Optimal water and fertilizer applications improve growth of Tamarix chinensis in a coal mine degraded area under arid conditions
Edited by M. Ahanger
Abstract
Coal-mined areas are often associated with hostile environmental conditions where the scarcity of water and key nutrient resources negatively affect plant growth and development. In this study we specifically addressed how different combinations of water (W), nitrogen (N) and phosphorus (P) might affect morpho-physiological and biochemical attributes of a native shrub species, Tamarix chinensis, grown on coal mine spoils. Our results show that under greenhouse conditions the application of moderate-to-high doses of W, N and P considerably improved growth-associated parameters (i.e. plant height, stem diameter, dry weight), as well as gas-exchange parameters, photosynthetic pigment contents and leaf water status of T. chinensis. Under field conditions high W and low N, P doses led to significant increases in plant growth-associated traits, gas-exchange parameters and leaf water status. Plant growth was generally higher under greenhouse conditions mainly because seedlings faced multiple stress when growing under field conditions. Low W-regime, regardless of N-P additions, improved osmotic adjustments in leaf tissues and also boosted the activity of several antioxidant enzymes to reduce the oxidative stress associated with W scarcity under greenhouse conditions. Importantly, our study shows how maximum growth performance of T. chinensis under field conditions was achieved at W, N and P doses of 150 mm year−1, 80 kg ha−1 and 40 kg ha−1, respectively. Our findings suggest that achieving optimal rates of W, N and P application is crucial for promoting the ecological restoration of coal-mined areas with T. chinensis under arid environmental conditions.
Abbreviations
-
- APX
-
- ascorbate peroxidase
-
- Cars
-
- carotenoids
-
- CAT
-
- catalase
-
- CCD
-
- central composite design
-
- Chl
-
- chlorophyll
-
- Ci
-
- intercellular concentration of CO2
-
- FC
-
- field capacity
-
- Gs
-
- stomatal conductance
-
- IGP
-
- integrated growth performance
-
- LWP
-
- leaf water potential
-
- MSI
-
- membrane stability index
-
- Pn
-
- net photosynthesis rate
-
- POD
-
- peroxidase
-
- RSM
-
- response surface methodology
-
- RWC
-
- relative water content
-
- SP
-
- soluble protein
-
- SS
-
- soluble sugar
-
- Tr
-
- transpiration rate
-
- W
-
- water
-
- WUE
-
- water use efficiency
Introduction
Opencast coal mining activities cause significant environmental degradation and are also associated with the problematic discharge of coal mine spoils, which further enhance environmental pollution and reduce the ecological status of coal mining areas (Kuka et al. 2013, Fan et al. 2018). In northwestern China there are abundant coal reserves, which contribute to approximately 70% of China's total coal production (Fan et al. 2018). Excessive coal mining activities in this region have caused severe ecological and environmental problems including landscape degradation (Lv et al. 2019), surface subsidence and coal gangue accumulation (Hu et al. 2015), modification of the natural environment (Lv et al. 2019), destruction of native vegetation and the transformation of coal-mining areas into deserts (Hu et al. 2015). Northwestern China is also considered an ecologically fragile zone due to the dominance of arid environments, which make ecological restoration more difficult to implement (Fan et al. 2018, Lv et al. 2019). Revegetation interventions in northwestern China remain crucial for restoring natural ecosystems and controlling or reducing desertification processes (Hu et al. 2015).
Water-shortage is one of the most important abiotic stress factors affecting vegetation restoration and ecological succession in abandoned coal mine ecosystems (Zhao et al. 2015). Plant species adapted to desert environments are more suitable for restoration programs because these plants have already evolved a number of physiological and biochemical mechanisms to cope with water stress (Roy et al. 2020). Physiological changes include, for example, a reduction of photosynthesis and transpiration rates (Zhang et al. 2016). Moreover, under water stress conditions, plants accumulate a large amount of reactive oxygen species (ROS), which are dangerous for cellular components such as proteins, nucleic acids and lipids. To counteract ROS-induced damages, plants enhance the response of antioxidant defense systems by upregulating the activities of several enzymes, including superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and ascorbate peroxidase (APX). Additionally, plants also accumulate a number of osmoprotectants, such as proline (Pro) and soluble sugars (SS) to protect the cellular compartments from water-deficiency associated adverse effects (Roy et al. 2020).
The adoption of sustainable and economically effective measures is essential to improve drought resistance of native plant species for the ecological restoration of coal mining areas. Previous studies suggest that suitable amounts of nitrogen (N) and phosphorus (P) fertilizers could improve plants' stress resistance in desert areas and also increase seedling establishment (Zhou et al. 2011, Li et al. 2019). However, it has been shown that the overuse of N and P in either grassland or agricultural areas can not only increase management costs but can also cause environmental contamination through leaching and volatilization of N and runoff of P (Hanifzadeh et al. 2017). Hence, the application of suitable amounts of water (W), N and P to support plant growth without creating adverse effects on seedlings establishment is a crucial step for promoting sustainable development in dryland coal mining areas, an issue which remains high on both the ecological and political agenda.
Suitable W, N and P application rates could be estimated by using optimization techniques that allow maximum growth and development of plants under specific W, N and P doses. For example, response surface methodology (RSM) is a useful mathematical and statistical method, which has been used to develop, improve, and optimize processes and to determine the optimum values of process variables (Roy et al. 2020).
RSM has been used to examine the combined effects of water and nutrient fertilizers on the growth and development of several plant species including Camellia sinensis (Wang et al. 2016), Catalpa bungei (Qiu et al. 2018) and Oryza sativa (Liu et al. 2019). However, very few studies have so far focused on the simultaneous effects that water and nutrient fertilizers might have on the growth performance of desert shrub species in coal mining areas. This is crucial if we want to develop appropriate strategies to support ecological restoration in coal mining areas using suitable plant species. To contribute to reduce this research gap, we set a number of experiments to test the performance of a native shrub species, Tamarix chinensis, which is commonly found in northwestern arid regions of China and plays a significant role in maintaining and restoring desert ecosystems (Zhang et al. 2016). This plant has ecological adaptation potentials for sand fixing, wind defense, and soil and water conservation in northwestern China (Zhang et al. 2016). Moreover, T. chinensis adopts a series of physiological and biochemical strategies to manage the detrimental effects of abiotic stresses (Zhang et al. 2016, Gao et al. 2017). Based on this evidence, we experimentally addressed morphological, physiological and biochemical responses of T. chinensis to different combinations of W, N and P doses where plants were grown on coal-mined spoils either in pot experiments or under field conditions. Finally, we searched for an optimal combination of rates of W, N and P application, which could improve the growth performance of T. chinensis during revegetation and ecological restoration in arid, degraded coal mining areas.
Materials and methods
Greenhouse experiment
A pot experiment was carried out inside a plastic shed (with no temperature controlling) at the Northwest Agriculture and Forestry University, (N 34°16′, E 108°4′), China to evaluate the growth performance of T. chinensis treated with different combination rates of W, N and P. Mean annual temperature, precipitation, relative humidity and photosynthetically active radiation of the experimental site (outside the plastic shed) were 13.7°C, 650 mm, 59% and 2450 MJ m−2, respectively. Coal mine spoil samples were collected from Yangchangwan coal mining area in Lingwu, Ningxia of China (106° 35′–106° 38′E, 37° 59′–38° 03´N). Average annual temperature and evaporation rates in Lingwu, Ningxia were 8.6°C and 2682 mm, respectively. Mean annual precipitation, relative humidity and photosynthetically active radiation of Lingwu, Ningxia were 245 mm, 50% and 2870 MJ m−2, respectively (https://www.timeanddate.com/weather/china/yinchuan/climate). Annual average and maximum wind speeds were 3.1 and 23.3 m s−1, respectively. The frost-free period lasted for 145 days. Mine spoils were sampled according to the method followed by Sikdar et al. (2020). Collected materials were homogenized followed by bulking, air drying, manual crushing and sieving through 2 mm mesh. The chemical properties of spoils are listed in Table S1.
One-year-old T. chinensis seedlings with similar height (105.55 ± 14.58 cm) and stem diameter (5.91 ± 0.98 mm) were collected from a forestry nursery, in Inner Mongolia, China. Each plastic pot (32 cm top diameter, 27 cm bottom diameter and 30 cm height) was filled with 14 kg of coal mine spoils and one seedling was planted in each pot in early March 2018. Sufficient water was supplied daily to the seedlings to ensure their normal growth in the first month and after that the water-stress treatment started according to the experimental design and continued up to October 2018. To maintain the water levels at field capacity for each water stress treatment, pots were watered daily by a weighing method following the process reported by Roy et al. (2020). The pots with seedling were randomly rearranged fortnightly to minimize the possible positional effects. Fertilizers (N and P) were added in holes close to the seedlings' root zone. The N fertilizer (urea-46% N) was added as granular form in four times (¼ May 6th, ¼ June 6th, ¼ July 6th and ¼ August 6th). P fertilizer (triple super phosphate-46% P2O5) was added also as granular form in two equals halves (1/2 May 6th and 1/2 July 6th) during the experimental period. We did not add K or other elements such as Mg, Ca or Fe in our experiment because our soils have relatively high levels of K (Table S1).
Experimental design
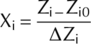 (())
(())| Independent variables | Codes | Coded and actual values | ||||
|---|---|---|---|---|---|---|
| −1.682 | −1 | 0 | +1 | +1.682 | ||
| Very low/ no dose | Low dose | Moderate dose | High dose | Very high dose | ||
| Soil water (W) (% FC) | A | 40 | 48.1 | 60 | 71.9 | 80 |
| Nitrogen (N) (g plant−1) | B | 0 | 0.34 | 0.84 | 1.34 | 1.26 |
| Phosphorus (P) (g plant−1) | C | 0 | 0.51 | 1.26 | 2 | 2.52 |
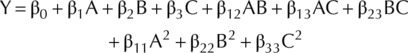 (())
(())| Treatments | Coded level of factors | Quantity applied | ||||
|---|---|---|---|---|---|---|
| Soil water (W) | Nitrogen (N) | Phosphorus (P) | W (% FC) | N (g plant−1) | P (g plant−1) | |
| W71.9N1.34P2 | 1 | 1 | 1 | 71.9 | 1.34 | 2 |
| W71.9N1.34P0.51 | 1 | 1 | −1 | 71.9 | 1.34 | 0.51 |
| W71.9N0.34P2 | 1 | −1 | 1 | 71.9 | 0.34 | 2 |
| W71.9N0.34P0.51 | 1 | −1 | −1 | 71.9 | 0.34 | 0.51 |
| W48.1N1.34P2 | −1 | 1 | 1 | 48.1 | 1.34 | 2 |
| W48.1N1.34P0.51 | −1 | 1 | −1 | 48.1 | 1.34 | 0.51 |
| W48.1N0.34P2 | −1 | −1 | 1 | 48.1 | 0.34 | 2 |
| W48.1N0.34P0.51 | −1 | −1 | −1 | 48.1 | 0.34 | 0.51 |
| W80N0.84P1.26 | 1.682 | 0 | 0 | 80 | 0.84 | 1.26 |
| W40N0.84P1.26 | −1.682 | 0 | 0 | 40 | 0.84 | 1.26 |
| W60N1.68P1.26 | 0 | 1.682 | 0 | 60 | 1.68 | 1.26 |
| W60N0P1.26 | 0 | −1.682 | 0 | 60 | 0 | 1.26 |
| W60N0.84P2.52 | 0 | 0 | 1.682 | 60 | 0.84 | 2.52 |
| W60N0.84P0 | 0 | 0 | −1.682 | 60 | 0.84 | 0 |
| W60N0.84P1.26 | 0 | 0 | 0 | 60 | 0.84 | 1.26 |
| W60N0.84P1.26 | 0 | 0 | 0 | 60 | 0.84 | 1.26 |
| W60N0.84P1.26 | 0 | 0 | 0 | 60 | 0.84 | 1.26 |
| W60N0.84P1.26 | 0 | 0 | 0 | 60 | 0.84 | 1.26 |
| W60N0.84P1.26 | 0 | 0 | 0 | 60 | 0.84 | 1.26 |
| W60N0.84P1.26 | 0 | 0 | 0 | 60 | 0.84 | 1.26 |
| Control | −1.682 | −1.682 | −1.682 | 40 | 0 | 0 |
Here, Y represents response variables; β0 determine constant-coefficient; β1, β2 and β3 represents interpret linear coefficients; β12, β13 and β23 represents interaction coefficients; β11, β22 and β33 determine quadratic coefficients; A, B and C represents coded value of W, N and P.
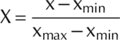 (())
(())In Eqn 3, X is the coded value for each treatment, x is the measured value of each treatment, xmin is the minimum, and xmax is the maximum value recorded for each parameter for different treatments.
Field experiment
A field experiment in the Yangchangwan coal mining area was conducted to assess the effects of W, N and P application rates on growth and development of T. chinensis. W, N and P application rates were selected based on evidence from our pot experiment as well as from several previous studies. Schachtsiek et al. (2014) recommended that the application of 155 mm year−1 is an effective strategy for early survival and growth of six afforestation desert shrub species including T. chinensis in abandoned degraded areas. Xu et al. (1998) concluded that effective water supply for artificial revegetation and desertification control with T. chinensis on the northwestern region of China was 115–150 mm year−1. Moreover, water levels from 80 to 160 mm year−1 were previously applied in several studies focusing on the establishment of desert shrub species in degraded areas of northwestern region of China (Khamzina et al. 2008, Li et al. 2016, Zhang et al. 2017). Specifically a L-4 orthogonal array design was assigned with two levels of W addition (W150, 150; and W100, 100 mm year−1), two levels of N addition (N160, 160; and N80, 80 kg ha−1) and two levels of P addition (P80, 80 kg ha−1; and P40, 40 kg ha−1). In May 2019, 20 plots measuring 4 × 4 m were established with five experimental treatments: (1) W at 150 mm year−1 + N at 160 kg ha−1 + P at 80 kg ha−1 (W150N160P80); (2) W at 150 mm year−1 + N at 80 kg ha−1 + P at 40 kg ha−1 (W150N80P40); (3) W at 100 mm year−1 + N at 160 kg ha−1 + P at 40 kg ha−1 (W100N160P40); (4) W at 100 mm year−1 + N at 80 kg ha−1 + P at 80 kg ha−1 (W100N80P80); and (5) W at 100 mm year−1 without N and P (control). In our study, we added low W level and no N and P fertilizers to the control treatment seedlings in both greenhouse and field experiments. We followed this approach to guarantee seedling survival based on indications from previous studies under similar environmental conditions (Li et al. 2016, Wang et al. 2016, Zhang et al. 2017). Under field conditions, each treatment was replicated four times (12 seedlings per each replicate i.e., 48 seedlings per treatment), separated by 2-m buffers and randomly assigned to plots within each block. N and P were applied in two halves (1/2 June 10th and 1/2 August 10th) during the experimental period. Beside natural precipitation, additional 100 and 150 mm water was added to experimental plots between early-May and end of October. Specifically, seedlings were watered every week in doses of 5–7.5 mm of water per meter square if an effective precipitation event (> 5 mm) did not occur within 7 days. Watering was suspended if significant rainfall occurred. During each of five consecutive monthly irrigation events we added 15, 20, 20, 20, 15 and 10 mm of water, respectively, to give a total of 100 mm for specific plots. Similarly, we added 22.5, 37.5, 37.5, 37.5, 22.5 and 15 mm of water each month to give a total of 150 mm to other plots between May and October 2019. Watering was carried out using a pump-line injector system for a total of 20 times during the experimental period. After 6 months of transplantation (October, 2019), eight representative seedlings from each treatment were randomly selected and harvested for determination of tap root length and biomass production (fresh and dry weight). The rest of the seedlings were allowed to grow further for revegetation and restoration of coal mine degraded spoils in Yangchangwan coal mining area of Ningxia, China.
Measurement of growth parameter
Plant morphological, physiological and biochemicals measurements for T. chinensis were taken at the end of the experimental period in both pot and field experiments.
Assessment of morphological parameters
Growth analysis of T. chinensis was performed by measuring plant height, stem diameter, maximum root length of the main tap root, plant fresh weight (FW) and plant dry weight (DW). The plant DW was measured by drying plant samples in an oven at 80°C for 72 h. The root-shoot (R/S) biomass ratio (dry weight basis) was calculated by dividing root and shoot biomass.
Measurements of physio-biochemical constituents and antioxidant enzyme activities
Leaf gas exchange parameters such as net photosynthesis rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), the intercellular concentration of CO2 (Ci) and water use efficiency (WUE, as Pn/Tr) were measured on sunny days between 08:30 and 11:30 h by a portable photosynthesis system CIRAS-3 (PP Systems). To avoid systematic bias in gas exchange parameter changes due to temporal differences, we measured leaf gas exchange parameters using an alternate model of different leaves in different plants, which ensured the comparability and accuracy of the measured data between different treatments. For three seedlings within each treatment, we randomly selected 2–3 healthy, mature, sun-exposed leaves in the mid-upper canopy of each plant as our sample for fixed observations. Each blade was measured three times. Given the irregular leaf shape of T. chinensis, we distributed the selected leaves throughout the leaf chamber as much as possible to obtain representative data. The plant leaves were removed and scanned with a scanner. The actual leaf surface area within the gas exchange cuvette was estimated using a LiCor 3100 leaf area meter (LiCor), and the photosynthetic parameters were recalculated based on the actual photosynthetically active area.
Chlorophyll (Chl) was extracted from fully expanded 0.1 g fresh leaves using a 10 ml mixture of ethanol, acetone and distilled water (4.5:4.5:1) according to the technique described by Roy et al. (2020). The absorbance of the extracts was recorded at 645, 663 and 470 nm, and the concentration of Chl a, Chl b, total Chls and catotenoids (Cars) were calculated as mg g−1 FW according to the formula of Arnon (1949).
Leaf water potential (LWP) was measured with the help of plant water potential meter (PMS-Model 1000, PMS Instrument Company) before morning 06:00. Relative water content (RWC) and membrane stability index (MSI) of T. chinensis leaves were measured according to the method described by Bandeppa et al. (2019). Free proline (Pro) from fresh leaf samples (0.1 g) was extracted using a sulphosalicylic acid solution and measured with a ninhydrin solution, according to Bates et al. (1973). The content of total soluble sugar (SS) and soluble protein (SP) were colorimetry-measured using anthrone-H2SO4 (Joseph 1955) and Bradford (1976) methods, respectively. Glucose and bovine serum albumin were used to develop standard curves for SS and SP, respectively.
The method from Roy et al. (2020) was adopted to extract, and measure the content of malondialdehyde (MDA) in leaf samples of T. chinensis. The superoxide anion (O2●–) was estimated by testing the formation of nitrite from hydroxylamine, as described by Ke et al. (2002). Hydrogen peroxide (H2O2) content was measured according to Velikova et al. (2000).
Fresh T. chinensis leaf samples (0.3 g) were homogenized in a mortar and pestle using 8 ml of 50 mM sodium phosphate buffer (pH 7.8), followed by centrifugation at 10 000g for 20 min at 4°C. The enzyme extracts obtained were used for the estimation of enzyme activities (Roy et al. 2020). The superoxide dismutase (SOD) activity was assayed following the method from Roy et al. (2020). The catalase (CAT) activity was measured based on the method of Beers and Sizer (1952). The peroxidase (POD) activity was carried out using the guaiacol oxidation method described by Ekmekci and Terzioglu (2005). Ascorbate peroxidase (APX) activity was determined following Nakano and Asada (1980).
Data analysis
Optimum water and fertilizer rates were obtained from an optimization process combined with the derringer's desired function approach using Design Expert statistical software version 11 (Stat-Ease). Origin 2018 (OriginLab Inc.) was used to perform principal component analysis of the traits. A heatmap was generated using the https://biit.cs.ut.ee/clustvis/online program package with Euclidean distance as the similarity measure, and hierarchical clustering with complete linkage heatmap summarized all the plant responses to the different combination of W, N and P treatments.
Results
Greenhouse experiment
Analysis of variance (anova) results are presented in Table S3. The models for different growth parameters had P-values of less than 0.0001, except for the root-shoot ratio (R/S). In contrast, lack of fit P-values was non-significant (greater than 0.05) for all models. Among different models (linear, interactive [2FI], quadratic, and cubic polynomial), the quadratic model was found to be the most suitable model for growth parameters and integrated growth performance (IGP) of T. chinensis (except plant dry weight (DW), R/S, leaf water potential (LWP) and catalase (CAT) activity) (Table S3). The differences between predicted R-squared (R2p) and adjusted R-squared (R2a) values were less than 0.2, indicating a good agreement between them (Roy et al. 2020; Table S3). A low CV value (less than 10%) for most of the growth parameters as well as IGP of T. chinensis (except H2O2 content) designated that the deviations between predicted and experimental values were very low, thus suggesting a reproducibility of the experimental model (Table S3). Finally, an adequate precision value above 4 suggests that the models predicted by the software could also be favorably used (Table S3).
Morphological parameters
The addition of W, N and P caused a significant increase in plant height and stem diameter in all experimental treatments compared to control. The maximum increase in plant height and stem diameter were observed under W60N1.68P1.26 treatment (W at 60% field capacity [FC], N at 1.68 g plant−1 and P at 1.26 g plant−1). Here plant height and stem diameter were 141.7 and 125.4% greater, respectively, than those of control seedlings (Table 3). DW dramatically increased from 23.75 to 34.18 g plant−1 when W level increased from 40% FC (W40N0.84P1.26) to 80% FC (W80N0.84P1.26; Table 3). The root: shoot ratio (R/S) significantly increased when seedlings were treated with W71.1N1.34P2 (+8.8%) and W71.1N1.34P0.51 (+10.5%) and decreased with W48.1N0.34P2 (−10.5%) compared to control seedlings (Table 3).
| Treatments | Plant height | Stem diameter | DW | Pn | Tr | WUE | Chl a | Chl b | Total Chls | Cars | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| cm | mm | g plant−1 | R/S | μmol m−2 s−1 | mmol m−2 s−1 | μmol mmol−1 | mg g−1 FW | mg g−1 FW | mg g−1 FW | mg g−1 FW | |
| W71.9N1.34P2 | 20.33 ± 1.39abc | 1.33 ± 0.09abc | 32.44 ± 2.66abc | 0.62 ± 0.06a | 11.5 ± 0.52bcde | 3.58 ± 0.02ij | 3.21 ± 0.15ab | 0.75 ± 0.05a | 0.31 ± 0.04a | 1.06 ± 0.08a | 0.28 ± 0.02ab |
| W71.9N1.34P0.51 | 21.13 ± 2.47ab | 1.37 ± 0.01ab | 31.86 ± 0.55abc | 0.63 ± 0.09a | 10.46 ± 0.92cde | 3.83 ± 0.07hi | 2.73 ± 0.24bcd | 0.69 ± 0.05ab | 0.29 ± 0.02ab | 0.98 ± 0.07ab | 0.23 ± 0.01cd |
| W71.9N0.34P2 | 14.23 ± 0.46efgh | 1.25 ± 0.01abcd | 32.98 ± 0.23ab | 0.58 ± 0.02ab | 9.75 ± 0.25de | 3.88 ± 0.03ghi | 2.52 ± 0.07def | 0.47 ± 0.02fgh | 0.21 ± 0.01defg | 0.68 ± 0.02fghi | 0.19 ± 0.01ef |
| W71.9N0.34P0.51 | 17.13 ± 1.14bcdef | 1.38 ± 0.02ab | 32.25 ± 0.7abc | 0.52 ± 0.03ab | 11.83 ± 0.96bc | 5.21 ± 0.1d | 2.27 ± 0.17defg | 0.43 ± 0.01h | 0.18 ± 0.01fg | 0.61 ± 0.01i | 0.16 ± 0.01f |
| W48.1N1.34P2 | 16.93 ± 0.98bcdef | 1.18 ± 0.01bcde | 26.83 ± 1.91defg | 0.53 ± 0.01ab | 9.95 ± 1.06cde | 3.03 ± 0.06k | 3.29 ± 0.38a | 0.46 ± 0.01fgh | 0.19 ± 0.01fg | 0.65 ± 0.01ghi | 0.17 ± 0.01ef |
| W48.1N1.34P0.51 | 16.6 ± 2.07cdef | 1.02 ± 0.07ef | 24.92 ± 0.22fg | 0.59 ± 0.01ab | 10.43 ± 0.27cde | 3.89 ± 0.08ghi | 2.68 ± 0.07bcde | 0.59 ± 0.01de | 0.24 ± 0.01bcde | 0.83 ± 0.01de | 0.22 ± 0.01cd |
| W48.1N0.34P2 | 11.83 ± 1.19ghi | 1.08 ± 0.05def | 27.66 ± 0.22defg | 0.47 ± 0.02b | 5.61 ± 0.45f | 2.21 ± 0.08l | 2.55 ± 0.27cdef | 0.49 ± 0.02fgh | 0.2 ± 0.01efg | 0.69 ± 0.02fghi | 0.17 ± 0.01ef |
| W48.1N0.34P0.51 | 13.43 ± 2.85fghi | 0.92 ± 0.07f | 25.82 ± 2.04efg | 0.55 ± 0.02ab | 9.69 ± 0.42de | 4.3 ± 0.28f | 2.26 ± 0.05defg | 0.61 ± 0.02cd | 0.23 ± 0.01cdef | 0.84 ± 0.02cde | 0.24 ± 0.01bc |
| W80N0.84P1.26 | 17.17 ± 1.67bcdef | 1.15 ± 0.07cde | 34.18 ± 0.73a | 0.6 ± 0.07ab | 12.53 ± 0.24ab | 4.08 ± 0.03fgh | 3.07 ± 0.07abc | 0.61 ± 0.02cd | 0.26 ± 0.01abcd | 0.87 ± 0.02cd | 0.24 ± 0.01c |
| W40N0.84P1.26 | 11.35 ± 1.82hi | 0.64 ± 0.02g | 23.75 ± 3.06gh | 0.54 ± 0.06ab | 9.54 ± 1.05e | 3.06 ± 0.02k | 3.11 ± 0.32ab | 0.51 ± 0.02fg | 0.21 ± 0.01defg | 0.72 ± 0.02fgh | 0.18 ± 0.01ef |
| W60N1.68P1.26 | 23.2 ± 1.15a | 1.42 ± 0.02a | 29.21 ± 0.2bcde | 0.6 ± 0.06ab | 12.68 ± 1.03ab | 4.16 ± 0.07fg | 3.06 ± 0.27abc | 0.45 ± 0.01gh | 0.17 ± 0.01g | 0.62 ± 0.01hi | 0.18 ± 0.01ef |
| W60N0P1.26 | 14.7 ± 0.52defgh | 1.26 ± 0.01abcd | 28.88 ± 0.18cde | 0.55 ± 0.11ab | 10.35 ± 0.75cde | 4.85 ± 0.03e | 2.14 ± 0.17fg | 0.24 ± 0.01i | 0.11 ± 0.01h | 0.35 ± 0.01j | 0.09 ± 0.01g |
| W60N0.84P2.52 | 15.83 ± 0.63defg | 1.41 ± 0.11a | 30.32 ± 1.41abcd | 0.56 ± 0.01ab | 7.32 ± 0.58f | 2.84 ± 0.05k | 2.58 ± 0.17cdef | 0.67 ± 0.01bc | 0.27 ± 0.01abc | 0.94 ± 0.01bc | 0.25 ± 0.02bc |
| W60N0.84P0 | 18.6 ± 0.68bcd | 1.34 ± 0.06abc | 28.51 ± 3.67cdef | 0.55 ± 0.01ab | 9.63 ± 1.19de | 4.94 ± 0.04de | 1.95 ± 0.25g | 0.73 ± 0.01ab | 0.29 ± 0.01ab | 1.02 ± 0.01ab | 0.29 ± 0.03a |
| W60N0.84P1.26 | 17.6 ± 1.16bcdef | 1.37 ± 0.15ab | 28.98 ± 2.07cde | 0.54 ± 0.01ab | 12.96 ± 1.39ab | 6.05 ± 0.11bc | 2.14 ± 0.22fg | 0.51 ± 0.01fg | 0.21 ± 0.01efg | 0.72 ± 0.01fgh | 0.19 ± 0.01de |
| W60N0.84P1.26 | 17.77 ± 1.3bcde | 1.38 ± 0.03ab | 30.13 ± 1.45bcd | 0.59 ± 0.03ab | 12.71 ± 0.24ab | 6.12 ± 0.03bc | 2.08 ± 0.04fg | 0.48 ± 0.01fgh | 0.19 ± 0.01efg | 0.67 ± 0.01fghi | 0.18 ± 0.01ef |
| W60N0.84P1.26 | 16.87 ± 1.34bcdef | 1.41 ± 0.04a | 29.57 ± 0.26bcde | 0.56 ± 0.01ab | 13.32 ± 0.25ab | 6.38 ± 0.05ab | 2.09 ± 0.05fg | 0.53 ± 0.03ef | 0.23 ± 0.01cdef | 0.76 ± 0.05ef | 0.16 ± 0.01ef |
| W60N0.84P1.26 | 18 ± 2.02bcde | 1.41 ± 0.01a | 28.63 ± 0.55cdef | 0.53 ± 0.01ab | 12.57 ± 0.07ab | 5.92 ± 0.11c | 2.12 ± 0.04fg | 0.5 ± 0.02fgh | 0.2 ± 0.02efg | 0.7 ± 0.04fghi | 0.18 ± 0.01ef |
| W60N0.84P1.26 | 17.27 ± 0.81bcdef | 1.35 ± 0.01abc | 31.77 ± 0.55abc | 0.58 ± 0.01ab | 11.6 ± 0.21bcd | 5.94 ± 0.21c | 1.95 ± 0.03g | 0.52 ± 0.03efg | 0.22 ± 0.02cdefg | 0.74 ± 0.05efg | 0.2 ± 0.01de |
| W60N0.84P1.26 | 17.43 ± 2.24bcdef | 1.39 ± 0.01ab | 29.34 ± 0.22bcde | 0.56 ± 0.01ab | 14.01 ± 0.12a | 6.46 ± 0.23a | 2.18 ± 0.09efg | 0.52 ± 0.02fg | 0.19 ± 0.04fg | 0.71 ± 0.05fghi | 0.18 ± 0.01ef |
| Control | 9.6 ± 0.39i | 0.63 ± 0.01g | 19.98 ± 0.95h | 0.57 ± 0.01ab | 6.33 ± 0.18f | 3.41 ± 0.11j | 1.86 ± 0.01g | 0.21 ± 0.01i | 0.09 ± 0.01h | 0.31 ± 0.01j | 0.11 ± 0.01g |
Gas-exchange attributes and photosynthetic pigment contents
Net photosynthesis rate (Pn) and transpiration rate (Tr) both increased rapidly with W, N and P application rates until reaching peak values of 14.01 and 6.46 mmol m−2 s−1, respectively at moderate W, N and P doses (W60N0.84P1.26). Then Pn and Tr decreased under greater W, N and P applications (Table 3). Leaf water use efficiency (WUE) significantly upregulated in all treatments, when compared to control. However, WUE reached maximum increase (+76.9% over control) under the W48.1N1.34P2 treatment (Table 3). Moderate-to-high W and N doses, regardless of P additions, caused significant increases in photosynthetic pigments compared to control. Therefore, plants treated with W71.9N1.34P2, W71.9N1.34P0.51, W60N0.84P2.52 and W60N0.84P0 showed a remarkable improvement in the contents of Chlorophyll (Chl) a by 257.1, 228.6, 219.0 and 247.6%; Chl b by 244.4, 222.2, 200.0 and 222.2%; total Chls by 241.9, 216.1, 203.2 and 229.0%; and Carotenoids (Cars) by 154.5, 109.1, 127.3 and 163.6%, respectively, over that of control treatment (Table 3).
Water-status, osmolytes and soluble proteins
The addition of W, N and P across the various treatments caused a remarkable increase in leaf water potential (LWP) values, which were 4.2–74.2% higher than those in control seedlings (Fig. 1A). Seedlings exposed to W71.9N1.34P2, W71.9N1.34P0.51 and W80N0.84P1.26 presented a significant enhancement in relative water content (RWC), which was 27.4, 29.2 and 29.6% higher, respectively, than the control. Also, membrane stability index (MSI) under these treatments increased by 82.8, 86.9 and 80.0%, respectively, over control seedlings (Fig. 1B,C). Addition of W, N and P remarkably reduced the contents of proline (Pro) and soluble sugar (SS) and increased soluble protein (SP) content over that of control (Fig. 1D–F). Seedlings exposed to W additions ≤48% FC, regardless of N and P additions, had greater Pro and SS and lower SP content. Pro and SS contents decreased by 17.7–54.9 and 6.7–57.9%, respectively, and SP content increased by 3.4–110.6%, across the various treatments compared to control (Fig. 1D–F).
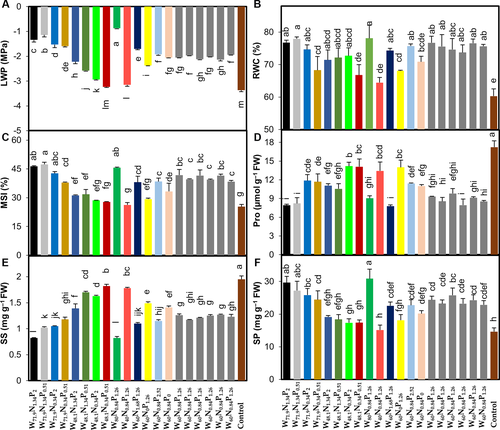
Lipid peroxidation, ROS production and activities of antioxidant enzymes
Leaf malondialdehyde (MDA), H2O2 and O2●– contents significantly decreased across treatments by 5.3–33.5, 9.7–81.8 and 16.7–77.8%, respectively, when compared to control (Table 4). Under the same N, P additions (N0.84P1.26), production rate of H2O2 and O2●– contents dramatically decreased from 0.65 to 0.13 (μmol g−1 FW) and 0.15 to 0.04 (nmol min−1 g−1 FW), respectively, when W levels increased from 40% FC (W40N0.84P1.26) to 80% FC (W80N0.84P1.26) (Table 4). Seedlings treated with very low- to low-W doses (≤48 %FC) such as W48.1N1.34P2, W48.1N1.34P0.51, W48.1N0.34P2, W48.1N0.34P0.51 and W40N0.84P1.26 showed less reduction in MDA contents compare to seedlings treated with moderate-to-very high W doses, regardless of N and P fertilization (Table 4). Treatments containing very low-to-low W and N doses such as W48.1N0.34P2, W48.1N0.34P0.51, W40N0.84P1.26 and W60N0P1.26 resulted in a notable increase in the activity of superoxide dismutase (SOD) (+11.6, +12.9, +2.2 and +8.1%, respectively, compared to control). Similarly, catalase (CAT) activity increased by 2.7, 6.2, 3.6 and 0.2% and ascorbate peroxidase (APX) activity by 15.9, 8.1, 4.7 and 5.9% respectively, compared to the control treatment (Table 4). In comparison with control, the peroxidase (POD) activity significantly decreased with the addition of W, N and P doses but the treatment W40N0.84P1.26 showed a slight improvement in POD activity (by 2.6%) compared to control (Table 4).
| Treatments | MDA | H2O2 | O2●– | SOD | CAT | POD | APX |
|---|---|---|---|---|---|---|---|
| μmol g−1 FW | μmol g−1 FW | nmol min−1 g−1 FW | U g−1 FW | U min−1 g−1 FW | U min−1 g−1 FW | μmol min−1 g−1 FW | |
| W71.9N1.34P2 | 3.54 ± 0.38cd | 0.17 ± 0.01ijk | 0.05 ± 0.01def | 113.87 ± 2.12fg | 80.81 ± 5.77h | 2.48 ± 0.02fgh | 17.35 ± 1.75hi |
| W71.9N1.34P0.51 | 3.45 ± 0.09d | 0.16 ± 0.01jk | 0.05 ± 0.01ef | 111.78 ± 3.81g | 86.22 ± 0.79fgh | 2.23 ± 0.1gh | 18.95 ± 3.99fghi |
| W71.9N0.34P2 | 3.62 ± 0.29cd | 0.19 ± 0.02hijk | 0.06 ± 0.01def | 123.94 ± 2.39ef | 85.4 ± 0.68gh | 2.58 ± 0.17fgh | 17.08 ± 0.36hi |
| W71.9N0.34P0.51 | 4.06 ± 0.49bcd | 0.16 ± 0.01jk | 0.07 ± 0.01de | 135.71 ± 7.29c | 90.81 ± 1.69cdefgh | 2.16 ± 0.09h | 18.44 ± 0.06ghi |
| W48.1N1.34P2 | 4.73 ± 0.31ab | 0.28 ± 0.01ghi | 0.08 ± 0.01d | 137.52 ± 4.23c | 98.72 ± 0.82abcd | 5.38 ± 0.11ab | 21.03 ± 0.14defgh |
| W48.1N1.34P0.51 | 4.14 ± 0.23bcd | 0.44 ± 0.02cde | 0.11 ± 0.01c | 131.94 ± 1.12cde | 97.15 ± 2.31abcd | 4.83 ± 0.08b | 20.05 ± 0.39efgh |
| W48.1N0.34P2 | 4.72 ± 0.16ab | 0.43 ± 0.04cde | 0.12 ± 0.01c | 152.82 ± 4.45a | 102.32 ± 1.78ab | 4.22 ± 0.67c | 27.94 ± 0.07a |
| W48.1N0.34P0.51 | 4.34 ± 0.17abc | 0.62 ± 0.04ab | 0.13 ± 0.02bc | 154.55 ± 1.32a | 105.73 ± 0.75a | 3.74 ± 0.26cd | 26.06 ± 0.57ab |
| W80N0.84P1.26 | 3.6 ± 0.13cd | 0.13 ± 0.01k | 0.04 ± 0.01f | 112.24 ± 2.22g | 84.41 ± 2.98gh | 2.12 ± 0.01h | 14.91 ± 1.85i |
| W40N0.84P1.26 | 4.87 ± 0.49ab | 0.65 ± 0.04a | 0.15 ± 0.01b | 139.91 ± 1.28bc | 103.13 ± 4.11ab | 5.57 ± 0.25a | 25.23 ± 2.03abcd |
| W60N1.68P1.26 | 3.84 ± 0.28cd | 0.15 ± 0.01k | 0.05 ± 0.01ef | 124.87 ± 1.01de | 88.43 ± 5.48defgh | 4.2 ± 0.01c | 23.14 ± 1.04bcdef |
| W60N0P1.26 | 4.19 ± 0.33bcd | 0.27 ± 0.01ghij | 0.07 ± 0.01de | 147.92 ± 2.76ab | 99.8 ± 2.66abc | 2.94 ± 0.13ef | 25.52 ± 2.25abc |
| W60N0.84P2.52 | 4.2 ± 0.31bcd | 0.37 ± 0.05defg | 0.11 ± 0.01c | 137.42 ± 2.65c | 86.53 ± 5.75efgh | 3.35 ± 0.09de | 21.54 ± 2.39cdefgh |
| W60N0.84P0 | 3.65 ± 0.26cd | 0.51 ± 0.02bc | 0.11 ± 0.01c | 132.41 ± 6.34cde | 96.82 ± 2.07abcde | 2.47 ± 0.02fgh | 21.85 ± 1.78bcdefg |
| W60N0.84P1.26 | 3.66 ± 0.29cd | 0.37 ± 0.04defg | 0.12 ± 0.01c | 134.31 ± 4.8cd | 93.88 ± 0.34bcdefg | 2.89 ± 0.05ef | 23.76 ± 0.16abcde |
| W60N0.84P1.26 | 3.51 ± 0.43cd | 0.33 ± 0.02efg | 0.11 ± 0.01c | 136.48 ± 4.54c | 88.45 ± 0.61defgh | 2.77 ± 0.2fg | 23.66 ± 0.22abcde |
| W60N0.84P1.26 | 3.57 ± 0.23cd | 0.29 ± 0.01fgh | 0.11 ± 0.02c | 135.43 ± 2.45c | 91.36 ± 5.18cdefg | 2.93 ± 0.05ef | 23.54 ± 1.33abcde |
| W60N0.84P1.26 | 3.42 ± 0.06d | 0.4 ± 0.05cdef | 0.12 ± 0.01c | 134.84 ± 1.09cd | 90.91 ± 6.21cdefgh | 2.83 ± 0.02ef | 22.34 ± 2.4bcdefg |
| W60N0.84P1.26 | 3.54 ± 0.39cd | 0.45 ± 0.05cd | 0.12 ± 0.01c | 135.3 ± 3.71c | 94.63 ± 6.22bcdefg | 2.92 ± 0.05ef | 21.47 ± 0.57cdefgh |
| W60N0.84P1.26 | 3.61 ± 0.29cd | 0.35 ± 0.01defg | 0.12 ± 0.01c | 130.04 ± 3.29cde | 89.58 ± 1.96cdefgh | 2.1 ± 0.01h | 22.34 ± 0.97bcdefg |
| Control | 5.14 ± 0.02a | 0.72 ± 0.02a | 0.18 ± 0.01a | 136.88 ± 3.63c | 99.59 ± 3.55abc | 5.43 ± 0.23a | 24.1 ± 0.77abcde |
The absolute value of the regression coefficient in the equation can be used to judge the significant degree of each factor on the different growth responses of T. chinensis (Table 5). The positive and negative values of the coefficient can indicate the synergistic or antagonistic effect of each factor on the different growth parameters. Coefficients of β1, β2 and β3 are positive values suggesting that W, N and P additions have synergistic effects on various growth traits of T. chinensis. For instance, W additions have synergistic effects on plant height, stem diameter, DW, R/S, Pn, Tr, Chl a, Chl b, total Chls, Cars, LWP, RWC, MSI and SP but antagonistic effects on other parameters. N additions have antagonistic effects on DW, Tr, Pro, SS, MDA, H2O2, O2●–, SOD, CAT and APX, but synergistic effects on other growth parameters (Table 5). P additions have synergistic effects on stem diameter, DW, WUE, LWP, RWC, MSI, Pro, SP, MDA, SOD and POD, but antagonism effects on other parameters (Table 5). Higher values of negative coefficients of β12, β22 and β32 imply that too much W, N and P additions can inhibit increases in stem diameter, Pn, Tr, and most growth traits (see Table 5). Absolute values of coefficients of β12, β13 and β23 show the coupling effects of W × N, W × P and N × P, respectively on different growth traits of T. chinensis (Table 5).
| Y | β0 | β1 | β2 | β3 | β12 | β13 | β23 | β12 | β22 | β32 | R2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant height | 17.500 | 1.744*** | 2.392*** | −0.704*** | 0.228ns | −0.304* | 0.504** | −1.209*** | 0.449*** | −0.163ns | 0.991*** |
| Stem diameter | 1.385 | 0.146*** | 0.039*** | 0.019** | −0.016ns | −0.061*** | 0.011ns | −0.173*** | −0.016* | −0.003ns | 0.994*** |
| DW | 29.401 | 3.064*** | −0.154ns | 0.593* | 0.936*** | ||||||
| R/S | 0.564 | 0.020** | 0.022*** | −0.002ns | 0.011ns | 0.019* | −0.011ns | 0.796*** | |||
| Pn | 12.870 | 0.944*** | 0.687** | −0.694** | −0.587* | 0.44ns | 0.84** | −0.706** | −0.537** | −1.611*** | 0.957*** |
| Tr | 6.149 | 0.350*** | −0.178** | −0.59*** | −0.261** | 0.171* | 0.289** | −0.934*** | −0.604*** | −0.821*** | 0.989*** |
| WUE | 2.093 | −0.001ns | 0.282*** | 0.197*** | −0.001ns | −0.021ns | 0.069** | 0.353*** | 0.179*** | 0.061** | 0.991*** |
| Chl a | 0.509 | 0.026*** | 0.062*** | −0.018** | 0.074*** | 0.044*** | 0.001ns | 0.023** | −0.053*** | 0.072*** | 0.986*** |
| Chl b | 0.206 | 0.016** | 0.023*** | −0.004ns | 0.026*** | 0.016** | −0.004ns | 0.013** | −0.021*** | 0.029*** | 0.951*** |
| Total Chls | 0.715 | 0.042*** | 0.085*** | −0.023* | 0.1*** | 0.059*** | −0.003ns | 0.035** | −0.074*** | 0.101*** | 0.981*** |
| Cars | 0.182 | 0.012** | 0.021*** | −0.007* | 0.022*** | 0.025*** | 0.005ns | 0.01* | −0.016*** | 0.031*** | 0.959*** |
| LWP | −2.042 | 0.673*** | 0.229*** | 0.054* | −0.09** | −0.09** | −0.025ns | 0.990*** | |||
| RWC | 75.462 | 2.461*** | 1.930*** | 1.365** | 0.964ns | 0.004 ns | −1.771** | −0.969* | −1.411** | −0.672ns | 0.938*** |
| MSI | 40.311 | 6.405*** | 2.533*** | 0.936ns | 0.821ns | 0.428 ns | −0.894ns | −1.175* | −1.976** | −1.199* | 0.960*** |
| Pro | 8.878 | −1.285*** | −1.797*** | 0.088ns | −0.096ns | −0.091ns | −0.006ns | 0.824*** | 0.701*** | 0.816*** | 0.974*** |
| SS | 1.231 | −0.299*** | −0.103*** | −0.092*** | −0.004ns | 0.021ns | −0.024ns | 0.031* | 0.029* | 0.024* | 0.989*** |
| SP | 23.927 | 4.490*** | 1.230*** | 0.653* | 0.473ns | 0.395ns | 0.237ns | −0.135ns | −1.073** | −0.684* | 0.972*** |
| MDA | 3.552 | −0.395*** | −0.108** | 0.113*** | −0.063ns | −0.165*** | 0.092* | 0.239*** | 0.161*** | 0.129*** | 0.981*** |
| H2O2 | 0.366 | −0.144*** | −0.040* | −0.039* | 0.039* | 0.049* | 0.001ns | 0.001ns | −0.063*** | 0.018ns | 0.950*** |
| O2●– | 0.116 | −0.029*** | −0.009** | −0.004ns | 0.004ns | 0.004ns | −0.001ns | −0.008** | −0.021*** | −0.003ns | 0.969*** |
| SOD | 134.38 | −10.109*** | −8.104*** | 0.19ns | 0.489ns | −1.691ns | 2.646* | −2.854** | 0.794ns | 0.271ns | 0.963*** |
| CAT | 92.754 | −6.748*** | −2.960*** | −2.194** | 0.899*** | ||||||
| POD | 2.7408 | −1.067*** | 0.321*** | 0.236** | −0.291** | −0.051ns | −0.006ns | 0.385*** | 0.288** | 0.055ns | 0.974*** |
| APX | 22.887 | −2.974*** | −1.182** | −0.045ns | 1.713*** | −0.727ns | −0.142ns | −1.219** | 0.286ns | −0.645* | 0.948*** |
| IGP | 0.528 | 0.049*** | 0.041*** | −0.003ns | 0.008* | 0.005 ns | 0.005ns | −0.015*** | −0.009** | −0.006* | 0.957*** |
Interactive effects of W, N and P on IGP of T. chinensis
To investigate the interactive effects of W, N and P on integrated growth performance (IGP) of T. chinensis, 3D surface plots were generated according to CCD. T. chinensis seedlings showed highest IGP of 0.60 when treated with high W, N and P doses (W71.9N1.34P2) (Table S2). The interaction between the two factors (x and y) with IGP of T. chinensis at the z-axis is shown in Fig. 2A–C. Each figure shows the effect of two factors when the third factor is kept at a central level (moderate dose).
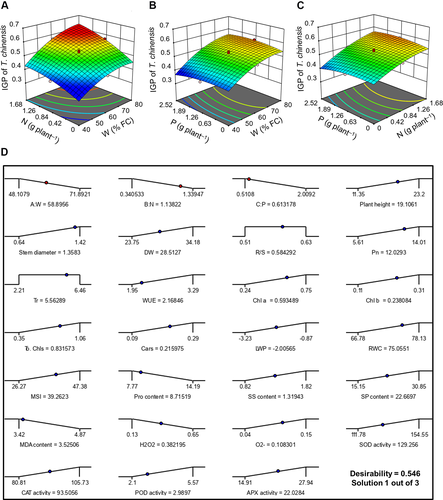
Fig. 2A shows that IGP of T. chinensis dramatically increased with the mutual increase of W and N additions. ANOVA results also show that the interactive effects of W × N had a significant effect (P < 0.05) on the IGP of T. chinensis (Table 5). We found that IGP rapidly increased as the W regime (Fig. 2B) and N doses (Fig. 2C) also increased either at low- or high-P doses. At very low-W and N doses, the growth performance of T. chinensis remarkably decreased and the addition of P did not reveal any positive effect. Therefore, no significant interactive effects (P > 0.05) of W × P and N × P were detected on the IGP of T. chinensis (Table 5). The magnitude of the effects of W, N and P on IGP of T. chinensis was W > N > P (Table 5).
Optimization of process variables and validation of the optimized condition
According to CCD analysis the optimal W, N and P application rate was 58.90% FC, 1.14 g plant−1, 0.61 g plant−1, respectively (Fig. 2D), which is equivalent to 160 and 85 kg P ha−1 and appropriate for a planting density of 1.4 × 105 seedlings ha−1 (1-year-old T. chinensis individuals) under field conditions (Su et al. 2014). The accuracy of the optimized outcome was tested by comparing mean experimental values with predicted values resulted by the RSM model. A desirability ramp was developed from optimal points presented in Fig. 2D.
Field experiment
We measured the effects of optimum W, N and P additions on multiple morpho-physiological features of field-grown T. chinensis including plant height, stem diameter, maximum root length, FW, DW, R/S ratio, Pn, Tr, Ci, WUE, RWC and MSI (Fig. 3A–L).
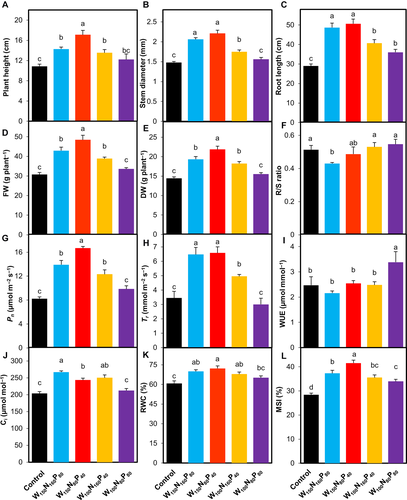
In comparison with control, W150N160P80 (W at 150 mm year−1, N at 160 and P at 80 kg ha−1), W150N80P40, W100N160P40 and W100N80P80 treatments markedly increased plant height (by 31.3, 57.3, 24.4 and 12.2%, respectively), stem diameter (by 39.2, 49.3, 18.2 and 5.4%, respectively), maximum root length (by 67.8, 74.7, 40.2 and 24.1%, respectively), FW (by 39.7, 57.6, 26.5 and 9.3%, respectively) and DW (by 33.8, 51.4, 26.2 and 7.3%, respectively) (Fig. 3A–E). However, the R/S ratio was considerably lower in the seedlings treated with W150N160P80 and W150N80P40 over control (Fig. 3F). T. chinensis treated with W150N160P80, W150N80P40, W100N160P40 and W100N80P80 showed increases in Pn by 68.9, 102.4, 49.5 and 19.5% and Ci by 31.2, 19.6, 23.0 and 4.2%, respectively, compared to control (Fig. 3G and J). The Tr was significantly higher in W150N160P80 (by 87.73%), W150N80P40 (by 90.92%) and W100N80P80 (by 43.67%) over control (Fig. 3H). Compared to control, W150N80P40, W100N160P40 and W100N80P80 enhanced WUE by 3.0, 0.5 and 37.0%, respectively, but W150N160P80 considerably reduced WUE by 12.6% (Fig. 3I). RWC in the leaf of T. chinensis increased by 15.13, 18.88, 11.75 and 7.13% and MSI increased by 31.2, 46.22, 25.04 and 19.52% when seedlings were treated with W150N160P80, W150N80P40, W100N160P40 and W100N80P80, respectively, as compared to control seedlings (Fig. 3K–L).
Growth performance of T. chinensis in response to combined applications of W, N and P assessed by heatmap and principal component analysis methods
Results from heatmap analyses show morphological, physiological and biochemical responses of T. chinensis to different W, N and P combinations under pot- (Fig. 4A) and field-grown (Fig. 4B) conditions. For both pot- and field-grown T. chinensis seedlings, measured traits that cluster together are strongly correlated to each other.
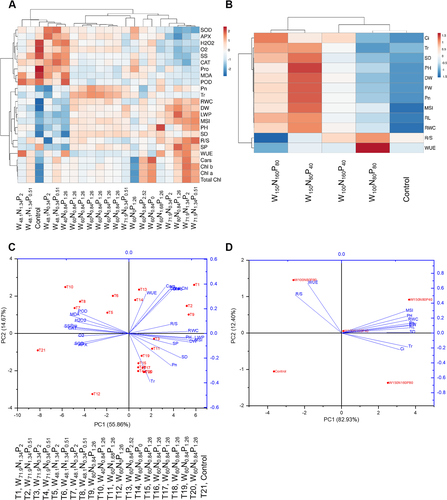
The aggregated data heatmap analysis (Fig. 4A) shows that treatments containing W doses ≤48% FC, regardless of N and P fertilization, clustered on the left while the rest of the treatments clustered on the right suggesting that W was the main clustering factor. Treatments containing moderate-to-very high W doses (≥ 48% FC; i.e. W71.9N1.34P0.51, W71.9N1.34P2, W80N0.84P1.26, W71.9N0.34P2 and W60N1.68P1.26, W60N0.84P0, W60N0.84P2.52 and W60N0.84P1.26) clustered together because of reduced antioxidant enzyme activities (i.e. SOD, APX, CAT and POD), ROS production (H2O2 and O2●–), osmolytes (SS and Pro) and MDA. The same treatments showed high levels of leaf water-status (RWC, LWP and MSI), morphological growth traits (DW, plant height [PH], R/S) and photosynthetic pigments contents (Chl a, Chl b, total Chls, and Cars) and gas-exchanges parameters (Pn and Tr). On the contrary, treatments containing very low- and low-W doses (≤48% FC) such as W48.1N1.34P2, W48.1N1.34P0.51, control, W48.1N0.34P2, W48.1N0.34P0.51 and W40N0.84P1.26, clustered on the left because of their high accumulation of ROS, MDA, antioxidative enzymes, osmolytes, but low levels of morphological growth traits, gas-exchanges parameters, photosynthetic pigments and leaf water-status (Fig. 4A).
For field grown T. chinensis, the heatmap identified two main clusters (Fig. 4B), where high-W containing treatments like W150N160P80 and W150N80P40 were separated from the rest of the treatments because of their high contents of Ci, Tr, SD (stem diameter), PH, DW, FW, Pn, MSI, RL (root length) and RWC. On the other hand, W100N160P40, W100N80P80 and control treatments clustered separately on the right because of their high contents of R/S ratio and WUE (Fig. 4B).
Principal component analysis (PCA) shows that for both pot and field experiments, the first five and first two principal components (PCs), respectively, had Eigen values higher than one. For pot and field experiments, the first two PCs explained 70.53 and 95.33% of the total variance, respectively, with PC1 and PC2 accounting for 55.86 and 14.67% for pot experiment (Fig. 4C) and 82.93 and 12.4% for field experiment (Fig. 4D).
Fig. 4C shows how T. chinensis treated with very low- to low-W doses such as W48.1N1.34P2 (T5), W48.1N1.34P0.51 (T6), W48.1N0.34P2 (T7), W48.1N0.34P0.51 (T8), W40N0.84P1.26 (T10) and control (T21), positioned on the negative side of PC1 of the PCA score plot, were strongly and positively correlated with MDA content, ROS production, osmolytes accumulation and antioxidant enzyme activities. On the contrary, moderate- to very high-W treated seedlings, regardless of N and P fertilization positioned on the positive side of PC1 of the PCA score plot showing positive correlations with photosynthetic pigments, leaf water-status, morphological parameters and gas-exchange parameters. Moreover, PC2 was positively correlated to WUE and negatively correlated to Tr (Fig. 4C).
Increases in W levels contributed to the sharp separation of PC1 in seedlings growing under field conditions (Fig. 4D). High W-treated seedlings under W150N160P80 and W150N80P40 treatments are distributed on the positive side of PC1 of the PCA score plot. These seedlings showed a strong positive correlation with MSI, PH, RWC, Pn, DW, RL, SD, Tr and Ci. Instead, low W-treated treatments like W100N80P80 and control treatments were distributed under the negative direction of PC1 showing a positive relationship with R/S ratio and WUE (Fig. 4D).
Discussion
Overall, our findings show how different growth traits of T. chinensis respond to different application rates of W, N and P under both greenhouse and field conditions. We found that plant height, stem diameter, DW and R/S ratio of T. chinensis seedlings all significantly increased with increasing W and N application rates either at low- or high-P doses under greenhouse conditions (Table 3). Under field conditions, however, T. chinensis seedlings showed significant increases in plant height, stem diameter, maximum root length, FW and DW associated with high W and low N and P levels (Fig. 3A–E). Overall, growth performance of T. chinensis in terms of plant height and DW was greater under greenhouse than field conditions (Table 3; Fig. 3A and E) possibly because water stress under field conditions is often accompanied by high temperature and irradiance, which contribute to increase the environmental stress (Correia et al. 1999). We found in general that low-W additions significantly reduced most growth-related traits of T. chinensis regardless of the addition of N and P fertilizers either under greenhouse or field conditions (Table 3; Fig. 3A–E). Findings from heatmap and PCA analyses further support the evidence that T. chinensis seedlings treated with low-W doses, regardless of N and P nutrient fertilizers, displayed stronger negative relationships with growth-associated parameters (Fig. 4A–D). This is possibly because under low-W availability, N and P forms in the soil did not mobilize well and plant roots could not uptake these nutrients adequately, thus ultimately inhibiting water and nutrients flow through the xylem to the surrounding cells (Zhang et al. 2016). This phenomenon can not only impair cell water relations, cell expansion, stomatal aperture and transpiration rate but also cause nutritional imbalance and oxidative stress, which together have resulted in severe negative consequences on plant morphological features. These effects were previously observed in T. chinensis, Amorpha fruticosa and Brassica juncea (Zhang et al. 2016, Bandeppa et al. 2019, Roy et al. 2020), under water-shortage conditions.
Photosynthetic traits have a significant role in maintaining and promoting plant growth and development (Wei et al. 2016). In this study, we found that moderate W, N and P doses led to significant improvements in Pn and Tr in pot-grown T. chinensis seedlings (Table 3). Recent experimental evidence also shows positive effects of W, N and P on gas-exchange parameters in Amorpha fruticosa growing under greenhouse conditions (Roy et al. 2020). Under field conditions we observed that Pn and Tr of T. chinensis seedlings significantly increased with high W and low N-P levels (Fig. 3G–H), indicating a positive W effect on photosynthesis performance, as also reflected in seedlings growth (Fig. 3A–E). It is likely that W availability is a key factor limiting plant photosynthetic capacity and growth (Myers et al. 2017). Several studies have suggested that the application of nutrients can scarcely offset the negative impact that water stress can have on plant growth associated with high temperatures and frequent drought events under field conditions (Lobell et al. 2014, Myers et al. 2017). In our study, Pn, Tr and Ci all significantly decreased with decreasing W, N and P applications under both greenhouse and field conditions (Table 3; Fig. 3G–J). This could be because water deficiency encourages plants to close their stomata in order to decrease Tr and prevent the entry of CO2 into the leaves thus reducing carboxylation rates and ultimately leading to reduction in Pn (Munjonji et al. 2017). WUE considerably increased under low-W regime in the presence of high N and P dose applications (Table 3; Fig. 3I). These results suggest that under low-W and high N, P additions, T. chinensis seedlings increased their WUE by enhancing water uptake and by decreasing water losses through lessening transpiration rates (Musembi et al. 2015). These findings were also supported by the results from heatmap and PCA, which show that seedlings treated with low-W and high N, P doses were strongly associated with high WUE in both greenhouse and field experiments (Fig. 4A–D). In accordance with our findings, Zhang et al. (2018) also found that drought led to adverse effects on transpiration rate in Solanum tuberosum. Moreover, Zhou et al. (2019) stated that under water-shortage conditions, the addition of N, P and K fertilizers had a positive effect on WUE in Malus domestica and Zea mays grown in the Loess Plateau of China.
The status of Chl content is usually considered an important tolerance index to understand how plants react to stressful conditions (Hosseinzadeh et al. 2016). In this study, contents of photosynthetic pigments (Chl a, Chl b, total Chls and Cars) significantly increased with W and N doses, while P did not exhibit any positive effect on parameters as mentioned above (Table 5), suggesting a positive impact of W and N in improving photosynthetic performance, as reflected in the growth of T. chinensis under greenhouse condition (Table 3). In our study, photosynthetic traits of T. chinensis showed positive responses to W and N supply because seedlings growing under water and nutrient-poor conditions can immediately benefit from increased availability of these key resources (Zhou et al. 2011). Greater leaf Chls content is a good indicator of higher photosynthetic capacity, which is in turn associated with increases in plant biomass production (Molinari et al. 2007). Similar results show that W and N additions have positive effects on Chls content in Amorpha fruticosa under greenhouse conditions (Roy et al. 2020). Since N is an essential component of Chl molecules, low N availability can lead to chlorosis-related symptoms in plants (Çelebi et al. 2010). Wei et al. (2016) observed that photosynthetic parameters decreased significantly under N deficiency and this response was associated with leaf senescence and production of lower dry matter in Zea mays. In contrast, low-W doses noticeably declined photosynthetic pigment contents (Table 3), possibly because of the excess production of ROS under water-shortage conditions, which then resulted in the degradation of pigmentation (Jajic et al. 2015) or in reduced synthesis of Chls and Cars (Khorasaninejad et al. 2018). These results are also supported by heatmap and PCA, which show that high-W and -N treated seedlings were also positively associated with photosynthetic pigments (Fig. 4A and C).
Leaf water potential (LWP) acts as an indicator of plant-water status, and it was shown, for example, in previous experiment that LWP increased under high W, N and P availability in Amorpha fruticosa under greenhouse conditions (Roy et al. 2020). Similarly, we observed that LWP of T. chinensis was positively influenced by W, N and P additions (Fig. 1A). RWC and MSI of N-fertilized seedlings considerably improved under both low- and high-W regimes (Fig. 1B,C), suggesting that N additions play a significant role in maintaining leaf water-status of T. chinensis under greenhouse conditions. The positive effects of N on increasing water-status was also found by Liao et al. (2017) in pot-grown Panax notogesing seedlings. Plants display a set of biochemical responses, such as accumulation of compatible solutes, namely proline (Pro), soluble sugars (SS) and soluble proteins (SP), which play significant roles in osmoregulation when plants are exposed to different stress situations (Ahmad et al. 2018a, b, Roy et al. 2020, Zhang et al. 2018). Our study reveals that very low- to low-W regimes resulted in the accumulation of high Pro and SS content in the leaf of T. chinensis (Fig. 1D,E). Under stressful conditions, changes in Pro and SS levels in plants indicate an adjustment or a self-protection mechanism to prevent protein denaturation and induce stress tolerance (Mohammadi et al. 2018). In accordance with our findings, Zhou et al. (2011) also demonstrated that an increase of W and N doses had a reversal effect on the accumulation of Pro and SS contents in Malcolmia africana and Bassia hyssopifolia under greenhouse conditions. However, SP content was lower in seedlings under water-shortage conditions (Fig. 1F). This could be due to enhanced activity of protease enzymes, proteolysis, or reduced protein synthesis along with lesser Pn (i.e. a smaller amount of carbon to build any metabolite) in seedlings growing under water-shortage conditions (Tariq et al. 2018). The findings of heatmap and PCA further supported these results, indicating that T. chinensis treated with high- to very high-W, and very high-N doses, displayed a stronger positive relationship with LWP, RWC, MSI and SP, and negative relationship with Pro and SS contents (Fig. 4A,C).
In our study, we found that T. chinensis showed significantly lower MDA, H2O2 and O2●− contents with the addition of W, N and P compared to control seedlings under greenhouse conditions (Table 4). However, MDA content was considerably higher under water-shortage conditions, probably due to a significant increase in O2●− and H2O2 contents induced by low-W regime, and caused severe damage to membrane permeability, resulting in oxidative damage at cellular levels (Hossain et al. 2015). Plants strengthen defensive mechanisms against ROS by increasing their ROS-detoxification capacity through the stimulation of the activities of several antioxidant enzymes such as SOD, CAT, POD and APX (Ahmad et al. 2018b, Ahanger et al. 2019, 2020, Pandey and Gautam 2020). In this study, T. chinensis increased the activities of SOD, CAT, POD and APX in response to excessive ROS under low W, N and P doses, suggesting that T. chinensis mounted antioxidant defense strategy to reduce oxidative damage under W shortage- and N, P-deficient conditions (Table 4). Results from heatmap and PCA further supported that H2O2 and MDA contents negatively corelated with antioxidant enzyme activities in the leaves of T. chinensis treated with low doses of W, N and P (Fig. 4A,C). Similar to our findings, water-shortage is known to increase antioxidant capacity to ensure higher tolerance to drought in several plant species, including Solanum tuberosum, Amorpha fruticosa and Brassica juncea (Zhang et al. 2018, Bandeppa et al. 2019, Roy et al. 2020).
Conclusions
Key findings from this study show how additions of W, N and P can greatly improve gas-exchange traits (Pn, Tr and Ci) and photosynthetic pigments contents (Chl a, Chl b, total Chls and Cars) thus ultimately contributing to increase plant height, stem diameter, root length, FW and DW of T. chinensis seedlings. However, growth-associated parameters drastically decreased under very low-W regimes despite the application of moderate rates of N and P under greenhouse conditions suggesting that without water availability, nutrient fertilizers are not properly dissolved in soils and cannot be taken up by plants. Similar effects were also observed under field conditions where applications of N and P could hardly improve plant growth under low-W regime. The leaf water-status and MSI of T. chinensis considerably increased with W and N doses under both greenhouse and field conditions. Overall, pot- grown T. chinensis seedlings performed better compared to field-grown seedlings because of high temperatures under field conditions. We suggest that the accumulation of high level of osmolytes (Pro and SS) and antioxidant enzymes (SOD, CAT, POD and APX), which inhibit the production of excessive ROS and reduce drought-induced oxidative damage, represent an important survival strategy of T. chinensis under low-W availability. Based on our findings we recommend that planting T. chinensis seedlings under applications of W = 150 mm year−1, N = 80 kg ha−1 and P = 40 kg ha−1 could be an effective strategy to support ecological restoration and sustainable agriculture in coal mine areas of China as well as in other coal-mined and/or semi-arid regions across the world.
Acknowledgements
This research was supported by National Key Research and Development Program of China “Eco-security technology for coal mining bases in the Northwestern arid desert regions in China” (2017YFC0504400), “Studies on the vegetation rehabilitation and conservation in abandoned coal mining land research” (2017YFC0504402), National Natural Science Foundation Project of China (No. 31670713) and Yulin City Forestry Science and Technology Plan Projects (K403021528).
Author contributions
R.R.: conceptualization, data curation, formal analysis, investigation, methodology, software, visualization, writing-original draft, writing – review and editing. J.X.W.: conceptualization, supervision, validation, writing – review and editing; resources, project administration, funding acquisition. M.G.M.: formal analysis, software, writing – review and editing. D.F.: software, writing – review and editing.
Open Research
Data availability statement
Data sharing is not applicable to this article as all new created data are already contained within this article.




