Nitrate reductase is required for salicylic acid-induced water stress tolerance of pepper by upraising the AsA-GSH pathway and glyoxalase system
Abstract
A trial was conducted to evaluate whether nitrate reductase (NR) participates in salicylic acid (SA)-improved water stress (WS) tolerance in pepper (Capsicum annuum L.) plants. Before starting WS treatment, 0.5 mM SA was applied to half of the well-watered (WW) plants as well as to WS-plants as a foliar spray once a day for a week. The soil water holding capacity was maintained at 40 and 80% of the full water storing capacity for WS and and well-watered (WW) plants, respectively. Water stress caused substantial decreases in total plant dry weight, Fv/Fm, chlorophyll a and b, relative water content, leaf water potential (ΨI) by 53, 37, 49, 21, 36 and 33%, respectively relative to control, but significant increases in malondialdehyde (MDA), hydrogen peroxide (H2O2), electrolyte leakage (EL), methylglyoxal (MG), proline, key antioxidant enzymes' activities, NO and NR activity. The SA reduced oxidative stress, but improved antioxidant defence system, ascorbate-glutathione (AsA-GSH) cycle enzymes, glyoxalase system-related enzymes, glyoxalase I (Gly I) and glyoxalase II (Gly II), plant growth, photosynthetic traits, NO, NR and proline. SA-induced WS tolerance was further improved by supplementation of sodium nitroprusside (SNP), a donor of NO. NR inhibitor, sodium tungstate (ST) was applied in conjunction with SA and SA + SNP to the WW and WS-plants to assess whether NR contributes to SA-improved WS tolerance. ST abolished the beneficial effects of SA by reducing NO and NR activity in WS-pepper, but the application of SNP along with SA + ST reversed negative effects of ST, showing that NO and NR are jointly needed for SA-induced WS tolerance of pepper plants.
Abbreviations
-
- APX
-
- ascorbate peroxidase
-
- AsA-GSH
-
- ascorbate-glutathione cycle
-
- CAT
-
- catalase
-
- DHAR
-
- dehydroascorbate reductas
-
- EL
-
- electrolyte leakage
-
- Fv/Fm
-
- maximum quantum efficiency of photosystem II
-
- Gly I
-
- glyoxalase I
-
- Gly II
-
- glyoxalase II
-
- GR
-
- glutathione reductase
-
- GST
-
- glutathione S-transferase
-
- H2O2
-
- hydrogen peroxide
-
- MDA
-
- malondialdehyde
-
- MDHAR
-
- monodehydroascorbate reductase
-
- MG
-
- methylglyoxal
-
- NO
-
- nitric oxide
-
- NR
-
- nitrate reductase
-
- POD
-
- peroxidase
-
- ΨI
-
- leaf water potential
-
- SA
-
- salicylic acid
-
- SNP
-
- sodium nitroprusside
-
- ST
-
- sodium tungstate
-
- WS
-
- water stress
-
- WW
-
- well-watered
Introduction
Sweet pepper (Capsicum annuum L.) is majorly cultivated in the Mediterranean region and frequently grown in glasshouses, which leads to greater yield and outstanding fruit quality over open field conditions (Serret et al. 2018). Sweet pepper is not tolerant to water stress (WS) conditions, which decreases plant biomass and fruit yield (Katerji et al. 1992).
Water stress suppresses plant growth, fruit yield and photosynthetic activity predominantly in arid and semi-arid regions without irrigation facilities (Patanè et al. 2011, Badr et al. 2018, Sharma et al. 2019a). Plant scientists have focused on investigating the detrimental effects of WS on plants, and the strategies to mitigate its negative effect on plants (Rolli et al. 2015, Blum 2017). Water resources used for irrigation purposes in agricultural production are gradually decreasing due to the increasing population and climate changes (Kahil et al. 2016). Water stress resulted in oxidative damage in plants by disturbing the equivalence between reactive oxygen species (ROS), e.g. H2O2, and O2·− and their scavengers, antioxidants (Mattos and Moretti 2015). Another metabolite synthesised in plant cells under stress is methylglyoxal (MG), which is produced in the glycolysis pathway, and can damage cell membranes at excess levels by degrading proteins and lipids (Askari-Khorasgani and Pessarakli 2019, Hasanuzzaman et al. 2019). Plants can also scavenge excess MG by upregulating the glyoxalase system regulating enzymes (Mostofa et al. 2018, Bhuyan et al. 2019). The injurious effect of several stressors, including water stress, can be alleviated by using bio-stimulators and thereby improving tolerance to WS in plants (Piotrowski and Romanowska-Duda 2018, Sharma et al. 2019b). In this respect, salicylic acid (SA) appears to be an enormous bio-stimulator against water stress among numerous defensive ingredients naturally generated within plants (Maghsoudi et al. 2019).
Plants grown in harsh environments produce hormones including salicylic acid (Khan et al. 2015). Stressed plants produce ROS which activate SA synthesis, and ROS and SA work together to enhance antioxidants, ultimately resulting in reduced ROS concentrations (Jahan et al. 2019). Furthermore, externally applied SA showed alleviation effect in various plants under various stressors, e.g. salt stress in mustard (Nazar et al. 2015a), cold stress in barley (Mutlu et al. 2016) and cadmium stress in Lemna minor (Lu et al. 2018). Externally applied SA has also shown to have a positive response to WS in various plants, e.g. wheat (Aldesuquy and Ghanem 2015, Noreen et al. 2017), soybean (Tang et al. 2017) and safflower (Chavoushi et al. 2019).
Nitrate reductase (NR) is known to mediate nitric oxide (NO) in plants (Chamizo-Ampudia et al. 2017, Pan et al. 2019). NR-induced NO improves tolerance to various stresses in plants (Pan et al. 2019, Kaya et al. 2020a,b). NO is a signalling molecule that plays a crucial role in enhancing plant tolerance in harsh environments (Fancy et al. 2017, Sharma et al. 2020), e.g. arsenic toxicity (Singh et al. 2017a), salt stress (Arora and Bhatla 2017), heat stress (Alamri et al. 2019) and sulphur deficiency (Siddiqui et al. 2020) as well as WS (Cechin et al. 2015, Hasanuzzaman et al. 2017a, Munawar et al. 2019, Khan et al. 2020). However, so far no investigation have focused on the function of NR-triggered NO in SA-induced WS tolerance in pepper plants by upraising the ascorbate-glutathione (AsA-GSH) cycle and glyoxalase system. Therefore, in this study the inhibitor of NR, sodium tungstate (ST) was applied to evaluate the possible role of NR-triggered NO in SA-enhanced WS tolerance of pepper plants.
Materials and methods
Cultivation and treatments
Greenhouse experimentation was carried out using pepper (Capsicum annuum L.) cv. ‘Semerkand’. After treating the seeds with 1% of NaOCl (v/v) solution for surface sterilisation, they were sown in a plate consisting of washed-sand an watered daily until seed germinating. One week after germination and before transferring seedlings, a related group of young seedlings' roots were treated with an inhibitor of nitrate reductase (NR), 0.1 mM sodium tungstate (ST) via supplying into an aqueous solution for just 3 h to get an insight into whether or not NR is involved in salicylic acid (SA)-improved tolerance water stress (WS). Thereafter, three seedlings of pepper were transferred to each pot containing 8 kg of dried soil. Furthermore, before WS treatment, half of the well-watered (WW) and WS seedlings were sprayed with 0.5 mM SA (2-hydroxybenzoic acid) solution consisting of tween-20 (0.01%) once a day for a week. However, control plants were sprayed with tween-20 (0.01%) solution alone. The WS treatment started after the SA pretreatment was completed. To get further evidence that NO participates in SA-improved WS tolerance, 0.1 mM sodium nitroprusside (SNP) was sprayed onto the SA and SA + ST treated plants once every week for 2 weeks. A detailed scheme of the treatments is given in Fig. 1. The concentration of SA (0.5 mM) selected for the current study is the same as used in other studies including soybean (Ardebili et al. 2014) and pepper (Kaya et al. 2020a). The 0.1 mM SNP concentration was selected based on the work of Cechin et al. (2015) in sunflower under WS and the use of 0.1 mM ST was selected based on the results of Kaya et al. (2020b) in pepper.
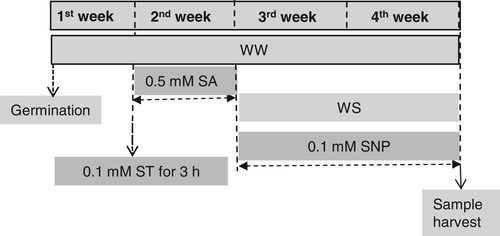
The soil was clay loam with CaCO3 25.2%, pH of 7.7, organic matter content 1.0%, available P 3.3 mg kg−1, and electrical conductivity 1.2 dS m−1. The exchangeable cation contents of K+, Ca2+, Mg2+ and Na+ were 1.25, 23.6, 10.4 and 0.67 cmol kg−1, respectively. Electrical conductivity and pH of irrigation water were 0.49 dS m−1 and 7.2, respectively. The soil was applied with nitrogen (100 mg kg−1) as granular urea, P2O5 (50 mg kg−1) as triple superphosphate, and K2O (120 mg kg−1) as potassium sulphate. Temperatures during the day and night period of the experiment were kept at 25 ± 2 and 15 ± 2°C, respectively. The ambient light period during the experimentation was 11 h. Relative humidity in the glasshouse was 65–70%.
Before initiating WS treatment, the full soil water holding capacity was determined by examining three pots with drainage holes. Each pot filled with air-dried soil (8 kg) was fully filled with distilled water and permitted to release excess water from pots for a day. After reweighting the pots, the full water storage capacity of the soil was measured according to the equation employed by Bonfim-Silva et al. (2015).
Each pot was weighed with an electronic scale twice a day to determine the amount of water evaporated, and the evaporated water plus water used by plants was re-added following the gravimetric procedure outlined by da Silva Leite et al. (2019). The soil holding water capacity of the pots were kept at 40 and 80% of the full water holding capacity for WS and WW plants, respectively, based on previous the results of an experiment done in pepper crop (Kaya et al. 2019).
Each replication had three pots and each treatment had three replications. The shoot and roots dry weights were quantified by choosing three pots or nine plants from each treatment. After the fresh weight of plants was recorded, they were subjected to 75°C in an oven for 3 days for quantification of dry weight. Completely extended youngest leaves of the remaining six plants from each replicate, i.e. 18 plants from each treatment were collected to obtain data of attributes below:
Chlorophyll contents and chlorophyll fluorescence
The quantification of chlorophyll was done using the protocol of Strain and Svec (1966). The extraction of 1 g of leaf material was done in 5 ml of acetone (90%). After filtering the extracts, they were kept in light-tight tubes. Absorbance values were noted at 663.5 and 645 nm for Chl a and Chl b, respectively.
For the quantification of chlorophyll fluorescence, the leaves of pepper were retained at a dark and light cycle employing the standard procedure. Thereafter fluorescence values were recorded with a chlorophyll fluorometer (Mini-PAM, Walz, Germany). Prior to recording readings, the leaves were retained in dark for 30 min. Values for minimum, maximal and variable fluorescence denoted by Fo, Fm and Fv, respectively, were noted and then maximum quantum efficiency of PSII (Fv/Fm) was calculated.
Quantification of leaf relative water content (RWC) and leaf water potential
The protocol of Weatherly and Barrs (1962) was followed to quantify leaf RWC. Leaves taken from the same position of the plant promptly were weighed to record the fresh weight (FW). Those samples were incubated into vials filled with distilled water for a day, and then surface water on the leaf was removed with a paper tissue and reweighed to obtain the leaf turgid weight (TW). Finally, those samples were dried in an oven at 65°C to note the leaf dry weight (DW). The RWC was calculated per the equation of Weatherly and Barrs (1962).
Leaf water potential was measured with a water potential apparatus (PMS model 600, USA) by using the third leaf from the top of each plant.
Leaf free proline content
The quantification of the leaf free proline was done according to Bates et al. (1973) in pepper plants. After extraction of 0.5 g of leaf material in 10 ml of sulfosalicylic acid (3%) solution, the filtrate (2 ml) was mixed with acid-ninhydrin (2.5% w/v) and glacial acetic acid (60% v/v) solutions, each 2 ml. Then the reaction solution was retained in a water bath at 100°C for 1 h. After cooling the mixture, it was mixed with 4 ml of toluene. The absorbance readings were recorded at 520 nm.
Electrolyte leakage (EL)
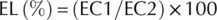
Hydrogen peroxide (H2O2) and malondialdehyde (MDA)
The quantification of leaf H2O2 was done per the protocol of Velikova et al. (2000). The extraction of fresh leaf tissue (0.5 g) was done in 3 ml of 1% TCA. After centrifugation at 12 000g for 15 min at 4°C, the filtrate (0.75 ml) was mixed with KI (1.5 ml, 1 M) and K buffer (0.75 ml, 10 mM) and then the absorbance readings were noted at 390 nm.
The protocol of Weisany et al. (2012) was employed to quantify leaf MDA. After 0.2 g of fresh leaf tissue was homogenised in trichloroacetic acid (TCA; 5 ml, 0.1%), it was centrifuged at 12 000 g at 4°C for 5 min. Thereafter, 1 ml of sample solution was mixed with 4 ml of 0.5% thiobarbituric acid (prepared in 20% [w/v] TCA). The mixture was retained at 90°C in a water bath for 30 min. Following cooling the sample, the absorbance values were noted at 532 nm.
Analysis of ascorbate (AsA) and glutathione (GSH)
A 500 mg fresh leaf tissue was extracted in 3 ml of meta-phosphoric acid (5%) and 1 M EDTA for the quantification of ASA and GSH. Thereafter, the extracted solution was centrifuged at 11 500 g for 12 min at 4°C. Thereafter, the aliquot was pipetted out for the determination of GSH and AsA.
The quantification of AsA level was done per the procedure of Huang et al. (2005). The neutralisation of aliquot (0.4 ml) was done by adding 600 μl of 500 mM K-phosphate buffer (pH 7.0), and then it was determined in K-phosphate buffer (100 mM, at pH 7.0) containing 0.5 unit ascorbate oxidase at 265 nm.
The oxidised GSH and glutathione disulfide (GSSG) were quantified employing the procedure of Yu et al. (2003). A 400 μl of aliquot was neutralised using 600 μl of K-phosphate buffer (0.5 M, pH 7.0). The calculation of GSH was done by the change in absorption rate at 412 nm wavelength for NTB (2-nitro-5-thiobenzoic acid) formed by the reduction of DTNB (5,5′-dithiol-bis [2-nitrobenzoic acid]). GSSG was quantified by eliminating GSH using a derivatizing agent, 2-vinyl pyridine.
Quantification of nitrate reductase (NR)
The NR activity was quantified employing the protocol of Sun et al. (2014). Briefly, a buffer containing 1,4-dithiothreitol (DTT, 5 mM), 0.1 M HEPES–KOH (pH 7.5), phenylmethylsulfonyl fluoride (0.5 mM), EDTA (1 mM), flavin adenine dinucleotide (20 μM), glycerol (10%), Triton X-100 (0.1%) and polyvinylpyrrolidone (PVP, 1%) was used to homogenise total soluble proteins. Following centrifugation of supernatant at 12 000 g at 4°C for 20 min, NR activity was assayed at 520 nm. The NR activity was quantified by adding 0.25 ml of aliquot into pre-warmed (25 °C) assay buffer (250 μl) consisting of DTT (1 mM), MgCl2 (10 mM), HEPES–KOH (50 mM, pH 7.5), 0.2 mM NADH and KNO3 (2 mM). After starting reaction, it was retained at 30°C for 30 min. A 50 μl of 0.5 M Zn-acetate was mixed with assay solution to last the reaction. The generated nitrite was quantified after adding 1 ml of 1% sulfanilamide in 3 M HCl plus 1 ml of N-(1-naphthyl) ethylenediamine (0.02%) in 0.2 M HCl.
Quantification of nitric oxide (NO)
The quantification of leaf NO was done employing the protocol of Zhou et al. (2005). The extraction of fresh leaf material (0.6 g) was done in 3 ml of 50 mM cold acetic acid buffer at pH 3.6 plus 4% zinc diacetate. Thereafter, the centrifugation of the extract was done at 10 000 g at 4°C for 15 min. The supernatant was pipette out, and the pellet resulted was washed with extraction buffer (1 ml). After both aliquots were mixed, a 0.1 g of charcoal was mixed with it. After filtrating and vortexing mixture, 1 ml of the Greiss reagent and 1 ml of the mixture were incubated at room temperature for 30 min. The absorbance readings were noted at 540 nm.
Enzyme extraction
A 500 mg of leaf material was homogenised in the extraction solution (1.0 ml) consisting of K–P buffer, AsA, KCl, glycerol and β-mercaptoethanol. The extract was centrifuged at 11 500 g for 10 min. This extract was also used for protein quantification (Bradford 1976) as well as a crude solution for the assay of enzyme activities.
Enzyme assay
The procedure of Nakano and Asada (1981) was followed to measure the activity of APX (EC: 1.11.1.11). Solution of enzyme was mixed with AsA (0.5 mM), EDTA (0.1 mM), K–P buffer (50 mM, pH 7.0) and H2O2 (0.1 mM). Absorbance readings were noted at 290 nm to monitor the changes in absorbance.
The protocol of Hossain et al. (2010) was followed to quantify the activity of MDHAR (EC: 1.6.5.4). Assay solution was added to 50 mM Tris–HCl buffer (pH 7.5), AsA (2.5 mM), NADPH (0.2 mM) and AO (0.5 U). A reduction in absorbance was noted at 340 nm for 60 s.
The protocol of Nakano and Asada (1981) was employed to quantify the activity of DHAR (EC: 1.8.5.1). The assay solution was added to a mixture solution of K–P buffer (50 mM, pH 7.0), GSH (2.5 mM) and DHA (0.1 mM). Thereafter the absorbance readings were noted at 265 nm to note the changes in absorbance.
The protocol of Foyer and Halliwell (1976) was followed to quantify the activity of GR (EC: 1.6.4.2). The enzyme assay solution (0.2 ml) was added to EDTA (0.1 mM), NADPH (200 μM), potassium-phosphate buffer (100 mM, pH 7.0) and GSSG (200 μM). Thereafter, the changes in absorbance were noted at 340 nm.
The protocol of Hossain et al. (2006) was followed to determine GST (EC: 2.5.1.18) activity. The assay solution was mixed with a solution of Tris–HCl buffer (0.1 M, pH 6.5), GSH (1.5 mM) and 1-chloro- 2,4-dinitrobenzene (1 mM) in a final quantity of 0.7 ml. Thereafter, the alterations in absorbance were noted at 340 nm.
The protocol of Chakravarty and Sopory (1998) was employed to quantify the activity of Gly I (EC: 4.4.1.5). A mixture solution consisting of magnesium sulphate (15 mM), K-phosphate buffer (100 mM, pH 7.0), MG (3.5 mM) and GSH (1.7 mM) was added to enzyme assay solution in a final volume of 0.8 ml. Absorbance readings were noted at 240 nm.
The procedure outlined by Principato et al. (1987) was employed to quantify the activity of Gly II (EC: 3.1.2.6) by monitoring the production of GSH at 412 nm for 1 min. A cocktail solution consisting of DTNB (0.2 mM), S-d-lactoylglutathione (SLG, 1 mM) and Tris–HCl buffer (100 mM, pH 7.2) was added to enzyme assay solution in a final volume of 1 ml.
Methylglyoxal (MG) levels
The procedure of Wild et al. (2012) was employed to determine leaf MG. After 0.5 g of leaf material was homogenised in perchloric acid (5%), the centrifugation of the extract was done at 11 000 g and 4°C for 10 min. The charcoal was added to the aliquot to decolorize, and it was also neutralised by adding a saturated solution of potassium carbonate at 25°C. The mixture was made up to a final volume of 1 ml with sodium dihydrogen phosphate and N-acetyl-l-cysteine (at the ratio of 24:1:25). The synthesis of N-α-acetyl-S-(1-hydroxy-2-oxo-prop-1-yl) cysteine was noted at 288 nm after 10 min.
Antioxidant enzymes
A 500 mg of fresh leaf tissue was extracted in a solution of 50 mM Na-P buffer and 1% of soluble polyvinylpyrrolidone and then the centrifugation of extracted solution was done at 20 000 g at 4°C for 15 min. The aliquot was collected for the quantification of catalase (CAT, EC: 1.11.1.6) and peroxide (POD, EC: 1.11.1.7) activities. Quantification of CAT activity was done in 3 ml of assay solution consisted of 50 mM potassium phosphate buffer (pH 7.0), H2O2 (5.9 mM) and enzyme extract (0.1 ml). The enzyme extract was added to start reaction. A decrease in absorbance at 240 nm was noted for 60 s following the disappearance of H2O2 (Chance and Maehly 1955). A change of 0.01 U per min in the absorbance reading was expressed as one unit CAT activity.
Quantification of the activity of POD was done in 3 ml of assay solution consisted of guaiacol (20 mM), potassium phosphate buffer (50 mM, pH 7.0), H2O2 (40 mM) and enzyme extract (0.1 ml). The enzyme extract was added to start reaction. The POD activity was determined by the rise in optical density at 470 nm due to the guaiacol oxidation by H2O2 after 60 s (Chance and Maehly 1955). A change of 0.01 U per min in the absorbance reading was defined as one unit POD activity.
Statistical analysis
The data for various parameters were worked out by using one way anova and mean data combined with the standard error were given in figures and compared at 0.05% probability level using the SAS version 9.1 (SAS Institute Inc., USA). Each treatment had three replications. Duncan's multiple range test was followed to determine significant differences among the mean values.
Results
SA-induced NR improves plant growth of pepper plants under WS
Water stress (WS, 40% of the full water store capacity) significantly (P ≤ 0.05) decreased the shoot, root and total plant dry mass by 60.5, 39.1 and 53.3%, respectively, compared to well-watered (WW, 80% of the full water store capacity) plants. However, exogenously applied salicylic acid (SA, 0.5 mM) noticeably elevated plant growth-related attributes, the shoot, root and total plant dry mass by 70.2, 37.4 and 51.8%, respectively, in comparison with plants subjected to WS alone (Fig. 2A,C), however, the enhanced plant growth traits due to SA were still lower than those in the WW-plants. Furthermore, to get additional proof for the involvement of NO in SA-improved WS tolerance, SNP, a donor of NO, was sprayed together with SA. The positive response of SA on the above mentioned parameters was further improved by 0.1 mM SNP in relation to those in the WS plants alone. Under WW conditions, those parameters were not significantly (P ≤ 0.05) altered by the other treatments.
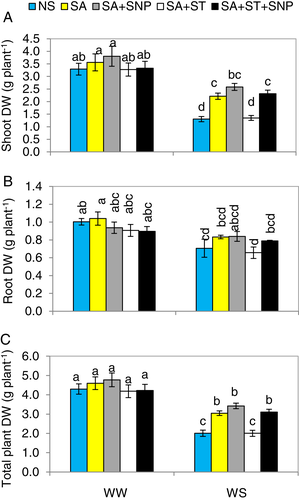
Application of the nitrate reductase (NR) inhibitor, sodium tungstate (ST, 0.1 mM), together with SA impaired the upregulated effect of SA on the above mentioned parameters in WS-plants. The supplementation of SNP reversed the damaging effects of ST on the plant growth under WS when applied in conjunction with SA (SA + ST + SNP). Those traits were not affected in the WW-plants by different treatments.
These data show that NR activity is needed for SA to be effective in enhancing plant growth. So, NR and SA together mutually participate in improved tolerance to WS in the pepper plants.
SA-induced NR enhances photosynthesis-related attributes in WS-pepper plants
Water stress substantially (P ≤ 0.05) reduced Chl a and b contents as well as photosystem II efficiency (Fv/Fm) in the pepper seedlings by 37.8, 49.1 and 21.3%, respectively, related to those in the WW-plants. Conversely, SA resulted in considerable elevations in the photosynthesis related-attributes mentioned above by 17.1, 44.9 and 26.1%, respectively compared to those seen in plants subjected to WS alone (Fig. 3A,C). The supplementation of SA + SNP resulted in further increases in the above mentioned parameters by 39.7, 60.01 and 33.3%, respectively, relative to those in the WS-plants, being not significantly different from WW plants. The beneficial effect of SA on those attributes was impaired by ST by blocking NR activity, showing that SA induces NR activity to enhance photosynthesis-related attributes in plants under WS conditions. The supplementation of SNP abolished the reversal effect of ST on the photosynthesis-related attributes under WS when applied in conjunction with SA (SA + ST + SNP). This also suggests that the effectiveness of SA is dependent on endogenous NO which is produced via externally as SNP or naturally produced by SA pre-treatment.
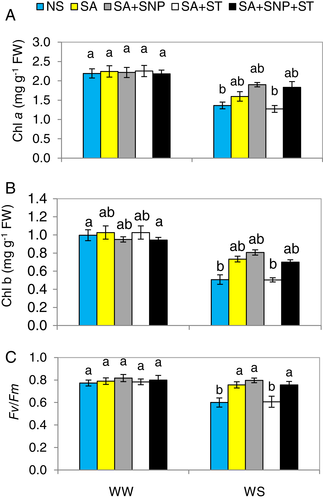
Plants subjected to WW conditions did not show any differences in those traits due to different treatments. Different treatments did not significantly change the above mentioned traits in the pepper plants under WW conditions.
SA-induced NR improves leaf water status and proline level under WS
Water stress markedly decreased leaf RWC and water potential (ΨI), which in turn considerably increased the proline content of pepper plants (Fig. 4A,C). However, SA significantly (P ≤ 0.05) increased leaf RWC, leaf ΨI and proline content by 36.5 and 32.8, 56.3%, respectively, when compared to WS-plants. The SNP supplementation along with SA caused a further increase in these parameters. The WW-plants were not affected by the various treatments. The beneficial effects of SA on the traits mentioned above were abolished, but those produced by SA + SNP were not eliminated by ST pre-treatment. This shows that NR activity is also necessary for SA-improvement of these parameters, and blocking NR activity makes SA ineffective in improving the water status of the plants. However, when NO is supplied externally along with SA + ST, high NR activity is not needed for SA be effective in improving water relation parameters under WS.
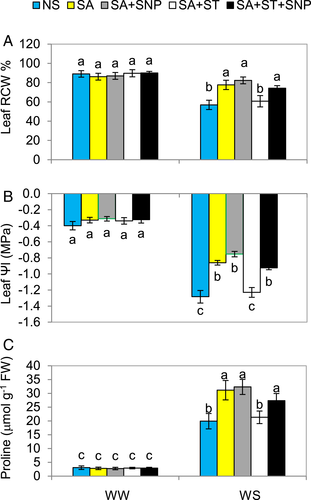
SA-induced NR and NO to enhance WS tolerance of pepper
To have an understanding if NR activity is needed for the SA-upregulation of the AsA-GSH cycle and glyoxalase to improve WS tolerance in pepper plant, alteration in leaf NO content and NR activity were quantified. Water stress resulted in elevations in both NO content and NR activity compared to WW-plants (Fig. 5A,B). Moreover, endogenous NO content and NR activity were considerably (P ≤ 0.05) enhanced by SA in WS-pepper seedlings. Supplementation of SNP along with SA (SA + SNP) resulted in further elevation in NO content, but reduced NR activity. The pretreatment of ST along with SA under WS totally abolished the NO content and NR activity, showing that ST blocks NR activity and thus inhibiting NO synthesis. So, SA could induce the synthesis of endogenous NO by activating NR in pepper seedlings. Endogenous NO could be a downstream signalling molecule of NR triggered by SA in improving WS tolerance of the pepper plants. The negative effect of ST on SA-induced NR and NO under WS was eliminated by the application of SNP by reinstalling NO synthesis without increasing NR. However, in WW-plants, ST reduced NR activity, but did not reduce NO synthesis.

SA-induced NR reduces oxidative stress in WS-pepper plants
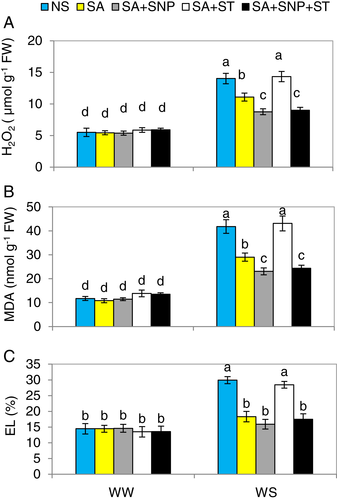
Water stress resulted in an increase in H2O2 and MDA contents and EL ratio, which are known as oxidative stress indicators, by 2.3-, 3.6- and 1.9-fold, respectively, over the levels seen in WW-plants. However, priming of SA reduced these oxidative stress parameters by 42.3, 30.6 and 38.8%, respectively, relative to those in WS-plants (Fig. 6A,C). Moreover, the supplementation of SNP along with SA (SA + SNP) resulted in higher decreases in oxidative stress-related traits in the WS-plants, and the data for EL did not differ compared to WW-treated plants. This is additional proof that externally applied NO as SNP can enhance the favourable effect of SA on WS tolerance in pepper plants. Application of ST abolished the reduced oxidative stress induced due to SA, but SNP application reversed the negative effect of ST, and reinstated the mitigation effect of SA on oxidative damage in the seedlings pre-treated with SA + ST. This shows that SA increases NR activity to reduce oxidative stress by increasing NO as a downstream signal molecule.
SA-induced NR improves AsA and GSH contents in WS-plants
Water stress markedly (P ≤ 0.05) elevated AsA and GSH contents by 141 and 61%, respectively, over the levels in the WW-plants (Fig. 7A,B). Plants supplemented with SA showed elevated AsA and GSH levels by 39 and 56%, respectively, over the-WS-plants. The spray of SNP together with SA resulted in further elevations in AsA and GSH by 68.7 and 97.4%, respectively, compared to the levels in WS-plants.
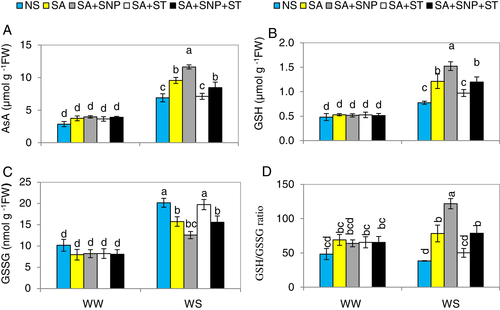
Water stress significantly (P ≤ 0.05) increased oxidised glutathione (GSSG) content by 98% compared to that in WW-plants, but SA and SA + SNP reduced its content by 22 and 37.6% over that in WS-plants (Fig. 7C). The GSH/GSSG ratio was reduced in the plants subjected to WS (Fig. 7D). An increase in GSH/GSSG ratio was obtained by the application of SA and SA + SNP.
Treatment with ST fully abolished the improvement in AsA and GSH, and the reduction in GSSG caused by SA under WS. This shows that NR activity is needed for SA to be effective in enhancing AsA and GSH contents and reducing GSSG content. Externally applied SNP eliminated the adverse effect of ST on the related attributes in plants treated with SA + ST. Conversely, different treatments showed no changes in these metabolites in unstressed plants.
SA-induced NR upregulates AsA-GSH cycle enzymes
To assess if NR activity is participated in the SA-upregulation of AsA-GSH cycle-related enzymes to elevate water stress tolerance in pepper plants, alterations in NR activity and activities of AsA-GSH cycle enzymes were quantified. Water stress substantially (P ≤ 0.05) increased the activities of GR and DHAR by 63.3 and 72.9%, respectively, but decreased APX, and MDHAR by 36.1 and 35.9%, respectively, relative to the activities seen in WW-plants (Fig. 8A–D). Furthermore, the enzyme activities related to AsA-GSH cycle were substantially (P ≤ 0.05) elevated in WS-pepper seedlings sprayed with SA and SA + SNP compared the plants that were only WS. The supplementation of ST to plants sprayed with SA under WS completely impaired the NR activity and the NO synthesis as well as AsA-GSH cycle-related enzyme activities. So, SA could have induced the activity of NR which is a source of production of endogenous NO, and thereby upregulating the AsA-GSH cycle to increase tolerance to WS in pepper. However, the negative effects of ST were eliminated by SNP application in the plants treated with SA + ST. This shows that as long as NO content was maintained in the plants treated with ST, SA can be effective in improving AsA-GSH cycle-related parameters.
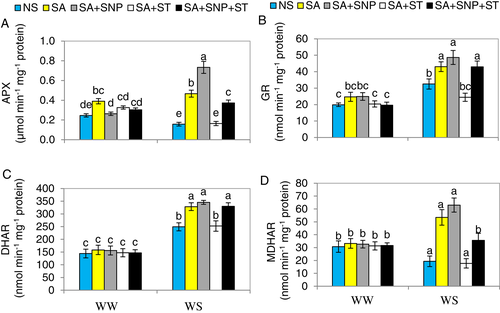
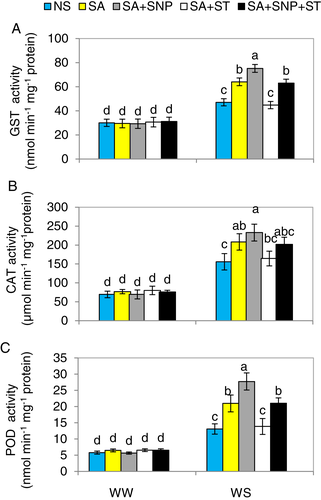
SA-induced NR upregulates antioxidant enzyme activities in pepper under WS
When pepper plants were subjected to WS, GST, CAT and POD enzyme activities were considerably (P ≤ 0.05) elevated by 57, 124 and 127%, respectively, over the activities seen in WW-plants (Fig. 9A–C). Further increases were obtained in the activities when WS-seedlings were supplied with SA and SA + SNP, while pre-treatment with ST plus SA completely eliminated the positive effect of SA on these enzymes. However, SNP application eliminated the impairment effect of ST on these parameters in the plants treated with SA + ST.
SA-induced NR upregulates glyoxalase system enzyme activities and reduces methylglyoxal content in pepper under WS
A significant increase (111%) in Gly I, but a slight reduction in Gly II was observed in plants under WS, compared to those in WW-plants (Fig. 10A,B). Methylglyoxal (MG) content was dramatically increased by 81% in response to WS, relevant to that in WW-plants (Fig. 10C).
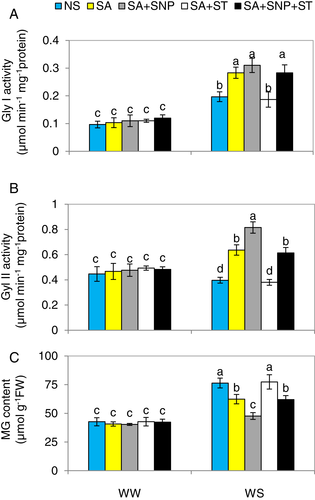
The WS plants pre-treated with SA had significantly higher Gly I and Gly II activities relevant to those in WS-plants alone. The pre-treatment of SA significantly reduced MG content in WS plants. The plants treated with SA + SNP showed higher Gly I and Gly II, and lower MG content than those in plants pretreated with SA alone under WS. However, the positive effects of SA were abolished by ST, demonstrating that NR activity functions together with SA to maintain the glyoxalase system and MG content under WS conditions. This reversing effect of ST was eliminated by SNP when it was applied along with SA + ST under WS conditions.
Discussion
NR mediates NO synthesis in SA-improved plant growth, water relation and proline under WS
This study was mainly aimed to evaluate the contribution of NR to SA-enhanced WS tolerance in pepper plants. The positive effects of SA have been confirmed in other plant species, e.g. in mustard (Nazar et al. 2015b), wheat (Maghsoudi et al. 2019) and barley (El-Samad et al. 2019) under WS, while no study appears to report the potential role of NR in SA-enhanced WS tolerance in plants.
Water stress has been shown to reduce metabolic processes in plants, e.g. plant growth (Mårtensson et al. 2017), water content (Shivakrishna et al. 2018) and oxidative stress (Chakraborty et al. 2015). The main effect of water stress on the plant is the impairment of plant–water relations (Dwivedi et al. 2018), and plants produce osmolytes, such as proline and glycine betaine to get more water from growth medium (Sadak et al. 2019). In this study, RWC and leaf water potential (Ψl) decreased in WS-plants. Accordingly, proline content was elevated under WS condition to sustain the water status within the plant, in accordance with previous studies (Jungklang et al. 2017).
However, pre-treatment with SA elevated leaf RWC, Ψl and proline content of plants subjected to WS, suggesting that SA could improve water content of plants subjected to WS by enhancing proline synthesis. Similarly, Nazar et al. (2015b) reported that SA enhanced the proline content and improved water potential, and thereby amended the osmoregulation in mustard plants under water stress. The possible reason for improved plant growth due to pre-treatment of SA under WS might have been due to fact that SA enhanced proline content and thereby improving water status of plants under WS conditions, as has also been speculated by Nazar et al. (2015b) in mustard plants. The present findings agree with others who also found that priming with SA elevated proline content under WS conditions (Verma et al. 2017, Lee et al. 2019). To provide further proof that the NO and SA work together to enhance WS tolerance of pepper plants, externally SNP, a donor of NO was applied together with SA. The alleviation effect of SA on plant growth and water relation was further improved when SNP was applied along with SA with added enhancement of endogenous NO as proof of that plant growth-related parameters were further increased compared the plants treated with SA alone.
To evaluate if NR participates in the SA-enhanced tolerance to WS in pepper plants, the plants sprayed with SA under WS were also treated with an inhibitor of NR, sodium tungstate (ST), to eliminate NO synthesis/accumulation due to SA. Application of ST reversed SA-induced increased plant growth, showing that ıncreased NR activity because of SA improves plant growth under WS and in the case of inhibiting NR activity, SA shows no positive effect on plant growth. Thus, NR is required for SA-induced improved plant growth under WS. Moreover, these findings reveal that inhibiting NR activity also impaired the synthesis of NO, indicating that NR could be the possible source of NO production under WS. Thus, NO could be a downstream signalling molecule of NR, being activated due to SA under WS. To get further evidence that NO has participated in SA-enhanced tolerance to WS in the pepper plants SNP was supplied in conjunction with SA + ST. Results show that when NO synthesis is provided externally, SA can be effective in improving WS tolerance in pepper plants. Earlier reports showed that exogenously applied NO improved WS stress tolerance of plants (Silveira et al. 2017, Hasanuzzaman et al. 2018a, Munawar et al. 2019) as well as to other stresses, e.g. salinity (Ahmad et al. 2016b), heat (Parankusam et al. 2017) and arsenic (Kushwaha et al. 2019). However, there seems to be no the literature dealing with the involvement of NR in SA-enhanced tolerance to WS.
NR mediates NO synthesis in SA-improved photosynthesis related traits under WS
Water stress impaired photosynthetic parameters, chlorophylls and maximum photochemical efficiency (Fv/Fm) of the pepper plants, as has been observed in Aloe vera L. (Hazrati et al. 2016), Pisum sativum L. (Embiale et al. 2016), Sorghum bicolor L. (Zhang et al. 2019a) and Ceratotheca triloba (Masondo et al. 2019). Chlorophyllase enzyme can dramatically be induced and damage to chlorophyll synthesis under WS conditions (Vanisri et al. 2017); this could be a potential reason for reduced chlorophyll under WS. Moreover, enhanced photosynthetic related attributes and SA-induced reduced H2O2, suggest that SA contributes to mitigating the detrimental effects of WS on photosynthetic attributes, perhaps by depressing the accumulation of H2O2 as reported in strawberry (Akbar Mozafari et al. 2018). Alike, SA has also been observed to increase chlorophyll synthesis in mustard (Nazar et al. 2015b) and wheat (Noreen et al. 2017) under WS. The SA-enhanced Fv/Fm possibly assists the plant to adapt to a harsh environment as reported in Salvia nemorosa plants subjected to drought stress (Habibi 2017).
SA activated the synthesis of NO in pepper plants subjected to WS by triggering NR as mentioned above. However, ST reversed the NR activity induced due to SA in pepper plants subjected to WS and thereby eliminated the synthesis of NO in plants primed with SA under WS, and like so, enhancements in chlorophyll content and Fv/Fm induced by SA were abolished, proposing that NR and NO might jointly contribute to SA-boosted chlorophyll content and Fv/Fm under WS. However, the supplementation of SNP, donor of NO, reversed the negative effect of ST on SA-induced photosynthetic-related parameters by restoring NO content without increasing NR activity. Externally supplied NO elevated chlorophyll content in Brassica napus L. (Akram et al. 2018) and Triticum aestivum L. (Hasanuzzaman et al. 2018a) plants subjected to WS as well as in Zea mays under salt stress (Kaya et al. 2015) and under cadmium toxicity (Yordanova et al. 2017).
NR mediates NO synthesis in SA-improved tolerance to WS of pepper plants
In this study, WS elevated endogenous NO in pepper seedlings, as previously observed in Cucumis sativus (Arasimowicz-Jelonek et al. 2009) and Brassica genotypes (Sahay et al. 2019). These results show that NO might participate as a signalling molecule in many critical physiological phenomena of WS-plants. Moreover, SA resulted in further increases in NO synthesis in plants subjected to WS, showing that NO may act as a scavenger of ROS or an antioxidant to improve WS tolerance of pepper plants. Although a reasonable level of NO is beneficial to improve stress tolerance, over-accumulation of NO can harm plants (Wang et al. 2017). So, a rationale level of NO should be maintained in the plant to attain improved stress tolerance of plants. SA-induced NO generation under WS has been stated by Shan and Wang (2017) in maize plants. In this study, the level of endogenous NO activated due to SA did not exceed the toxic levels to be harmful to physiological processes plants under WS. The treatment of SA + SNP also led to further increase NO contents without any damaging effects as evidently showed that reduced oxidative stress-related parameters and improved antioxidant defence system-related parameters. This data evidently show that SA might mediate endogenous NO synthesis by triggering NR activity. However, the mitigating effect of SA was eliminated by application of ST along with SA pre-treatment by blocking NR activity and thereby reducing the synthesis of NO. As mentioned above, the harmful effect of ST was inverted by externally supplied SNP in the pepper plants treated SA + ST under WS conditions by reinstalling endogenous NO content without increasing NR activity. This suggests that SA is needed NO triggered by NR activity or that provided through externally supplied SNP in improving tolerance to WS. Similar results have been reported that SA promoted NR activity in mustard plants under WS (Nazar et al. 2015b) and rice plants subjected to arsenate toxicity (Singh et al. 2017b).
NR mediates NO synthesis in SA-reversed oxidative stress under WS
Water stress leads to oxidative stress primarily by disturbing electron transfer during the photosynthesis process (Zhang et al. 2019b). The MDA (lipid peroxidation) and H2O2 are well-known indicators of oxidative impairment, which were both found to be higher under WS in this experiment, relevant to WW-plants, indicating higher oxidative impairment in plants under WS. It has similarly been observed that WS-induced oxidative stress, indicating higher MDA and H2O2 by Borjas-Ventura et al. (2019) and Zhang et al. (2019b). Furthermore, under WS condition, electrolyte leakage was higher due to higher MDA and H2O2 as reported previously in durum wheat by Slama et al. (2018). Chances of interrelated traits may cause disruption of ion exchange capacity of the cell membrane and metabolic events connected to the function of the cell membrane (Zahra et al. 2018).
Remarkably, pretreatment of SA and SA + SNP reversed the oxidative stress in the plants under WS by reducing MDA and H2O2 content. NO-induced mitigation of oxidative damage in the plants subjected to WS has been stated in mustard (Nazar et al. 2015b) and wheat (Abbadi et al. 2015). However, the supply of ST eliminated the promoting effect of SA on reversing oxidative stress, since it reduced the activity of NR and synthesis of NO in WS-plants, indicating that NR and NO contribute to SA-induced lowered oxidative stress. This negative effect of ST was eliminated by SNP, showing that restoring NO content can make SA effective in reducing oxidative stress under WS. The response of plants to NO on reduced oxidative stress has earlier been investigated in WS-plants (Zhang et al. 2016, Akram et al. 2018).
NR mediates NO synthesis in SA-upregulated non-enzymatic antioxidants under WS
Further critical mechanism evolved by plants to adopt under WS is to regulate the antioxidant defence system by raising the levels of AsA and GSH metabolites, which in turn act as scavengers of ROS, and maintaining the redox status of the cell (Akram et al. 2017, Han et al. 2020). It has earlier been stated that AsA and GSH are the key scavenging metabolites for scavenging H2O2 in cells of plants (Asgher et al. 2017, Hasanuzzaman et al. 2018b). The current findings obviously reveal that the content of GSH and AsA enhanced to counteract the accumulation of H2O2 to reverse oxidative stress in plants exposed to WS. GSH contributes to scavenging ROS and is converted into GSSG and which in turn increases GSSG levels under WS as found in this experiment. However, SA lowered GSSG and further enhanced AsA and GSH contents similar to which is observed in Arabidopsis (Csiszár et al. 2018) and Vigna angularis (Ahanger et al. 2020) plants under salinity stress. The GSH/GSSG ratio is another key indicator for assessment of stress tolerance of plants. It means that the higher the GSH/GSSG ratio, the higher the stress tolerance of the plant (Nguyen et al. 2019) as observed in this experiment with exogenous supplementation of SA and SA + SNP.
Supplementation of ST reversed the beneficial effect of SA on non-enzymatic antioxidant levels; this was achieved by lowering the content of NR activity and NO content. This adverse effect of ST was abolished by the application of SNP, showing that NO supplied externally leads to reinstall NO content and thereby making SA effective in increasing non-enzymatic antioxidants under WS. The response of plants to NO on ascorbate has earlier been investigated in plants under WS (Munawar et al. 2019).
NR mediates NO synthesis in SA-upregulated the AsA-GSH cycle under WS
Plants can activate the antioxidant defence system by upregulating AsA and GSH to survive under stress conditions (Nanda and Agrawal 2016). These metabolites scavenge ROS and sustain the cellular redox status (Noctor et al. 2018). Water stress increased both AsA and GSH levels in the plants, compared to WW-plant.
The higher glutathione reductase (GR) activity leads to a high level of GSH in cellular organelles under drought conditions (Hasanuzzaman et al. 2017b) as observed in this experiment. Analogous findings were reported in maize subjected to WS by Ahmad et al. (2016a). Interestingly, SA application resulted in a further elevation in the GR activity and the GSH content under WS conditions as similarly observed in mustard by Nazar et al. (2015b). GSH and GSSG ratio maintains physiological processes under optimum and harsh conditions (Nahar et al. 2016). However, an accumulation of oxidised glutathione (GSSG) was observed under environmental stress, and so the GSH/GSSG ratio is lower (Nahar et al. 2016), as observed in this experiment under water stress. SA application enhanced the GSH/GSSG ratio by decreasing GSSG and increasing GSH content under water stress.
Ascorbate peroxidase (APX) needs AsA for scavenging H2O2 to water (Sharma 2016). Water stress reduced APX activity in plants, as similarly observed in rapeseed plants (Hasanuzzaman et al. 2017a) and maize (Ahmad et al. 2017) under WS, but some conflict data have also been observed in Brassica napus (Lotfi et al. 2015) and soybean (Xing et al. 2016) wherein WS enhanced the activity of APX. However, SA noticeably increased APX activity in Thymus daenensis under WS conditions (Bahari et al. 2015) as well as in Nitraria tangutorum plants under salinity (Yan et al. 2018).
DHAR is a AsA-GSH cycle-related enzyme which converts DHA to AsA (Ding et al. 2020). Water stress enhanced the activity of DHAR in the pepper seedlings. Similar results have already been reported in Agropyron cristatum (Shan and Liang 2010) and Artemisia annua (Soni and Abdin 2017) under WS. The application of SA caused further elevation in DHAR activity in mustard (Alam et al. 2013) as is also seen in this study.
Monodehydroascorbate reductase (MDHAR) plays a critical role in sustaining a reduced pool of AsA and ascorbate redox state (Wang et al. 2018). (MDHAR) was decreased in the WS-pepper plants, similarly, observed in rapeseed (Hasanuzzaman et al. 2017a, b). The application of SA increased the activity of MDHAR in the pepper seedlings, as observed similarly in mustard plants (Alam et al. 2013).
Treatment of ST, the inhibitor of NR, together with SA eliminated the regulation of the AsA-GSH pathway-allied enzymes presented above. This obviously confirms that NR activity is required for SA-enhanced water stress tolerance in the pepper seedlings. This seems to be the first study in the literature, reporting that SA induces NR activity and so it elevates the AsA-GSH cycle to enhance WS tolerance. Externally applied NO improved the AsA-GSH cycle in tomato under water stress (Wu et al. 2011) and wheat (Shan et al. 2015). These findings suggest the idea that NR activity induced by SA and so NR triggered NO could increase water stress tolerance of pepper plants.
NR mediates NO synthesis in SA-upregulated antioxidant defence system under WS
Plants have evolved the antioxidant defence system to control the over-generation of ROS within the cell (Nahar et al. 2016, Hasanuzzaman et al. 2017b). So, in this experiment, the role of SA in upregulating the antioxidant defence system by appraising antioxidant enzyme activities has also been assessed. Glutathione S-transferase (GST) activity increased in this experiment, as observed previously in rapeseed (Hasanuzzaman et al. 2017a) under WS. GST can scavenge H2O2 by using glutathione as a substrate (Wahibah et al. 2018). Further increases in GST were obtained due to SA under WS, suggesting an affirmative role of GST in scavenging H2O2 as stated in rapeseed treated with NO under WS (Hasanuzzaman et al. 2017a). Abiotic stress tolerance can be improved by the upregulation of antioxidant enzymes to retain ROS levels below the threshold level (Arora et al. 2016). So, tolerance to WS in pepper plants could be linked with SA-induced upregulation of antioxidant enzymes. SA may play a key role in scavenging ROS by activating NR, source of NO, as has been shown that using ST reduced GST activity, but increased H2O2 levels.
CAT activity increased under WS in pepper plants, similarly, reported in Coffea canephora (Lima et al. 2002) and Curcuma alismatifolia (Jungklang et al. 2017) under WS. Plants sprayed with SA had higher CAT activity under water stress. These findings are similar to those reported in Thymus daenensis (Bahari et al. 2015) and cowpea plants (Dutra et al. 2017). Another enzyme participating in scavenging H2O2 is CAT (Das and Roychoudhury 2014, in this study we show that SA application increased CAT activity and reduced H2O2.
Another antioxidant enzyme tested in this experiment is POD which showed an increase in plants subjected to WS, as previously observed in Brassica napus (Lotfi et al. 2015) and wheat (Qayyum et al. 2018). The application of SA resulted in further increase in POD activity under water stress. It has previously been shown that SA increased POD activity in wheat (Yavas and Unay 2016, Maghsoudi et al. 2019) under WS.
The upregulated effect of SA on the antioxidant enzyme's activities was eliminated by pretreatment with ST by reducing NR activity and endogenous NO content, suggesting that endogenous NO accumulation and NR activity participate in SA-upregulated antioxidant defence system to improve tolerance to WS in pepper plants. Previous results showed that exogenously applied NO upregulated GST enzyme activity in rapeseed (Hasanuzzaman et al. 2017a) and wheat (Hasanuzzaman et al. 2018a) under water stress. Furthermore, it has been shown that NO enhanced the activity of CAT in Cakile maritima seedlings (Jday et al. 2016) and POD activity in broccoli (Munawar et al. 2019) under water stress.
NR mediates NO synthesis in SA-upregulated glyoxalase system under WS
The Gly I and Gly II enzymes are part of the glyoxalase system scavenging over-accumulation of methylglyoxal (MG) under stress conditions (Li et al. 2017, Hasanuzzaman et al. 2019). Under water stress conditions, MG accumulation in plants has well been reviewed by Hasan et al. (2016) and recently reported in wheat (Hasanuzzaman et al. 2018a). Water stress elevated the activity of Gly I but reduced that of Gly II, as is also reported in rapeseed (Hasanuzzaman et al. 2017a) and mung beans (Nahar et al. 2015). The application of SA enhanced both enzyme activities, showing the involvement of SA in tolerance to WS by improving the glyoxalase system. SA-upregulated Gly I and Gly II have been stated in mustard seedlings under WS (Alam et al. 2013) as similarly observed in this experiment.
The pretreatment with ST eliminated the upregulation effect of SA on the glyoxalase system by blocking NR activity and NO synthesis in plants under WS. It has well been established the positive role of exogenously applied NO in upregulating glyoxalase system in rapeseed (Hasanuzzaman et al. 2017a) and wheat (Hasanuzzaman et al. 2018a) under WS.
Conclusions
Overall, the pretreatment with ST together with SA reversed the beneficial effects of SA, and caused SA being ineffective in enhancing WS tolerance. Both NR and NO are required for SA to enhance WS tolerance in pepper plants. Furthermore, NR induced by SA could play a key role in upregulating AsA-GSH cycle and glyoxalase system as well as reversing oxidative stress. In the future, the involvements of other biomolecules in SA-induced tolerance to WS and other stressors also need to be studied.
Author Contributions
C.K. set up the experiment, carried out data analysis and also wrote up the manuscript.
Acknowledgements
The author wishes to thank the University of Harran (Turkey) for partly supporting this study, particularly for supplying all chemicals required for the experimentation.
Open Research
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




