae family | ||||||||||||
| Moringa | Sadak et al., 2020 | |||||||||||
| Musaceae family | ||||||||||||
| Banana | Wei et al., 2017 | |||||||||||
| Plumbaginaceae family | ||||||||||||
| Rosemary | Li, Zhao, et al., 2019 | |||||||||||
| Poaceae family | ||||||||||||
| Rice | Kang et al., 2010 | |||||||||||
| Rice | Han et al., 2017 | |||||||||||
| Rice | Byeon & Back, 2014a | |||||||||||
| Rice | Lee & Back, 2017a, 2017b | |||||||||||
| Rice | Lee & Back, 2016 | |||||||||||
| Rice | Wei et al., 2016 | |||||||||||
| Rice | Byeon, Lee, et al., 2015 | |||||||||||
| Wheat | Qiao et al., 2019 | |||||||||||
| Poaceae family | ||||||||||||
| Wheat | Ni et al., 2018 | |||||||||||
| Wheat | Li, Brestic, et al., 2018 | |||||||||||
| Wheat | Kaya et al., 2019 | |||||||||||
| Wheat | Turk et al., 2014 | |||||||||||
| Wheat | Cui et al., 2017 | |||||||||||
| Wheat | Zuo et al., 2017 | |||||||||||
| Wheat | Zafar et al., 2019 | |||||||||||
| Maize | Fleta-Soriano et al., 2017 | |||||||||||
| Maize | Chen et al., 2018 | |||||||||||
| Maize | Okant & Kaya, 2019 | |||||||||||
| Maize | Ye et al., 2016 | |||||||||||
| Maize | Huang et al., 2019 | |||||||||||
| Maize | Ahmad et al., 2019 | |||||||||||
| Maize | Cao, Li, et al., 2019 | |||||||||||
| Maize | Jiang, Cui, et al., 2016; Jiang, Li, et al., 2016 | |||||||||||
| Ray grass | Zhang, Shi, et al., 2017 | |||||||||||
| Tall fescue grass | Alam et al., 2018 | |||||||||||
| Naked oat | Gao, Zhang, et al., 2018 | |||||||||||
| Barley | Arnao & Hernández-Ruiz, 2009b | |||||||||||
| Barley | Li, Tan, et al., 2016 | |||||||||||
| Bermudagrass | Xie et al., 2018 | |||||||||||
| Creeping bentgrass | Merewitz & Liu, 2019 | |||||||||||
| Bermudagrass | Shi, Jiang, et al., 2015 | |||||||||||
| Creeping bentgrass | Ma et al., 2018 | |||||||||||
| Creeping bentgrass | Gao et al., 2019 | |||||||||||
| Rosaceae family | ||||||||||||
| Crabapple | Gong et al., 2017 | |||||||||||
| Crabapple | Li et al., 2012; Li, Liang, et al., 2016 | |||||||||||
| Apple | Wang, Sun, Li, et al., 2013 | |||||||||||
| Malus | Li et al., 2015 | |||||||||||
| Malus | Wei et al., 2019 | |||||||||||
| Apple | Liang, Ma, et al., 2018 | |||||||||||
| Peach | Gao, Lu, et al., 2018 | |||||||||||
| Rutaceae family | ||||||||||||
| Citrus | Kostopoulou et al., 2015 | |||||||||||
| Rubiaceae family | ||||||||||||
| Coffea | Campos et al., 2019 | |||||||||||
| Musaceae family | ||||||||||||
| Banana | Wei et al., 2017 | |||||||||||
| Solanaceae family | ||||||||||||
| Tomato | Zhou, Zhao, et al., 2016 | |||||||||||
| Tomato | Karaca & Cekic, 2019 | |||||||||||
| Tomato | Siddiqui et al., 2019 | |||||||||||
| Tomato | Li, Hasan, et al., 2016 | |||||||||||
| Tomato | Jahan et al., 2020 | |||||||||||
| Tomato | Umapathi et al., 2018 | |||||||||||
| Solanaceae family | ||||||||||||
| Tomato | Yin et al., 2019 | |||||||||||
| Tomato | Altaf et al., 2020 | |||||||||||
| Tomato | Cai et al., 2017 | |||||||||||
| Tomato | Liu, Jin, et al., 2015 | |||||||||||
| Tomato | Liu, Wang, et al., 2015 | |||||||||||
| Tomato | Yan, Jing, et al., 2019 | |||||||||||
| Tomato | Martinez et al., 2018 | |||||||||||
| Tomato | Xu et al., 2016 | |||||||||||
| Tomato | Hasan et al., 2018 | |||||||||||
| Tomato | Ding et al., 2017 | |||||||||||
| Tomato | Ding et al., 2018 | |||||||||||
| Tomato | Hasan et al., 2015 | |||||||||||
| Tomato | Debnath et al., 2018 | |||||||||||
| Tomato | Yan, Sun, et al., 2019 | |||||||||||
| Tomato | Hasan et al., 2019 | |||||||||||
| Tomato | Jahan et al., 2019 | |||||||||||
| Tomato, Tabcoo | Lee & Back, 2019 | |||||||||||
| Pepper | Kaya et al., 2020 | |||||||||||
| Pepper | Sarafi et al., 2017 | |||||||||||
| Potato | Zhang, Zheng, et al., 2017 | |||||||||||
| Tobacoo | Wang, Duan, et al., 2019 | |||||||||||
| Tamarillo | Lin et al., 2018 | |||||||||||
| Theaceae family | ||||||||||||
| Tea | Li, Wei, et al., 2018 | |||||||||||
| Vitaceae family | ||||||||||||
| Grape | ||||||||||||
| Grape | ||||||||||||
| Grape | Jiao et al., 2016 | |||||||||||
| Grape | Wang, An, et al., 2019 | |||||||||||
| Grape | Boccalandro et al., 2011 | |||||||||||
| Valerianceae family | ||||||||||||
| Valerian | Hodzic et al., 2018 | |||||||||||
2.5 Endogenous application of melatonin to plants
As described in the above section, the protective role of exogenously applied melatonin in plants is pointed out by many studies. However, recently it has been demonstrated that environmental conditions can vary the endogenous melatonin levels. In the study of Afreen et al. (2006), after UV-B radiation treatment, endogenous melatonin content of roots of Glycyrrhiza uralensis multiplied to about 80 L g−1 FW, which is about a sevenfold increase as compared to control plants. These data suggest the accumulation of melatonin as a protective molecule in the plant tissue, toward different abiotic stressors like chemical agents, UV radiation, cold, water deficiency, and light–dark cycle.
Recently, under salt-stress conditions in sunflower seedlings, melatonin levels have been determined (Mukherjee et al., 2014). A sixfold increase in melatonin in cotyledons and a twofold increase in roots were shown by seedlings grown by adding NaCl compared to untreated plants, despite very high quantification of melatonin (on the order of L g g−1 FW). Salt-treated plants also have a higher level of serotonin. In two Malus species, drought conditions upregulated four biosynthetic genes of melatonin, i.e., T5H, HIOMT, TDC, and SNAT (Li et al., 2015). All up to date available data showed evidence that the melatonin biosynthesis is induced by abiotic stressors, and in response to the abiotic stressor, melatonin plays the role of signal intermediate.
In freezing tissues or cold conditions, the protective role of melatonin has been demonstrated by a number of studies (Bajwa et al., 2014; Zhao et al., 2011). On the contrary, limited studies worked on heat stress. On germination, the high temperature’ inhibitory effect was reversed by melatonin treated seeds of thermos-sensitive Phacelia tanacetifolia (Tiryaki & Keles, 2012). Also, the resistance against heat stress in cucumber seedlings was improved, as activities of enzymes related to nitrogen metabolism at high temperatures were significantly increased by exogenous melatonin, thus restricting the ammonium content and increasing the nitrate content.
Recently, in Arabidopsis, the effect of high temperatures on thermo-tolerance factors and endogenous melatonin levels has been studied (Shi, Tan, et al., 2015). In Arabidopsis seedlings, endogenous melatonin content was increased by twofold to fivefold, provoked by heat stress (37°C). Also, under heat stress (45°C for 120 min), the survival rate of plants was enhanced (~50%) by exogenous treatment of melatonin (20 μM) as compared to untreated plants in which the survival rate was 5%.
A range of transgenic plants having an expression for ectopic genes, primary genes for melatonin synthesis from animal origin, has been utilized, to study the potential role of melatonin in the physiology of plants. As a rule, in overexpressing plants enriched with melatonin, a higher resistance toward abiotic stressors like UV-B, cold, and drought can be observed compared to wild type plants. It is demonstrated by these results that endogenous melatonin plays its role in defense against stressors and is critical for scavenging of ROS, even at lower concentrations (nano or picograms measured per gram of fresh weight). In transgenic modified plants, higher root biomass, root growth rate, and robustness were observed.
3 CONCLUSION AND FUTURE PERSPECTIVE
In the past 20 years, researchers have conducted hundreds of researches and published articles about melatonin, which reveals that it has a primary role in the plant's defense system. Being an excellent antioxidant, the appropriate concentration of melatonin alleviates oxidative stress caused by abiotic stress. The protective mechanism of melatonin has been detailed by various researchers.
Nevertheless, melatonin requires a lot of further research to uncover its full potential. Besides, the exact position of melatonin biosynthesis in plants, it still needs thorough investigation. As melatonin is highly unstable, its transportation in plant organs and organelles is intricate to monitor (Van-Tassel et al., 2001).
The role of melatonin in promoting root growth is widely accepted; however, its nutrient uptake ability still needs investigation since no such report has been published, which clarifies the role of melatonin in nutrient uptake and transportation. Likewise, brief information is available regarding the absorption of melatonin in plants through foliar application and its enhancement on plant growth and development.
Yet, numerous major issues are needed to be explored. Detailed investigations are required regarding the usage of exogenous melatonin and endogenous melatonin against insects, nematodes, or viruses. Also, there is an information lack regarding the precise regulation of genes and core pathways by melatonin. Concluding the whole thing, across the entire plant kingdom, there is enormous potential for research in finding the melatonin impact in essential life functions as well as new approaches development for progressing in industrial agriculture and plant cultivation.
In conclusion, the role of melatonin in strengthening plants against abiotic/biotic stress has broadened the field of study regarding plant improvement and protection against pathogens.
Concluding dynamic literature available related to melatonin, significant improvements in both plants and animals by melatonin may be hypothesized, as many aspects support this hypothesis. The mitigating role of melatonin against biotic and abiotic stresses in plants is vastly unraveled. It acts as a natural protector and antioxidant and enhances tolerance against any adversity, mainly by enhancing ROS and RNS scavenging; improves photosynthesis system by preserving chlorophyll against oxidative stress; recovers leaf ultrastructure and retards leaf senescence induced by stress; stimulates plant growth regulation; enhances seed germination; promotes roots regeneration and better root architecture; hence proving its role as a biostimulator in plants. Thus melatonin in plants lies amidst recent frontiers studied and explored by researchers, focusing on its supporting role in crops' resistance against adverse environmental conditions, which otherwise affects plants' development throughout its lifecycle.
AUTHOR CONTRIBUTIONS
Ming-Xun Ren, Freddy Mora-Poblete, Marino B. Arnao, Jen-Tsung Chen conceived the study; Muhammad Ahsan Altaf, Rabia Shahid, Safina Naz, Hamza Sohail wrote the manuscript; Muhammad Mohsin Altaf, Muhammad Anwar, Sidra Shahid, Awais Shakoor helped in making tables and figures; Sunny Ahmar, Muhammad Anwar finalized the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




