Impact of exogenously applied trehalose on leaf biochemistry, achene yield and oil composition of sunflower under drought stress
Abstract
This study was carried out to assess the influence of trehalose, a non-reducing disaccharide involved in improving plant stress tolerance, on two cultivars (Hysun 33 and FH 598) of sunflower (Helianthus annuus L.) grown under control and drought stress conditions. At pre-flowering stage, varying concentrations (10, 20 and 30 mM) of trehalose were applied to the foliage. Drought stress significantly suppressed the plant growth, total soluble proteins, chlorophyll, achene yield per plant, oil percentage, organic contents, as well as oil palmitic and linoleic acids in both sunflower cultivars. External application of trehalose significantly reduced RMP (relative membrane permeability), and the accumulation of H2O2 (hydrogen peroxide), while a considerable improvement was recorded in shoot fresh and shoot and root dry weights, total soluble proteins, glycinebetaine, AsA (ascorbic acid), total phenolics, achene yield per plant, oil contents, inorganic and organic contents, and the activities of catalase (CAT), superoxide dismutase (SOD) and peroxidase (POD) enzymes under water-limited regimes. The cultivar Hysun 33 was superior to the other cultivar in plant growth, RMP, glycinebetaine, proline, achene yield per plant, oil contents, and palmitic and linoleic acids. Overall, foliar-applied trehalose improved plant growth, oxidative defense system, yield and oil composition of sunflower under drought stress conditions.
Abbreviations
-
- AsA
-
- ascorbic acid
-
- CAT
-
- catalase
-
- EC
-
- electrical conductivity
-
- GB
-
- glycinebetaine
-
- H2O2
-
- hydrogen peroxide
-
- MDA
-
- malondialdehyde
-
- OD
-
- optical density
-
- PEG
-
- polyethylene glycol
-
- POD
-
- peroxidase
-
- RMP
-
- relative membrane permeability
-
- ROS
-
- reactive oxygen species
-
- SOD
-
- superoxide dismutase
-
- TBA
-
- thiobarbituric acid
-
- TCA
-
- trichloroacetic acid
Introduction
Major environmental stresses such as drought, salinity and temperature impose a negative impact on plant's growth and development, ion uptake, primary and secondary metabolites, and oxidative system. Of them, drought is the most injurious one, because it markedly impairs growth and development of crops and their yield (Ashraf et al. 2011, Rabert et al. 2014, Akram et al. 2016). Drought stress influences adversely the rate of photosynthesis, water transport and nutrition system, and formation of seeds (Aslam et al. 2013). Several studies show that drought stress affects plant growth due to several factors including a decrease in uptake and accumulation of inorganic nutrients, e.g. Ca, K, N and P. Drought stress also perturbs the synthesis of vital photosynthetic pigments including carotenoids and chlorophylls a and b (Akram and Ashraf 2011, Shafiq et al. 2014). Furthermore, water deficiency exacerbates the negative effects of other stresses such as high salinity and temperature (Ashraf 2010, Ashraf et al. 2013).
Drought stress causes the production of ROS (reactive oxygen species) that are very injurious to cellular membrane structure, which in turn impairs the mechanism of photosynthesis (Fariduddin et al. 2009). The free radicals of ROS cause lipid peroxidation, inactivate some key enzymes, and destroy the nucleic acids resulting in cell/tissue death (Ahmad et al. 2007, Ashraf 2009). Under oxidative stress conditions, plants adopt a variety of scavenging mechanisms such as the activation of antioxidant enzymes (catalase, superoxide dismutase, peroxidase, etc.), and formation of antioxidant metabolites (ascorbic acid, glutathione, phenolics, etc.) which can effectively counteract the drought-induced ROS.
Organic compounds including proline, glycinebetaine (GB), sugars, and sugar alcohols accumulate in various cellular compartments and act as compatible solutes or osmolytes to protect them from the injuries of drought stress (Kamran et al. 2009, Akram et al. 2016). Among the different sugars involved in stress tolerance, trehalose is considered as the most important one. It is a stable non-reducing disaccharide that is found commonly in bacteria, fungi, animals and plants (Trevelyan and Harrison 1956, Chen and Haddad 2004, Iturriaga et al. 2009, Vandesteene et al. 2010, Lunn et al. 2014, Shafiq et al. 2015). Numerous studies have shown that trehalose has the potential to minimize the effects of environmental stresses, particularly water deficiency and salinity (Fatemeh et al. 2012). Trehalose has a very important role in carbon assimilation, carbohydrate reservoir, energy source and osmoprotection in plants under abiotic stresses (Lunn et al. 2014). For example, foliar-applied trehalose significantly enhanced the growth of Brassica plants exposed to drought stress induced by PEG (polyethylene glycol) (Alam et al. 2014). In another study with radish, Shafiq et al. (2015) assessed the effect of both foliar and pre-sowing modes of external application of trehalose at the rate of 25 and 50 mM. They found that foliar-applied trehalose considerably enhanced the oxidative defense system in the edible part of radish. The external use of trehalose proved helpful in minimizing the adverse effects of an abiotic stress on plants (Ma et al. 2013).
Sunflower is an important crop due to its considerably important vegetable oil (Cancalon 1971). Chemically, sunflower oil comprises four beneficial fatty acids, namely linoleic, palmitic, stearic and oleic acids (Baydar and Erbas 2005). Moreover, it contains vitamins, micronutrients and antioxidants, which preserve the cells from oxidative damages. The sunflower oil also contains alpha-tocopherol that plays an important role against cardiovascular diseases (Kirsh et al. 2006). The sunflower oil is very low in cholesterol and consists of saturated and unsaturated fatty acids (Francois 1996, Akram and Ashraf 2011). Sunflower is a drought sensitive crop (Tyagi et al. 2018) that requires three optimized irrigations after the flowering stage to ensure a reasonable sunflower seed yield (Kaya et al. 2018). Moreover, in the absence of seasonal precipitation, at least one additional irrigation is thought to be required (Göksoy et al. 2004). Considering its need for water, sunflowers are often subjected to drought stress. Trehalose is considered effective in ameliorating the adverse effects of water deficiency. So, it was hypothesized that exogenous application of trehalose could improve growth, achene yield and oil composition of sunflower plants when subjected to drought stress conditions. The primary objective of this study was to assess the effect of foliar application of trehalose on growth, chlorophyll pigments, secondary metabolites, antioxidative defense system and yield components of drought-stressed sunflower plants. Furthermore, it was assessed whether or not foliar-applied trehalose was effective in improving the growth, oil yield and composition of sunflower plants exposed to water stress.
Materials and methods
Plant and growth conditions
Seeds of two cultivars (X and Y) of Helianthus annuus L. were planted in a field (District Jhang, Punjab, Pakistan) in Mid-February and growth continued up to Mid-May 2016. Soil texture was sandy loam having nitrogen, 70 mg g−1 soil; phosphorus, 7.13 mg g−1 soil; potassium, 167.57 mg g−1 soil; pH, 8.2; EC, 1.7 dS m−1; 35% saturation percentage 0.89% and organic matter. In this area, the average rainfall calculated was 185 mm during the study period. The trial was arranged in a completely randomized block design including two main plots (control and drought treatments) with four subplots each replicated four times. The distance between two plants was 50 cm. During the entire growth period, the sunflower plants were subjected to two irrigation regimes: control (normal watering plots received 10 irrigations) and drought-treated (six irrigations). Sunflower achenes were soaked in distilled water for 2 h before being sown 1 cm deep in the soil by the hand-drill method. During the whole period of growth, thinning and weeding of the crop were regularly done. After 50 days of seedling emergence, and at the pre-flowering stage, three levels of trehalose (10, 20 and 30 mM) along with control (0 mM) mixed with Tween-20 (0.1%) were sprayed, with a plastic sprayer, to the leaves of all plants. Fifteen milliliter per plant of each concentration of trehalose were applied as a foliar spray. Two weeks after the trehalose spray, two plants from each replicate were harvested to determine plants growth. The fresh weights (shoots and roots separately) of the two plants were recorded after washing them well with water. Then they were placed in an oven at 65°C for 72 h and their dry weights recorded. Average of these two plants were used to overcome human handling error. The remaining plants from each replicate were used for all others biochemical analyses. When the plants had mature capitula, the achenes were plucked and used to analyze yield, oil and related parameters. During the whole study, the following attributes were measured.
Relative membrane permeability (RMP)
The fully expanded third leaf from each plant was cut into 1 cm2 discs that were placed in test tubes each containing 20 ml deionized distilled water. After vortexing the samples for 30 s, initial electrical conductivity (EC0) of each sample was measured using an EC meter. The samples were then stored at 4°C for 24 h and the conductivity (EC1) was measured again. The samples were then autoclaved for 10 min, cooled to room temperature and the conductivity was measured for the third time (EC2). The relative permeability of cell membrane was calculated using the formula proposed by Yang et al. (1996).
Chlorophyll (a, b) contents
The third fresh leaf from the top (0.15 g) was triturated in 5 ml of 80% acetone as described by Arnon (1949). The absorbance of the supernatants was read at 663, 645 and 480 nm using UV–visible spectrophotometer.
Free proline determination
The third fresh leaf from the top (0.25 g) was ground in a pestle and mortar containing 5 ml of 3% sulfosalicylic acid following Bates et al. (1973). The reading of the extracted sample was taken at 520 nm using a spectrophotometer.
Glycinebetaine (GB) content
The third fresh leaf from the top (0.25 g) was ground in 5 ml distilled water and left it for overnight at 4°C following the protocol of Grieve and Grattan (1983), GB contents were measured at 365 nm.
Hydrogen peroxide
Fresh leaf (0.25 g of the third leaf) was ground and hydrogen peroxide was extracted using 2.5 ml trichloroacetic acid (TCA; 0.1%) solution following Velikova et al. (2000). The samples were placed in ice and centrifuged for 15 min at 12 000g. To a sample aliquot of 0.5 ml derived from the supernatant, 0.5 ml of buffer (potassium phosphate) and 1.0 ml of KI were added. The absorbance of each sample was read at 390 nm.
Malondialdehyde (MDA)
Malondialdehyde contents were estimated following Cakmak and Horst (1991). Fresh leaf (0.25 g of the third leaf) was homogenized in 2.5 ml of 5% TCA solution. After this, the samples were centrifuged for 15 min at 15 000g. One milliliter of the supernatant was treated with 4 ml of 0.5% thiobarbituric acid (TBA). All samples were heated in a water bath at 95°C and cooled in an ice bath before recording the absorbance.
Ascorbic acid (AsA)
Fresh leaf (0.12 g) was ground in 6% TCA solution following the method of Mukherjee and Choudhuri (1983). The extract (2.0 ml) was treated with 1.0 ml of 2% dinitrophenyl hydrazine in 9 N H2SO4 and 1.0 ml of thiourea (10%). All samples were heated for 1 h at 60°C and cooled before adding 2.5 ml of 80% H2SO4 and OD was measured.
Total phenolics
Fresh leaf (0.1 g of the third leaf) was ground in 5 ml of 5% acetone in an ice bath. To 0.1 ml of the extract were added 2 ml of distilled water and 1 ml of the Folin–Ciocalteu's phenol reagent. The mixture was shaken and 5 ml of 20% Na2CO3 were added to it and then total volume (20 ml) was prepared by adding distilled water as described by Julkunen-Tiitto (1985). The samples were measured at 750 nm to determine total phenolic content.
Total soluble proteins
Fresh leaf (0.1 g of the third leaf) was ground using phosphate buffer in an ice bath. Then, 0.1 ml of the mixture was mixed with 2 ml of the Bradford reagent in test tubes and then OD was read at 595 nm (Bradford 1976).
Estimation of antioxidant enzymes
Fresh leaf (0.5 g of the third leaf) was ground in 10 ml of buffer (sodium phosphate 50 mM, pH 7.8) using a pestle and mortar.
Superoxide dismutase (SOD): The supernatant (50 μl) was taken in a cuvette and 400 μl of distilled water, 250 μl potassium phosphate buffer, 10 μl methionine, 100 μl of Triton-X, 50 μl of NBT and 50 μl of riboflavin were added. The samples were placed in light for 15 min as described by van Rossum et al. (1997) before measuring the absorbance at 560 nm using a spectrophotometer.
Catalase (CAT)
Following the protocol of Luck (1974), 1.9 ml of potassium phosphate buffer was mixed with 1.0 ml of 5.9 mM hydrogen peroxide. Then, 0.1 ml of the enzyme extract was added to the mixture. The OD was measured at 240 nm using a spectrophotometer.
Peroxidase (POD)
The plant extract (50 μl) was mixed in 1.8 ml phosphate buffer (pH 7.0), 100 μl of hydrogen peroxide (H2O2) and 100 μl of guaicol (20 mM). The absorbance was recorded at 400 nm following the method of Chance and Maehly (1955).
Quantification of the yield per plant
The achenes of the mature sunflower plants were plucked from each head and achene yield per plant (g) was recorded.
Achene analyses
Organic and inorganic contents

Extraction of oil
Oil was extracted from the sunflower achenes following the IUPAC Paquot (1979) method. The Soxhlet apparatus and n-hexane (Merck, HPLC grade) as an extractant were used for extracting the oil. The volume of n-hexane (MW 86.18) used was 500 ml. The extraction of oil was completed after 10 h and all oil samples were stored in a refrigerator until further analysis.
Refractive index (RI)
An oil drop was placed on a prism and the refractive index was read using a refractometer. The temperature of the prism used to complete this process was kept at 40°C.
Oil contents

Determination of α-tocopherol
The alpha-tocopherol in the oil was determined using an HPLC following the method of Lee et al. (2003) by (i) relating the retention time, (ii) using peak areas and (iii) using the computer SRI peak simple chromatography.
Fatty acid composition
The Paquot (1979) IUPAC standard method was used for preparing fatty acid methyl esters (FAMEs). FAMEs were identified through authentic standards by matching with relative and absolute retention times.
Experimental design and statistical analysis
The experimental design used was randomized block design with four replicates. The MSTAT computer package (MSTATC Statistical Package 1990) was employed for the analysis of variance of data for each variable. The significant difference among the mean values was determined using LSD at 5% probability (Snedecor and Cochran 1980). A correlation analysis was also carried-out among different attributes and values presented in Supporting Information Table S1.
Results
Trehalose application improved plant growth and biomass yield under drought stress
Shoot and root weights (fresh and dry) of both sunflower cultivars significantly decreased (P ≤ 0.001) upon drought stress conditions applied as withholding of irrigation water (Table 1; Fig. 1). Application of trehalose as a foliar spray caused a significant improvement in shoot fresh (P ≤ 0.05) and shoot and root dry weights (P ≤ 0.01; 0.001), but it remained non-significant for root fresh weight. Both sunflower cultivars varied significantly in terms of all growth attributes measured. Of both sunflower cultivars, cv. Hysun 33 performed better than FH 598 in terms of root and shoot weights under both water regimes.
| Source of variations | df | Shoot fresh weight | Shoot dry weight | Root fresh weight | Root dry weight |
|---|---|---|---|---|---|
| Drought stress (D) | 1 | 2823*** | 312.5*** | 1367*** | 9623*** |
| Trehalose (Tre) | 3 | 4437* | 8032** | 3504 ns | 227.2*** |
| Cultivars (Cv) | 1 | 1490*** | 1679*** | 34233*** | 2225*** |
| D × Tre | 3 | 3126 ns | 514.1 ns | 193.0 ns | 20.38 ns |
| D × Cv | 1 | 2168 ns | 382.2 ns | 2400*** | 41.16 ns |
| Tre × Cv | 3 | 2578 ns | 285.9 ns | 177.1 ns | 25.03 ns |
| D × Tre × Cv | 3 | 4588 ns | 155.6 ns | 920.5 ns | 9.865 ns |
- *, ** and *** indicates significant at 0.05, 0.01 and 0.001 levels, respectively.
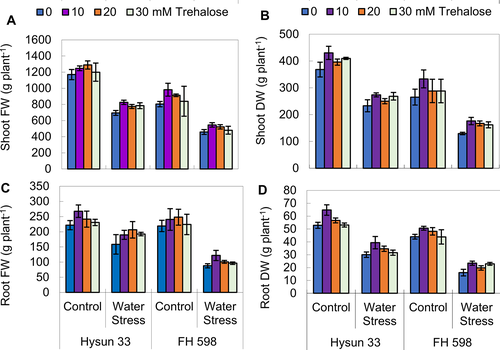
Supplementation of trehalose reduced relative membrane permeability under drought stress
Relative membrane permeability increased significantly (P ≤ 0.001) in both sunflower lines under water stress conditions. The application of trehalose caused a marked reduction in the RMP of both sunflower cultivars. The cultivar Hysun 33 was better in RMP than FH 598 under drought stress regimes (Table 2; Fig. 2).
| Source of variations | df | RMP | Chlorophyll a | Chlorophyll b | GB |
|---|---|---|---|---|---|
| Drought stress (D) | 1 | 2188*** | 0.303*** | 1.409*** | 8750*** |
| Trehalose (Tre) | 3 | 269.7** | 0.053 ns | 0.050 ns | 648.0** |
| Cultivars (Cv) | 1 | 817.3*** | 0.077 ns | 0.111* | 3817*** |
| D × Tre | 3 | 12.16 ns | 0.011 ns | 0.022 ns | 206.8 ns |
| D × Cv | 1 | 10.74 ns | 0.0001 ns | 0.037 ns | 1336** |
| Tre × Cv | 3 | 37.29 ns | 0.007 ns | 0.013 ns | 78.69 ns |
| D × Tre × Cv | 3 | 41.51 ns | 0.006 ns | 0.0294 ns | 109.3 ns |
| Proline | MDA | H2O2 | |||
| Drought stress (D) | 1 | 9.459* | 576.7** | 4069 ns | |
| Trehalose (Tre) | 3 | 2.849 ns | 226.3** | 2190 ns | |
| Cultivars (Cv) | 1 | 8.431* | 66.74 ns | 1271.3 ns | |
| D × Tre | 3 | 1.0137 ns | 28.70 ns | 1709 ns | |
| D × Cv | 1 | 0.247 ns | 0.390 ns | 2379 ns | |
| Tre × Cv | 3 | 0.331 ns | 13.69 ns | 593.8 ns | |
| D × Tre × Cv | 3 | 0.503 ns | 17.12 ns | 58.97 ns |
- *, ** and *** indicates significant at 0.05, 0.01 and 0.001 levels, respectively.
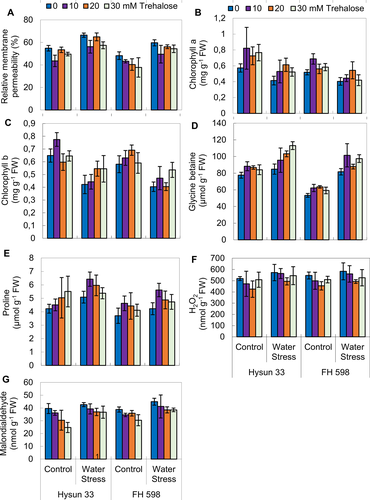
External application of trehalose improved chlorophyll (a, b) contents under drought stress
Drought stress conditions caused a marked reduction in chlorophyll a and b in both sunflower cultivars. No significant change was observed in the accumulation of chlorophyll a and b contents due to foliar-applied varying levels of trehalose in both sunflower cultivars (Table 2; Fig. 2). Both sunflower cultivars showed a similar response for chlorophyll a, whereas Hysun 33 was found better in chlorophyll b content.
Supplementation of trehalose enhanced the leaf free proline and glycinebetaine content under drought stress
Leaf free proline and glycinebetaine accumulation increased significantly in both sunflower lines on exposure to water stress (Table 2; Fig. 2). The trehalose-treated plants depicted a non-significant response for proline, but a significant (P ≤ 0.01) increase in glycinebetaine accumulation was recorded. Of all three levels of trehalose, 10 and 30 mM of trehalose were most effective in enhancing glycinebetaine contents in FH598 and 30 mM was more effective for Hysun 33 under drought stress as compared to the controls. Among both sunflower cultivars, Hysun 33 had a higher proline content, while FH 598 was better in GB accumulation under drought stress conditions.
External application of trehalose decreased accumulation of H2O2 and MDA content under drought stress
No effect of drought stress and trehalose application was observed on the accumulation of hydrogen peroxide (H2O2) in both sunflower cultivars. Both sunflower cultivars showed a similar pattern of accumulation of H2O2 under water stress (Table 2; Fig. 2).
A significant increase was observed in the malondialdehyde contents in both sunflower cultivars under drought stress (Table 2; Fig. 2). The application of trehalose significantly increased the MDA content in both sunflower cultivars subjected to drought stress.
Exogenous application of trehalose enhanced the accumulation of total soluble proteins under drought stress
The total amount of soluble proteins decreased significantly (P ≤ 0.001) in both sunflower cultivars under water stress. Owing to exogenous application of trehalose, a considerable (P ≤ 0.001) improvement was observed in total soluble proteins in drought-stressed sunflower plants. Of all concentrations of trehalose used, 20 mM was more effective for cv. Hysun 33 and 30 mM for cv. FH 598 under drought stress (Table 3; Fig. 3).
| Source of variations | df | TSP | AsA | Total phenolics | SOD |
|---|---|---|---|---|---|
| Drought stress (D) | 1 | 2958*** | 23.42* | 68.57** | 76.22*** |
| Trehalose (Tre) | 3 | 2068*** | 16.46** | 21.60* | 4.169*** |
| Cultivars (Cv) | 1 | 8215 ns | 4.624 ns | 46.75* | 1.264 ns |
| D × Tre | 3 | 1419** | 0.4953 ns | 1.968 ns | 2.750** |
| D × Cv | 1 | 2557** | 3.543 ns | 22.20 ns | 2.182* |
| Tre × Cv | 3 | 1436 ns | 1.111 ns | 5.367 ns | 0.175 ns |
| D × Tre × Cv | 3 | 241.7 ns | 1.642 ns | 0.754 ns | 0.473 ns |
| CAT | POD | ||||
| Drought stress (D) | 1 | 28.44*** | 1546** | ||
| Trehalose (Tre) | 3 | 3.635*** | 1387*** | ||
| Cultivars (Cv) | 1 | 2.074 ns | 100.8 ns | ||
| D × Tre | 3 | 4.137** | 28.12 ns | ||
| D × Cv | 1 | 6.913*** | 16.07 ns | ||
| Tre × Cv | 3 | 0.027 ns | 32.52 ns | ||
| D × Tre × Cv | 3 | 0.303 ns | 21.54 ns |
- *, ** and *** indicates significant at 0.05, 0.01 and 0.001 levels, respectively.
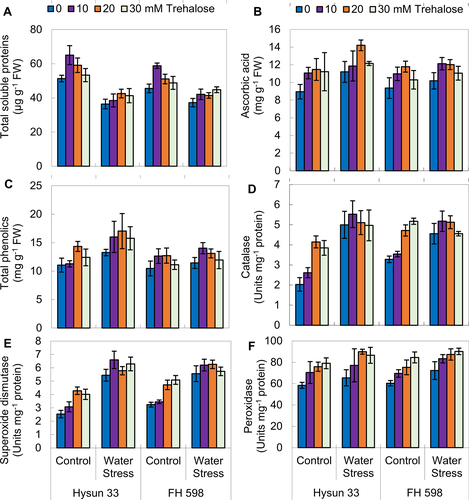
Supplementation of trehalose increased the levels of non-enzymatic antioxidants under drought stress
A significant increase (P ≤ 0.05) in ascorbic acid content was observed in both sunflower cultivars under drought stress conditions as well as in the trehalose-treated plants (Table 3; Fig. 3). Of all levels, trehalose sprayed at 20 mM was better under drought stress conditions, particularly for cv. Hysun 33.
Water stress resulted in a significant increase (P ≤ 0.01) in total phenolic content in both sunflower cultivars (Table 3; Fig. 3). The externally applied trehalose also significantly (P ≤ 0.05) raised the total phenolic contents. All three levels of trehalose applied were highly effective in promoting the phenolic content in both cultivars under drought stress conditions. Of both cultivars, Hysun 33 was better than FH 598 in this parameter.
External application of trehalose enhanced the activities of enzymatic antioxidants under drought stress
The activities of enzymatic antioxidants such as superoxide dismutase, peroxidase and catalase were significantly (P ≤ 0.001) promoted under reduced water contents in the soil. The external use of trehalose significantly (P ≤ 0.001) enhanced the activity of all three antioxidant enzymes. No prominent change was observed in the performance of both sunflower cultivars under both water stress and trehalose application (Table 3; Fig. 3).
Exogenous application of trehalose promoted yield attributes under drought stress
Drought stress had an adverse effect on achene yield per plant and oil percentage in both sunflower cultivars (Table 4; Fig. 4). In contrary, foliar-applied trehalose (10, 20 and 30 mM) significantly improved the achene yield per plant in both sunflower cultivars, while the oil contents only in cv. Hysun 33. Of both sunflower cultivars, Hysun 33 performed better than the other cultivar under water stress.
| Source of variations | df | Achene fresh weight | Achene dry weight | Achene yield/plant | Inorganic contents |
|---|---|---|---|---|---|
| Drought stress (D) | 1 | 4336*** | 3743*** | 3037*** | 7.770*** |
| Trehalose (Tre) | 3 | 1422** | 708.8** | 837.2*** | 1.062** |
| Cultivars (Cv) | 1 | 1151*** | 1159*** | 5043*** | 4.050*** |
| D × Tre | 3 | 686.4 ns | 44.12 ns | 116.45 ns | 1.140** |
| D × Cv | 1 | 3616*** | 95.25 ns | 203.8 ns | 0.045 ns |
| Tre × Cv | 3 | 2514 ns | 120.0 ns | 22.93 ns | 0.888* |
| D × Tre × Cv | 3 | 239.5 ns | 47.71 ns | 71.61 ns | 0.998* |
| Organic contents | |||||
| Drought stress (D) | 1 | 7.664*** | |||
| Trehalose (Tre) | 3 | 1.025* | |||
| Cultivars (Cv) | 1 | 0.760 ns | |||
| D × Tre | 3 | 1.307* | |||
| D × Cv | 1 | 0.080 ns | |||
| Tre × Cv | 3 | 1.084* | |||
| D × Tre × Cv | 3 | 0.8614 ns |
- *, ** and *** indicates significant at 0.05, 0.01 and 0.001 levels, respectively.
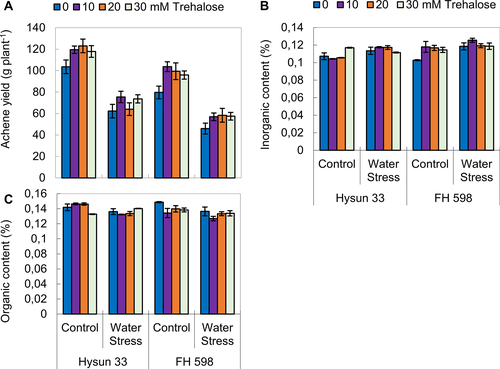
Supplementation of trehalose enhanced the yield and oil quality attributes under drought stress
Water stress increased the achene inorganic contents in both sunflower cultivars but reduced (P ≤ 0.001) the organic contents only in FH 598 under stress and non-stress conditions (Table 4; Fig. 4). The trehalose treatment significantly improved (P ≤ 0.05) both organic and inorganic contents. The cultivar Hysun 33 was better in organic content, while cv. FH 598 in inorganic contents under stress conditions.
Drought caused a significant increase in palmitic and linoleic acids, but no significant effect was observed on stearic and oleic acids (Table 5; Fig. 5). Foliar-applied trehalose had a non-significant effect on all these parameters. The amount of palmitic and linolenic acid was affected by drought stress in both sunflower cultivars. The amounts of stearic acid and oleic acid were not affected by drought stress. However, cv. Hysun 33 accumulated more palmitic acid, stearic acid and linoleic acid, while cv. FH598 accumulated more oleic acid under drought stress.
| Source of variations | df | Oleic acid | Linoleic acid | Oil contents/plant | Alpha-tocopherol |
|---|---|---|---|---|---|
| Drought stress (D) | 1 | 4.182 ns | 6.432* | 125.7*** | 8977*** |
| Trehalose (Tre) | 3 | 2.207 ns | 1.805 ns | 0.507 ns | 234.5 ns |
| Cultivars (Cv) | 1 | 7927*** | 6499*** | 162.0*** | 35203** |
| D × Tre | 3 | 1.504 ns | 1.545 ns | 0.343 ns | 53.18 ns |
| D × Cv | 1 | 10.85** | 15.41** | 1.606* | 1032* |
| Tre × Cv | 3 | 1.239 ns | 1.042 ns | 2.021*** | 126.9 ns |
| D × Tre × Cv | 3 | 2.285 ns | 2.819 ns | 1.896*** | 108.7 ns |
| Palmitic acid | Steric acid | ||||
| Drought stress (D) | 1 | 0.047* | 0.074 ns | ||
| Trehalose (Tre) | 3 | 0.004 ns | 0.027 ns | ||
| Cultivars (Cv) | 1 | 14.41*** | 21.36*** | ||
| D × Tre | 3 | 0.005 ns | 0.028 ns | ||
| D × Cv | 1 | 0.040* | 0.184* | ||
| Tre × Cv | 3 | 0.006 ns | 0.220** | ||
| D × Tre × Cv | 3 | 0.001 ns | 0.025 ns |
- *, ** and *** indicates significant at 0.05, 0.01 and 0.001 levels, respectively.
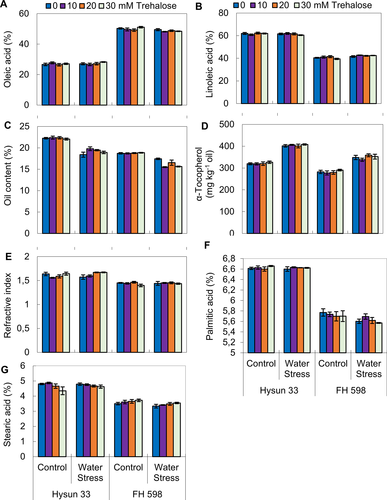
A significant increase in α-tocopherol level was observed due to drought stress in sunflower plants (Table 5; Fig. 5). Foliar-applied trehalose proved ineffective for this parameter. Of both sunflower cultivars, Hysun 33 was better in α-tocopherol contents than the other cultivar.
A significant positive association among growth and yield attributes, while no significant association was found among other physiological and biochemical attributes measured.
Discussion
Generally, plant growth and various plant processes are affected by water shortage, soil salinity, extremes of temperature, etc. (Choi et al. 2011, Hasanuzzaman et al. 2013, Aziz et al. 2018). To survive under such adverse cues, plants employ different defensive adaptive mechanisms including osmotic adjustment/osmoregulation, ion homeostasis, high level/activity of antioxidants, etc. (Holmström et al. 2000, Khan et al. 2015). Under drought stress conditions, osmoregulation is considered as an important mechanism, for which certain low molecular weight carbohydrates, proteins and amino acids play an important role in improving plant stress tolerance (Hasegawa et al. 2000, Akram et al. 2016). To enhance the endogenous levels of osmoprotectants, they can be applied exogenously to bring their intrinsic concentrations to optimum levels. Therefore, foliar spray of such organic compounds has been advocated as a viable strategy for plants to thrive well under unfavorable stress conditions (Teixeira et al. 2017). Trehalose is known to effectively stabilize the desiccated enzymes, proteins, antioxidants and lipids as well as protect the biological membranes from damages during abiotic stresses (Garg et al. 2002, Agrawal and Rathore 2007, Aldesuquy 2015). In this investigation, drought stress conditions significantly impaired the growth parameters (shoot and root fresh and dry weights) of both sunflower cultivars (Hysun 33 and FH 598). These results are similar to some earlier findings on radish, cowpea and canola (Shafiq et al. 2015, Rebouças et al. 2017, Akram et al. 2018). However, foliar application with 10 mM trehalose significantly improved these growth parameters of sunflower plants. These results can be related to some earlier studies in which exogenous application of trehalose improved growth of maize (Zeid 2009), Catharanthus roseus (Chang et al. 2014), Brassica species (Alam et al. 2014), wheat (Ibrahim and Abdellatif 2016) and radish (Akram et al. 2016) under stress conditions. The trehalose-induced plant growth improvement was suggested to be associated with the upregulation of the oxidative defense system, maintenance of turgor potential and nutritional balance as well as suppression in the levels of reactive oxidants under stress conditions (Alam et al. 2014, Akram et al. 2016).
Upon exposure to abiotic stresses, the photosystems I and II are damaged, which negatively affect chlorophyll contents (Baker et al. 2007, Athari and Talebi 2014). Drought stress has been reported to reduce the chlorophyll a and b contents (Amira and Qados 2014),which we confirm in this study, particularly a reduction in chlorophyll a content due to water stress. However, foliar-applied trehalose did not influence the accumulation of chlorophyll a and b under stress and non-stress field conditions. In contrast to our results, Akram et al. (2016) reported that in radish plants, seeds pretreated with trehalose caused a significant increase in chlorophyll a content. Similarly, Sadak (2016) reported enhanced levels of photosynthetic pigments in trehalose-treated fenugreek plants as compared to those in non-treated plants. The variation between these results and those reported earlier can be due to the concentration of trehalose used, the type of sunflower cultivars, the water irrigation levels as well as the mode of trehalose application.
Withholding of irrigation water significantly raised the relative membrane permeability of both sunflower cultivars, a common phenomenon taking place under stress conditions (Akram and Ashraf 2011, Akram et al. 2012, Hammad and Ali 2014). The fortification of trehalose significantly decreased the RMP in both sunflower cultivars. Similarly, Akram et al. (2015) reported that application of trehalose decreased RMP in dehydrated radish plants. So, it can be suggested that trehalose plays a role in stabilizing membrane integrity, thus acting as an important membrane stabilizer. Hydrogen peroxide (H2O2) is one of the potent metabolites among oxidative species that deteriorate the structure of biological membranes during abiotic stresses (Quan et al. 2008, Jajic et al. 2015). In this study, drought stress and trehalose showed no significant effect on hydrogen peroxide content in both sunflower cultivars. Analogous to our results, Akram et al. (2016) found that the exogenous application of trehalose did not affect H2O2 in radish plants under drought stress. Contrary to this, Alam et al. (2014) observed a positive role of trehalose in lowering the H2O2 levels in Brassica species under dehydration-induced oxidative stress. When abiotic stresses accentuate, oxidative free radicals are produced which disrupt membranous fatty acids, thereby increasing the extent of lipid peroxidation and levels of malondialdehyde (Shafiq et al. 2015). Higher concentration of MDA was observed in both sunflower cultivars, which is similar to that reported in wheat plants (Farooq et al. 2013). A number of reports published earlier show the positive role of trehalose in decreasing the MDA content in different crops, e.g. maize (Ali and Ashraf 2011b), rice (Theerakulpisut and Gunnula 2012), wheat (Ma et al. 2013), Brassica (Alam et al. 2014) and radish (Shafiq et al. 2015). All these studies suggest that high MDA accumulation is the indication of lipid peroxidation which is commonly low in stress-tolerant plants.
Osmoprotectants such as glycinebetaine and proline play a significant role in plants to endure prevailing abiotic stress conditions (Anjum et al. 2011). These solutes upregulate plant key physiological processes under stress conditions and make the plants acclimatized (Nounjan et al. 2012). In this study, leaf proline and GB accumulation increased considerably in both sunflower cultivars under drought stress, similar to what observed by Razaji et al. (2014). The GB accumulation in trehalose-treated plants of both sunflower cultivars was significantly higher than in the untreated ones, particularly under drought stress conditions. However, trehalose application had a non-significant effect on proline accumulation. Our results contradict to those of Alam et al. (2014), who observed an enhanced accumulation of proline in Brassica campestris seedlings during a combined treatment of trehalose and drought stress. Not only foliar spray but also pre-sowing seed treatment with trehalose induced higher levels of proline in water-stressed radish plants (Akram et al. 2016). So, it can be suggested that the trehalose-induced increase in GB accumulation. Particularly in sunflower cv. Hysun-33, plays an important role in plant drought stress tolerance.
The high content of ascorbic acid (AsA) in plants acts as an antioxidant and it can minimize the adverse effects of free radicals (Farouk 2011, Ye et al. 2012). This study indicates that drought stress significantly increased the ascorbic acid content, similar to those reported by Sečenji et al. (2009) in wheat plants. Furthermore, the exogenous use of trehalose increased the ascorbic acid concentration, agreeing with Alam et al. (2014) who reported a beneficial role of trehalose in dehydrated Brassica. Previously, the promoting role of trehalose in AsA accumulation has also been reported in drought-stressed radish plants (Shafiq et al. 2015, Akram et al. 2016). In contrast, Ali and Ashraf (2011a) reported a non-significant effect of trehalose on AsA concentration in drought-stressed maize plants.
Plants accumulate phenolic compounds as an adaptative mechanism against the adverse effects of oxidative free radicals during abiotic stress conditions (Akram et al. 2017, Sadiq et al. 2017). During this study, it was observed that moisture deficiency increased the total phenolic content in sunflower plants, which is similar to the findings of Gharibi et al. (2016) in Achillea species. In our study trehalose played a significant role in increasing phenolic compounds in water-stressed sunflower plants. An earlier study conducted by Ibrahim and Abdellatif (2016) showed that phenolic compounds increased in wheat plants when treated with trehalose under drought stress. Similar results were found in radish (Akram et al. 2016) and maize (Ali and Ashraf 2011b). It is likely that phenolic compounds activate the signaling pathways to stimulate non-enzymatic antioxidants against a stress adversity, thereby resulting in improved plant growth.
The occurrence of moisture deficit conditions in cellular compartments leads to the generation of reactive oxygen species which damage the cellular organelles and their membranes (Ashraf 2009, Aziz et al. 2018). Antioxidant enzymes, particularly superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) suppress the adverse effects of ROS (Khan and Panda 2008, Akram et al. 2017). In this experimentation, the activity of SOD, POD and CAT increased significantly in water-stressed sunflower cultivars, which positively relate to the studies of Selote and Khanna-Chopra (2010) on wheat and Darvishan et al. (2013) on corn under stress conditions. The application of trehalose to sunflower leaves significantly increased the activities of SOD, CAT and POD enzymes under water stress regimes. In earlier studies, a similar enhancement in the activities of SOD, CAT and POD upon trehalose treatment was reported in maize (Ali and Ashraf 2011a), wheat (Ma et al. 2013, Aldesuquy 2015, Ibrahim and Abdellatif 2016), Brassica (Alam et al. 2014) and radish (Shafiq et al. 2015, Akram et al. 2016) under varying water regimes. These studies suggested that exogenous application of trehalose upregulates the oxidative defense system, which might have a considerable role in improving plant growth under stress conditions.
In this study, water stress had adverse effects on yield parameters such as achene yield per plant and achene oil contents. Stress-induced yield reduction has already been widely reported (Shahbaz et al. 2008, Akram et al. 2011, Akram and Ashraf 2011). Little literature is available on trehalose-related yield attributes in oil-seed-crops, but trehalose application was shown to have prominent effects on yield in diverse crops, e.g. ajwain (Ashraf and Orooj 2006), cotton (Ahmad et al. 2007), sunflower (Akram et al. 2011) and Moringa oleifera (Anwar et al. 2006). In this study, trehalose external application significantly improved the achene yield in both sunflower cultivars under drought stress conditions. The drought stress-induced reduction in achene yield could have been due to the deterioration of photosynthetic pigments, disturbance in stomatal conductance, enhanced lipid peroxidation and injurious effects of ROS (Amira and Qados 2014, Akram et al. 2017).
Drought stress is known to adversely affect the quality, yield and chemical composition of different oils (Angioni et al. 2006, Akram et al. 2011). In this study, the field moisture deficit conditions caused a significant decrease in palmitic and linoleic acids, but no significant effect was recorded on stearic acid and oleic acid concentrations. Trehalose application had no considerable effect on all these parameters. Not a single report is available in the literature on the effect of trehalose on oil composition of oil-seed crops under stress and non-stress conditions. Anwar et al. (2006) reported that drought conditions caused increased concentrations of palmitic acid and oleic acid in the seed oil of Moringa oleifera. Tocopherols, being important non-enzymatic antioxidants, retain the fatty acid profile in seed oil contents due to the alleviation of the adverse effects of reactive oxygen species (Israr et al. 2011). However, their contents differ in many oil-crops. In this investigation, a marked increase was observed in α-tocopherol level under field withholding irrigations. Foliar-applied trehalose proved ineffective for this parameter, independently of the concentration used. Similar reports from the literature showed an increased in tocopherol in safflower seed oil and sunflower (Noreen and Ashraf 2010, Akram and Ashraf 2011) under stress conditions. In contrast, Anwar et al. (2006) reported a significant reduction in the tocopherol content in the seed oil of Moringa oleifera. Reports on stress-induced increase or decrease in α-tocopherol are available but cannot be compared with this study due to change in the level or type of the stress applied, type of plant species, type or mode of exogenous application of a solute and experimental conditions.
Conclusions
In conclusion, drought stress significantly decreased the shoot and root fresh and dry weights, chlorophyll pigments, total soluble proteins, achene yield per plant, oil percentage, organic contents, oil palmitic and linoleic acids, while it enhanced RMP, leaf free proline, GB, hydrogen peroxide, malondialdehyde, ascorbic acid, total phenolics, achene inorganic contents, α-tocopherol levels as well as the activities of antioxidant enzymes (SOD, POD and CAT) in sunflower plants. The external application of trehalose decreased the lipid peroxidation by decreasing the accumulation of H2O2 and RMP, while a significant improvement was observed in plant growth, glycinebetaine, ascorbic acid, total phenolics, total soluble proteins, achene yield per plant, oil contents, inorganic and organic contents as well as the activities of SOD, POD and CAT enzymes under drought stress conditions. Among both sunflower cultivars, Hysun 33 had a higher tolerance to drought stress than FH598 as reflected by a better plant growth, reduced RMP, and high glycinebetaine, proline, achene yield per plant, oil contents, and palmitic and linoleic acids. So, it can be suggested that exogenous application of trehalose can be tested on different plants grown in stress-prone areas.
Author contributions
F.K. conducted the experiment, A.A. analyzed the data, N.A.A. designed the research and supervised, M.A. critically checked the manuscript, M.N.A. and P.A. help in manuscript writing and results interpretations.
Acknowledgements
The authors acknowledge the GUCF for supporting through project no. 43-Bot-2 and the Researchers Supporting Project Number (RSP-2020/116), King Saud University, Riyadh, Saudi Arabia.
Open Research
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




