Pleiotropic effects of enhancing vacuolar K/H exchange in tomato
Abstract
Cation antiporters of the NHX family are widely regarded as determinants of salt tolerance due to their capacity to drive sodium (Na) and sequester it into vacuoles. Recent work shows, however, that NHX transporters are primarily involved in vacuolar potassium (K) storage. Over-expression of the K/H antiporter AtNHX1 in tomato increases K accumulation into vacuoles and plant sensitivity to K deprivation. Here we show that the appearance of early leaf symptoms of K deficiency was associated with higher concentration of polyamines. Transgenic roots exhibited a greater sensitivity than shoots to K deprivation with changes in the composition of the free amino acids pool, total sugars and organic acids. Concentrations of amides (glutamine), amino acids (arginine) and sugars significantly increased in root, together with a reduction in malate and succinate concentrations. The concentration of pyruvate and the activity of pyruvate kinase were greater in the transgenic roots before K withdrawal although both parameters were depressed by K deprivation and approached wild-type levels. In the longer term, the over-expression of the NHX1 antiporter affected root growth and biomass partitioning (shoot/root ratio). Greater ethylene release produced longer stem internodes and leaf curling in the transgenic line. Our data show that enhanced sequestration of K by the NHX antiporter in the vacuoles altered cellular K homeostasis and had deeper physiological consequences than expected. Early metabolic changes lead later on to profound morphological and physiological adjustments resulting eventually in the loss of nutrient use efficiency.
Abbreviations
-
- ACC
-
- 1-aminocyclopropane-1-carboxylic acid
-
- ADC
-
- arginine decarboxylase
-
- CPA1
-
- cation/proton antiporter 1
-
- FV
-
- fast-activating vacuolar channel
-
- HAK5
-
- high-affinity K transporters
-
- lsd
-
- Least Significant Differences
-
- PK
-
- pyruvate kinase
-
- ROS
-
- reactive oxygen species
-
- SAMDC
-
- S-adenosyl-methionine synthetase
-
- SV
-
- slow-vacuolar channel
-
- WT
-
- wild-type
Introduction
By accumulating large quantities of potassium (K) between 2 and 10% of plant dry weight, plants secure a series of biophysical and biochemical process in which K is fundamental (Wyn Jones and Gorham 1983, Marschner 1995). Potassium uptake occurs through specific transporters and channels and it is stored in vacuoles where it plays a dual role: maintenance of cytosolic K homeostasis and contribution to cell turgor (Wang and Wu 2013, 2017, Anschutz et al. 2014, Nieves-Cordones et al. 2014). The cytosolic K activity is tightly regulated (at ca. 100 mM) through the integrated regulation of K uptake and efflux at the plasma membrane and K import and export at the tonoplast (Walker et al. 1996). Vacuoles release stored K when external concentrations decline to keep cytosolic concentration constant (Walker et al. 1996), thereby securing basic metabolic functions, like pH regulation, protein synthesis, the activation of enzymes related to carbohydrate metabolism, and the control of electrical membrane potential (Wyn Jones and Pollard 1983, Marschner 1995, Amtmann and Rubio 2012). Genomic and genetic studies related to genes encoding K transporters have unraveled additional functions of cellular K in stomatal movements, cell cycle progression, plant development, and in stress signaling and responses (reviewed in Pardo and Rubio 2011, Anschutz et al. 2014, Wang and Wu 2017), including plant immunity to pathogens (Jeworutzki et al. 2010, Brauer et al. 2016). Retention of cellular K is key component in successful adaptation to a saline environment since salinity stress elicits a significant depolarization of the root plasma membrane and ROS production, both of which open outward-rectifying K channels that discharge K in an attempt to re-build the membrane potential (Shabala and Pottosin 2014). The salinity-induced K loss through the plasma membrane implies the need to replenish the cytosolic K pool by drawing K stored in vacuoles (Cuin et al. 2003, Leidi et al. 2010). Uncontrolled K loss results in ion imbalance, the collapse of cell turgor and the transition to programed cell death (Huh et al. 2002, Demidchik et al. 2010).
The protein AtNHX1 of Arabidopsis thaliana was initially described as a main source of salt tolerance in plants by driving Na accumulation into vacuoles (Apse et al. 1999, Zhang and Blumwald 2001). Many subsequent reports confirmed the ability of NHX-like proteins from various origins to convey salt and drought tolerance to transgenic plants of several species, including crops (Ma et al. 2017), but the underlying mechanism remained uncertain because salt tolerance not always correlated with enhanced Na accumulation (Jiang et al. 2010). A meta-analysis of a large number of publications reporting tolerance phenotypes of plants overexpressing exchangers of the cation/proton antiporter 1 family (CPA1, which includes NHX proteins) concluded that the transgenic effect on K status was generally more pronounced than on Na content (Ma et al. 2017). Indeed, AtNHX1 catalyzes K/H and Na/H exchange in biochemical assays with purified vacuoles (Venema et al. 2002, Hernandez et al. 2009), and a reverse genetic approach has conclusively shown that the main role for Arabidopsis transporters AtNHX1 and AtNHX2 is the thermodynamically active accumulation of K in cell vacuoles (Bassil et al. 2011, Barragan et al. 2012, Andres et al. 2014). In transgenic tomato overexpressing AtNHX1, the increase of the K/H antiporter activity leads to greater K storage in vacuoles and, consequently, to K deficiency symptoms even when leaves showed greater K concentration than control plants (Leidi et al. 2010). The sequestration of K in vacuoles of NHX1-transgenic plants reduced cytosolic K activity from 98 ± 1.3 mM in control plants to 55 ± 2.2 mM in the transgenics and elicited the switch to high-affinity K uptake system by roots (Leidi et al. 2010). Similar results have been shown with the over-expression in tomato of its own tonoplast exchanger NHX2 (Huertas et al. 2013). Because the large central vacuole is involved in cytosolic pH homeostasis (Martinoia et al. 2007), the vacuolar NHX antiporters could play a role in this process by exchanging protons from vacuoles with cytosolic K (Kurkdjian and Guern 1989, Rodríguez-Rosales et al. 2009). A decrease in cytosolic K caused by K starvation was associated with the acidification of the cytosol and the inhibition of protein synthesis and root growth (Walker et al. 1998), and thus depletion of cytosolic K by NHX overexpression could also alter the cytosolic pH. In agreement with this notion, the vacuolar pH in the nhx1 nhx2 mutant of Arabidopsis was more alkaline than in control plants (Bassil et al. 2011, Andres et al. 2014), albeit the impact on cytosolic pH was not determined.
Increases in concentration of putrescine appear in K deficient leaves (Basso and Smith 1974) but there are other changes induced by low K, e.g. production of reactive oxygen species (ROS) and phytohormones such as auxin, ethylene and jasmonic acid (Shin and Schachtman 2004, Shin et al. 2005, Ashley et al. 2006). Root cells sensing the low external K increase their plasma membrane potential and activate high-affinity K transport proteins (Wang et al. 2002, Nieves-Cordones et al. 2008, Wang and Wu 2013, 2017). Ethylene signaling would work upstream of ROS in the signaling of K starvation, thereby increasing the expression of high-affinity K transporters (HAK5) and promoting root hair elongation (Jung et al. 2009). Among the metabolic changes induced by K starvation, the accumulation of sugars and N-rich amino acids in roots is a direct consequence of the inhibition of glycolysis and upregulation of the GS/GOGAT/GDH cycle for ammonia assimilation (Armengaud et al. 2009). There are a number of enzymes that regulate carbon and nitrogen metabolism and are known to be affected either by cytosolic K availability or pH (Plaxton 1996, Sakano 1998, Stitt et al. 2002, Armengaud et al. 2009). Among these enzymes, pyruvate kinase (PK) might play a primary role in controlling glycolysis since this enzyme shows a strict K requirement for maximum activity (Besford 1978, Smith et al. 2000). The activity of PK in the glycolytic flux affects C and N metabolism (Plaxton 1996, Armengaud et al. 2009) and its regulation by N metabolites (glutamate and aspartate) is critical for providing energy and C skeletons for N assimilation (Smith et al. 2000). In fact, PK is induced by nitrate and its activity increases following nitrate reduction (Scheible et al. 2000). However, in conditions of limited K the uptake and transport of nitrate are inhibited (Peuke et al. 2002) as well as root nitrate reductase activity (Armengaud et al. 2009).
Since the discovery of NHX proteins, many reports have asserted the use of these proteins as determinants of salt tolerance (Ma et al. 2017). However, as indicated above, the NHX1-overexpression in tomato reduces K cytosolic activity (Leidi et al. 2010) and this also might interfere with key metabolic processes in which K is involved as cofactor or signaling element. The sequestration of K in vacuoles induced by the antiporter could impair K cellular homeostasis with deeper consequences than expected. Considering the need of controlling cytosolic K activity for normal development of cellular processes, we addressed the question of how NHX1 overexpression may affect plant metabolism and physiology.
Materials and methods
Plant cultivation
The transgenic line N367 of tomato (Solanum lycopersicum) cultivar Microtom overexpressing the AtNHX1 antiporter has been described elsewhere (Leidi et al. 2010). The representative line N367 was selected among four independent transgenic events yielding comparable phenotypes because of its transgenerational stability. Plants were cultivated in hydroponic systems using a modified nutrient solution based in Long Ashton nutrient solution (Hewitt 1966), which we refer to from now onwards as LAK medium. The solution contained (in mM): NO3− (4); H2PO4− (1); SO42−(3); K+ (4); Ca2+ (2); Mg2+ (1); and (in μM): Fe (50 as FeEDDHA); Mn (10); Cu (1); Zn (5); B (30). Ten plants (five replicates per line) grew in 10-l plastic containers with nutrient solutions continuously aerated with an aquarium pump in a completely randomized design. The solutions were changed weekly. Plants were kept in a growth chamber with the following conditions (day/night): 26/20 ± 2°C; 40/60% relative humidity; 14 h light; photosynthetically active radiation, 250 μmol m−2 s−1.
Responses to K withdrawal and K availability
Potassium withdrawal experiments were performed transferring plants grown in 4 mM K for 2 weeks into the LAK nutrient solutions devoid of K. Changes induced by K withdrawal were also studied in shorter-terms (days), by transferring 1 month grown plants at 4 mM K into nutrient solutions without K and sampling at 1, 2, 3, 4 and 8 days after transfer.
To study the effect of K availability, plants of lines WT and N367 were grown at 0.1, 1 or 10 mM K. For the 0.1 mM K treatment, the K source was K2SO4 and the P source (KH2PO4) was replaced by NaH2PO4. For the 10 mM K treatment, the additional K was provided as K2SO4 and the concentration of Mg in the nutrient solution was increased to 5 mM (in the form of MgSO4) to provide a K:Mg ratio of 2:1 and prevent physiological disorders (Mg deficiency) (Sainju et al. 2003).
Effect of salinity
Short-term experiments with sublethal saline stress (50 mM NaCl) were used as a preliminary approach on the effects of Na on K uptake and similar metabolic processes affected by low K (amino acids, organic acids, PK). Plants grown in 4 mM K for 14 days were salinized with 50 mM NaCl. Salt was added gradually reaching its final concentration after 6 h. Roots were harvested at times 0, 1, 2 and 4 days.
Root growth
Analysis of root morphology was performed in plants grown at different K concentration by staining root samples with toluidine blue for obtaining high contrast 300 dpi images (Primax, Colorado Direct, Utrecht, The Netherlands) and analyzed with Delta-T Scan (Cambridge, UK). Root hair length and density was estimated from images obtained with a Zeiss microscope (Axio Scope, Jena, Germany) with a 2.5× objective in dark field and analyzed with AxioVision software.
Determination of ethylene
For the determination of ethylene evolution, whole plants were placed in hermetically sealed 150-ml glass flasks and incubated under similar conditions of the growth chamber 24 h with 10 ml nutrient solution bathing the roots to avoid desiccation. After incubation, ethylene production was analyzed in a Perkin Elmer 8600 gas chromatograph (Waltham, MA) equipped with a Porapak Q column and nitrogen carrier at 80°C.
Analysis of free amino acids and polyamines
Leaves and roots were frozen in liquid N2 and stored at −70°C until analysis. Frozen leaves and roots were then ground and centrifuged (9200 g 5 min at 4°C). Clear supernatants were used for the analysis of free amino acids, which were separated and quantified after derivatization with phenylisotiocyanate by reversed-phase high-performance liquid chromatography (Heinrikson and Meredith 1984) using a Waters HPLC (Pico. Tag free amino acids column) (Milford, MA). Free polyamines in the similar extracts were quantitated as dansyl derivatives following Marcé et al. (1995).
Determination of K, sugars and organic acids
Potassium was determined by flame photometry (Jenway model PFP7, Felsted, UK). Root or leaf supernatants (obtained as described above), or aqueous extractions from dried and milled samples were used for K analysis. Content of organic acids in roots were measured either by enzymatic kits (for malate, citrate, and succinate, R-Biopharm AG, Darmstadt; for pyruvate, Greiner Diagnostics, Bahlingen, Germany). Total sugar content was estimated by the phenol-sulfuric assay using sucrose as standard (Ashwell 1969).
PK activity assay
PK activity in root homogenates was assayed according to Besford (1978). Tissue samples were ground with Ten-Broek (Wheaton, Millville, NJ) on ice with 100 mM Tris–HCl buffer pH 7.4 containing 50% glycerol and 0.1 mM DTT (1:10 w/v ratio) and centrifuged at 20 000 g for 10 min at 4°C (Heraeus Biofuge Primo R, Thermo Scientific, Osterode am Harz, Germany). The standard reaction mixture for the measurement of PK activity contained the following (μmoles): Tris–HCl buffer (pH 7.4), 50; phosphoenolpyruvate (PEP) (tricyclohexylammonium salt), 1.5; ADP (Na salt), 2.5; MgSO4, 10; KCI, 50; and enzyme solution, 0.5 ml (equivalent to about 1 mg protein) in a total volume of 1 ml. The reaction was stopped by the addition of 1 ml of 0.025% 2,4-dinitrophenylhydrazine in 2 N HCl. After 10 min at RT, 5 ml of 0.6 N NaOH was added and after a further 10 min the absorption at 510 nm of the solution was determined.
Antiport activity in root tonoplast vesicles
Isolation of root vacuolar membranes was performed as described by Barkla et al. (1995) and the measurement of cation/proton exchange was monitored by fluorescence quenching of ACMA (9-amino-6-chloro-2-metoxy-acridine) as described by Leidi et al. (2010) using purified tonoplast vesicles in 10 mM BTP-MES buffer pH 8.0 with 250 mM mannitol, 100 mM tetramethyl ammonium chloride and 3 mM MgSO4. Changes in fluorescence were recorded with a Hitachi fluorescence spectrophotometer (F 2500).
Statistical analyses
The analysis of data (plant growth, metabolites concentration, etc) was performed with IBM SPSS Statistics v.21 (Armonk, NY, USA) using a completely randomized design model. Mean comparisons were made when significant differences were found (F test) using the lsd (Least Significant Differences) test at P < 0.05 level.
Results
Responses to K withdrawal and K availability
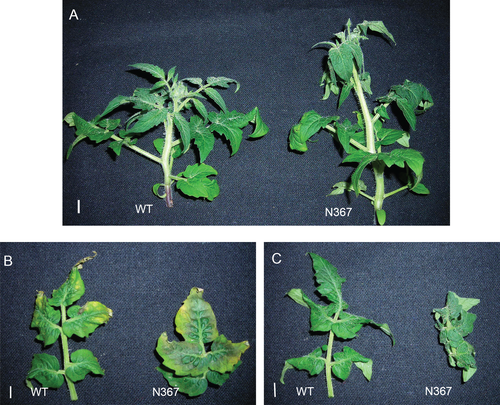
After 2 weeks of K withdrawal, N367 plants were significantly taller than wild-type (WT) plants (Fig. 2, Table 1) but no significant difference in shoot biomass between lines was found. Stem internodes were longer in the transgenic than in WT plants and the leaves of the N367 line presented severe epinasty resembling the effects of ethylene action, while symptoms of K deficiency (leaf scorching) appeared in the lower leaves (Fig. 2). Ethylene production was only detected in shoots from N367 plants, even under sufficient K conditions (Table 1).
| Shoot (g FW plant−1) | Stem length (cm) | Ethylene (pmol g−1 FW h−1) | |
|---|---|---|---|
| 4 mM K | |||
| WT | 12.6 ± 1.6a | 9.7 ± 0.5a | 0.0a |
| N367 | 9.7 ± 2.5a | 9.0 ± 0.6a | 1.1 ± 0.10b |
| 0 mM K | |||
| WT | 4.7 ± 0.6a | 8.3 ± 0.5a | 0.0a |
| N367 | 4.5 ± 0.5a | 11.5 ± 0.5b | 1.9 ± 0.11b |
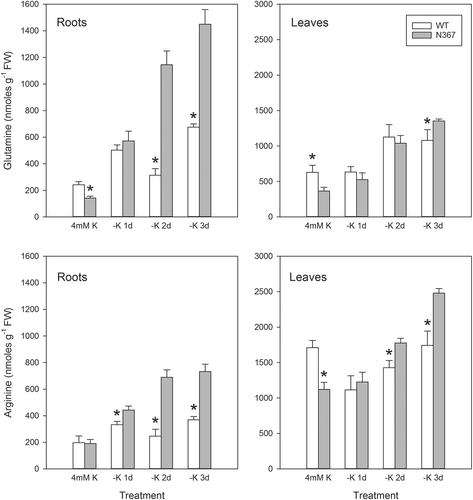
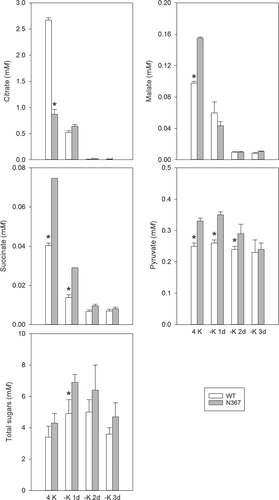
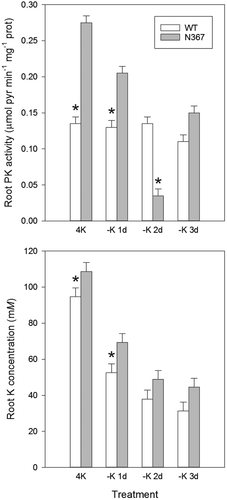
The nutritional K status of the plant affects the pool of polyamines, free amino acids, organic acids and sugars. Two weeks after transfer into a K-deprived nutrient solution, the N367 line showed a significantly greater putrescine concentration in lower and medial leaves than the WT (Table 2), although the accumulation of polyamines depended on the days in K-deficient conditions and the leaf position (Table S1). The response to K deficiency was remarkable in roots shortly after transfer into nutrient solution without K (1–3 days), and significant differences in the composition of the free amino acid pool (Fig. 1) and the concentration of organic acids and sugars (Fig. 3) were found between transgenic and WT lines. The concentration of glutamine and arginine in the transgenic line showed a significant increase relative to the WT (Fig. 1). However, after longer periods (8 days) of K deficiency, a general reduction in the concentration of amino acids occurred, with no remarkable differences between lines (Fig. S1). Major differences between N367 and WT lines were also found in root sugars and organic acids concentration (Fig. 3). The K withdrawal led to a transient increase in root sugar concentration and to the depletion of organic acids from the citric-acid-cycle (Fig. 3). A modest decrease of pyruvate content that was more pronounced in N367 plants was also recorded. Accordingly, the activity of PK in roots of N367 plants was significantly reduced by the diminished K supply (Fig. 4) shortly after K withdrawal. The comparatively higher pyruvate concentration and PK activity in transgenic roots (Figs 3 and 4) suggested a greater glycolytic flux required either for maintaining mitochondrial respiration or anabolic processes, and is in agreement with the greater sugar concentration found in NHX1 transgenic roots. In all root samples the K concentration remained higher in the N367 line than in the WT (Fig. 4). Under control conditions, a significantly higher cation/H+ exchange was observed in root tonoplast vesicles from the N367 transgenic line in comparison with the WT line (Fig. S2).
| Put (nmol g−1 FW) | Spd (nmol g−1 FW) | Nspd (nmol g−1 FW) | Spm (nmol g−1 FW) | |
|---|---|---|---|---|
| Lower leaves | ||||
| WT | 370.2a | 76.4a | – | 15.9a |
| N367 | 1635.0b | 72.4a | – | 22.4a |
| Medial leaves | ||||
| WT | 342.5a | 122.3a | – | 26.1a |
| N367 | 832.5b | 95.6b | – | 23.2a |
| Upper leaves | ||||
| WT | 226.9a | 141.4a | 27.2a | 25.0a |
| N367 | 213.8a | 127.8b | 62.2b | 23.4a |
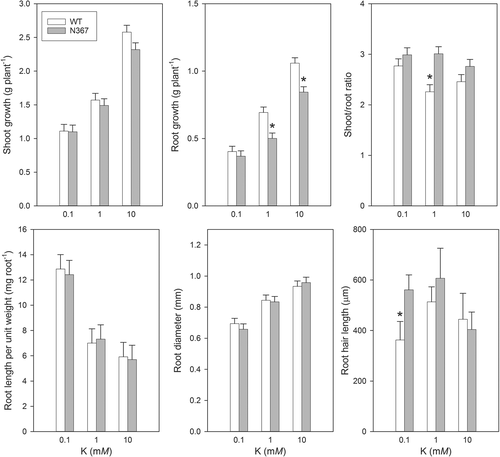
As K withdrawal stress led to rapid metabolic changes, we also tested whether a differential K availability during growth affected WT and NHX1-overexpressing lines differently. The main differential feature was the less developed root systems in N367 leading to greater shoot/root ratio and greater root hair length (Fig. 5). Root length per unit of root weight, root diameter or root hair length were all affected by K supply but only root hair length at low K showed significant differences between lines (Fig. 5).
Responses to salinity
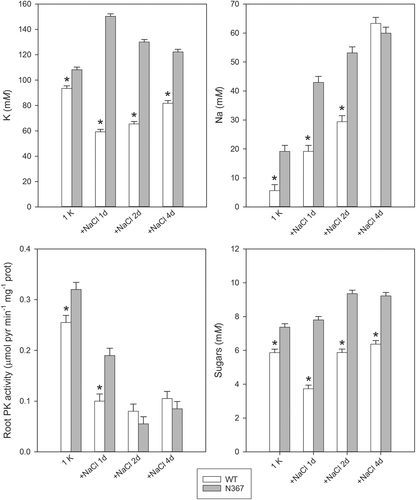
Salinity may restrict K uptake as well as modify the cytosolic K contents (Cuin et al. 2003). Notably, while root K concentration decreased in the WT after the salinity treatment, in the transgenic line the K concentration increased transiently and then declined as Na accumulated (Fig. 6). However, K concentrations were always greater in the transgenic line compared with WT. A moderate salt stress (50 mM NaCl) imposed for a short time also led to differences in the free amino acids of roots from WT and N367 lines (Fig. S3). Salt stress produced the reduction of amino acid concentration in both tomato lines, but the transgenic line maintained greater concentrations of glutamate, arginine, proline and glutamine than the WT (Fig. S3). Salinity also produced a significant decrease in PK activity in both lines (Fig. 6). The concentration of total sugars in the roots was also affected by salinity with an increasing trend in N367 while in the WT it only decreased transiently after 1 day of saline treatment (Fig. 6).
Discussion
We show here that the enhanced accumulation of K in vacuoles of tomato plants overexpressing the cation/proton antiporter NHX produced an acute responses to K deprivation and had long-term consequences on plant growth. Under sustained K deficiency transgenic plants released ethylene, increased the length of stem internodes and showed early symptoms of K deficiency associated to putrescine accumulation. These features are in agreement with the twofold reduction of cytosolic K activity described in NHX-overexpressing plants (Leidi et al. 2010), i.e. transgenic plants may be constitutively ‘sensing’ K deprivation even though the total K content is always greater than in control plants. Enhanced ethylene production by N367 plants even under K-replete conditions (4 mM KCl; Table 1) supports this notion as ethylene has been shown to play a key role in the transduction of the low-K signal (Schachtman 2015). Interestingly, significant metabolic changes occurred in NHX1-plants shortly after imposing K deprivation, which might explain late metabolic disorders. Rapid metabolic changes included greater levels of glutamine, asparagine and sugars, but diminished concentration of organic acids. The significant increase in amides (glutamine), positively charged amino acids (arginine) and sugars in the roots occurred shortly after transferring plants into K-minus solutions (Figs 1, 3) while a significant diminution in organic acids (malate, citrate, succinate, pyruvate) concentration occurred (Fig. 3). The changes in tomato xylem sap composition under K deficiency described by Sung et al. (2015) agree with our results. PK, a cytosolic enzyme requiring K as co-factor, reduced its activity in roots after K deprivation in the transgenic line while it was kept relatively constant in the WT (Fig. 4). However, in all cases, K remained at higher concentration in root tissues from line N367 than in the WT (Fig. 4). The product of PK activity, pyruvate, reduced its concentration when K was withdrawn from the nutrient solution likewise other organic acids from the tricarboxylic acid cycle (citrate, succinate, malate) (Fig. 3). The simultaneous increase in sugar concentration (Fig. 3) suggests the interruption of the glycolytic flux, which may occur at the regulatory step of PK (Plaxton 1996). Similar changes were shown in Arabidopsis submitted to K deprivation and were attributed to inhibition of PK by low cytoplasmic K (Armengaud et al. 2009). Considering the strict requirement of PK for K as co-factor, the analysis of its activity for sensing plant K status had been suggested (Besford 1978, Armengaud et al. 2009). Our data with NHX1-overexpressing tomato are in agreement with Armengaud et al. (2009) positing that low cytosolic K could produce the inhibition of glycolytic flow by affecting PK activity. PK activity shows a hyperbolic dependence on K concentration, with a typical threshold of maximal activation around 50 mM K. This threshold value is near to the cytosolic K activity measured in root epidermal cells of N367 seedlings under K-limited (0.1 mM KCl) conditions (55 ± 2.2 mM vs 98 ± 1.3 mM in the WT) (Leidi et al. 2010). Thus, the cytosolic K should be expected to fall sooner below the set value for maximal PK activation in the NHX1 line than in the WT, thereby reaching K cytosolic concentrations well within the range of the steepest response of PK to K availability (Armengaud et al. 2009). The enzymatic activity of PK in the transgenic line was significantly reduced already after 1 day of K deprivation, coherent with the lower cytosolic K activity produced by enhanced sequestration of K in vacuoles. Notably, PK activity in plants grown at 4 mM K was greater in the N367 line relative to the WT (Fig. 4), but even though PK requires K as co-factor, PK has a complex regulation that may be affected by repressors and activators like the amino acids glutamate and aspartate (Baysdorfer and Bassham 1984, Plaxton 1996, Smith et al. 2000) whose concentrations are also altered in the transgenic plants at K-replete conditions (Fig. S1). In fact, among the changes observed in K-starved tomato roots, in all experiments there was always a decrease in the aspartate/glutamate ratio (Fig. S1), which has been described as an effective inhibitor of PK (Baysdorfer and Bassham 1984). Such ratio might alternatively (or jointly to low K) downregulate enzyme activity.
The inhibition of the glycolytic carbon flow affects N uptake and metabolism (Stitt et al. 2002) and such decreased C flow is suspected to occur under K deficiency when a built-up of sugars correlates with a significant reduction in organic acids of the TCA (tricarboxylic acid)-cycle. The accumulation of N-rich amino acids also indicates to which extent the reduced glycolytic C flow in K deficient plants may alter N metabolism. Changes in C and N metabolites (sugars, organic acids, amino acids) detected in tomato roots after removing K from the nutrient solution were more rapid in NHX1-overexpressing plants, indicating their greater sensitivity to K limitation compared with WT plants. Our results indicate that under sudden changes in K supply, the over-expression of the tonoplast exchanger NHX1 in tomato significantly affects C and N metabolism. Transgenic plants were more sensitive to nutrient stress even though they presented higher tissue K contents than the control. The metabolic differences between WT and N367 leading to greater sugar and proline accumulation in the transgenic line were already pointed out as contributing factors of its greater salt tolerance (Leidi et al. 2010).
Plants are very plastic for adapting to K deficiency, re-establishing cytosolic K progressively by using more efficiently vacuole K reserves (Walker et al. 1996). The N367 line might have greater difficulty for providing K to the cytosol if K release by vacuolar channels is short-circuited by the NHX1 antiporter (Jiang et al. 2010). The metabolic and physiological changes in the WT might occur later than in the transgenic line when vacuolar K is really exhausted for providing cytosol requirements. In this regard, the accumulation of polyamines in N367 plants is particularly telling. There are three known gateways for releasing vacuolar K into the cytosol. Slow- (SV) and fast-activating (FV) vacuolar channels are ubiquitous and prominent tonoplast channels with poor K/Na selectivity. FV channels are active at the resting Ca2+ levels, whereas SV channels are activated by membrane depolarization and regulated by luminal and cytosolic Ca2+. The VK channels (a.k.a TPK, two pore K channels) are highly selective for K and mediate cation release mainly in guard cells in response to changes in cytosolic Ca2+, but are far less abundant in other cell types (Hedrich 2012). These K-conductive tonoplast channels are differentially inhibited by polyamines (reviewed by Pottosin and Shabala 2014). FV channels are inhibited by μM concentrations of spermine and spermidine, and by mM amounts of putrescine, all polyamines block SV channels and vacuolar TPKs are rather insensitive to polyamines (Brüggemann et al. 1998, Dobrovinskaya et al. 1999, Hamamoto et al. 2008). Therefore, the increased levels of putrescine in N367 plants (Table 2) could exacerbate the disproportionate accumulation of K in the vacuolar lumen at the expense of the cytosolic pool by inhibiting the release of K through FV and SV channels (Pottosin and Shabala 2014). Of note is that the accumulation of polyamines that has been often reported under salinity stress in various species, including tomato (Botella et al. 2000), would primarily inhibit the activity of non-selective FV and SV channels, thereby enabling the effective sequestration of Na inside vacuoles with no leak currents towards the cytosol (Pottosin and Shabala 2014), which may also contribute to the salt tolerance of plants overexpressing NHX-like transporters.
After a prolonged period of K deficiency, plants over-expressing the K+/H+ exchanger released ethylene and showed significant differences in morphology (Fig. 2), and accumulated greater putrescine concentration than the WT (Table 2, Table S1). The early metabolic alterations in C and N metabolites shown above led later on to these more profound changes like leaf epinasty, marginal chlorosis and necrosis (Fig. 2) or modifications in shoot and root architecture (Figs 2 and 5). These late changes induced by K deficiency like epinasty, increased internode length and plant height, are typical modifications induced by ethylene while those of leaf chlorosis and necrosis have been related to polyamine accumulation (Basso and Smith 1974). The increase of putrescine detected in leaves of N367 was only significant after 2 weeks under K-deficiency (Table S1). Such accumulation of putrescine in N367 might be related to the greater arginine concentration found in its roots and leaves (Fig. 1) albeit the direct relationship between arginine concentration and putrescine synthesis by arginine decarboxylase (ADC) remains to be proved. In mutants of Arabidopsis defective in arginase an increased accumulation of putrescine was observed (Flores et al. 2008, Shi et al. 2013). In Arabidopsis, the increase in ADC activity is observed after 2 weeks in K deficiency and greatly increases after 4 weeks (Watson and Malmberg 1996). As it was found in the N367 tomato line (Table 2, Table S1), a significant increase in putrescine occurred when K deficiency affected plants for at least 1 week. Such an increase in putrescine biosynthesis might have been induced by ethylene, which is able to stimulate ADC and SAMDC (S-adenosyl-methionine synthetase) activities as well as putrescine accumulation (Lee and Chu 1992). In a similar way, the application of the ethylene-precursor ACC (1-aminocyclopropane-1-carboxylic acid) leads to ethylene production and induces putrescine accumulation (Tamai et al. 1999). Interestingly, nutrition with ammonia, which greatly interferes with K uptake, may produce ethylene release and putrescine accumulation (Barker and Corey 1991, Feng and Barker 1993) similar to the symptoms described above for K deficiency. Roots responded to K availability by reducing their biomass while increasing root length per unit of root weight and diminishing root diameter in both lines at low K concentration (Fig. 5). Morphological changes observed in roots may be attributed to ethylene production in response to K deficiency (Jung et al. 2009, García et al. 2015). Interestingly, low K promoted significantly greater root hair length in the transgenic line (Fig. 5), which might also be related to greater ethylene production by their roots.
Interestingly, a short NaCl treatment also produced a reduction in root PK activity, an increase in sugar concentration and modified the free amino acid pool in roots (Fig. S3). The PK activity in N367 roots showed a significant reduction in spite the root K concentration did not decrease with the salt treatment. The increased Na concentration in the roots (Fig. 6) may reduce cytosolic K activity (Carden et al. 2003) affecting PK activity, as the enzyme is less active with Na as cofactor (Tomlinson and Turner 1973, Besford and Maw 1975, Oria-Hernández et al. 2005). Our results on the inhibitory effect of salinity on PK activity agree with previous reports on different species (Porath and Poljakoff-Mayber 1968, Kalir and Poljakoff-Mayber 1976, Muscolo et al. 2003). Imposing salt stress led to some expected changes in tomato plants as a reduction in N and C metabolism (Cramer et al. 1995) but the differential response of tomato NHX-lines apparently resulted from the way K was partitioned in cytosolic and vacuolar pools. In a range of salt treatments, the vacuolar K content in the transgenic tomato was maintained higher than in the WT (Leidi et al. 2010). The greater vacuolar allocation of K in N367 probably hindered the re-establishment of an adequate cytosolic K-Na ratio (Maathuis and Amtmann 1999), as evidenced by the finding that the NHX-overexpressing line showed more evident signs of metabolic K deficiency (accumulation of sugars, proline, arginine and glutamine) than the WT. However, the integration of all the features still lead to salt tolerance of the transgenic plant and hence our data provide further evidence that salt-tolerance associated to antiporter over-expression may mostly depend on protective compounds (such as sugars, proline, etc) rather than a differential Na compartmentation (Leidi et al. 2010).
In summary, the cytosolic K deficiency induced by the activity of the K+-Na+/H+ antiporter NHX1 produces severe metabolic alterations whenever root cells do not meet sufficient K for uptake. When the external K concentration fulfills the root demands, the transgenic plant grows normally although spending more C for building shoots than roots. Vacuolar K+ sequestration by the antiporter overexpression makes many K-related processes more sensitive to nutrient deficiency altering sooner metabolic pathways and leading to earlier appearance of K-deficiency symptoms.
Author contributions
A. D. L. performed research; E. O. L. designed research and analyzed data; J. M. P. and E. O. L. wrote the paper.
Acknowledgements
This work was supported by grant BFU2012-35060 from Ministerio de Economia y Competitividad (MINECO, co-financed by the European Regional Development Fund) to J. M. P. with additional support from the SSAC grant PJ01105105 from the Rural Development Administration, Republic of Korea. The technical assistance of M.A. Parrado is greatly appreciated.




