Transcriptome profiles of soybean leaves and roots in response to zinc deficiency
Abstract
Zinc (Zn) deficiency is a widespread agricultural problem in arable soils of the whole world. However, the molecular mechanisms underlying Zn-deficiency response are largely unknown. Here, we analyzed the transcriptomic profilings of soybean leaves and roots in response to Zn deficiency through Illumina's high-throughput RNA sequencing in order to understand the molecular basis of Zn-deficiency response in the plants. A total of 614 and 1011 gene loci were found to be differentially expressed in leaves and roots, respectively, and 88 loci were commonly found in both leaves and roots. Twelve differentially expressed genes (DEGs) were randomly selected for validation by quantitative reverse transcription polymerase chain reaction, and their fold changes were similar to those of RNA-seq. Gene ontology enrichment analysis showed that ion transport, nicotianamine (NA) biosynthetic process and queuosine biosynthetic process were enriched in the upregulated genes, while oxidation–reduction process and defense response were enriched in the downregulated genes. Among the DEGs, 20 DEGs are potentially involved in Zn homeostasis, including seven ZRT, IRT-related protein (ZIP) transporter genes, three NA synthase genes, and seven metallothionein genes; 40 DEGs are possibly involved in diverse hormonal signals such as auxin, cytokinin, ethylene and gibberellin; nine DEGs are putatively involved in calcium signaling; 85 DEGs are putative transcription factor genes. Nine DEGs were found to contain zinc-deficiency-response element in their promoter regions. These results could provide comprehensive insights into the soybean response to Zn deficiency and will be helpful for further elucidation of the molecular mechanisms of Zn-deficiency response and Zn-deficiency tolerance in plants.
Abbreviations
-
- ABA
-
- abscisic acid
-
- ADH
-
- alcohol dehydrogenase
-
- BR
-
- brassinosteroid
-
- bZIP
-
- basic leucine zipper
-
- CA
-
- carbonic anhydrase
-
- Ca2+
-
- calcium ion
-
- CDPK
-
- calcium-dependent protein kinase
-
- CIPK
-
- CBL-interacting protein kinase
-
- CK
-
- cytokinin
-
- CSD
-
- copper/Zn superoxide dismutase
-
- DEG
-
- differentially expressed gene
-
- FPKM
-
- fragments per kilobase of transcript per million mapped reads
-
- G2-like
-
- Golden 2-like
-
- GA
-
- gibberellin
-
- GO
-
- gene ontology
-
- HMA
-
- heavy metal tolerance family protein
-
- JA
-
- jasmonate
-
- KEGG
-
- Kyoto encyclopedia of genes and genomes
-
- MTP
-
- metal tolerance protein
-
- NA
-
- nicotianamine
-
- OXS3
-
- oxidative stress 3
-
- RNA-seq
-
- RNA sequencing
-
- ROS
-
- reactive oxygen species
-
- SnRK
-
- SNF1-related protein kinase
-
- TF
-
- transcription factor
-
- ZDRE
-
- zinc-deficiency response element
-
- ZFP
-
- Zn-finger domain-containing protein
-
- ZIFL
-
- zinc-induced facilitator
-
- ZIP
-
- ZRT, IRT-related protein
-
- Zn
-
- Zinc
Introduction
Zinc (Zn) is an essential micronutrient for all organisms, which in its oxidized Zn2+ form acts as a catalytic, regulatory or structural co-factor for a large number of enzymes and regulatory proteins. Zinc is also the only metal present in enzymes of all six major classes (oxidoreductases, transferases, hydrolases, lyases, isomerases and ligases; Broadley et al. 2007). About 5 to 6% of prokaryotic proteome and around 9% of the eukaryotic proteome are Zn-binding proteins (Andreini et al. 2006). In plants, the well-known proteins associated with Zn include the enzymes like carbonic anhydrase (CA), alcohol dehydrogenase (ADH) and copper/zinc superoxide dismutase (CSD), and the abundant Zn-finger domain-containing proteins (ZFPs), which have functions in transcriptional regulation (Broadley et al. 2007). Zinc deficiency is a serious and widespread agricultural problem in arable soils worldwide (Alloway 2009). Only a small portion of the total Zn in soils, which includes all of the Zn present in structural minerals and adsorbed in various forms to other soil components, is available for plant uptake. In addition, the plant-available Zn in soils is strongly influenced by factors such as high pH (>7), low redox potential, prolonged flooding, and high contents of bicarbonate, organic matter and phosphorus (Alloway 2009). Low available Zn concentration in soils affects plant growth and development, and further influences the crop yield and the Zn nutritional quality for humans and animals (Wissuwa et al. 2006, Cakmak 2008, Impa and Johnson-Beebout 2012). The characteristic symptoms of Zn deficiency include chlorosis of young leaves, reduced leaf size and stunted and thin stems. Older leaves also show wilting and curling with extensive chlorosis and stunted growth under severe Zn-deficiency stress (Hacisalihoglu and Kochian 2003).
Plants have evolved several mechanisms to adapt to the low levels of soil Zn and to maintain the intracellular levels of Zn within optimal ranges (Cakmak 2000, Hacisalihoglu and Kochian 2003, Ghandilyan et al. 2006, Rose et al. 2012, Sinclair and Krämer 2012, Ricachenevsky et al. 2015). These include increasing the availability of Zn for root uptake by changing the root system architecture, formation of arbuscular mycorrhiza symbiosis, and releasing of phytosiderophores and organic acids; enhancing root uptake and translocation of Zn from roots to shoots with the involvements of transporters such as ZRT, IRT-related proteins (ZIPs), zinc-induced facilitators (ZIFLs), metal tolerance proteins (MTPs) and heavy metal tolerance family proteins (HMAs); controlling cellular Zn homeostasis by a combination of transport, chelation, trafficking and sequestration; and biochemical adaptation by regulating the expression of key Zn-requiring enzymes (Hacisalihoglu et al. 2003, Ishimaru et al. 2005, Cavagnaro 2008, Widodo et al. 2010, Tiong et al. 2014).
Because Zn is critical for diverse biochemical and physiological processes, and excess Zn accumulation is toxic to plants, cellular Zn homeostasis has to be tightly regulated to ensure suitable level within the cells and tissues of plants. When suffering low Zn stress, plants undergo an adaptive response to maintain Zn homeostasis. Recently, a significant step toward unraveling the molecular mechanisms of Zn homeostasis was made. Two F-group basic leucine zipper (bZIP) transcription factors (TFs), bZIP19 and bZIP23, were identified to be essential, because they function redundantly in transcriptional regulation of Zn-deficiency adaptation in Arabidopsis (Assunção et al. 2010). The binding sites of bZIP19 and bZIP23 are the cis-regulatory element named zinc-deficiency response element (ZDRE), and the potential target genes are involved in Zn homeostasis, such as the Zn transporter genes ZIP4, ZIP9 and ZIP12, and the nicotianamine synthase (NAS) genes NAS2 and NAS4 that encode enzymes catalyzing the synthesis of nicotianamine (NA; Assunção et al. 2010, Inaba et al. 2015). The F-group bZIP genes that are closely related to Arabidopsis bZIP19 and bZIP23 also exist in other plant species (Castro et al. 2017). Recently, their orthologous genes in cereals such as wheat and barley were found to have a conserved function in Zn-deficiency response (Evens et al. 2017, Nazri et al. 2017). Although some important progress has been made, overall knowledge about the molecular mechanisms underlying Zn-deficiency adaptation and Zn homeostasis regulation is still far from complete.
Transcriptomic analysis by high-throughput RNA sequencing (RNA-seq) is a useful tool for identifying differentially expressed genes (DEGs) under stress conditions or in tissues of different developmental stages at a genome-wide level (Lu et al. 2016, Zeng et al. 2016b, Ashoub et al. 2018). Recently, transcriptomic analyses were conducted in rice tissues under Zn deficiency for the identification of genes associated with Zn-efficiency and Zn-deficiency responses (Bandyopadhyay et al. 2017, Nanda et al. 2017). Four genes upregulated in Zn-efficient rice varieties by Zn deficiency, which encode a Zn-finger CCHC-type domain-containing protein, a plasma membrane polypeptide family protein, a gibberellin (GA)-stimulated protein and a monosaccharide transporter, were found to be potential candidate genes conferring Zn efficiency (Nanda et al. 2017). However, to the best of our knowledge, the transcriptomic responses to Zn deficiency in other plant species are still unclear.
Soybean is an important food crop providing protein-rich food for the increasing world population. Zinc deficiency is recognized as one of the most critical micronutrient deficiency problems in world crops, especially for crops grown in calcareous and saline soils with high pH values (Alloway 2009). Because soybean is frequently grown in these problem soils, low. Zn stress is a non-negligible factor, which could limit the productivity of soybean (Payne et al. 1986). In this study, the transcriptome changes in response to Zn deficiency were analyzed by RNA-seq in soybean leaves and roots. A total of 1537 loci were found to be differentially expressed; they were classified into several groups according to their potential functions in Zn-deficiency responses. The RNA-seq results and the possible signaling components identified here provide gene candidates for understanding the molecular mechanisms of Zn-deficiency stress response and tolerance in soybean.
Materials and methods
Plant material and growth conditions
Williams 82 is the soybean cultivar used for the reference soybean genome (Schmutz et al. 2010). Soybean (Glycine max var. Williams 82) seeds were soaked in sterilized water for 4 h, and then incubated at room temperature in the dark between two layers of moistened filter paper (Zeng et al. 2010). Four days later, seedlings were grown hydroponically in a 10-l tank filled with half-strength-modified Hoagland nutrient solution containing 2.5 mM Ca(NO3)2, 2.5 mM KNO3, 0.5 mM KH2PO4, 1.25 mM MgSO4, 10.0 μM Fe-EDTA, 3.4 μM MnSO4, 0.16 μM CuSO4, 0.38 μM ZnSO4, 23.0 μM H3BO3, 0.25 μM Na2MoO4, with pH adjusted to 5.6 (Zeng et al. 2016b). Nutrient solution was changed every 2 days. Plants were grown in a growth chamber with a photoperiod set at 16/8 h light/dark at 26/22°C and light intensity set at 150 μmol m−2 s−1. After 8 days of cultivation, soybean seedlings were transferred into Zn-sufficient (with ZnSO4) or Zn-deficient (without ZnSO4) nutrient solutions, respectively. Roots and leaves of soybean seedlings were separately sampled after treatment of Zn deficiency for 12 days, frozen in liquid nitrogen, and then stored at −80°C until RNA preparation.
Determination of chlorophyll contents and metal concentration
For chlorophyll (Chl) determination, about 0.5 g of the leaf blades were ground into homogenate along with a small amount of quartz sand and calcium carbonate, and 10 ml 95% ethanol. The extract was then filtered with filter paper into in a volumetric flask, and the mortar and pestle were rinsed with 95% ethanol for several times. The volume of the clean extract was brought to 25 ml with 95% ethanol, and the absorbance of the extract was measured at 665 and 649 nm, respectively. Chl contents were calculated based on the absorbance, the volume of the extract and the fresh weight of leaf blades: Chl a = 13.95A665–6.88A649, Chl b = 24.96A649–7.32A665.
For metal concentration assays, plant tissues were collected and dried in an oven at 80°C for 3 days. The roots were thoroughly rinsed with deionized water before sample collection. The dried roots or shoots were ground, and about 0.05 to 0.10 g sample was used to be digested with HNO3/HClO4 (4:1, v/v) at 180°C for 4 h. After diluting five times with 1% HNO3, the concentrations of Zn, Fe, Cu, Mn, Ca and Mg were determined by Inductively Coupled Plasma Mass Spectroscopy (Perkin-Elmer NexION 300X, Waltham, MA).
Library construction and RNA sequencing
Soybean leaves (the first trifoliate true leaves) and roots were collected after Zn-deficiency treatment for 12 days. Eight samples (Zn-sufficient leaves, Zn-deficient leaves, Zn-sufficient roots and Zn-deficient roots, each with two biological replicates) were used for mRNA library construction and sequencing. Each sample was mixed from three biological replicates. Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer's instruction. The total RNA quantity and purity were analyzed by Agilent Bioanalyzer 2100 with RNA 6000 Nano LabChip Kit (Agilent, Santa Clara, Redwood, CA), and the RNA integrity number (RIN) of all RNA samples were more than 7.0. Approximately, 10 μg of total RNA was subjected to Poly(A) mRNA isolation using poly-T oligo attached magnetic beads (Invitrogen). The mRNA is fragmented into small pieces using divalent cations under elevated temperature after purification. The cleaved RNA fragments were then reverse transcribed to create cDNA libraries according to the protocol for the mRNA-Seq sample preparation kit (Illumina, San Diego, CA). The average size of inserts for the paired-end libraries was 300 ± 50 bp. The paired-end sequencing was performed on an Illumina Hiseq 2000 at LC-BIO (Hangzhou, China) following instructions from Illumina.
Mapping of RNA-seq reads and differential counting
The initial base calling and quality filtering of the reads generated with the Illumina analysis pipeline (Fastq format) were performed using a custom Perl script and the default parameters of the Illumina pipeline (http://www.illumina.com). Additional filtering for poor-quality bases was performed using the FASTX-toolkit available in the FastQC software package (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). To facilitate the read mapping, the G. max reference genome (Gmax2.0 version) was indexed by Bowtie2 (http://www.phytozome.net) (Langmead and Salzberg 2012). The read mapping was performed using the Tophat software package (Trapnell et al. 2009). Tophat allows multiple alignments per read (up to 40) and a maximum of two mismatches when mapping the reads to the reference genome. Reads were first mapped directly to the genome using indexing, and the unmapped reads were used to identify novel splicing events. The aligned read files were processed by Cufflinks to measure the relative abundances of the transcripts by using the normalized RNA-seq fragment counts. The estimated gene abundance was measured in terms of the fragments per kilobase of transcript per million mapped reads (FPKM). The DEGs between the two sets of samples were identified using Cuffdiff. Only the genes with a log2 fold change (FC) ≥1 or ≤−1, and a P-value ≤0.05 were considered as significantly DEGs. The RNA-seq datasets of this study were deposited in NCBI's Gene Expression Omnibus and are accessible through GEO accession number GSE111799 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE111799).
Quantitative reverse transcription polymerase chain reaction analysis
Total RNA was extracted from soybean tissues using RNApure Plant Kit (with DNase I; CoWin Biotech, Beijing, China) and digested with DNase I to eliminate genomic DNA contamination according to the manufacturer's instruction. cDNA was synthesized from 1.0 μg total RNA by SuperRT Reverse Transcriptase (CoWin Biotech) using oligo(dT) primers in a 20-μl reaction system. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed on an MyiQ Single Color Real-time PCR system (Bio-Rad) as described previously (Zeng et al. 2016a). Relative expression levels were normalized to that of two internal control genes ACTIN11 (Glyma.18G290800) and EF1b (Glyma.02G276600), using the Pfaffl method (ratio = (Etarget)ΔCTtarget(control-sample)/(Eref) ΔCTref(control-sample)) (Pfaffl 2001). The calculated efficiency (E) of all primers was between 1.7 and 2.3. The gene-specific primers are designed through the qPCR primer database (Lu et al. 2018). The sequences of primers are listed in Table S1.
Functional annotation and gene ontology enrichment
The DEGs were annotated for gene ontology (GO) terms (Ashburner et al. 2000) and categorized into molecular function, cellular component and biological process categories. GO term enrichment was performed using single enrichment analysis (SEA) tool on AgriGo (http://bioinfo.cau.edu.cn/agriGO/) (Du et al. 2010). GO category (http://geneontology.org/) or Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (http://www.genome.jp/kegg/) with a P-value ≤0.05 was regarded as significantly enriched (Kanehisa et al. 2016).
Identification of ZIP, NAS and metallothionein gene families in soybean genome
The sequences of ZIP, NAS and MIT proteins from Arabidopsis and rice were used as queries to blast against the soybean genome database using the BlastP program. The amino acid sequences were obtained and analyzed for amino acid similarities of proteins in the same family. For phylogenetic analysis, sequences of proteins in Arabidopsis, rice and soybean were obtained from TAIR (http://www.arabidopsis.org/), RAP-DB (http://rapdb.dna.affrc.go.jp/) and Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html), respectively. The amino acid sequences of proteins were aligned using ClustalW, and a phylogenetic tree was constructed by the neighbor-joining method using the software MEGA6 (Tamura et al. 2013).
Promoter cis-element analysis
Promoter sequences (1500-bp upstream) of the transcription start sites of all DEGs were extracted from the SoyBase database (http://www.soybase.org/). The presence of the ZDRE (RTGTCGACAY) (Assunção et al. 2010) was analyzed using Regulatory Sequence Analysis Tools (http://floresta.eead.csic.es/rsat/) (Medina-Rivera et al. 2015). The prediction of novel cis-elements enriched in the promoters of the DEGs was analyzed using the DREAM tool in MEME (based on E-value <0.05) (Bailey et al. 2015).
Results
Transcriptome profilings of soybean leaves and roots in response to Zn deficiency
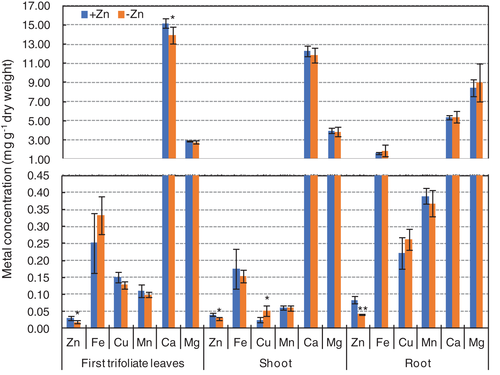
To identify Zn-deficiency-responsive genes in soybean, RNA-seq analyses were conducted in leaves and roots after treatments with or without Zn for 12 days. Physiological analyses were performed to confirm the effectiveness of the Zn-deficiency treatment. After Zn-deficiency for 12 days, there was no significant differences in the chlorophyll contents of the unifoliolate, the first trifoliolate and the second trifoliolate leaves or in the root and shoot biomass and primary root length (Fig. S1). However, the Zn concentrations in the first trifoliolate leaves, the shoots, with the exception of the first trifoliolate leaves, and the roots of Zn-deficient seedlings were all significantly lower than that of control plants. In addition, the calcium (Ca) concentration was significantly decreased in the first trifoliolate leaves of Zn-deficient seedlings, and the copper (Cu) concentration was significantly increased in the shoot of Zn-deficient seedlings (Fig. 1). Although no significant morphological difference was observed, the endogenous Zn concentration of the Zn-deficient seedlings decreased dramatically confirming the effectiveness of Zn-deficiency treatment. For RNA-seq analyses, two biological replicates were included for each treatment, and in total eight libraries were constructed. By Illumina high-throughput sequencing, each library obtained reliable clean reads ranged from 40.8 to 50.6 million, and most of the clean reads (70.4–77.7%) from each library can be perfectly mapped to the soybean reference genome (Wm82.a2.v1; https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Gmax) (Table S2). Reads that could not be mapped to the soybean genome were discarded, and only the mapped reads were analyzed further. The abundance of each gene mapped was measured in terms of the FPKM. A total of 40 035 and 42 157 gene loci were detected in leaves and roots, respectively (Tables S3 and S4). The correlation of the two biological replicates for each treatment was calculated to inquire the variability between the replicates. The Pearson's correlation (R-value) of each comparison all exceeded 80% (Fig. S2), indicating a high correlation between these biological replicates.
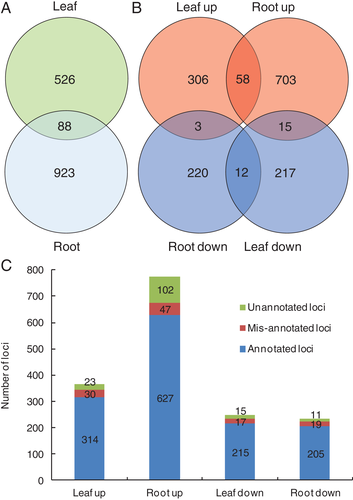
Differential expression analysis showed that a total of 614 and 1011 gene loci were significantly differentially expressed under Zn deficiency in leaves and roots, respectively (Fig. 2A, Tables S5 and S6), and 88 loci were commonly responsive to Zn deficiency in both leaves and roots (Fig. 2A, B, Table S7). Among the differentially expressed loci, 367 loci and 776 loci were upregulated in leaves and roots, respectively. Notably, 102 loci were putatively mis-annotated, and 147 loci were unannotated (Fig. 2C, Tables S8 and S9). Here the mis-annotated loci were considered as loci assembled by Cufflinks but spanning two or more loci annotated in the current soybean genome assembly. Unannotated loci are an assembled group of reads not overlapping with any known loci annotated in the current soybean genome assembly. At least 54 loci, which possess functional annotations, were commonly upregulated or downregulated by Zn deficiency in leaves and roots of soybean (Table S10), suggesting their important roles in the adaptation of leaves and roots to Zn-deficiency stress. Interestingly, some loci exhibited a discrepant expression pattern in response to Zn deficiency in leaves and roots (Fig. 2B, Table S7), suggesting the differential responses of leaves and roots to Zn deficiency.
Validating RNA-seq data by qRT-PCR
To validate the reliability of the RNA-seq data, 12 DEGs were randomly selected and analyzed by qRT-PCR. The FCs of these genes under Zn deficiency observed by qRT-PCR were almost similar to that shown by RNA-seq (Fig. 3). This result suggests that the RNA-seq data obtained here are reliable.
Enrichments of functions and pathways of Zn-deficiency-responsive genes
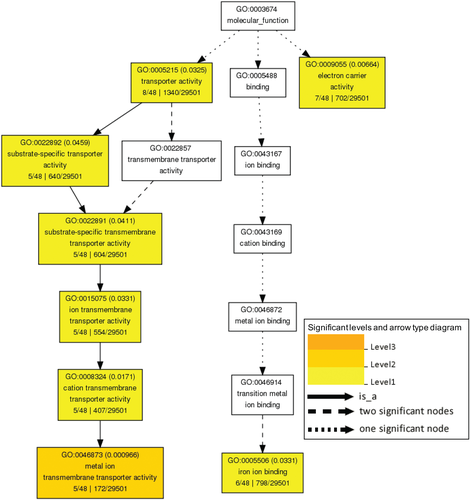
To understand the functions of Zn-deficiency-responsive genes in leaves and roots, GO term enrichment analysis was performed (P ≤ 0.05). In total, 52 GO terms of biological process group, 74 GO terms of molecular function group and 16 GO terms of cellular component group were significantly enriched (Figs 4, S3 and S4). GO terms like metal ion transport (GO:0030001), NA biosynthetic process (GO:0030418), transmembrane transport (GO:0055085), and queuosine biosynthetic process (GO:0008616) were both enriched in upregulated genes of leaves and roots, while oxidation–reduction process (GO:0055114) and defense response (GO:0006952) were both enriched in downregulated genes of leaves and roots (Fig. 4). Some GO terms were enriched in the common DEGs in leaves and roots. These include four terms of biological process group (metal ion transport, cation transport, ion transport and transmembrane transport), and eight terms of molecular function group (such as metal ion transmembrane transporter activity, iron ion binding and electron carrier activity; Figs 5 and S5), indicating a significant over-representation of DEGs with ion transport functions in both leaves and roots of soybean. In addition, 34 KEGG pathways were significantly enriched in the DEGs of leaves and roots, including mineral absorption, photosynthesis, oxidative phosphorylation, peroxisome, carbon metabolism and plant hormone signal transduction (Fig. S6). Some pathways were also commonly enriched in leaves and roots, such as circadian rhythm (KO04710), isoquinoline alkaloid biosynthesis (KO00950) and flavonoid biosynthesis (KO00941) (Fig. S6).
Transcriptional responses of genes potentially involved in Zn homeostasis
The expression levels of 20 genes that are potentially involved in Zn uptake, translocation, reallocation and homeostasis were found to be significantly changed by Zn deficiency in leaves or roots of soybean (Table 1). Among them, eight genes were commonly responsive to Zn deficiency in both leaves and roots, including five genes encoding ZIP (ZRT, IRT-related protein) transporters (Gm15g262800, Gm13g004400, Gm08g164400, Gm20g063100, and Gm06g052000), two genes encoding NAS (Gm19g228400 and Gm09g036400), and one gene encoding MTP (Gm18g050500).
| Tissue | Gene | Description | −Zn FPKM | +Zn FPKM | Log2(FC) | P-value |
|---|---|---|---|---|---|---|
| Leaf | Gm15g262800 | Zinc transporter GmZIP3 | 60.52 | 1.23 | 5.62 | 5.0E-05 |
| Gm13g004400 | Zinc transporter GmZIP4 | 350.38 | 8.57 | 5.35 | 5.0E-05 | |
| Gm08g164400 | Zinc transporter GmZIP2 | 49.18 | 1.47 | 5.06 | 5.0E-05 | |
| Gm20g063100 | Zinc transporter GmZIP1 | 314.02 | 11.32 | 4.79 | 5.0E-05 | |
| Gm06g052000 | Zinc transporter GmZIP11 | 318.10 | 54.39 | 2.55 | 5.0E-05 | |
| Gm18g050500 | MTP | 79.62 | 0.29 | 8.09 | 3.5E-03 | |
| Gm19g228400 | NAS GmNAS2 | 187.25 | 40.26 | 2.22 | 5.0E-05 | |
| Gm09g036400 | NAS GmNAS3 | 28.04 | 6.41 | 2.13 | 5.0E-05 | |
| Root | Gm15g262800 | Zinc transporter GmZIP3 | 110.84 | 15.07 | 2.88 | 5.0E-05 |
| Gm08g164400 | Zinc transporter GmZIP2 | 48.39 | 7.52 | 2.69 | 5.0E-05 | |
| Gm13g004400 | Zinc transporter GmZIP4 | 830.13 | 192.14 | 2.11 | 5.0E-05 | |
| Gm20g063100 | Zinc transporter GmZIP1 | 243.21 | 57.57 | 2.08 | 5.0E-05 | |
| Gm06g052000 | Zinc transporter GmZIP11 | 567.72 | 142.94 | 1.99 | 5.0E-05 | |
| Gm20g022500 | Zinc transporter GmIRT2 | 195.47 | 69.82 | 1.49 | 5.0E-05 | |
| Gm07g223200 | Zinc transporter GmIRT1 | 7.08 | 2.97 | 1.26 | 5.0E-04 | |
| Gm16g088300 | Heavy metal ATPase 5 | 1.25 | 0.47 | 1.40 | 1.2E-03 | |
| Gm18g050500 | MTP | 551.11 | 0.56 | 9.93 | 6.3E-03 | |
| Gm09g122600 | MTP | 92.21 | 40.30 | 1.19 | 5.0E-05 | |
| Gm19g228400 | NAS GmNAS2 | 121.30 | 16.92 | 2.84 | 5.0E-05 | |
| Gm03g231200 | NAS GmNAS1 | 85.25 | 31.14 | 1.45 | 5.0E-05 | |
| Gm09g036400 | NAS GmNAS3 | 344.80 | 140.98 | 1.29 | 5.0E-05 | |
| Gm18g180800 | MT GmMT2e | 387016.00 | 23008.20 | 4.07 | 5.0E-05 | |
| Gm12g154300 | MT GmMT3b | 18846.00 | 2101.34 | 3.16 | 5.0E-05 | |
| Gm03g082100 | MT GmMT2d | 213.51 | 25.49 | 3.07 | 7.5E-04 | |
| Gm06g242900 | MT GmMT3a | 31385.00 | 4169.65 | 2.91 | 5.0E-05 | |
| Gm17g230500 | MT GmMT2b | 31467.80 | 9637.59 | 1.71 | 1.0E-03 | |
| Gm14g093100 | MT GmMT2c | 6035.76 | 2140.41 | 1.50 | 2.0E-03 | |
| Gm07g132000 | MT GmMT2a | 1924.23 | 742.97 | 1.37 | 1.0E-04 |
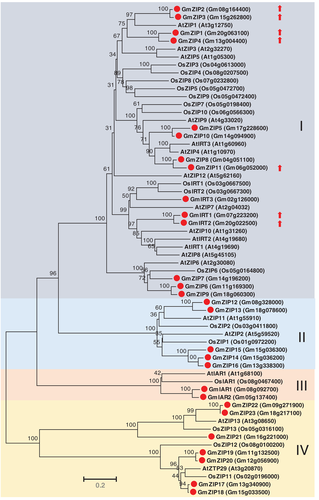
Based on the homologous search using Arabidopsis and rice ZIP proteins, a total of 28 ZIP transporter genes were identified in soybean genome (Table S11). All of these ZIP proteins were predicted to be localized in the plasma membrane. Phylogenetic analysis indicated that these soybean ZIP proteins are closely related to their homologs in Arabidopsis or rice (Fig. 6), suggesting their conserved functions during land plant evolution. In addition, gene families of NAS and metallothionein (MT) were also identified in soybean genome; five members of NAS family and nine members of MT family were found based on homologous search genome-wide level (Tables S12 and S13, Figs S7 and S8). Interestingly, a large proportion of these three families were upregulated by Zn deficiency in leaves or roots (Table 1), including seven ZIP family members (25%), three NAS family members (60%) and seven MT family members (78%). All of the seven ZIP genes belong to clade I of the family.
In addition to ZIP transporter genes, there were 60 DEGs potentially encoding transporters or channels of other nutrients or organic compounds, such as organic acids, amino acids, peptides, sugar, copper, phosphate, potassium, molybdate, sulfate, ammonium and nitrate (Table S14). Among these DEGs, Gm09g138600 (a putative copper transporter), Gm05g145000 (a putative multidrug resistance-associated protein), Gm13g086700 (a putative dicarboxylate transporter) and Gm01g055700 (a putative HCO3− transporter) were all upregulated in both leaves and roots. The responses of these genes encoding transporters or channels suggest the possible involvement of these transporters and channels in the interactions between Zn and other nutrients.
Regulation of Zn-binding protein genes by Zn deficiency
Forty-eight genes encoding putative Zn-binding proteins were found to be responsive to Zn deficiency in leaves or roots of soybean, such as Gm05g007100 and Gm19g007700 (encoding CA), Gm04g240800 and Gm06g122600 (encoding ADH), Gm03g242900 (encoding CSD), and Gm02g181200 (encoding nuclear RNA polymerase) (Table 2). It is interesting that one gene encoding Zn-binding ribosomal protein (Gm01g203600) was upregulated in roots but downregulated in leaves under Zn deficiency, suggesting the complicated regulation of this Zn-binding protein in different tissues of Zn-deficient plant.
| Tissue | Gene | Description | −Zn FPKM | +Zn FPKM | Log2(FC) | P-value |
|---|---|---|---|---|---|---|
| Leaf | Gm15g262900 | Zinc knuckle (CCHC-type) family protein | 60.52 | 1.23 | 5.62 | 5.0E-05 |
| Gm09g080100 | Cytidine/deoxycytidylate deaminase family protein | 3.92 | 1.11 | 1.82 | 2.6E-02 | |
| Gm02g205000 | Zinc ion binding; nucleic acid binding | 6.67 | 1.95 | 1.78 | 1.5E-02 | |
| Gm12g131800 | RING/U-box superfamily protein | 1.81 | 0.67 | 1.44 | 4.5E-02 | |
| Gm16g104500 | DZC domain-containing protein | 1.60 | 0.63 | 1.34 | 1.5E-02 | |
| Gm08g273400 | Zinc-finger (C3HC4-type RING finger) family protein | 13.11 | 6.17 | 1.09 | 5.0E-05 | |
| Gm19g014400 | Matrixin family protein | 8.61 | 4.11 | 1.07 | 1.3E-03 | |
| Gm03g166800 | SET-domain-containing protein lysine methyltransferase | 2.84 | 1.36 | 1.06 | 3.5E-04 | |
| Gm03g082300 | Tim10/DDP family zinc-finger protein | 0.54 | 4.92 | −3.19 | 1.4E-02 | |
| Gm02g027800 | Matrixin family protein | 0.68 | 3.20 | −2.23 | 2.6E-03 | |
| Gm01g203600 | Zinc-binding ribosomal protein family protein | 10.79 | 31.71 | −1.56 | 4.4E-02 | |
| Gm06g110400 | Cold shock domain protein 1 | 12.08 | 34.95 | −1.53 | 5.0E-05 | |
| Gm02g028800 | Matrixin family protein | 1.02 | 2.86 | −1.49 | 6.8E-03 | |
| Gm17g130300 | Cytidine/deoxycytidylate deaminase family protein | 0.49 | 1.24 | −1.34 | 2.4E-02 | |
| Gm16g152700 | GATA TF 9 | 0.99 | 2.36 | −1.26 | 4.0E-02 | |
| Gm03g161300 | Poly(ADP-ribose) polymerase 2 | 2.50 | 5.77 | −1.21 | 5.0E-05 | |
| Gm19g180900 | RING/U-box superfamily protein | 0.70 | 1.54 | −1.13 | 2.5E-02 | |
| Gm06g045400 | Salt tolerance zinc finger | 17.98 | 38.60 | −1.10 | 5.0E-05 | |
| Gm18g225500 | RING/U-box superfamily protein | 1.13 | 2.26 | −1.00 | 4.1E-02 | |
| Root | Gm05g007100 | CA 1 | 199.45 | 6.57 | 4.92 | 5.0E-05 |
| Gm19g007700 | CA 1 | 110.18 | 5.16 | 4.42 | 5.0E-05 | |
| Gm06g193700 | Mini zinc finger | 35.78 | 4.41 | 3.02 | 4.7E-02 | |
| Gm09g230200 | C2H2-like zinc-finger protein | 44.69 | 6.95 | 2.68 | 1.0E-04 | |
| Gm07g222600 | Zinc ion binding;nucleic acid binding | 1.53 | 0.52 | 1.57 | 1.8E-03 | |
| Gm02g242600 | Zinc-finger protein 4 | 7.11 | 2.40 | 1.57 | 1.4E-02 | |
| Gm14g130700 | CHY-type/CTCHY-type/RING-type Zinc-finger protein | 102.24 | 35.21 | 1.54 | 5.0E-05 | |
| Gm05g188900 | RING/U-box superfamily protein | 23.28 | 8.23 | 1.50 | 5.0E-05 | |
| Gm10g149400 | RING/U-box protein | 42.17 | 16.26 | 1.37 | 1.2E-03 | |
| Gm19g008500 | Oxidoreductase, zinc-binding dehydrogenase | 10.64 | 4.14 | 1.36 | 5.0E-05 | |
| Gm05g035600 | Zinc-finger (C3HC4-type RING finger) family protein | 527.45 | 207.02 | 1.35 | 1.1E-02 | |
| Gm13g248000 | B-box type zinc-finger family protein | 7.53 | 3.09 | 1.28 | 3.5E-02 | |
| Gm02g181200 | Nuclear RNA polymerase A1 | 1.16 | 0.51 | 1.18 | 1.5E-03 | |
| Gm14g177900 | RING/U-box superfamily protein | 2.74 | 1.21 | 1.18 | 1.3E-02 | |
| Gm17g202700 | CHY-type/CTCHY-type/RING-type Zinc-finger protein | 31.79 | 14.10 | 1.17 | 5.0E-05 | |
| Gm09g212800 | DZC domain-containing protein | 2.07 | 0.97 | 1.10 | 3.9E-02 | |
| Gm12g061400 | FTSH protease 10 | 2.59 | 1.24 | 1.07 | 9.0E-04 | |
| Gm07g170200 | Zinc-finger, C3HC4 type (RING finger) family protein | 51.57 | 24.74 | 1.06 | 1.0E-04 | |
| Gm01g203600 | Zinc-binding ribosomal protein family protein | 150.57 | 74.32 | 1.02 | 7.5E-03 | |
| Gm12g198400 | B-box type zinc-finger family protein | 6.31 | 3.14 | 1.01 | 2.7E-02 | |
| Gm02g029400 | C2H2 and C2HC zinc-finger superfamily protein | 0.28 | 1.69 | −2.62 | 4.5E-02 | |
| Gm05g197400 | GroES-like zinc-binding ADH family | 4.05 | 15.20 | −1.91 | 5.0E-05 | |
| Gm03g242900 | CSD 1 | 104.23 | 354.80 | −1.77 | 5.0E-05 | |
| Gm19g201800 | RING-H2 group F2A | 9.25 | 26.33 | −1.51 | 5.0E-05 | |
| Gm04g240800 | ADH 1 | 30.69 | 80.17 | −1.39 | 5.0E-05 | |
| Gm20g005800 | C2H2-like zinc-finger protein | 0.31 | 0.81 | −1.37 | 2.3E-02 | |
| Gm06g185000 | GATA type zinc-finger TF | 18.66 | 46.60 | −1.32 | 5.0E-05 | |
| Gm02g308400 | Tim10/DDP family zinc-finger protein | 9.74 | 23.80 | −1.29 | 2.2E-02 | |
| Gm06g122600 | ADH 1 | 219.68 | 467.40 | −1.09 | 5.0E-05 | |
| Gm06g310400 | Zinc ion binding | 0.86 | 1.73 | −1.01 | 3.8E-02 |
Regulation of genes involved in reactive oxygen species production and scavenging by Zn deficiency
Oxidative stress resulting from high levels of reactive oxygen species (ROS) is considered to be a major factor for the development of Zn-deficiency symptoms such as leaf chlorosis (Cakmak 2000). Here, at least 21 genes potentially involved in ROS scavenging were identified to be differentially expressed in leaves or roots of soybean under Zn deficiency (Table 3), suggesting their involvement in Zn-deficiency tolerance. These genes encode many kinds of proteins, such as thioredoxin, glutathione peroxidase, glutaredoxin, iron superoxide dismutase, CSD and copper amine oxidase. Two genes encoding OXIDATIVE STRESS 3 (OXS3) were upregulated in leaves (Gm07g125600 and Gm01g006000). Six genes encoding potential thioredoxin proteins were responsive to Zn deficiency in leaves or roots; three were upregulated (Gm10g021100, Gm15g078600 and Gm16g220500), and three were downregulated (Gm05g179500, Gm03g146000 and Gm04g167500). In addition, one gene (Gm19g233900, encoding respiratory burst oxidase GmRbohF) possibly related to ROS production was repressed by Zn deficiency in soybean leaves. However, there were no common DEGs for ROS scavenging between leaves and roots, suggesting the different molecular mechanisms of different tissues in the response to Zn-deficiency-mediated oxidative stress.
| Tissue | Gene | Description | −Zn FPKM | +Zn FPKM | Log2(FC) | P-value |
|---|---|---|---|---|---|---|
| Leaf | Gm10g021100 | Thioredoxin superfamily protein | 9.99 | 1.59 | 2.65 | 1.5E-03 |
| Gm01g219400 | Glutathione peroxidase 6 | 4.60 | 1.34 | 1.78 | 2.8E-02 | |
| Gm07g125600 | OXS3 | 9.97 | 3.38 | 1.56 | 2.5E-03 | |
| Gm01g006000 | OXS3 | 10.50 | 3.80 | 1.47 | 1.8E-03 | |
| Gm19g021300 | Glutaredoxin family protein | 1.90 | 0.76 | 1.32 | 1.4E-02 | |
| Gm16g164400 | Peroxidase superfamily protein | 1.73 | 0.70 | 1.31 | 4.1E-02 | |
| Gm14g152000 | Protein disulfide isomerase like 1–1 | 0.79 | 0.35 | 1.18 | 3.2E-02 | |
| Gm18g211100 | Peroxidase superfamily protein | 4.38 | 12.46 | −1.51 | 5.0E-05 | |
| Gm16g056300 | Ferritin/ribonucleotide reductase-like protein | 1.34 | 3.06 | −1.19 | 2.5E-02 | |
| Gm10g193500 | Fe superoxide dismutase 2 | 393.98 | 815.15 | −1.05 | 5.0E-05 | |
| Gm19g233900 | Respiratory burst oxidase GmRbohF | 0.47 | 0.94 | −1.02 | 2.7E-02 | |
| Root | Gm03g214500 | NADH–ubiquinone oxidoreductase-related | 392.35 | 154.56 | 1.34 | 8.0E-04 |
| Gm15g078600 | Thioredoxin superfamily protein | 12.93 | 5.13 | 1.33 | 2.3E-02 | |
| Gm18g025000 | Glutaredoxin family protein | 8.07 | 3.52 | 1.20 | 9.3E-03 | |
| Gm17g085500 | Copper amine oxidase family protein | 24.69 | 12.15 | 1.02 | 5.0E-05 | |
| Gm16g220500 | Thioredoxin H-type 9 | 23.80 | 11.88 | 1.00 | 5.7E-03 | |
| Gm05g179500 | Thioredoxin superfamily protein | 0.00 | 0.94 | +Zn only | 4.2E-03 | |
| Gm03g242900 | CSD 1 | 104.23 | 354.80 | −1.77 | 5.0E-05 | |
| Gm03g146000 | WCRKC thioredoxin 1 | 0.99 | 3.24 | −1.70 | 3.3E-02 | |
| Gm05g055000 | Copper chaperone for SOD1 | 12.42 | 36.30 | −1.55 | 5.0E-05 | |
| Gm17g053000 | Peroxidase superfamily protein | 3.00 | 8.04 | −1.42 | 2.5E-04 | |
| Gm04g167500 | Atypical CYS HIS rich thioredoxin 4 | 5.29 | 10.87 | −1.04 | 1.7E-03 |
Regulation of genes associated with calcium (Ca2+), sugar and hormonal signals by Zn deficiency
At least 49 DEGs in Zn-deficient leaves or roots of soybean were found to be possibly related to the signaling of Ca2+ and hormones such as auxin, cytokinin (CK), ethylene, GA, abscisic acid (ABA), polyamine, brassinosteroid (BR) and jasmonate (JA) (Table 4). Among them, about two third of these genes (33/49) were upregulated by Zn deficiency, including seven genes likely involved in auxin signaling (Gm15g113300, Gm07g043000, Gm03g088900, Gm13g361200, Gm03g167400, Gm05g196300 and Gm08g322300), six genes (Gm03g160200, Gm15g236200, Gm19g154400, Gm09g225400, Gm19g051800 and Gm17g087900) possibly involved in CK signaling, three genes encoding GA-regulated family proteins (Gm17g237100, Gm14g087200 and Gm06g185300), two genes encoding polyamine oxidases (Gm09g227500 and Gm15g169600), and one gene encoding calcium-dependent protein kinase (CDPK) GmCDPK8 (Gm10g217000). In contrast, 16 genes were repressed by Zn deficiency in soybean leaves or roots, including three genes encoding ethylene response factors (Gm02g006200, Gm05g063600 and Gm19g248900), two genes encoding ADHs (Gm04g240800 and Gm06g122600), two genes encoding response regulators (Gm04g137600 and Gm07g079000), and one gene encoding EF-hand-containing calmodulin-like protein GmCML109 (Gm13g163900).
| Tissue | Signal | Gene | Description | −Zn FPKM | +Zn FPKM | Log2(FC) | P-value |
|---|---|---|---|---|---|---|---|
| Leaf | ABA | Gm19g069200 | Highly ABA-induced PP2C gene 3 | 1.10 | 0.27 | 2.03 | 0.04 |
| Auxin | Gm15g113300 | Indole-3-butyric acid response 1 | 2.71 | 0.79 | 1.78 | 0.02 | |
| Auxin | Gm07g043000 | Dormancy/auxin associated family protein | 27.27 | 8.92 | 1.61 | 0.00 | |
| Auxin | Gm03g088900 | Auxin-responsive family protein | 7.37 | 3.23 | 1.19 | 0.02 | |
| Auxin | Gm13g361200 | AUX/IAA transcriptional regulator family protein | 15.69 | 7.18 | 1.13 | 0.02 | |
| CK | Gm03g160200 | Cytochrome P450 family 94, subfamily B, polypeptide 1 | 1.75 | 0.19 | 3.22 | 0.01 | |
| CK | Gm15g236200 | Response regulator 3 | 1.04 | 0.24 | 2.14 | 0.04 | |
| CK | Gm19g154400 | Isopentenyltransferase 3 | 3.68 | 1.17 | 1.65 | 0.00 | |
| CK | Gm09g225400 | CKX 6 | 2.16 | 0.75 | 1.53 | 0.00 | |
| CK | Gm07g079000 | Response regulator 2 | 0.49 | 1.38 | −1.50 | 0.01 | |
| Ethylene | Gm02g006200 | Ethylene response factor 1 | 0.49 | 2.56 | −2.38 | 0.05 | |
| Ethylene | Gm05g063600 | Ethylene-responsive element-binding factor 1 | 2.67 | 8.00 | −1.58 | 0.00 | |
| Ethylene | Gm14g049200 | Ethylene-forming enzyme | 0.61 | 1.79 | −1.56 | 0.04 | |
| Ethylene | Gm19g248900 | Ethylene response factor 1 | 1.52 | 4.40 | −1.53 | 0.02 | |
| GA | Gm11g003200 | GA 2-oxidase 8 | 1.18 | 0.30 | 1.96 | 0.04 | |
| Polyamine | Gm15g169600 | Polyamine oxidase 5 | 0.90 | 0.44 | 1.03 | 0.03 | |
| Calcium | Gm10g217000 | CDPK GmCDPK8 | 2.39 | 0.79 | 1.60 | 0.00 | |
| Calcium | Gm05g090900 | CDPK-related kinase GmCRK6 | 1.30 | 42.09 | −5.01 | 0.00 | |
| Calcium | Gm19g233900 | Respiratory burst oxidase GmRbohF | 0.47 | 0.94 | −1.02 | 0.03 | |
| Root | ABA | Gm06g040400 | ABA-responsive elements-binding factor 2 | 88.70 | 32.93 | 1.43 | 0.00 |
| ABA | Gm04g240800 | ADH 1 | 30.69 | 80.17 | −1.39 | 0.00 | |
| ABA | Gm06g122600 | ADH 1 | 219.68 | 467.40 | −1.09 | 0.00 | |
| Auxin | Gm03g167400 | Auxin-induced protein 13 | 1.35 | 0.32 | 2.09 | 0.05 | |
| Auxin | Gm05g196300 | SAUR-like auxin-responsive protein family | 32.11 | 9.77 | 1.72 | 0.02 | |
| Auxin | Gm08g322300 | IAA carboxylmethyltransferase 1 | 2.79 | 0.90 | 1.63 | 0.04 | |
| Auxin | Gm13g284600 | Auxin-responsive GH3 family protein | 0.10 | 0.75 | −2.92 | 0.02 | |
| Auxin | Gm20g080000 | YUCCA 3 | 0.18 | 0.84 | −2.23 | 0.03 | |
| Auxin | Gm09g251600 | Auxin efflux carrier family protein | 1.12 | 2.36 | −1.07 | 0.03 | |
| Auxin | Gm10g031800 | AUX/IAA transcriptional regulator family protein | 1.33 | 2.73 | −1.04 | 0.04 | |
| BR | Gm13g252200 | BAK1-interacting receptor-like kinase 1 | 7.10 | 3.25 | 1.13 | 0.05 | |
| CK | Gm19g051800 | Response regulator 1 | 2.36 | 0.39 | 2.61 | 0.04 | |
| CK | Gm17g087900 | Histidine kinase | 1.61 | 0.60 | 1.44 | 0.00 | |
| CK | Gm04g137600 | Response regulator 9 | 0.76 | 2.44 | −1.69 | 0.01 | |
| Ethylene | Gm11g239000 | Ethylene insensitive 3 family protein | 1.94 | 0.54 | 1.85 | 0.01 | |
| Ethylene | Gm18g069300 | Ethylene sensor | 3.84 | 1.33 | 1.52 | 0.00 | |
| Ethylene | Gm09g026200 | Ethylene-responsive nuclear protein (ERT2) | 4.14 | 2.00 | 1.05 | 0.01 | |
| Ethylene | Gm14g049000 | Ethylene-forming enzyme | 0.36 | 1.11 | −1.61 | 0.03 | |
| GA | Gm17g237100 | GA-regulated family protein | 253.56 | 57.90 | 2.13 | 0.00 | |
| GA | Gm14g087200 | GA-regulated family protein | 106.45 | 38.86 | 1.45 | 0.01 | |
| GA | Gm09g036500 | RGA-like 2 | 20.60 | 8.51 | 1.28 | 0.00 | |
| GA | Gm06g185300 | GA-regulated family protein | 38.65 | 18.39 | 1.07 | 0.04 | |
| JA | Gm05g098600 | Lipoxygenase 1 | 2.40 | 0.97 | 1.31 | 0.04 | |
| Polyamine | Gm09g227500 | Polyamine oxidase 1 | 5.71 | 2.43 | 1.23 | 0.00 | |
| Calcium | Gm09g029600 | Annexin 8 | 1.40 | 0.41 | 1.78 | 0.03 | |
| Calcium | Gm11g127500 | EF-hand calcium-binding protein GmCML32 | 29.32 | 9.84 | 1.58 | 0.01 | |
| Calcium | Gm08g028800 | Phospholipase A2 family protein | 5.68 | 1.93 | 1.56 | 0.04 | |
| Calcium | Gm09g003400 | C2 calcium/lipid-binding plant phosphoribosyltransferase | 0.83 | 0.40 | 1.04 | 0.02 | |
| Calcium | Gm15g187400 | CIPK 1 | 4.43 | 2.19 | 1.01 | 0.00 | |
| Calcium | Gm13g163900 | Calcium-binding EF-hand protein GmCML109 | 0.56 | 1.22 | −1.13 | 0.02 |
In addition, there were at least 25 DEGs that are potentially involved in sugar metabolism and signaling (Table S15). Among these genes, Gm15g105900 which likely encodes a glucose-6-phosphate/phosphate translocator, and Gm08g240400 which likely encodes an SNF1-related kinase were all induced by Zn deficiency in leaves and roots. These results suggested that Ca2+, sugar and hormonal signals are involved in plant response and tolerance to Zn-deficiency stress.
Regulation of genes linked to protein phosphorylation and dephosphorylation by Zn deficiency
At least 51 protein kinase genes and 6 protein phosphatase genes were found to be differentially expressed under Zn deficiency in leaves or roots of soybean; most of the protein kinase genes (39/51), and all of the six protein phosphatase genes were upregulated (Table S16). The protein kinases genes belong to several subfamilies, such as receptor-like protein kinase, SNF1-related protein kinase, CDPK, CBL-interacting protein kinase (CIPK) and casein kinase. These results suggested that protein phosphorylation and dephosphorylation are involved in plant adaptation to Zn deficiency.
Identification of TFs responding to Zn deficiency
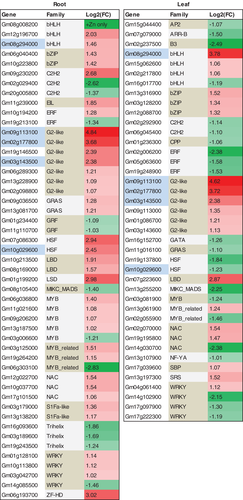
The expression levels of at least 85 TFs genes were changed under Zn deficiency in leaves or roots of soybean; five of them were commonly responsive to Zn deficiency in both leaves and roots (Fig. 7). By searching the plant TF database (Jin et al. 2016), these Zn-deficiency-responsive TF genes were found to belong to a diverse range of families, such as G2-like (Golden 2-like), WRKY, bHLH, MYB and MYB-related, NAC, bZIP, ERF, C2H2, HSF, Trihelix, GRAS and LBD. The DEGs belonging to the same TF family largely showed a similar response to Zn deficiency. For example, the 10 G2-like TF genes, the five bZIP TF genes and the three LBD TF genes were all upregulated by Zn deficiency, while the three Trehelix TF genes and the two GRF TF genes were all downregulated by Zn deficiency.
Motifs enriched in promoters of DEGs and the DEGs potentially regulated by F-group bZIP TFs
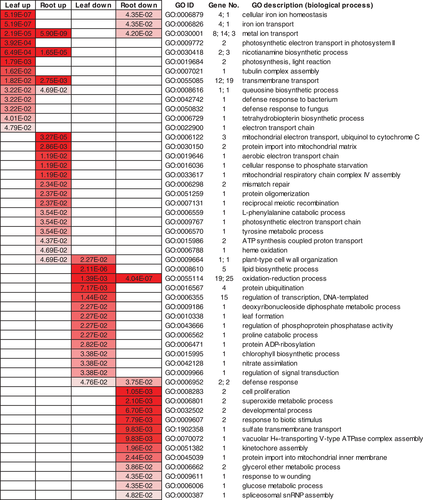
The motifs enriched in the promoter regions of upregulated and downregulated DEGs in roots and leaves were analyzed. About 16 to 43 motifs were significantly enriched in each of the four groups (leaf upregulated, root upregulated, leaf downregulated and root downregulated; Table S17, Figs S9 and S10). Only one motif (CWCGTS) was commonly enriched in the upregulated DEGs of leaves and roots, but there were some other motifs with similar sequences enriched in both leaf DEGs and root DEGs. This result suggests the potential involvement of these enriched motifs in the regulatory network of Zn-deficiency responses in the different tissues of soybean.
ZDRE (RTGTCGACAY) is a known binding motif of the F-group bZIP TFs, such as bZIP19 and bZIP23, which are critical and conserved regulators of Zn-deficiency responses (Assunção et al. 2010). Here, the presence of the 10-bp ZDRE in the 1500-bp promoter regions of all the Zn-deficiency-responsive genes was searched. Only nine DEGs (all upregulated) were found to exist with a single copy of ZDRE in their promoters; GmZIP1, GmZIP4 and GmNAS3 were included (Table 5). This result suggests that these genes are potential targets of the F-group bZIP TFs under Zn deficiency. Here, we did not analyzed the existence of ZDRE in the promoter regions of other genes in soybean genome with the exception of the Zn-deficiency-responsive DEGs. According to a previous study (Assunção et al. 2010), 141 Arabidopsis genes and 219 rice genes were predicted to contain one to three copies of the ZDRE motif in their promoter regions. There should also be a lot of other soybean genes containing ZDRE in their promoter regions. But whether the genes containing ZDRE in their promoters would be regulated by F-group bZIP TFs require experimental validation. Some of the genes containing ZDRE in their promoters may not be the authentic targets of F-group bZIP TFs and could not be bound and regulated by the TFs because the existance of the ZDRE sequence in their promoter regions would be accidental.
| Gene | ZDRE location | −Zn FPKM | +Zn FPKM | Log2(FC) | P-value | Description | |
|---|---|---|---|---|---|---|---|
| Root | Glyma.13G004400 | −133 | 830.13 | 192.14 | 2.11 | 5.00E-05 | Zinc transporter GmZIP4 |
| Glyma.20G063100 | −143 | 243.21 | 57.57 | 2.08 | 5.00E-05 | Zinc transporter GmZIP1 | |
| Glyma.15G240300 | −480 | 15.23 | 3.73 | 2.03 | 1.76E-02 | Uncharacterized protein | |
| Glyma.18G239500 | −45 | 3.18 | 0.97 | 1.71 | 5.00E-05 | Subtilisin-like serine endopeptidase | |
| Glyma.05G188900 | −875 | 23.28 | 8.23 | 1.50 | 5.00E-05 | RING/U-box superfamily protein | |
| Glyma.09G036400 | −509 | 344.80 | 140.98 | 1.29 | 5.00E-05 | NAS GmNAS3 | |
| Glyma.03G078600 | −36 | 13.72 | 6.53 | 1.07 | 5.00E-05 | Glycerol-3-phosphate acyltransferase | |
| Glyma.09G003700 | −11 | 13.47 | 6.66 | 1.02 | 1.70E-03 | RAB GTPase homolog A4D | |
| Leaf | Glyma.09G255700 | −1105 | 1.28 | 0.20 | 2.68 | 5.60E-03 | Protein kinase superfamily protein |
Discussion
Distinct transcriptome profiling of leaves and roots in responses to Zn deficiency
In this study, we investigated the transcriptome profilings of soybean leaves and roots in responses to Zn deficiency using Illumina's high-throughput RNA-seq, and a total of 1537 loci were found to be differentially expressed. However, only a small portion of them (88 loci, 5.7%) were commonly responsive to Zn deficiency in both leaves and roots (Fig. 2A); many of the common DEGs are possibly involved in Zn homeostasis control (Fig. 5). Some loci were found to have a converse expression pattern in leaves and roots under Zn deficiency (Fig. 2B). In addition, most of the GO terms and pathways enriched in the DEGs of leaves and roots were distinct (Figs 4 and S6). These results suggest that Zn deficiency triggers a distinct transcriptomic response in leaves and roots of soybean. Discrepant global expression in leaves and roots was also indicated in plant responses to other nutrient stresses, such as phosphorus deficiency and magnesium deficiency (Wu et al. 2003, Hermans et al. 2010).
Upregulation of Zn transporters that are possibly involved in Zn uptake and translocation
Zn ions are taken up by root epidermal cells, then migrate by diffusion to xylem parenchyma cells and then are transported into the xylem vessel for translocation to plant shoots (Palmgren et al. 2008). These processes involve diverse Zn transporters. Here, the expression of some genes potentially involved in Zn transportation, translocation, and reallocation, was triggered by Zn deficiency in roots and leaves of soybean (Table 1). This result is consistent with the enhancement of the uptake and root-to-shoot translocation of Zn under Zn-deficient condition (Tiong et al. 2015). The alternations of genes potentially involved in Zn homeostasis control is in line with previous studies of Zn-deficiency response (Widodo et al. 2010, Bandyopadhyay et al. 2017, Nanda et al. 2017), and is also similar to the deficiencies of some major elements (such as N, P, K and S), which trigger the expression of genes encoding transporters of these ions in order to increase the capacity of uptake and translocation of the ions (Maruyama-Nakashita et al. 2003, Ma et al. 2012, Zeng et al. 2018).
ZIP transporters are the well-known Zn-responsive transporters and are involved in Zn uptake and translocation (Grotz et al. 1998, Ishimaru et al. 2005). Here, 28 ZIP transporter genes were identified in soybean genome, and 7 of them belonging to clade I were found to be induced by Zn deficiency (Fig. 6). The number of ZIP proteins in soybean is more than that in Arabidopsis, rice, maize and some other plants (Mäser et al. 2001, Li et al. 2013a, Tiong et al. 2015, Evens et al. 2017), which may be associated with the whole-genome duplication events that occurred about 59 and 13 million years ago (Schmutz et al. 2010). In addition to ZIP transporters, several genes encoding potential Zn transporters, such as HMA transporter (Gm16g088300) and MTP transporters (Gm18g050500 and Gm09g122600) were also differentially expressed (Table 1). Based on the functions of their orthologous genes in model plants (Hussain et al. 2004, Arrivault et al. 2006, Palmgren et al. 2008), these genes might also play a role in Zn translocation and allocation in soybean.
Enhancement of the synthesis of Zn chelators, such as NA and MT under Zn-deficiency condition
NA and its derivative phytosiderophores (e.g. mugineic acid and deoxy-mugineic acid) are known to chelate metals such as Zn, and are important for their long-distance transport in plants (Clemens et al. 2013). Phytosiderophore production only exists in grasses, while NA is ubiquitous throughout vascular plants (Ricachenevsky et al. 2015). Here, five members of NAS genes were identified in soybean genome, and three of them were found to be induced by Zn deficiency (Fig. S7). This is in line with previous reports showing that some NAS genes (like OsNAS3 and HvNAS1) are also induced by Zn deficiency in other plants (Suzuki et al. 2006, Suzuki et al. 2008, Clemens et al. 2013). It has been shown that enhanced expression of rice NAS2 elevates NA levels, results in higher Zn accumulation and improves plant tolerance to Zn deficiency (Lee et al. 2011). Our data suggest a role of the Zn-induced GmNAS1/2/3 in Zn homeostasis and Zn-deficiency tolerance.
MTs, another kind of metal chelators, are small cysteine-rich proteins capable of binding transition metals including Zn (Guo et al. 2008, Sinclair and Krämer 2012). Over-expression of AtMT4a improved Zn tolerance in Arabidopsis (Rodríguez-Llorente et al. 2010). Over-expression of OsMT1a resulted in higher accumulation of Zn in rice tissues and enhanced resistance to drought stress, which is possibly related to higher ROS scavenging capacity and higher expression of Zn-induced CCCH zinc-finger TFs (Yang et al. 2009). Recently, some studies also suggest that MTs are involved in the scavenging of ROS (Xue et al. 2009, Kumar et al. 2012). We identified nine members of MT family in soybean genome and seven of them were found to be induced by Zn deficiency (Fig. S8). These GmMTs may play a role in maintaining Zn homeostasis and also in Zn-deficiency stress tolerance.
Involvement of Zn-binding proteins in plant response to Zn deficiency
Nearly, 10% of the protein-coding genes in eukaryotic are predicted to encode Zn-binding proteins (Andreini et al. 2006, Broadley et al. 2007). Here, at least 48 genes encoding potential Zn-binding proteins were found to be responsive to Zn deficiency, and many of these genes encode putative ZFPs (Table 2). ZFPs can act in transcriptional regulation, either directly by DNA/RNA binding or indirectly by site-specific modification and/or regulation of chromatin, and can also participate in RNA metabolism and other cellular functions (Englbrecht et al. 2004). Recently, ZFPs were found to be responsive to many stresses and are involved in tolerance to various stresses in plants (Davletova et al. 2005, Huang et al. 2009, Luo et al. 2012). Whether the Zn-responsive ZFPs can sense and respond to Zn-deficiency stress remains to be answered.
In addition to ZFPs, some DEGs possibly encoding Zn-requiring metabolic enzymes, such as CA, CSD and ADH were responsive to Zn deficiency (Table 2). CA is a catalytic metalloenzyme that reversibly catalyzes the hydration of [CO2] to yield HCO3−, and is involved in photosynthesis by facilitating the diffusion of [CO2] (Randall and Bouma 1973). Here, the expression of two potential CA genes (Gm05g007100 and Gm19g007700) was induced in Zn-deficient roots, which may compensate for the reduced CA activity under Zn deficiency (Sasaki et al. 1998, Li et al. 2013b). The activities of ADH, which is involved in alcohol fermentation and lignin biosynthesis, and of CSD, which are involved in ROS scavenging, are considered to be reduced by Zn deficiency (Moore and Patrick 1988,Hacisalihoglu et al. 2003 , Li et al. 2013b). Here, the corresponding mRNAs of these enzymes were downregulated by Zn deficiency, which is in conformity with previous studies (Hacisalihoglu et al. 2003, Li et al. 2013b). Maintaining higher activities of these Zn-containing enzymes such as CA, CSD and ADH under low Zn conditions have been considered to be an important factor in Zn efficiency (Rengel 1995, Hacisalihoglu et al. 2003, Chen et al. 2008). In addition to the transcriptional regulation of Zn-binding protein genes, post-transcriptional regulation is also involved, such as the negative regulation of CSD by microRNA528 and microRNA398 (Li et al. 2013b).
Possible involvements of hormones and Ca2+ in plant response to Zn deficiency
Plant hormones have been depicted in plant responses to various nutrient stresses such as deficiencies of nitrogen, phosphorus, potassium, sulfur and iron (López-Bucio et al. 2003, Rubio et al. 2009, Krouk et al. 2011). In this study, at least 40 Zn-deficiency-responsive genes were possibly linked to the signaling of hormones such as auxin, CK, ethylene, GA and polyamine (Table 4), suggesting the interrelationships between hormones and Zn-deficiency response. A gene (Gm20g080000) encoding a likely YUCCA family protein, which is a flavin monooxygenase-like enzyme involved in auxin biosynthesis by catalyzing the hydroxylation of the amino group of tryptamine (Zhao et al. 2001), was repressed by Zn deficiency in roots. Another downregulated gene (Gm14g049000) in Zn-deficient roots encodes a putative ethylene-forming enzyme, which is possibly involved in ethylene biosynthesis by catalyzing the oxidation of 1-aminocyclopropane-1-carboxylic acid, the last step in ethylene biosynthesis (Wang et al. 2002). It would be interesting to investigate whether the downregulation of the biosynthesis of auxin and ethylene is associated with the modification of root development and morphology under Zn deficiency.
CKs regulate cell division as well as many developmental events, such as shoot branching and leaf development. CK oxidases/dehydrogenases (CKX) are responsible for irreversible CK degradation (Schmülling et al. 2003). Here, a gene encoding putative CKX (Gm09g225400) was induced by Zn deficiency in leaves. Whether the CK content of leaves is reduced by Zn deficiency and whether the shoot growth inhibition upon Zn deficiency is related to the upregulation of CKX genes deserve further investigations. Bioactive GAs regulate diverse developmental processes such as stem elongation, leaf expansion and flowering time. GA 2-oxidases (GA2ox) are responsible for the conversion of bioactive GAs to inactive forms (Olszewski et al. 2002). Here, a gene (Gm11g003200) encoding a potential GA2ox was induced by Zn deficiency in leaves. Whether the upregulation of GA2ox is associated with the development of Zn-deficiency symptoms such as reduced leaf size, stunted stems and late flowering (Marschner 1995, Chen and Ludewig 2018), remains to be examined.
Ca2+ is a universal signal which plays an important role in plant responses to diverse environmental stresses (Poovaiah et al. 2013, Yuan et al. 2014). External environmental stimuli can quickly trigger specific spatial–temporal patterns of changes in cytosolic Ca2+ concentration, which are perceived and decoded by a series of EF-hand-containing Ca2+ sensors (Zeng et al. 2015). It has been well documented that Ca2+ signal has a critical role in homeostasis controls of nitrate, potassium and sodium (Kudla et al. 2010). Here, nine DEGs are potentially related to Ca2+ signaling, and six of them were upregulated by Zn deficiency (Table 4). Some of them were also responsive to other stresses. For example, GmCML109 (Gm13g163900) were downregulated by cold, flooding, phosphorus deficiency and iron deficiency (Zeng et al. 2017), suggesting its role in multiple stress responses. Further research is needed in order to investigate whether Ca2+ signal is involved in the regulation of Zn-deficiency response and/or Zn homeostasis.
Transcriptional regulation of plant response to Zn deficiency
Transcriptional control of the expression of stress-responsive genes is an important part of plant responses to a series of environmental stresses. Arabidopsis F-group bZIP TF bZIP19 and bZIP23 have been shown to be essential for the Zn homeostasis regulation (Assunção et al. 2010, Inaba et al. 2015). The F-group bZIP TFs in other plants such as wheat and barley were also found to have a similar function in Zn-deficiency response (Castro et al. 2017, Evens et al. 2017, Nazri et al. 2017). There are also two members of F-group bZIP TFs in soybean genome, GmbZIP121 (Gm11g108100) and GmbZIP122 (Gm12g033000) (Liao et al. 2008). Their expression levels were not changed under Zn deficiency in this study. However, five genes encoding other subgroups of bZIP TFs were found to be induced by Zn deficiency (Fig. 7). It would be interesting to examine their roles in Zn-deficiency response. In addition, nine DEGs were found to contain ZDRE in their promoter regions; these genes including GmZIP1, GmZIP4 and GmNAS3 were all upregulated by Zn deficiency (Table 5). This is consistent with the previous report that a set of Zn-responsive genes including AtZIP3, AtZIP4, AtZIP9, AtIRT3, AtNAS2 and AtNAS4 are possibly regulated by AtbZIP19/bZIP23 (Assunção et al. 2010). Therefore, the role of F-group bZIP TFs in Zn-deficiency response should be conserved in land plants. Whether and how the orthologs in soybean function in the regulation of Zn-deficiency response deserve further investigations.
In addition to bZIP TF, 80 TF genes belonging to other families, such as G2-like, WRKY, bHLH, MYB, NAC, ERF, HSF and GRAS were also responsive to Zn deficiency (Fig. 7). G2-like TFs are required for chloroplast development and can regulate the expression of a suite of photosynthesis-related genes (Waters et al. 2009). Here, 10 G2-like TF genes were all upregulated by Zn deficiency. Whether the induction of G2-like TFs could partially compensate the reduced chlorophyll content and photosynthesis rate under long-term Zn-deficiency stress should be investigated in the future. Further investigations are also needed to understand the potential roles of Zn-responsive TFs in transcriptional regulations of Zn signaling, Zn homeostasis, physiological response, metabolic adaptation, growth adjustment and stress tolerance under Zn deficiency.
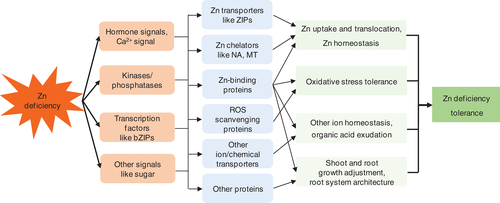
In conclusion, this study provides a genome-wide transcriptome analysis of soybean leaves and roots in response to Zn deficiency. A lot of genes were identified to be responsive Zn deficiency, which contain various different biological functions, such as Zn uptake and translocation, ROS homeostasis, Ca2+ signaling, sugar metabolism and signaling, hormonal signaling, protein phosphorylation and dephosphorylation and transcriptional regulation mediated by TFs. Based on the transcriptome analysis, we proposed a hypothetic working model involving the signaling pathways underlying Zn-deficiency response and tolerance (Fig. 8). Our transcriptome results would provide a platform for functional characterizations of these Zn-deficiency-responsive genes in Zn-deficiency stress sensing, signaling and tolerance. Further genetic modifications of some candidates could improve Zn use efficiency and Zn-deficiency tolerance in plants.
Author contributions
H.Z. and Y.Z. conceived the study and designed the experiments. H.Z., X.Z., M.D. and X.Z. performed the experiments. H.Z. and Y.Z. analyzed the data. H.Z. wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31201679) and the Zhejiang Provincial Natural Science Foundation of China (LY15C020006).




