Abscisic acid phytohormone estimation in tubers and shoots of Ipomoea batatas subjected to long drought stress using competitive immunological assay
Edited by M. Ahanger
Abstract
Sweet potato (Ipomoea batatas L.), typically cultivated in temperate climates under low inputs, is one of the most important crops worldwide. Abscisic acid (ABA) is an important plant stress-induced phytohormone. Hitherto, few works analyzed the ABA function in sweet potato tissue growth. Very scarce information is available concerning the ABA role in sweet potato response to water scarcity conditions. Here, we show the ABA content variation in shoots and tubers of eight sweet potato accessions subjected to drought stress. ABA was also related to other resistance traits, such as chlorophyll content index (CCI), carbon isotopic discrimination (Δ13C), oxalic acid (OA) and water use efficiency (WUE), to assess stress response mechanisms to water deficit between their organs. The most resilient drought-stressed sweet potato plants accumulated ABA-shoot, and significantly decreased the ABA-tuber content. ABA signaling was related to Δ13C and CCI decrease and WUE increment, as an attempt to cope with water stress by partially closing the stomata. The partial closure of stomata could be in part due to the presence of OA-shoots, known to affect the intensity of the ABA-shoot signal in stomatal closure. Higher CCI content and minimal Δ13C-shoot differences indicated good carboxylation fractionation, with higher Δ13C-tuber content as an indicator of efficient tuber 13C fixation and growth. Our work demonstrated that ABA could be used in conjunction with the other traits studied for the assessment of sweet potato whole-plant responses to environmental stresses, and thus aid the selection of the best drought tolerant genotypes for breeding programs.
Abbreviations
-
- δ13C
-
- carbon isotope composition
-
- Δ13C
-
- carbon isotope discrimination
-
- ABA
-
- abscisic acid
-
- Acc.
-
- accession
-
- CAN
-
- Canary Islands
-
- CCI
-
- chlorophyll content index
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- GUI
-
- Guinea-Bissau
-
- HRP
-
- Horseradish Peroxidase
-
- MAD
-
- Madeira Island
-
- OA
-
- oxalic acid
-
- PAR
-
- photosynthetic active radiation
-
- PCA
-
- principal component analysis
-
- RHa
-
- relative air humidity
-
- VWC
-
- volume water content of soil
-
- WUE
-
- water use efficiency
Introduction
Sweet potato [Ipomoea batatas (L.) Lam.], after potatoes (Solanum tuberosum L.) and cassava (Manihot esculenta Crantz) root tubers, is one of the most important staple crops in the world (Lebot 2009, FAOSTAT Statistical Database 2018). Sweet potato is an important food source supply in tropical and developing countries owing to the storage of 80–90% carbohydrates in root dry matter content (Lebot 2009). Some countries also use the sweet potato leaves as a vegetable source, providing high content in protein, soluble dietary fiber, minerals (iron), polyphenols and vitamins (Bradbury and Holloway 1988, Ishida et al. 2000).
The major sweet potato production occurs in Asia, with 60.7 Mt in 2018, representing 66% of worldwide production (FAOSTAT Statistical Database 2018). Its production is usually done in temperate climates, under low input conditions. With the characteristic low plant growth habit and extensive root system, the sweet potato can be moderately tolerant to water scarcity (Smittle et al. 1990, Ekanayake and Collins 2004, Motsa et al. 2015a, 2015b). The exposure to an extended period of abiotic stress leads to plant physiological modifications, changing the crop quality and biochemical composition to cope and survive stress (Wang and Frei 2011).
Abscisic acid (ABA) is an important isoprenoid stress-induced phytohormone present in sweet potato (Nakatani and Komeichi 1991, Nagata and Saitou 2009, Wani et al. 2016, Lau et al. 2018). This isoprenoid plays a pivotal role in numerous plant biochemical and physiological processes, including stomatal closure, lipid synthesis, protein storage and starch accumulation, related to development, growth and signaling pathways (Mengel et al. 2001, Firon et al. 2009, Danquah et al. 2013, Wani et al. 2016, Saddhe et al. 2017, Vishwakarma et al. 2017). Proline and ethylene synthesis, which have a protective function under both water and saline stress, are also stimulated by ABA (Mengel et al. 2001). ABA is considered one of the most effective plant hormones due to its quick synthesis under abiotic stresses, such as drought, salinity, temperature and nutrient imbalance (Mengel et al. 2001, Firon et al. 2009, Osakabe et al. 2014, Sah et al. 2016, Salehi-Lisar and Bakhshayeshan-Agdam 2016). Plants also abscises organs, such as leaf abscission, to promote senescence as stress response (Mengel et al. 2001, Sah et al. 2016). ABA can be quantitated by the Enzyme-Linked Immunosorbent Assay (ELISA), a sensitive method that can detect low concentrations of this phytohormone in plant tissues. Currently, it is one of the most affordable techniques that allow fast detection of ABA without the need for complex purification steps and uses a combination of the specificity of antigen–antibody reaction with the same sensitivity of enzymatic assays (Huang et al. 2014).
Under a tenacious dry environment, root ABA signalizes the plant shoots that they are facing stressful conditions around the roots, leading to stomatal closure to avoid water loss (Wani et al. 2016). Drought could induce the stomatal closure through a root-to-shoot signaling with an ABA synthesis occurring mainly in the chloroplasts (Mengel et al. 2001, Osakabe et al. 2014, Salehi-Lisar and Bakhshayeshan-Agdam 2016). Other works also indicate that the leaves are the predominant location for ABA biosynthesis during drought stress, and also that the ABA from leaves has a greater effect over root development (McAdam et al. 2016). The cellular dehydration that triggers ABA biosynthesis in the chloroplast mesophyll tissues involves various enzymes and the utilization of β-carotene (Mengel et al. 2001, Wani et al. 2016, Vishwakarma et al. 2017). The stomatal responses are closely related to soil water potential, with its closure inhibiting transpiration and increasing the water flow in the plant through hydraulic conductivity (Mengel et al. 2001, Ma and Qin 2014, Osakabe et al. 2014, Salehi-Lisar and Bakhshayeshan-Agdam 2016). The plasma membrane and tonoplast use the ion and water transport systems to control turgor pressure changes in the guard cell. The guard cells detect the increase of ABA levels, leading to a subsequent reduction of turgor and volume to avoid water loss, and trigger the stomatal closure (Ma and Qin 2014, Osakabe et al. 2014).
The atmospheric CO2 also acts as a signaling molecule in stomatal responses, whereas increased CO2 concentrations in leaves induce the stomatal closure, leading to a reduction in the photosynthesis rate in leaves, and affecting the water use efficiency (WUE; Salehi-Lisar and Bakhshayeshan-Agdam 2016, Osakabe et al. 2014). WUE reflects how plants manage the water use for vital activities and plant production during scarcity conditions, with the most tolerant ones usually displaying higher WUE (Mengel et al. 2001, Ganança et al. 2018, Gouveia et al. 2019). The chlorophyll measured in the full-grown leaves could be associated with the rate of photosynthesis, which is one of the most common parameters used as indicator of plant performance under drought stress (Shao et al. 2015, Salehi-Lisar and Bakhshayeshan-Agdam 2016, Gouveia et al. 2020). The carbon isotopic discrimination (Δ13C), that represents the photosynthetic depletion of 13C in field-grown plants, is linked with stomatal aperture and is commonly used in C3 plants for the assessment of drought resilience (Farquhar et al. 1989, Zhang et al. 2015, Gouveia et al. 2019). Oxalic acid (OA) is an ethanedioic acid that inhibits ABA-induced stomatal closure in Arabidopsis thaliana plants (Guimarães and Stotz 2004). OA is a free acid that can form an equilibrium between soluble (potassium or sodium oxalate) or insoluble (calcium oxalate) salts, which variation plays an important role in the plant ion balance and osmoregulation under drought conditions (Franceschi and Horner 1980, Gouveia et al. 2020).
The chlorophyll content index (CCI), Δ13C, OA and WUE traits were previously used in the evaluation of the crop resilience to water scarcity environments (Gouveia et al. 2019, Gouveia et al. 2020). To date, a few works reported the ABA effects on sweet potato cultivars, including tuber yield of two accessions grown in fully irrigated pots (Nakatani and Komeichi 1991), the inhibitory growth effects of exogenous application of ABA to in vitro plantlets of one accession (Jarret and Gawel 1991), sink activity through leaf-petiole cuttings from one accession grown in a growth chamber and treated with ABA solution (Nagata and Saitou 2009), and growth of in vitro shoots of two accessions exposed to osmotic pressure induced by polyethylene glycol (Lau et al. 2018). These experiments revealed that ABA was one of the factors that regulated the sweet potato sink activity and strength. The higher the sink activity, the higher sugar uptake and metabolic activity observed in the growing tuberous roots (Nagata and Saitou 2009). The thickening of the tuberous root diameter by cell division activity in the secondary cambium of sweet potato was mainly promoted by ABA located around the primary cambium and meristem in the xylem (Nagata and Saitou 2009, Ravi and Saravanan 2012). The heaviest tubers that show the highest ABA content under control conditions had a decreased tissue growth under osmotic pressure (Nakatani and Komeichi 1991, Nagata and Saitou 2009, Lau et al. 2018). These experiments were performed on a model scale to assess ABA importance in the growth of sweet potato plantlets without involving the real field conditions, and determining ABA throughout the whole plant subjected to water stress. Therefore, our study aimed to better elucidate the ABA involvement in the responses of field full-grown sweet potatoes subjected to long water scarcity conditions. We used a set of eight accessions from diverse geographical provenances to seek if drought leads to endogenous ABA accumulation due to stress as a physiological response to drought stress conditions. The specific goals were: (1) determining the ABA content in shoots and tubers of field-grown sweet potato plants subjected to long-term drought stress, and (2) relating ABA with other resistance traits (CCI, Δ13C, OA and WUE) evolved in crop tolerance to better understand the sweet potato responses to water deficit.
Materials and methods
Sweet potato accessions
Eight accessions of sweet potato [I. batatas (L.) Lam.] from Madeira Island (MAD), Canary Islands (CAN) and Guinea-Bissau (GUI) (Table 1) were submitted to water scarcity conditions in the present study.
| Accession ID | Variety local name | Origin |
|---|---|---|
| 1036 | Brasileira | Madeira Island (MAD) |
| 1038 | 5 Bicos | Madeira Island (MAD) |
| 2927 | de Flor | Madeira Island (MAD) |
| 3126 | Inglesa | Madeira Island (MAD) |
| 2937 | Roja | Canary Islands – Tenerife (CAN) |
| 2938 | Cubana | Canary Islands – Tenerife (CAN) |
| 3124 | Vermelha | Guinea-Bissau – Bafatá (GUI) |
| 3125 | Branca | Guinea-Bissau – Bafatá (GUI) |
Experimental field assay
Five months of field trials were established in 2017 at the ISOPlexis experimental field (32°39′N, 16°55′W, at Funchal, Madeira, Portugal), using a split-plot design. In the main plot, the sweet potato accessions were grown in two independent blocks, one under regular open field conditions (control) and the other under an open rain shelter (experimental variant). The shelter aimed to avoid rain feed and ensure the water deficit conditions. Each accession was planted in three subplots (replicates): eight independent rows per block, 30 vine cuttings per accession in total, with 70 × 80 cm in and between the rows. This makes five vine cuttings per accession, added in each row per subplot, that were fully irrigated in both open and shelter environments until the beginning of the third month of the trial. Three vines were also added as test samples, in both open and shelter blocks, exempt from water stress throughout the trial duration by keeping full irrigation. Two distinct water regimes were then applied, through drip irrigation system, with 1.6 mm for control and 0.9 mm for water deficit variants, per subplot, three times a week during the remaining 3 months. During this period, control block received approximately 77 mm of water, while the drought block received approximately 54 mm. Controls were also fed with 117.5 mm of rainfall during this period. During rain periods, irrigation was suspended on control block. Both control and drought variants were also assessed periodically for the photosynthetic active radiation (PAR, 400–700 nm) with a ceptometer (AccuPAR LP-80), volume water content of soil (VWCs) with a soil moisture sensor (WaterScout SM10), air temperature (Ta) and relative air humidity (RHa) with a data logger (Testo 174H). During the assay, we registered a 24.6% PAR decrease under the rain shelter relative to open field environment, on average, with 1514.5 μmol m−2 s−1 for control and 1142.0 μmol m−2 s−1 for drought. The VWCs measured at 10 cm of depth of homogenized field soil shown on average 12.8% VWCs for control, representing 35% of field capacity, and 3.5% VWCs for drought, representing equal or less than 10% of field capacity. During the assay, an average of 19.46°C Ta and 68.07% RHa were observed for control; an average 22.25°C Ta and 66.40% RHa were registered for drought. Throughout the entire agronomic trial, neither fertilizers nor pesticides were applied, and manual weeding was performed regularly.
Preparation of sweet potatoes whole-plant flour samples
At the end of the agronomic trial, 384 root tubers and shoots (stem, stalk and leaves) samples from control and drought plots were collected. All samples were washed, weighed (Sartorius Basic BA2100S), chopped on a mandolin cutter (2–3 mm thick), subsequently placed in an air oven to dehydrate until constant weight during approximately 48 h at 65°C (Memmert UF260), and finally ground into flour (IKA-Werke M20). The flour was placed in bags (Termofilm PA/PE), vacuum sealed (Audionvac VMS153) and stored at −35°C (Liebherr ProfiLine GGPV6570) until analysis.
Quantitative detection of abscisic acid in plant tissues
ABA, a weak acid, is a sesquiterpene with an α,β-unsaturated ketone in the ring and a conjugated diene side chain (Huang et al. 2014). Its content was determined in the root tuber and shoot flours by the ELISA technique, using the ABA ELISA Kit (MBS 282218, 96 tests, MyBioSource Inc.) and a microplate reader (Tecan Sunrise Remote A-5082; software Magellan™ V7.1, Tecan). Flour samples were previously extracted with 0.940 μl 1× PBS, and centrifuged at 9838 g during 5 min, with supernatant collected for analysis. Lyophilized ABA standard was diluted (from 1.56 to 100 ng ml−1) and used as calibration standards. Specifically, 50 μl of standards and samples were individually added to the microplate wells pre-coated with an antibody specific to ABA, followed by 50 μl Horseradish Peroxidase (HRP)-conjugated ABA, and incubated for 1 h at 37°C, protected from light. The competitive inhibition reaction occurs between HRP-labeled ABA and unlabeled ABA with the antibody. One hundred microliter of substrate solution was added to the wells protected from light and incubated for 20 min at 37°C. The reaction was terminated by adding 50 μl of stop solution. The obtained data at 450 nm, corrected at 620 nm, were subjected to logarithmic transformations. The analyses were performed in triplicate and the values were expressed in ng g−1 of dry flour.
The method accuracy was validated through the recovery index percentage of ABA (Van Reeuwijk et al. 1998). Three distinct flour samples were spiked with 25 ng ml−1. The samples and the spikes were analyzed five times, showing 102% of recovery index, a high sensitivity and excellent specificity for ABA detection and quantification in sweet potato tissues.
Carbon isotope discrimination
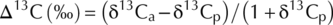
The analysis was made in triplicate with results presented in ‰ units of dry flour.
Chlorophyll content index
The relative chlorophyll content of the sweet potato fresh leaves was measured and expressed as the CCI. We employed a non-destructive measurement method using a chlorophyll content meter (Opti-Sciences CCM-200 PLUS). The CCI values were obtained through the absorbance in transmittance mode, at 653 and 931 nm, whose values were proportional to the amount of chlorophyll in the tissue. Three measurements were performed in the morning on the adaxial leaf surface, avoiding the branching veins. An average CCI value was recorded for each replicate.
Oxalic acid
OA was quantitated in flour samples from root tubers and shoots (Gouveia et al. 2020). Exactly 0.4 g of flour was extracted with hydrochloric acid (HCl, 6.0 M) to allow the reduction of oxalic into glyoxylic acid, with a further reduction into glycolic acid. A potassium permanganate solution (KMnO4, 0.05 M) was used to precipitate and titrate the sample extracts for OA quantitation. We used Dye (1956) calculation for the total OA quantification. The analyses were made in triplicate, with values presented as mg 100 g−1 of dry flour.
Water use efficiency
WUE was calculated as the ratio of the whole-plant dehydrated biomass to total water used per subplot and expressed in g l−1 (Ganança et al. 2018).
Statistical methods
The results were expressed on a dry weight basis, as the main average of sweet potato root tubers and shoots, for control vs. drought plots. IBM spss Statistics V24 for Mac was used for one-way anova, Tukey hsd test and Pearson correlations. The statistically significant differences were expressed with P-value lower than 0.05. The mvsp V3.1 for Windows was used to perform the principal component analysis (PCA).
Results
ABA variation in root tubers and shoots during drought
A wide range of strategies of ABA synthesis and accumulation in both organs was observed among the eight sweet potato accessions. The data of ABA variation in root tubers and shoots for both control and drought experimental variants are shown in Table 2.
| ABA content (ng g−1) | Δ13C content (‰) | OA content (mg 100 g−1) | WUE †† (g l−1) | CCI†† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tuber†† | Shoot†† | Tuber†† | Shoot†† | Tuber†† | Shoot†† | |||||
| MAD | 1036 | Control | 38.8 ± 3.6a | 297.8 ± 51.5bcd | 19.3 ± 0.7h | 20.5 ± 0.4g | 13.8 ± 0.3c | 47.0 ± 7.1hij | 3.6 ± 0.9abc | 18.5 ± 2.4ab |
| Drought | 26.9 ± 0.0a | 483.5 ± 14.5d | 16.6 ± 0.0bcd | 17.7 ± 0.0b | 26.8 ± 4.8d | 80.7 ± 8.5k | 6.3 ± 1.3abcd | 31.3 ± 7.2abcde | ||
| Variation | −11.9 | +185.7 | −2.7 | −2.8 | +13.0 | +33.8 | +2.6 | +12.9 | ||
| 1038 | Control | 58.8 ± 38.8ab | 231.2 ± 30.2ab | 18.1 ± 0.4fg | 19.8 ± 0.3efg | 7.4 ± 1.5abc | 7.5 ± 1.2ab | 9.3 ± 2.2cde | 37.9 ± 5.3de | |
| Drought | 11.5 ± 9.4a | 290.6 ± 68.2abc | 17.6 ± 0.9defg | 19.3 ± 1.0cdefg | 4.4 ± 1.4a | 4.4 ± 1.2a | 11.1 ± 3.4def | 28.6 ± 3.5abcde | ||
| Variation | −47.3 | +59.43 | −0.5 | −0.5 | −3.1 | −3.1 | +1.8 | −9.3 | ||
| 2927 | Control | 5.8 ± 3.2a | 180.6 ± 11.0ab | 17.9 ± 0.1efg | 19.9 ± 0.3efg | 13.3 ± 1.4bc | 12.9 ± 2.1abc | 4.8 ± 1.8abc | 41.6 ± 4.8e | |
| Drought | 13.5 ± 4.4a | 227.4 ± 57.5ab | 16.8 ± 0.1bcde | 18.2 ± 0.4bc | 29.1 ± 5.9d | 29.0 ± 5.3abc | 7.9 ± 3.7cd | 35.9 ± 0.7de | ||
| Variation | +7.7 | +46.8 | −1.1 | −1.7 | +15.8 | +16.1 | +3.0 | −5.7 | ||
| 3126 | Control | 53.3 ± 8.0ab | 301.2 ± 41.1bcd | 17.1 ± 0.3cdef | 19.0 ± 0.3cdef | 10.6 ± 3.5abc | 23.6 ± 0.9cdef | 6.9 ± 3.0bcd | 25.8 ± 1.5abcd | |
| Drought | 44.3 ± 33.6ab | 446.2 ± 60.3cd | 14.9 ± 0.2 a | 16.0 ± 0.5a | 15.2 ± 0.2c | 31.4 ± 1.9defg | 4.6 ± 0.8abc | 31.5 ± 5.6abcde | ||
| Variation | −9.1 | +145.0 | −2.2 | −3.0 | +4.7 | +7.8 | −2.4 | +5.7 | ||
| CAN | 2937 | Control | 40.2 ± 27.2a | 178.9 ± 38.1ab | 17.8 ± 0.5efg | 20.0 ± 0.6efg | 5.0 ± 1.1ab | 36.5 ± 3.2efgh | 0.8 ± 0.3a | 21.0 ± 2.5abc |
| Drought | 31.0 ± 0.0a | 250.8 ± 6.8ab | 16.4 ± 0.0bc | 18.6 ± 0.0bcd | 7.7 ± 0.0abc | 49.2 ± 6.5hij | 1.1 ± 0.4a | 18.3 ± 2.4a | ||
| Variation | −9.2 | +71.9 | −1.3 | −1.4 | +2.7 | +12.7 | +0.3 | −2.7 | ||
| 2938 | Control | 54.8 ± 24.1ab | 107.4 ± 14.7a | 18.0 ± 0.2fg | 19.7 ± 0.2defg | 12.3 ± 3.3abc | 54.4 ± 2.7j | 1.7 ± 0.8ab | 32.3 ± 3.7bcde | |
| Drought | 26.3 ± 7.1a | 261.0 ± 44.4abc | 15.8 ± 0.0ab | 17.5 ± 0.1b | 7.1 ± 3.6abc | 39.2 ± 5.5ghi | 1.6 ± 1.3ab | 25.6 ± 0.8abcd | ||
| Variation | −28.6 | +153.6 | −2.2 | −2.2 | −5.2 | −15.2 | −0.2 | −6.7 | ||
| GUI | 3124 | Control | 6.6 ± 0.5a | 287.5 ± 96.1abc | 18.6 ± 0.2gh | 20.2 ± 0.5fg | 10.1 ± 1.6abc | 53.2 ± 9.1ij | 5.0 ± 1.6abc | 31.2 ± 6.7abcde |
| Drought | 21.5 ± 1.7a | 295.2 ± 145.4abcd | 18.3 ± 0.3gh | 20.3 ± 0.4g | 12.5 ± 3.7abc | 38.2 ± 5.1fgh | 16.5 ± 2.2f | 26.8 ± 3.0abcd | ||
| Variation | +14.9 | +7.8 | −0.4 | +0.1 | +2.4 | −15.0 | +11.4 | −4.4 | ||
| 3125 | Control | 98.1 ± 13.5b | 132.5 ± 78.2ab | 18.1 ± 0.4fg | 19.8 ± 0.3defg | 15.2 ± 1.9c | 18.9 ± 4.9abcd | 5.1 ± 1.2abc | 35.1 ± 8.2de | |
| Drought | 50.5 ± 28.7ab | 175.1 ± 73.2ab | 17.6 ± 0.3defg | 18.9 ± 0.4cde | 9.8 ± 3.3abc | 22.0 ± 2.4bcde | 14.2 ± 1.3ef | 32.5 ± 5.7cde | ||
| Variation | −47.7 | +42.6 | −0.4 | −0.9 | −5.4 | +3.2 | +9.2 | −2.6 | ||
| Mean | Control | 44.6 | 214.6 | 18.1 | 19.9 | 11.0 | 31.8 | 4.7 | 30.4 | |
| Drought | 28.2 | 303.7 | 16.7 | 18.3 | 14.1 | 36.8 | 7.9 | 28.8 | ||
| Variation | −16.4 | +89.1 | −1.4 | −1.6 | +3.1 | +5.0 | +3.2 | −1.6 | ||
Overall, the ABA mean content in the shoots was almost 5-fold higher than in the tubers for control and reached almost 11 folds under drought. At the same time, the ABA mean content decreased by 37% in the tubers, from 45 to 28 ng g−1, meanwhile it increased by 41% in the shoots, from 215 to 304 ng g−1, under water scarcity.
The variation of ABA-shoot content submitted to drought ranged from 175.1 (acc. 3125) to 483.5 ng g−1 (acc. 1036). The variations of ABA-tuber content were between 11.5 (acc. 1038) and 50.5 ng g−1 (acc. 3125). The highest values of ABA content were recorded in the shoots, but the highest variations were observed in the tubers, with 4.4-folds (drought) and 16.9-folds (control), respectively.
The analysis of ABA variation in tubers shows that acc. 3125 holds a significant highest content under both control and stress variants, decreasing during stress from 98 to 51 ng g−1 (−48%). However, the highest decrease was observed in the acc. 1038, where ABA-tuber content changes from 59 to 12 ng g−1 (−80%) under stress. That is, under stress, the ABA content varied according to the following series: acc. 3125 (−48 ng g−1), 1038 (−47 ng g−1), 2938 (−29 ng g−1), 1036 (−12 ng g−1), 2937 (−9 ng g−1), 3126 (−9 ng g−1), with acc. 2927 (+8 ng g−1) and 3124 (+15 ng g−1) as exceptions. These last two accessions had the lowest ABA-tuber content in control conditions, but were the only ones that recorded an ABA increase when submitted to drought, from 6 to 14 ng g−1 (133%), and 7 to 22 ng g−1 (214%), respectively.
Still, in the shoots, the acc. 1036 had significantly the highest variation of ABA content between control and stress conditions, increasing from 298 to 484 ng g−1 (+62%). Meanwhile, the ABA-shoot suffers the lowest variation in acc. 3124, only ranging from 288 to 295 ng g−1 (+2%). Contrariwise, acc. 3125 showed the lowest ABA-shoot content under stress, with 175 ng g−1. The ABA variation in shoots, due to stress, increased according to the following series: acc. 3124 (+7.8 ng g−1), 3125 (+42.6 ng g−1), 2937 (+46.8 ng g−1), 1038 (+59.5 ng g−1), 2937 (+71.9 ng g−1), 3126 (+145.0 ng g−1), 2938 (+153.6 ng g−1) and 1036 (+185.7 ng g−1).
Δ13C and OA in root tubers and shoots during drought
Table 2 shows the variation of carbon isotope discrimination (Δ13C) and OA content in the control and experimental (stress) variants.
Water scarcity slightly decreased the Δ13C content in both organs. On average, drought decreased Δ13C-shoot from 20 to 18‰ (−10.0%), while the Δ13C-tuber decreased from 18 to 17‰ (−6%). The acc. 3124 was the exception, by slightly increasing the Δ13C-shoot during drought, but remaining at 20‰ under both experimental conditions. However, acc. 1036 had the highest Δ13C-tuber content but also exhibited the highest loss due to drought, ranging from 19 to 17‰ (−11%). Conversely, both the acc. 1038 and 3124 showed the lowest difference and significantly higher Δ13C content in both organs under both experimental conditions. The acc. 3126 showed the lowest Δ13C content in both organs under drought stress, displaying 15‰ for Δ13C-tuber and 16‰ for Δ13C-shoot.
The OA content in shoots was about threefold higher than in the tubers, under both experimental conditions. Also, the OA variation on average is higher in tubers than in the shoots. On average, the OA in tubers and shoots increased, respectively, from 11 to 14 mg 100 g−1 (+27%), and from 32 to 37 mg 100 g−1 (+16%), between control and drought stress. However, in the acc. 1038, the OA content decreased to the same extend in both organs, clocking the same response to drought at 4 mg 100 g−1. Inversely, acc. 1036, 2927, 3126 and 2937 increased the OA content in both organs under both conditions. The acc. 1036 showed significantly higher OA content, reaching 27 mg 100 g−1 for OA-tuber and 81 mg 100 g−1 for OA-shoot under water scarcity conditions.
WUE and CCI content variation to drought
Data regarding the whole-plant WUE and the CCI from shoots are presented in Table 2. On average, drought led to a 60% increase of WUE, from 5 to 8 g l−1. The acc. 1038 showed significantly higher WUE (9.34 g l−1) under control conditions. The acc. 3124 exhibited the highest WUE range, from 5 to 17 g l−1 (+240%) under drought. Contrariwise, acc. 2937 had the lowest WUE under both experimental environments, ranging between 0.77 and 1.09 g l−1 (+42%). Meanwhile, acc. 3126 and 2938 were the only ones that decreased WUE under drought conditions.
Drought decreased the CCI by 3% on average, from 30 to 29. The acc. 1036 and 3126 were the only ones that increased the CCI during drought, from 19 to 31 (+63%) and 26 to 32 (+23%), respectively. However, acc. 2927, 3124 and 3125 decreased the CCI under drought, but they managed to keep the highest chlorophyll content under both experimental conditions. Accession 1038 recorded the highest CCI decrease under drought, from 38 to 29 (−24%), meanwhile acc. 2937 showed the smallest CCI among all accessions, ranging from 21 to 18 (−14%).
Variance and parameters associations
Significant differences (P ≤ 0.01) were recorded between the eight sweet potato accessions (cases) by the one-way anova and Tukey hsd multiple comparisons between control and drought environments for ABA, CCI, Δ13C, OA and WUE (Table 2). Nine significant correlations (P ≤ 0.05) were detected among the five variables in the study, using the Pearson correlation coefficient (Table 3). One strong significant correlation was observed between Δ13C-shoot and Δ13C-tuber (r = 0.92). Modest correlations were registered between ABA-shoot with Δ13C-shoot (r = −0.45), Δ13C-tuber (r = −0.40), OA-shoot (r = 0.36) and with ABA-tuber (r = −0.30). Other moderate correlations between OA-shoot with OA-tuber (r = 0.39) and WUE (r = −0.33) were also detected. Similarly, the OA-tuber with CCI (r = 0.32) and with Δ13C-shoot (r = −0.32) were moderately correlated.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. WUE | – | ||||||
| 2. ABA-tuber | −0.11 | – | |||||
| 3. OA-tuber | −0.12 | −0.09 | – | ||||
| 4. Δ13C-tuber | 0.23 | 0.03 | −0.19 | – | |||
| 5. CCI | 0.24 | −0.04 | 0.32* | 0.01 | – | ||
| 6. ABA-shoot | 0.07 | −0.30* | 0.26 | −0.40** | −0.12 | – | |
| 7. OA-shoot | −0.33* | −0.15 | 0.39** | −0.08 | −0.28 | 0.36* | – |
| 8. Δ13C-shoot | 0.15 | 0.01 | −0.32* | 0.92** | −0.06 | −0.45** | −0.15 |
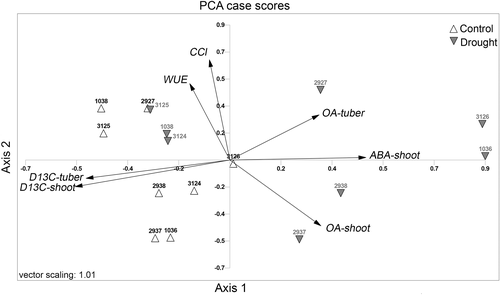
The data from cases (samples) and variables (traits) were transformed into linearly uncorrelated variables through a PCA (Fig. 1). The PCA analysis explained 84.4% of total cumulative variance along with four principal components, i.e. axis. The first two axis explained 54.6% of the cumulative variance. The axis 1 had 34.1% of variance with eigenvalues of 2.7, while the axis 2 had 20.5% of variance with eigenvalues of 1.6. The ABA-shoot, Δ13C-shoot and Δ13C-tuber were the main variables that show a strong correlation relative to axis 1; meanwhile, the CCI and WUE variables were highly correlated with axis 2. The accessions spatially distributed near to the end of the main variable vectors, as referred above for both axis, are the ones with the highest variability. When the main variable vectors are in opposed directions, it can show a reverse relation between them. For example, the drought-stressed acc. 1036 and 3126 were situated at the end of the ABA-shoot variable vector, which was in the opposed direction relative to the Δ13C-shoot and Δ13C-tuber vectors. It showed that their spatial distribution associated them, among all acc., as the ones with the highest ABA-shoot, and with the lowest Δ13C-shoot and Δ13C-tuber for drought conditions. As both accessions showed the highest spatial distance between the control and drought variants, they apparently also denoted the highest sensitivity to drought. On the other hand, acc. 3124, 3125 and 1038 shared practically the same spatial distribution between control and drought environments, displaying a more efficient response to drought.
Discussion
ABA-shoot and ABA-tuber interaction at drought
ABA naturally accumulates during plant growth and its accumulation is enhanced under stress conditions (Nakatani and Komeichi 1991). When plants experience water scarcity, they respond with ABA-shoot accumulation by initiating an adaptive response through the regulation of the plant water status as a strategy to survive under the hostile conditions (McAdam et al. 2016). In fact, we observed an ABA-shoot accumulation in all accessions tested in our study.
Under control conditions, the content of ABA-shoot was approximately fivefold higher than ABA-tuber, which agrees with Li and Jia (2014) findings. These authors argued that under stress-free conditions, plant leaves usually show a much higher ABA content than the roots and stems, due to a higher ABA content located in the chloroplasts (up to 68% of total ABA). The 5-fold difference registered between the shoot and tuber organs increased up to 11-fold under drought stress. This increase could be attributed to the general ABA accumulation in sweet potato shoots in detriment to the decrease of ABA in tubers as a response to drought. In case the underground organs are unable to synthesize ABA during drought conditions, either because of biochemical inability or collapse of carotenoid precursor reserves (β-carotene), it was found that plants could have a normal increase in foliar ABA level, with normal stomatal responses to drought (Danquah et al. 2013, McAdam et al. 2016). The significant negative correlation between ABA in both organs allow us to corroborate the presence of a root-to-shoot ABA signaling path in sweet potato during water privation (Mengel et al. 2001, Osakabe et al. 2014, Salehi-Lisar and Bakhshayeshan-Agdam 2016). The drought is signalized to the shoots, where the signal is amplified in the leaf chloroplasts to accumulate ABA (Mengel et al. 2001, Osakabe et al. 2014, McAdam et al. 2016, Salehi-Lisar and Bakhshayeshan-Agdam 2016, Wani et al. 2016). However, the increase of the ABA-shoot biosynthesis can lead to a higher ABA catabolism due to the depletion of the ABA xanthophyll precursors. Drought may compromise the delivery of ABA precursors from the shoots to the roots, which could be one of the reasons for the decrease of ABA-tubers content (Li and Jia 2014, McAdam et al. 2016). We reported that only two accessions (2927 and 3124) increased the ABA-tuber during stress. This suggests that both exhibit a dynamic equilibrium between ABA biosynthesis and catabolism, which remains in agreement with the conclusions of Li and Jia (2014). Taking into consideration reports of Duman (2012) and Tuberosa (2012), we hypothesize that the ABA-tuber increase in acc. 2927 and 3124 could have facilitated the plant water uptake into the roots during drought, which also led to a better diffusion of nutrients taken from the soil into the plant. Such a pattern of response to drought was not present in the remaining accessions which show a decreased ABA-tuber content. However, the increasing levels of ABA in maize roots during drought conditions stimulated root elongation and hence facilitated the pursuit of water and nutrients in surrounding soil environments (Sah et al. 2016). However, our observations do not support Sah et al. (2016) findings in maize plants, since the reduction of sweet potato total biomass appeared to be a way for them to cope with water stress (Gouveia et al. 2019). Likewise, Lau et al. (2018) observed a downregulation of tissue growth with higher ABA accumulation in in vitro sweet potato plantlets. Jarret and Gawel (1991) also demonstrated that sweet potato plantlets exposed to four concentrations of ABA, ranging from 0.01 to 10 mg l−1 (i.e. 10–10 000 ng g−1), had lag development of root and axillary buds from nodal segments. Considering that we dealt with fully grown tubers and shoots that were subjected to a long water scarcity period, the ABA values naturally varied among the accessions and their organs. Although, the highest ABA values obtained for the shoots (484 ng g−1 for acc. 1036) and for tubers (51 ng g−1 for acc. 3125) under drought were not significantly correlated with the total plant biomass loss (data not shown). The ABA accumulation in response to water scarcity stress ought to be considered merely a conjecture of the mechanism of plants' resilience to water stress. We incline to hypothesize that ABA was predominantly involved in the regulation of plant water status in association with the other traits investigated in this study rather than playing a direct role in the downregulation of tissue growth during drought. However, our results also show that ABA evolvement depends on accessions skills, because they demonstrated to have differentiated behavior under drought.
ABA variation and its relationship with other drought tolerance traits
ABA modulates the plant responses including the regulation of stomatal opening, growth and development, and contributes to the stress signal transduction pathways during drought (Ramakrishna and Ravishankar 2014). Under normal conditions of ample water availability, stable ABA content delays leaf senescence and allows cumulative photosynthesis and transpiration processes over the crop cycle (Tardieu and Davies 1992, Tuberosa 2012, Osakabe et al. 2014). Open stomata allows higher photosynthesis activity and higher CCI content, which improves the carboxylation fractionation due to an equilibrate CO2 diffusion in leaf chloroplasts, with Δ13C values reaching near 31‰ (or δ13C = −38‰; O'Leary 1993, Osakabe et al. 2014, Shao et al. 2015). The higher the plant transpiration, the greater is the water potential gradient between the root tubers and shoot cells. The scarce water availability in the roots reduces the water status in shoots, i.e. the ABA increased content in the leaves induces stomata closure to reduce transpiration as a water-conserving mechanism (Tardieu and Davies 1992, Osakabe et al. 2014). Reduced transpiration rates compromise nutrient transport from the roots to the shoots, while nutrient stress could be another possible factor stimulating the accumulation of ABA (Firon et al. 2009, Duman 2012). Lower transpiration reduces water loss and leads to WUE increase (Black et al. 2015). Nonetheless, the stomatal closure restricts the photosynthetic activity and constrains the leaf CO2 uptake, which leads to a decrease of carboxylation fractionation and drives to Δ13C values near to 4‰ (or δ13C = −12‰; O'Leary 1993).
We observed that the ABA-shoot increase was significantly correlated with Δ13C-tuber decrease, and both factors were connected to stomatal closure. The available data of Δ13C, CCI, and WUE point to a partial closure of the stomata in sweet potato as a response to drought. Lau et al. (2018) also showed that in vitro sweet potato plantlets registered an ABA level increment during drought, leading to stomatal closure to avoid water loss by transpiration. The CCI decrease could indicate that drought slightly restricted the sweet potato photosynthesis activity, while the Δ13C decrease indicates that the carboxylation fractionation was lowered due to lower leaf CO2 availability (Farquhar et al. 1989, O'Leary 1993, Shao et al. 2015, Zhang et al. 2015, Gouveia et al. 2019). Both organs decreased Δ13C due to drought, with the amount of Δ13C-shoot slightly greater than Δ13C-tuber in both control and drought conditions, which was also observed by Zhang et al. (2015). This indicates that the Δ13C-tuber still had a greater 13C fixation relatively to Δ13C-shoot as a result of the transport of photo-assimilates from shoots to tubers, which improved plant growth during drought stress (Wegener et al. 2015, Zhang et al. 2015).
The partial closure of stomata could be also connected to the increment of oxalic acid, affecting the ABA signal to induce stomatal closure. With the increase of oxalic acid content, the shoot accumulates osmotically active molecules that induce the stomatal opening instead of closing it as signaled by ABA (Guimarães and Stotz 2004). We found that OA-shoot increased during drought, which was significantly correlated with ABA-shoot accumulation. It may suggest that the ABA capacity to induce stomata closure was affected. The biosynthesis of OA organic osmolytes is the key for plant resistance to drought, helping to resist prolonged harsh water scarcity and permitting a better recovery upon rehydration (Tuberosa 2012). Plant accumulation of OA occurs from the glycolate oxidized in glyoxylate derived from photosynthesis activity, which is then oxidized into oxalic acid (Igamberdiev and Eprintsev 2016). However, drought slightly downregulated photosynthesis due to the CCI decrease, perchance to protect the chloroplasts from photoinhibition and subsequent oxidative damage (Prasad et al. 2008, van Heerdena and Laurie 2008, Osakabe et al. 2014). Nonetheless, the partial stomatal closure still allowed for reasonably high CCI and organic osmolytes synthesis and permitted the increase of WUE during drought to minimize biomass loss.
The plants that were the most resilient to drought had greater ABA-shoot production, higher photosynthesis activity, higher chlorophyll content and increased WUE (Tardieu and Davies 1992, Tuberosa 2012, Black et al. 2015, Lau et al. 2018). Taking into consideration all studied traits, the acc. 3124 could be considered the most resilient to drought, followed by accessions 3125 and 1038. The acc. 3124 managed to retain high CCI content and a good carboxylation fractionation resulting from an equilibrate CO2 diffusion in leaf chloroplasts, with minimal Δ13C difference. This may point to a good photosynthesis rate under both experimental conditions. This acc. also had the highest Δ13C-tuber content, an indicator of efficient tuber 13C fixation and growth during drought. It maintained a balanced OA equilibrium in both organs and showed the best WUE under both experimental conditions. Finally, a high ABA production sustains in both organs in response to stress, allowing for a better osmotic and nutrient equilibrium.
Conclusions
Using multivariate analysis, analysis of variance and correlations, it was shown how the differences of ABA content in eight sweet potato accessions grown under water scarcity were related to the CCI, Δ13C, OA and WUE. Drought triggered ABA-shoots biosynthesis, while it significantly decreased the ABA-tubers content. The ABA-shoot accumulation was correlated with Δ13C-shoot and OA decrease. The ABA-shoot increase seemed to be a root-to-shoot ABA signaling attempt to cope with water stress by stomatal closure, which was directly related to the Δ13C and CCI decrease, and higher WUE. The presence of OA-shoot may have affected the intensity of the ABA-shoot signal in stomatal closure, contributing to only a partial stomatal closure during the harsh water scarcity conditions. These combined factors appeared to be suitable tools to identify sweet potato accessions with higher resilience to drought. Among all accessions in the study, acc. 3124 exhibited the best trait combination in response to water scarcity. Therefore, it should be considered as a potential candidate for sweet potato drought tolerance improvement programs, that can be used in culture adaptation to climate changes.
Author contributions
C.G. participated to the drought assay and samples preparation, performed the ABA and oxalic analysis, interpreted and summarized all data generated from those experiments, and wrote the manuscript. J.G. quantified the WUE and helped C.G. in CCI. J.S. coordinated the δ13C analysis. V.L. and M.C. coordinated the work and revised the manuscript.
Acknowledgements
The authors thank J.G.R. de Freitas and H.G.M. de Nóbrega from the ISOPlexis Genebank from the Madeira University (Portugal) who provided valuable assistance with the management of the field trials and meaningfully contributed to the harvest and samples preparation. This work was supported by the Programa Operacional Madeira 14–20, Portugal 2020 and the European Union through the European Regional Development Fund (grant number M1420-01-0145-FEDER-000011, CASBio), and Agência Regional para o Desenvolvimento da Investigação Tecnologia e Inovação, Portugal 2020 and the European Union through the European Social Fund (grant number M1420-09-5369-FSE-000001, ARDITI).
Open Research
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




