Interaction between the signaling molecules hydrogen sulfide and hydrogen peroxide and their role in vacuolar H+-ATPase regulation in cadmium-stressed cucumber roots
Abstract
Vacuolar H+-ATPase (V-ATPase; EC 3.6.3.14) is the main enzyme responsible for generating a proton gradient across the tonoplast. Under cadmium (Cd) stress conditions, V-ATPase activity is inhibited. In the present work, hydrogen sulfide (H2S) and hydrogen peroxide (H2O2) cross-talk was analyzed in cucumber (Cucumis sativus L.) seedlings exposed to Cd to explain the role of both signaling molecules in the control of V-ATPase. V-ATPase activity and gene expression as well as H2S and H2O2 content and endogenous production were determined in roots of plants treated with 100 μM CdCl2 and different inhibitors or scavengers. It was found that H2S donor improved photosynthetic parameters in Cd-stressed cucumber seedlings. Cd-induced stimulation of H2S level was correlated with the increased activities of the H2S-generating desulfhydrases. Increased H2O2 and lowered H2S contents in roots were able to reduce V-ATPase activities similar to Cd. H2O2 and H2S-induced modulations in V-ATPase activities were not closely related to the transcript level of encoding genes, suggesting posttranslational modifications of enzyme protein. On the other hand, exogenous H2O2 raised H2S content in root tissues independently from the desulfhydrase activity. Although treatment of control plants with H2S significantly stimulated NADPH oxidase activity and gene expression, H2S did not affect H2O2 accumulation in roots exposed to Cd. The results suggest the existence of two pathways of H2S generation in Cd-stressed cucumber roots. One involves desulfhydrase activity, as was previously demonstrated in different plant species. The other, the desulfhydrase-independent pathway induced by H2O2/NADPH oxidase, may protect V-ATPase from inhibition by Cd.
Abbreviations
-
- AG
-
- aminoguanidine
-
- Asc
-
- ascorbic acid
-
- CAS
-
- β-cyanoalanine synthase
-
- CO
-
- carbon monoxide
-
- DAB
-
- 3,3′-diaminobenzidine
-
- DAO
-
- diamine oxidase
-
- D-CD
-
- D-cysteine desulfhydrase
-
- DMTU
-
- N,N′-dimethylthiourea
-
- DPI
-
- diphenyleneiodonium chloride
-
- L-CD
-
- L-cysteine desulfhydrase
-
- MB
-
- methylene blue
-
- NO
-
- nitric oxide
-
- OAS
-
- O-acetylserine
-
- OAS-TL
-
- O-acetyl-L-serine(thiol)lyase
-
- PAG
-
- propargylglycine
-
- PAM
-
- pulse amplitude modulation
-
- PCR
-
- polymerase chain reaction
-
- PSII
-
- photosystem 2
-
- ROS
-
- reactive oxygen species
-
- SIR
-
- sulfite reductase
-
- TCA
-
- trichloroacetic acid
-
- V-ATPase
-
- vacuolar H+-transporting ATPase
Introduction
H2S, a colorless gas with greater solubility in lipophilic solvents than in water, can permeate through plasma membranes. H2S has been known as an environmental toxin harmful to living organisms for about 300 years. At high levels, i.e. 3000 parts per billion (ppb), H2S causes leaf damage and decreases plant growth ( Thompson and Kats 1978). This results from its ability to inhibit cytochrome c oxidase in mitochondria, similar to cyanide, and to reduce cell energy production (Lisjak et al. 2013). On the other hand, lower levels of exogenous H2S, such as 100 ppb, could promote plant growth (Wang 2012). Most recently, it has been suggested that endogenous H2S acts as a biologically active molecule that, along with nitric oxide (NO) and carbon monoxide (CO), is a member of the gasotransmitters (gasomediators) controlling intracellular processes in animals (Wang 2002, Pae et al. 2009, Li et al. 2011).
Similar to animals, plant cells possess a tight regulatory mechanism controlling endogenous H2S content within the physiological range. The enzymes responsible for H2S generation (cysteine-degrading enzymes) and removal (cysteine-synthesizing enzymes) have been identified in plants (Papenbrock et al. 2007). The first group includes L-cysteine and D-cysteine desulfhydrases (L-CD and D-CD, respectively), which specifically metabolize L-cysteine or D-cysteine, respectively, to form H2S, pyruvate and ammonium. This group also includes sulfite reductase (SIR), producing H2S from sulfite in the process of sulfate assimilation, and β-cyanoalanine synthase (CAS), converting cysteine and cyanide to H2S and β-cyanoalanine (Papenbrock et al. 2007, Li 2013). The presence of L-CD and D-CD in several cell compartments, including the cytosol, chloroplasts and mitochondria, has been reported in various plant species (Wang 2012, Lisjak et al. 2013). On the other hand, O-acetyl-L-serine(thiol)lyase (OAS-TL) consumes H2S during cysteine biosynthesis and may be responsible for H2S depletion and detoxification. Three OAS-TL isoforms have been distinguished: cytosolic OAS-TL A1, plastid OAS-TL B and mitochondrial OAS-TL C (Burandt et al. 2002, Wirtz et al. 2004). In Arabidopsis thaliana, the protein DES1 catalyzing the desulfhydration of L-cysteine was identified and is located in the cytosol, which is the major site of cysteine biosynthesis (Álvarez et al. 2010). It was proposed that cysteine production and degradation (H2S consumption and release) in the cytosol, and consequently generation of the H2S pool with a potential signaling purpose, is coordinated via lyase OAS-TL A1 and DES1 activities (Álvarez et al. 2010, Romero et al. 2014).
The ability to freely penetrate the cell membranes, to have an endogenous production on demand and a rapid degradation indicate that H2S can function as an important cellular signal also in plants. Current knowledge about H2S emphasizes its physiological functions in regulation of stomatal movement, senescence, autophagy, seed germination, organogenesis and photosynthesis (Li 2013, Lisjak et al. 2013, Calderwood and Kopriva 2014). H2S has been proposed to mediate plant responses to stress conditions, including fungal infections, salt, drought, heat, hypoxia and heavy metals (Li 2013, Lisjak et al. 2013, Calderwood and Kopriva 2014). H2S is now considered as a secondary messenger similar to other well-known small reactive compounds, such as H2O2 and NO (Neill et al. 2002, Hancock et al. 2011, Lisjak et al. 2013).
Cd is a strongly toxic environmental factor, easily taken up by roots and transported to aerial parts of plants. To minimize the damage related to exposure to this heavy metal, plants have evolved a complex network of homeostatic mechanisms (DalCorso et al. 2008). The early reaction to the presence of Cd in the environment includes modification of plasma membrane NADPH oxidase and, consequently, changes in H2O2 production, as well as accumulation of other signals, such as NO, calcium, polyamines or plant hormones (Chmielowska-Bąk et al. 2014). A lot of data indicates that Cd can promote the generation of H2O2 in plant cells and this molecule may function as a second messenger in the cascade of events leading to plant protection against Cd toxicity (Hossain et al. 2015). More recently, it was shown that H2S is able to activate protective mechanisms mitigating Cd stress in plants (Li et al. 2012a, Sun et al. 2013, Cui et al. 2014, Shi et al. 2014, Mostofa et al. 2015, Zhang et al. 2015). Furthermore, the H2S-cysteine cycle system was found to be a key regulator of plant response to Cd stress (Jia et al. 2016). Although Cd can affect generation of H2S and H2O2 in plant tissues, it is not clear yet how these molecules cooperate with each other in the Cd-stressed plants (Jin and Pei 2015). Recent studies suggest that H2S may function as a downstream as well as upstream component of the H2O2 signaling pathways activated during cellular processes, such as seed germination (Li et al. 2012b, Li and He 2015), and in response to stress factors, such as high temperature (Li et al. 2015).
Under heavy metal stress conditions, both the central vacuole and vacuolar proton pumps seem to be involved in cytosol detoxification. Trans-tonoplast proton gradient generated by V-ATPase and H+-pyrophosphatase (V-PPase) can be used as a driving force by active antiporters, such as CDF (cation diffusion facilitators) and CAX (cation/proton exchangers) proteins, to remove harmful heavy metals from the cytoplasm (Singh et al. 2016). On the other hand, V-ATPase, as the main tonoplast proton pump of mature plant cells, takes part in growth processes by regulating expansion of the central vacuole, and consequently cell turgor (Seidel et al. 2013), as well as stomatal density and opening (Zhang et al. 2013). Surprisingly, we have demonstrated previously that Cd inhibits activity of V-ATPase, indicating that this enzyme is not an essential element of Cd tolerance in Cucumis sativus roots (Kabała et al. 2010, 2013, 2014). Our results suggested that under Cd stress V-ATPase is subjected to posttranslational regulation. As yet, the mechanism of Cd action on V-ATPase has not been fully elucidated. It is obvious that V-ATPase as a multimeric complex of 13 different subunits, playing house-keeping functions in plant cells, must be subjected to tight as well as multifaceted control. Modulation of enzyme activity may result from biochemical modifications (e.g. reversible disulfide bridge formation and phosphorylation), interaction with other molecules (e.g. aldolase) or disturbance in complex stability (Seidel 2009).
Therefore, in the current study, we aimed to explain the role of H2S, recently identified as a signaling molecule, and possible cross-talk between H2S and H2O2 in the regulation of V-ATPase in Cd-treated plants. It was found that H2S improves photosynthetic fluorescence parameters in Cd-stressed cucumber seedlings exhibiting beneficial effect on plant condition. We determined the content of H2S as well as the activities of H2S-generating and -consuming enzymes in roots of plants subjected to different Cd concentrations for a short and long time of exposure. We assayed V-ATPase activity, measured as both ATP hydrolysis and ATP-dependent H+-transport, in cucumber seedlings treated with generators and scavengers/inhibitors of both signaling molecules. These analyses were performed in parallel with the expression of eight genes encoding four V-ATPase subunits essential for ATP hydrolysis and proton translocation (VHA-A, VHA-B, VHA-a, VHA-c). Moreover, H2O2 production and its interaction with H2S were determined.
Materials and methods
Plant material and treatment
Cucumber seedlings (C. sativus L. var. Wisconsin), after germination in darkness for 2 days at 25°C, were grown in medium containing 1.7 mM KNO3, 1.7 mM Ca(NO3)2 × 4 H2O, 0.33 mM KH2PO4, 0.33 mM MgSO4 × 7 H2O and micronutrients: 75 μM ferric citrate, 10 μM MnSO4 × 5 H2O, 5 μM H3BO4, 1 μM CuSO4 × 5 H2O, 0.01 μM ZnSO4 × 7 H2O, 0.05 μM Na2MoO4 × 2 H2O (pH 5.5). After 5 days seedlings were transferred to fresh medium without (control) or with 10, 50, 100 or 150 μM CdCl2 for the next 2 or 24 h. Cd concentration used in the present work is related to our earlier studies (Kabała et al. 2010, 2013). The following inhibitors or scavengers were added to the control nutrient solution and the 100 μM-CdCl2 solution for 24 h: 100 μM NaHS (H2S donor), 1 mM propargylglycine (PAG, H2S biosynthesis inhibitor), 5 mM H2O2 (based on Janicka-Russak and Kabała 2012), 1 mM ascorbic acid (Asc, free radical scavenger), 5 mM dimethylthiourea (DMTU, H2O2 scavenger), 50 μM diphenyleneiodonium chloride (DPI, plasma membrane NADPH oxidase inhibitor), 100 μM aminoguanidine (AG, diamine oxidase inhibitor) Alternatively, plants were pretreated with 100 μM NaHS and 5 mM H2O2 for 24 h before Cd exposure to avoid CdS precipitation. All reagents were purchased from Sigma-Aldrich (Saint Louis, MO). The plants were grown hydroponically under a 16 h photoperiod (light intensity: 180 μmol m−2 s−1) at 25°C by day and 22°C by night. The relative humidity in the light and dark was 70%.
Chlorophyll fluorescence measurement
Chlorophyll a fluorescence was measured according to the method described by Burzyński and Żurek (2007) using the pulse amplitude modulation (PAM) system (FMS2 fluorometer, Hansatech Instruments, King's Lynn, UK). Before each measurement, the cotyledons were dark-adapted for 30 min to complete reoxidation of photosystem 2 (PSII).
H2S content
H2S was quantified according to the method described by Li (2015) with some modifications. Cucumber root tissue (2 g), frozen and ground into fine powder with a mortar and pestle under liquid nitrogen, was homogenized with 2 ml of 20 mM hydroxymethylaminomethane (Tris)-HCl buffer (pH 8.0) containing 10 mM EDTA (ethylene diamine tetraacetic acid) and 20 mM Zn acetate (zinc trap). The samples were centrifuged at 15 000 g for 15 min at 4°C. One milliliter of the supernatant was added to 1 ml of 30 mM FeCl3 dissolved in 1.2 N HCl and 1 ml of 20 mM N,N-dimethyl-p-phenylenediamine dihydrochloride dissolved in 7.2 N HCl. The samples were incubated at room temperature for 15 min and the absorbance of formed methylene blue (MB) was measured at 670 nm. In MB method, a strongly acidic condition is used to liberate H2S from zinc sulfide complex. The amount of H2S was quantified based on a standard curve using NaHS as the standard.
H2O2 content
H2O2 was quantified according to the method of Sergiev et al. (1997) described by Velikova et al. (2000) with some modifications. Cucumber root tissue (1 g), frozen and ground into fine powder with a mortar and pestle under liquid nitrogen, was homogenized with 2 ml of 0.1% trichloroacetic acid (TCA) and centrifuged at 12 000 g for 20 min at 4°C. To the supernatant (0.5 ml) was added 0.5 ml of 10 mM K-phosphate buffer (pH 7.0) and 1 ml of 1 M KI. The samples were incubated at room temperature in darkness for 60 min and the absorbance was measured at 390 nm. The amount of H2O2 was quantified based on a standard curve.
H2O2 visualization
H2O2 was detected by immersing whole 6-day-old cucumber plants in a 1 mg ml−1 solution of DAB (3,3′-diaminobenzidine) in 0.33 mM 2-(N-morpholino)ethanesulfonic acid (MES)-NaOH, pH 5.5, at room temperature for 8 h in dark tubes, according to Thordal-Christensen et al. (1997) with some modifications. After incubation, the segments (3 mm) of main roots were fixed in formalin/acetic acid/alcohol, FAA (40% formalin, 80% glacial acetic acid and 50% ethanol) for 24 h. After fixation, roots were washed twice in 50% ethanol, dehydrated in a graded ethanol-butanol solution series, and embedded in paraffin at 56°C. Sections 12 μM thick were cut using a rotary Leica RM213 microtome (Leica Biosystems, Wetzlar, Germany), mounted on glass slides with Haupt's adhesive, deparaffinized in xylene and embedded in Euparal. Images of the DAB-stained roots were made using an Olympus BX50 microscope and Olympus DP71 camera (Tokyo, Japan).
Determination of activity of H2S-generating and -consuming enzymes
Cucumber root tissue (1 g), frozen and ground into a fine powder with a mortar and pestle under liquid nitrogen, was homogenized with 5 ml of 20 mM Tris–HCl (pH 8.0) and centrifuged at 13 000 g for 10 min at 4°C. The protein content of the supernatant was adjusted to 50 μg ml−1 to obtain equal amounts of protein in each sample.
L-CD and D-CD activity (EC 4.4.1.1 and 4.4.1.15, respectively) was assayed according to the method of Siegel (1965) described by Riemenschneider et al. (2005a) with some modifications. L-CD activity was measured as H2S released from L-cysteine. The assay mixture contained 100 mM Tris–HCl (pH 9.0), 0.8 mM L-cysteine, 2.5 mM DTT (dithiothreitol) and 50 μg protein in a total volume of 1 ml. The reaction was started by the addition of L-cysteine. The samples were incubated at 37°C for 15 min. The reaction was terminated by adding 100 μl of 30 mM FeCl3 dissolved in 1.2 N HCl and 100 μl of 20 mM N,N-dimethyl-p-phenylenediamine dihydrochloride dissolved in 7.2 N HCl. After incubation at room temperature for 30 min, formed MB was determined spectrophotometrically at 670 nm. D-CD activity was measured as H2S released from D-cysteine in the same way with one exception: the pH of the Tris–HCl buffer was 8. Solutions with different concentrations of Na2S, treated in the same way as the assay samples, were used as the standard for the quantification of H2S.
The OAS-TL activity (EC 4.2.99.8) was measured as cysteine formation during the reaction according to Riemenschneider et al. (2005b) with some modifications. The assay mixture contained 100 mM Tris–HCl (pH 7.5), 5 mM OAS (O-acetylserine), 5 mM Na2S, 33.4 mM DTT and 50 μg protein in a total volume of 0.5 ml. The reaction was started by the addition of Na2S. The samples were incubated at 37°C for 30 min. The reaction was terminated by adding 0.5 ml of acidic ninhydrin reagent [0.8% ninhydrin (w/v) in 1:4 HCl:acetic acid]. The samples were heated at 100°C for 10 min and then cooled on ice for 10 min. Finally, 0.9 ml of 70% ethanol was added to 0.1 ml of the sample. The absorbance of the formed color complex was determined spectrophotometrically at 560 nm. Solutions with different concentrations of L-cysteine, treated in the same way as the assay samples, were used as the standard. The solutions of cysteine, DTT, OAS, Na2S and ninhydrin reagent were prepared freshly before use.
Membrane fraction isolation
Tonoplast fractions were prepared from cucumber root tissues according to the method described by Kabała and Kłobus (2001) using a discontinuous sucrose density gradient consisting of 20, 28, 32 and 42% (w/w) sucrose.
Plasma membrane fractions were prepared from cucumber root tissue according to the method described by Kłobus (1995) using a two-phase system containing 6.2% PEG (polyethylene glycol) 3350 and 6.2% dextran T500.
Determination of activity of vacuolar H+-ATPase and plasma membrane NADPH oxidase
The hydrolytic activity of V-ATPase (EC 3.6.3.14) was determined in tonoplast fractions according to the method of Gallagher and Leonard (1982) described by Kabała et al. (2013) and expressed as the difference between the activity measured in the absence and presence of specific inhibitor NaNO3. Inorganic pyrophosphate released during the reactions was assayed according to Ames (1966). ATP-dependent proton transport catalyzed by V-ATPase was measured spectrophotometrically by monitoring a decline in absorbance of acridine orange at 495 nm according to Kabała et al. (2013).
NADPH oxidase activity (EC 1.6.3.1) was assayed in plasma membrane fractions according to the method of Sagi and Fluhr (2001) described by Jakubowska et al. (2015) using 0.5 mM XTT [2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt] and 0.1 mM NADPH.
Determination of protein level
Protein content in all fractions was estimated according to the method of Bradford (1976) using bovine serum albumin as the standard.
RNA isolation and cDNA synthesis
Isolation of total RNA and synthesis of cDNA were performed according to the method described by Kabała et al. (2014) using Tri Reagent (Sigma-Aldrich, Saint Louis, MO) and a high-capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), respectively, according to the manufacturer's instructions.
Real-time PCR reaction
The expression profile of V-ATPase (CsVHA) and NADPH oxidase (CsRboh) genes in cucumber roots was performed with the LightCycler 480 system (Roche, Basel, Switzerland). The cDNA was used as a template in a real-time polymerase chain reaction (PCR) reaction with the Real-Time 2 × PCR Master Mix SYBR kit (A&A Biotechnology, Gdynia, Poland). The reaction conditions for VHA genes were as follows: 95°C for 30 s; 40 cycles of 95°C for 10 s, 58°C (for CsVHA-A, B, c2, c3, a1, a3) or 60°C (for CsVHA-c1) or 62°C (for CsVHA-a2) for 10 s, 72°C for 12 s, 15 s of final melting at 65°C (for CsVHA-A, B, a1, a3, c2, c3) or 68°C (for CsVHA-a2 and c1). Two genes, one encoding cucumber elongation factor 1-alpha (CsEF1, EF446145), the other encoding cucumber tonoplast intrinsic protein (CsTIP41, GW881871), were used as the internal controls (Migocka and Papierniak 2011). Some primer sequences have been previously described by Kabała et al. (2014) for CsVHA-A, c1, c2, c3, CsEF1 and CsTIP41 genes. Primer sequences specific to other amplified genes, i.e. CsVHA-B, a1, a2, a3, were designed by LightCycler ProbeDesign software (Roche, Basel, Switzerland). All primers used in qRT-PCR analysis are listed in Table S1. Analysis of melting curve was performed to confirm the specificity of amplification and the absence of non-specific by-products. The PCR reactions for CsRboh genes were conducted according to Jakubowska et al. (2015).
Statistics
All experiments were independently repeated at least three times, with each sample performed with two or three replications. Data are expressed as means ± sd. The Tukey's multiple range test (significant at P < 0.05) was used for statistical analysis.
Results
To determine whether endogenous H2S concentration changes in cucumber roots under Cd stress, seedlings were treated with 10, 50, 100 or 150 μM CdCl2 for 2 and 24 h. A short time of exposure (2 h) did not significantly affect the H2S content (Fig. 1A). A more pronounced effect of Cd was observed when plants were subjected to this heavy metal for a longer time (24 h). In such conditions, the H2S amount increased at all Cd concentrations, reaching the highest level (145% of the control) in roots treated with 100 μM Cd (Fig. 1A).
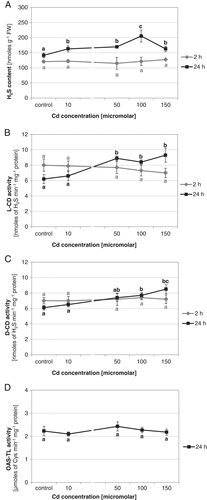
Since desulfhydrases are the main enzymes responsible for H2S generation in plant tissues, the activities of both L-CD and D-CD were assayed in cucumber roots grown under different Cd conditions. As Fig. 1B,C shows, activities of desulfhydrases changed in a similar manner to the previously determined H2S content. Two-hour treatment with Cd did not have an important effect on enzyme actions. In contrast, 24-h exposure to Cd ions stimulated both L-CD and D-CD activities. These activities increased to a similar degree at 50, 100 and 150 μM Cd concentrations, reaching 143, 139, 151% for L-CD, and 121, 127, 140% for D-CD, respectively (Fig. 1B,C). OAS-TL, as an H2S-consuming enzyme, can affect the amount of H2S in plant tissues. For this reason, its activity was also estimated in roots, which showed elevated H2S content (plants treated with Cd for 24 h). OAS-TL activity remained at a level similar to the control, regardless of Cd concentration (Fig. 1D). Based on the above results and our earlier studies, further analyses were performed in plants subjected to 100 μM Cd for 24 h. Under such stress conditions, V-ATPase activity has been shown to be downregulated.
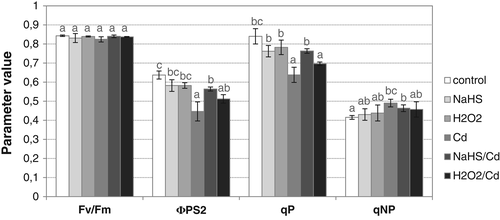
To verify whether exogenous H2S is able to improve condition of cucumber plants under Cd stress, photosynthetic fluorescence parameters were determined in cotyledons of seedlings treated with CdCl2, NaHS (a H2S donor) or NaHS and CdCl2 (NaHS/Cd; Fig. 2). Metal and NaHS did not change the potential efficiency of PSII, calculated as Fv/Fm. Otherwise, the actual quantum efficiency of PSII (ΦPS2), diminished after metal treatment (70% of control), was restored by NaHS addition (NaHS/Cd), achieving 89% of the control level. Similarly, the photochemical quenching (qP) parameter, expressing the utilization of the excitation energy to drive the photosynthetic electron transport, decreased in seedlings exposed to Cd (76% of control) and increased in plants treated with NaHS and Cd (91% of control). In contrast, NaHS did not affect the non-photochemical quenching (qNP) value, which increased after Cd treatment (118% of control). Application of H2O2 did not have an as pronounced effect as NaHS on improving the photosynthesis parameters ΦPS2 and qP (Fig. 2).
To explain the role of signaling molecules in the regulation of V-ATPase in cucumber roots, the effect of NaHS and H2O2 as well as their scavengers/inhibitors on both ATP hydrolysis and ATP-driven H+-transport was analyzed. Both signaling molecules differently regulated enzyme activities in cucumber plants grown under normal conditions (Fig. 3A). Treatment of seedlings with NaHS caused visible stimulation of ATP hydrolysis and proton transport (about 25% over control), whereas exposure to H2O2 significantly reduced them (by 25 and 40%, respectively). Opposite results were observed when PAG (an inhibitor of H2S biosynthesis) or ascorbate/DMTU (H2O2 scavengers) were applied. PAG inhibited V-ATPase to the same extent as H2O2. On the other hand, ascorbic acid (Asc) and DMTU had a stimulatory effect, similar to H2S. This indicates that H2S can act as a positive regulator of V-ATPase while H2O2 acts as a negative regulator.
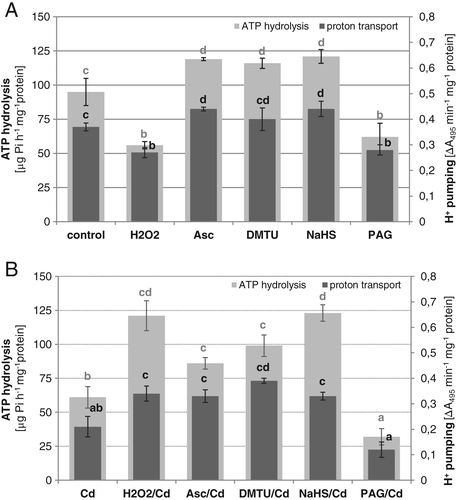
As we observed previously in C. sativus var. Krak and in this study in C. sativus var. Wisconsin, V-ATPase is inhibited by 40% in roots of seedlings exposed to Cd. However, application of H2O2 scavengers to Cd-treated plants resulted in recovery of enzyme activities to nearly the control level. In contrast, under Cd stress, PAG addition caused a further decrease in V-ATPase activities, which reached the level of about 30% of the control (Fig. 3B). Such results are consistent with those described above for cucumber grown under normal conditions. They suggest that Cd-induced downregulation of V-ATPase could be at least in part caused by endogenous H2O2, whereas H2S biosynthesis prevents loss of V-ATPase activity. As predicted, pretreatment of Cd-stressed plants with the H2S donor alleviated the inhibitory effect of Cd on V-ATPase. However, ATP hydrolysis increased to a greater extent (about 30% over the control value) than ATP-dependent proton transport (about 90% of the control), changing the H+/ATP stoichiometry. Surprisingly, similar changes in enzyme activities were observed when H2O2 was introduced into the plant medium before Cd exposure (Fig. 3B).
The transcription of eight genes encoding four important V-ATPase subunits, i.e. catalytic subunit A and regulatory subunit B of the V1 complex as well as transmembrane subunits a and c of the V0 sector, was analyzed in roots of cucumber seedlings treated with H2S, H2O2, their scavengers/inhibitors and Cd. CsVHA-A and CsVHA-B were maintained at nearly control levels under all experimental conditions (Fig. 4A,B). Likewise, transcript levels of the three genes encoding CsVHA-a subunits did not change significantly under control and stress conditions except for CsVHA-a2, which was upregulated in roots treated with DMTU and Cd (Fig. 4B).
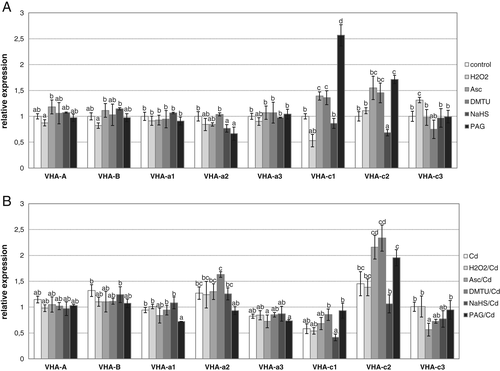
The most important changes were found in the transcript levels of CsVHA-c isoforms. Expression of CsVHA-c1 was significantly reduced after plant exposure to H2O2 and somewhat increased after treatment with H2O2 scavengers (Fig. 4A). Similarly, transcription of CsVHA-c1 was diminished by Cd and remained at a decreased level in most Cd-induced conditions, reaching the lowest value after H2S pretreatment (Fig. 4B). On the other hand, the level of CsVHA-c1 transcript showed a pronounced increase in control roots subjected to PAG (Fig. 4A). In the case of CsVHA-c2, it was demonstrated that its expression was stimulated in Cd-stressed roots, achieving the highest value after application of DMTU (Fig. 4B). Moreover, CsVHA-c2 was also upregulated in control plants treated with PAG, but to a lesser extent than CsVHA-c1, as well as by DMTU and ascorbate (Fig. 4A). CsVHA-c3 transcript level showed less visible changes but tended to be stimulated by H2O2 in control conditions (Fig. 4A) and inhibited by ascorbate under Cd stress (Fig. 4B).
In the next steps, the possible interrelation between H2O2 and H2S generation was analyzed in cucumber roots stressed with Cd. H2S content increased in roots of heavy metal-exposed plants after their pretreatment with H2O2, reaching 15% over the Cd-induced level. Addition of Asc or DMTU to the nutrient medium reduced the H2S amount by 18 and 27%, respectively, in seedlings exposed to Cd (Fig. 5A). Stimulation of H2S accumulation, similar to the H2O2-induced one (H2O2/Cd), was observed when plants were pretreated with NaHS. Moreover, application of PAG lowered H2S production to a similar extent as DMTU (Fig. 5A). Addition of H2O2, however, did not affect the activities of desulfhydrases. Similarly, when ascorbic acid was introduced into the nutrient solution, levels of both L-CD and D-CD activity did not change significantly in Cd-stressed roots (Fig. 5B).
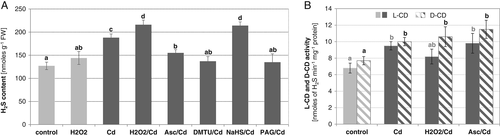
H2O2 level in plant tissues is partially related to the activities of H2O2-producing enzymes, including plasma membrane NADPH oxidase and cell wall-bound diamine oxidase (DAO). The effect of DPI (an NADPH oxidase inhibitor) and AG (a DAO inhibitor) on H2S content was analyzed in cucumber roots. It was found that, under Cd stress, H2S level was significantly diminished after seedling exposure to DPI, reaching about 64% of that determined in the presence of Cd (Fig. 6A). It was also demonstrated that addition of DPI or AG to the nutrient solution had no significant effect on the activity of desulfhydrases in roots of metal-stressed cucumber plants (Fig. 6B).
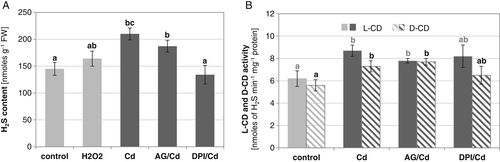
On the other hand, the involvement of H2S in H2O2 production was tested in plants treated with Cd. Detection of H2O2 using DAB-based histochemical staining revealed that higher H2O2 content was accumulated after Cd exposure than after NaHS treatment (Fig. S1). Measurement of H2O2 concentration in root tissues confirmed that H2O2 increased in roots of plants treated with Cd (reaching about 130% of the control). However, exposure of seedlings to NaHS prior to Cd stress did not affect importantly the H2O2 production. No effect was also observed when an H2S inhibitor, PAG, was applied to seedlings growing under stress conditions (Fig. 7). In contrast, a slight increase in H2O2 amount was determined in seedlings pretreated with exogenously provided H2O2. Furthermore, a visible reduction in H2O2 level occurred in stressed roots after application of H2O2 scavengers, especially DMTU. H2O2 content decreased to a value similar to the control (Fig. 7).
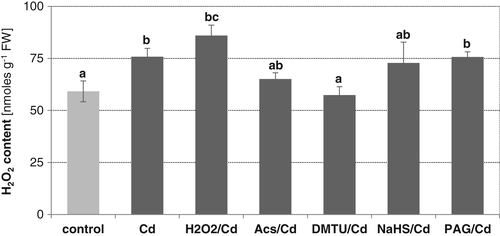
When control plants were treated with NaHS, visible stimulation of plasma membrane NADPH oxidase activity (above 200% of the control value) was observed in roots (Fig. 8A). This effect was related to a significant increase in transcript levels of two from nine NADPH oxidase isoforms, CsRbohF3 and CsRbohB (Fig. 8B).
Additionally, changes in H2S and H2O2 levels were monitored for 24 h after plant transfer to Cd-containing medium. The results revealed differences in the behavior of the two signaling molecules. H2S content did not change significantly during the first 8 h compared to the level determined in roots of seedlings subjected to Cd for 24 h (Fig. S2A). In contrast, H2O2 showed the highest amount after 4 h of Cd treatment. It diminished in the next 4 h, reaching a level close to that assayed after 2 as well as 24 h of Cd exposure (Fig. S2B).
Discussion
A growing body of evidence demonstrates that H2S, beside H2O2 and NO, acts as an important signaling molecule. At a low physiological range, it affects physiological functions of mammals at molecular, cellular, tissue and systemic levels (Wang 2002). It participates in many biological processes, such as smooth muscle relaxation, hippocampal long-term potentiation, brain development, blood pressure and inflammation (Wang 2002). In contrast to the extensive studies on H2S functions in animals, the molecular mechanisms underlying the action of endogenous H2S in plant cells have not been fully explained. Nowadays, H2S is considered as an important molecule involved in both the regulation of plant physiological processes and plant tolerance to abiotic stresses (Lisjak et al. 2013).
Several studies have indicated that exogenously supplied H2S is able to alleviate Cd toxicity mainly through the regulation of Cd transport and accumulation (Sun et al. 2013), ROS detoxification and reduction of oxidative damage (Li et al. 2012a, Ali et al. 2014, Shi et al. 2014), or modifications in cell structures including mitochondria, ER and Golgi (Ali et al. 2014). Our results showed that application of a H2S generator improved photosynthetic parameters in seedlings subjected to Cd. Earlier studies indicated that H2S promotes chloroplast development and modulates the expression of genes involved in photosynthesis (Chen et al. 2011). Less is known, however, about the endogenous generation of H2S in plant tissues under Cd stress. H2S content increased in roots of cucumber seedlings exposed to Cd in a concentration- and time-dependent manner, with the most efficient effect observed for 100 μM Cd applied for 24 hours. This increase was related to the enhanced activity of both L-CD and D-CD, suggesting that endogenous production of H2S in cucumber occurs enzymatically in response to Cd. The present results are consistent with those demonstrating elevated H2S synthesis upon Cd stress in Medicago sativa (Cui et al. 2014) and A. thaliana (Qiao et al. 2016) as well as increased expression of genes encoding H2S synthesizing enzymes in Cd-treated Brassica rapa (Zhang et al. 2015, Lv et al. 2017).
The active concentration of H2S is the result not only of its production, but also of its metabolism, storage and associated release. It may change rapidly and extensively (Wang 2002, Li et al. 2011, Kimura 2012). In plant tissues, OAS-TL seems to be the main enzyme responsible for H2S consumption, which takes place during sulfur assimilation and cysteine formation (Burandt et al. 2002, Wirtz et al. 2004). However, the endogenous H2S level determined in Cd-stressed cucumber roots was not affected by OAS-TL activity. The enzyme activity remained at a similar level in both control and heavy metal-treated seedlings. Earlier studies using Arabidopsis and tobacco overexpressing OAS-TL genes revealed that Cd tolerance is associated with increased OAS-TL activity and, as a consequence, enhanced cysteine biosynthesis and supply of sulfur-containing compounds (Domínguez-Solís et al. 2004, Ning et al. 2010).
On the other hand, it is widely accepted that H2O2 and other ROS are important players in the plant response to heavy metals. Enhanced generation of H2O2 under Cd stress has been observed in plants. Moreover, it was reported that exogenous application of H2O2 increased plant tolerance to this metal (Hossain et al. 2015, Hasanuzzaman et al. 2017). Recent evidence suggests that H2S can play a role in the H2O2 signaling pathways. Li et al. (2012b) reported that H2S is a mediator in H2O2-induced germination of Jatropha curcas seeds and acts downstream of H2O2. Similarly, in seeds of mung bean, it was shown that both molecules could promote germination but H2O2 seems to be a downstream signal of H2S (Li and He 2015).
Transport of heavy metals, including Cd, across the tonoplast and their accumulation inside the vacuolar lumen are mediated by primary active ABC proteins and P1B-ATPases, energized directly by ATP hydrolysis, or by secondary active antiporters using a transmembrane H+ gradient (Krämer et al. 2007, Martinoia et al. 2007). Two tonoplast proton pumps, V-ATPase and V-PPase, with different structure and energy source are responsible for the generation of the electrochemical H+ gradient required for secondary transport processes. In Populus euphratica cells, H2S-stimulated vacuolar Cd accumulation was observed (Sun et al. 2013). Cd flux across the tonoplast was proton gradient-dependent and probably, at least in part, mediated by the Cd2+/H+ antiporter (Sun et al. 2013). A significant role of V-ATPase in plant adaptation to Cd stress has been confirmed in Arabidopsis seedlings overexpressing the ThVHA-c1 gene from tamarisk (Yang et al. 2016). However, in cucumber, V-ATPase activity is inhibited in roots of seedlings exposed to Cd (Kabała et al. 2010, 2013), suggesting that other tonoplast proteins or other cell compartments are involved in Cd sequestration in this organ. In the present work, we examined the possible role of the signaling molecules H2S and H2O2 in Cd-induced downregulation of the enzyme. Under control conditions, V-ATPase was stimulated by H2S as indicated using NaHS and PAG as H2S generator and inhibitor, respectively. In contrast, H2O2 and its scavengers had the opposite effects. These results suggested that elevated H2O2 level and lowered H2S content reduce both ATP-dependent proton pumping and ATP hydrolysis, catalyzed by V-ATPase, similar to Cd. The same effects were observed when H2O2 scavengers or a H2S biosynthesis inhibitor were applied to Cd-stressed plants, confirming the up- and downregulation of V-ATPase activity by the tissue content of H2S and H2O2, respectively.
Only one study to date has demonstrated direct interaction between endogenous H2S and V-ATPase (Mikami et al. 2011). In mouse retina, it was found that H2S generated in horizontal cells activates vacuolar H+-ATPase to release protons and suppress calcium channels in photoreceptor cells. This mechanism protects retinal neurons from light-induced degeneration (Mikami and Kimura 2012). On the other hand, inhibition of V-ATPase by H2O2 was postulated several years earlier by Tavakoli et al. (2001) in barley. They demonstrated that H2O2 introduced into the reaction medium is responsible for the reversible oxidation of the vacuolar enzyme. Tavakoli et al. (2001) suggested that similar redox control of V-ATPase exists in vivo in plants. The above results are consistent with our observations that H2S can act as a positive regulator and H2O2 as a negative regulator of the ATP-dependent proton pump. It was assumed in both studies that the action of H2S or H2O2 is related to reversible disulfide bond formation within VHA subunits, where H2S could reduce the inhibitory -S-S- bond in the catalytic site (Mikami et al. 2011) while H2O2 promotes its formation (Tavakoli et al. 2001). It is well established in mammalian cells that H2S can physiologically modify cysteine residues in proteins by S-sulfhydration ( persulfidation). Endogenous H2S converts cysteine thiol (-SH) groups to persulfide (-SSH) bridge, resulting in functional changes in enzymatic activities, structures and subcellular localizations of the target proteins. The effect of S-sulfhydration can either activate or inhibit enzymatic activities (Mustafa et al. 2009). Most recently, it was showed that S-sulfhydration reversibly regulates the functions of plant proteins in a similar manner (Aroca et al. 2015, 2017). Aroca et al. (2017) revealed that at least 5% of the entire Arabidopsis proteome might be persulfidated. Currently, it is postulated that protein persulfidation functions as a mechanism that reduces oxidized cysteine residues. This enhances the antioxidant capacity of thiols (Filipovic and Jovanović 2017). Filipovic and Jovanović (2017) suppose that H2S cannot interact with cysteine residues directly. As a result of ROS production, thiols are oxidized forming sulfenic acid (-SOH), which reacts with H2S. Created persulfide prevents further oxidation to sulfinic and sulfonic acids. The actual effect of S-sulfhydration on V-ATPase activity in cucumber roots remains to be examined.
Unexpectedly, pretreatment of cucumber with H2O2 before Cd exposure had a similar beneficial effect on V-ATPase as H2S donor application. This result was contrary to that obtained for control plants. It is likely that under Cd stress, H2O2 application can induce signaling pathways leading to the mitigation of detrimental effects of metal including restoration of V-ATPase activity. Alleviation of Cd-induced proton pump inhibition by both signaling molecules was related to the visible changes in its coupling efficiency, i.e. H+/ATP stoichiometry. Such changes imply posttranslational modification of enzyme protein, such as reversible disassembly, and play a role in controlling vacuolar acidification (Cipriano et al. 2008).
Although some mechanisms of H2S and H2O2-dependent V-ATPase regulation at the protein level have been postulated, changes in transcription of VHA genes under all experimental conditions were also monitored. It is well known that the expressional regulation of the vacuolar proton pump might be related to plant adaptation to unfavorable environmental conditions (Dietz et al. 2001). Plant vacuolar H+-ATPase is a complex of 13 different subunits and at least some of them may be encoded by multigene families (Schumacher and Krebs 2010). Therefore, its regulation seems to be multifaceted. In the C. sativus genome, 20 VHA genes have been identified (Kabała et al. 2014). It was shown that the expression profile of genes encoding subunit A, B, a and c, involved in ATP hydrolysis and proton translocation, was not closely related to the earlier observed modulation in V-ATPase activity. This confirms that biochemical modifications of enzyme protein are responsible for the H2S- and H2O2-induced regulation rather than changes in gene level. Nevertheless, it is worth noting that a positive relationship between CsVHA-c1 expression and V-ATPase activity was observed in cucumber roots treated with H2O2, H2O2 scavengers and Cd. No analogous dependence has been found for the H2S effect. On the other hand, CsVHA-c2 transcription was always enhanced under Cd stress conditions, especially when the H2O2 level decreased (Asc and DMTU treatments), as well as under conditions of lowered H2S content (PAG treatment), but it was not correlated with V-ATPase activity. Similarly, CsVHA-c1 mRNA significantly increased in roots treated with a H2S inhibitor. It was proposed by Kluge et al. (2003) that an increase in the number of proteolipid c subunits could modulate the rotor size of V0 sector and in this way affect the coupling ratio and activity of V-ATPase. Upregulation of VHA-c and VHA-E genes upon Cd stress was also showed in barley (Finkemeier et al. 2003, Sharma et al. 2004). Sharma et al. (2004) proposed that enhanced transcription, without an effect on protein level, might compensate protein turnover induced by stress conditions.
To explain the relationship between H2S and H2O2 in signaling pathways leading to the modification of V-ATPase activity, scavengers and inhibitors of both molecules were also used to monitor changes in their endogenous levels. It was found that treatment of cucumber seedlings with H2O2 increased, whereas exposure to ascorbic acid and DMTU decreased, the level of H2S in roots of Cd-stressed seedlings (Fig. 5A), indicating that H2O2 stimulates H2S production. However, the observed H2O2-dependent modifications in H2S level were not a result of changes in activities of H2S releasing desulfhydrases (Fig. 5B), suggesting other ways of H2S generation. As both desulfhydrase activity and H2O2 level increased in cucumber roots stressed with Cd, we suggest that two pathways responsible for H2S production exist under these conditions: one L-CD/D-CD-dependent and one H2O2-dependent. In plant cells, the homeostasis of H2S is also regulated by other H2S-synthesizing enzymes, including SIR and CAS (Li 2013). However, under heat stress, pretreatment of maize seedlings with H2O2 raised the endogenous H2S content by activating L-CD activity, whereas SIR activity remained unaffected (Li et al. 2015). Moreover, it was postulated that H2S produced within chloroplasts and mitochondria does not diffuse to the cytosol (Romero et al. 2014). In animal cells, it was shown that endogenous production of H2S could also come from non-enzymatic reduction of elemental sulfur using reducing equivalents obtained from the oxidation of glucose. H2S may be stored in proteins in the form of divalent sulfur bound known as bound sulfane sulfur, which releases H2S under reducing conditions (Wang 2002, Li et al. 2011, Kimura 2012). A similar mechanism of H2S generation has not been described yet in plants.
Among several possible sources of ROS in plant cells, plasma membrane-bound NADPH oxidase seems to play the most important role under stress conditions. It catalyzes oxygen reduction to the superoxide radical (O2.−), which is rapidly converted to H2O2 by superoxide dismutase (Glyan'ko and Ischenko 2010). In addition, apoplastic H2O2 generation can be derived from amine (putrescine, spermidine, spermine) oxidation mediated by diamine (DAO) and polyamine (PAO) oxidases (Toumi et al. 2010). The produced H2O2 is then found to actively participate in the signaling network, with numerous targets including stomata (Paschalidis et al. 2010). Treatment of plants with inhibitors of H2O2-producing enzymes showed that repression of NADPH oxidase significantly lowered the accumulation of H2S in cucumber roots exposed to Cd (Fig. 6A). Such results suggest that NADPH oxidase-dependent endogenous production of H2O2 is involved in the Cd-induced increase of H2S content. Similarly, the observed effect of inhibitors of H2O2-generating enzymes on H2S level was not related to the changes in L−/D-cysteine desulfhydrase activities (Fig. 6B). Thus, it remains to be resolved in the future whether NADPH oxidase (H2O2)-induced H2S generation is enzymatic or non-enzymatic in nature.
On the other hand, in Arabidopsis des1 mutants, defective in desulfhydrase activity, a significant decrease in ROS production occurred (Álvarez et al. 2010). Experiments using exogenously applied H2S donors showed that H2S is able to enhance the beneficial effect of H2O2 on plant tolerance to high temperature (Li et al. 2015) and UV-B stress (Li et al. 2016). It was reported in salt-stressed Arabidopsis roots that H2S induces H2O2 accumulation by alteration of NADPH oxidase activity (Li et al. 2014). Our earlier work has shown that treatment of cucumber with Cd stimulates the activity of NADPH oxidase as well as induces the expression of several encoding genes (Jakubowska et al. 2015). In this study, exogenous application of NaHS to control plants also caused a significant increase in enzyme activity as well as in transcript levels of some CsRboh genes, confirming that H2S can promote H2O2 generation via NADPH oxidase and suggesting that H2S-dependent regulation of H2O2 content may also occur in cucumber subjected to Cd. However, modulation of the H2S level by adding NaHS or PAG did not significantly change Cd-induced accumulation of H2O2. In the other plants subjected to Cd stress, it was found that H2S could be responsible for both an increase (Lv et al. 2017) as well as a decrease in H2O2 level (Mostofa et al. 2015, Zhang et al. 2015). Mostofa et al. (2015) suggested that reduced H2O2 production, together with a lowered malondialdehyde level, indicates that H2S alleviates Cd-induced oxidative damage. In Cd-stressed cucumber roots, H2O2 accumulation and H2O2-induced signaling pathways probably do not depend on changes in H2S level. It is possible that the form of H2S and H2O2 crosstalk depends on plant species and its sensitivity to environmental stresses. In some cases, these molecules can synergistically regulate many common targets as it was confirmed for H2O2 and NO. Moreover, it was also proposed that H2S as a reductant can directly interact with ROS, including H2O2, and in this way affect the level of oxidants (Li and Lancaster 2013). H2S capacity to react with oxygen has been suggested as the second mechanism of H2S action beside S-sulfhydration.
All our observations were made after 24 h of Cd application. At this time, H2S showed a higher level than during the first 8 h of exposure. On the other hand, H2O2 content was reduced in cucumber relative to the maximum determined in the fourth hour. This indicates that H2O2 as a strong oxidizing agent is metabolized faster, probably to protect the cell against oxidative damage. It is therefore possible that under shorter Cd exposure both messengers will induce different effects.
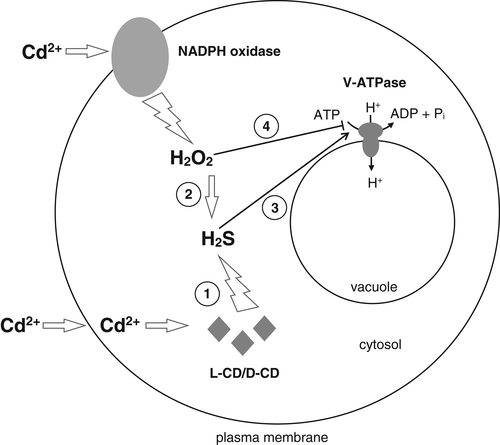
In conclusion, our results revealed that V-ATPase activity in cucumber roots is affected by H2S and H2O2, and that the two signaling molecules have opposite effects. H2S was shown to upregulate V-ATPase activity whereas H2O2 is responsible for enzyme downregulation. It was found that changes in V-ATPase activity induced by both messengers are not related to the gene expression, suggesting that H2S and H2O2 affect the proton pump at the posttranslational level, e.g. via a well-known mechanism involving regulatory disulfide bond formation. Cd stress induced an elevated level of both signaling molecules and inhibited V-ATPase activity as a result of a high content of H2O2 and/or lowered H2S concentration. Moreover, we have shown that cross-talk between H2S and H2O2 occurs in cucumber roots exposed to Cd. H2O2 and NADPH oxidase seem to function as upstream signals in H2S-mediated pathways. All H2O2-modulated changes in H2S level appear not to involve desulfhydrase activities, suggesting that other enzymatic or non-enzymatic reactions are responsible for H2S production. Summing up, we propose that Cd stress increased the H2S level in two ways: by stimulation of desulfhydrases and via the H2O2/NADPH oxidase-induced pathway independent of desulfhydrase activity. The second way can protect V-ATPase from the negative effect of Cd (Fig. 9).
Author contributions
K.K. and M.J. designed this research. M.Z., D.G., M.R. and D.J. performed the experiments. K.K. and M.J. conducted the data analysis. K.K. wrote the manuscript. All the authors approved the final manuscript.
Acknowledgements
This work was partly supported by the Polish Ministry of Science and Higher Education, grant no. OPUS 2012/05/B/NZ3/00422. We thank Anna Szawłowska-Kubik and Beata Kuligowska for their excellent technical assistance.




