TaMDAR6 acts as a negative regulator of plant cell death and participates indirectly in stomatal regulation during the wheat stripe rust–fungus interaction
Abstract
We identified a new monodehydroascorbate reductase (MDAR) gene from wheat, designated TaMDAR6, which is differentially affected by wheat–Puccinia striiformis f. sp. tritici (Pst) interactions. TaMDAR6 is a negative regulator of plant cell death (PCD) triggered by the Bax gene and Pst. Transcript levels of TaMDAR6 are significantly upregulated during a compatible wheat–Pst interaction, indicating that TaMDAR6 may contribute to plant susceptibility. In addition, H2O2 production and PCD are significantly induced and initial pathogen development is significantly reduced in the TaMDAR6 knocked-down plants upon Pst infection. Thus, the suppression of TaMDAR6 enhances wheat resistance to Pst. Besides, the suppression of TaMDAR6 during an incompatible interaction induces a change in the morphology of stomata, which leads to poor stoma recognition and as a consequence to reduced infection efficiency. The percentage of infection sites that develop substomatal vesicles decreases in the TaMDAR6 knocked-down plants during the incompatible interaction presumably due to the increase in ROS accumulation, which is likely to activate other resistance mechanisms that have a negative effect on substomatal vesicle formation. TaMDAR6 can therefore be considered a negative regulator of PCD and of wheat defense to Pst.
Abbreviations
-
- AA
-
- ascorbic acid
-
- APX
-
- ascorbate peroxidase
-
- Bax
-
- mammalian Bax gene
-
- Bgh
-
- Blumeria graminis
-
- BSMV
-
- barley stripe mosaic virus
-
- CAT
-
- catalase
-
- CYR
-
- Chinese races of Pst
-
- DFCI
-
- computational biology and functional genomics laboratory database
-
- GPX
-
- glutathione peroxidase
-
- GSH
-
- glutathione
-
- HR
-
- hypersensitive response
-
- MDAR
-
- monodehydroascorbate reductase
-
- NCBI
-
- National Center for Biotechnology Information
-
- ORF
-
- open reading frame
-
- PCD
-
- programmed cell death
-
- PDS
-
- phytoene desaturase
-
- PR proteins
-
- pathogenesis-related proteins
-
- Pst
-
- Puccinia striiformis f. sp. tritici
-
- pyr_redox 2
-
- pyridine nucleotide-disulfide oxidoreductase 2 domain
-
- pyr_redox
-
- pyridine nucleotide-disulfide oxidoreductase domain
-
- PEG
-
- polyethylene glycol
-
- qRT-PCR
-
- quantitative real-time PCR
-
- ROS
-
- reactive oxygen species
-
- SOD
-
- superoxide dismutase
-
- TaCAT
-
- catalase
-
- TaMDAR6
-
- chloroplastic monodehydroascorbate reductase
-
- TaPOD
-
- class III peroxidase
-
- TaPR2
-
- β-1,3-glucanase
-
- TaPR5
-
- thaumatin-like
-
- TOCs
-
- tocopherols
-
- VIGS
-
- virus-induced gene silencing
Introduction
Wheat (Triticum aestivum) is one of the most important cereal crops in the world, which is the primary source of vegetable protein in human food (Chandra et al. 2014, Langridge 2012). Therefore, research on wheat has provided a basic understanding of this food crop to improve its productivity. By contrast, plant diseases have a negative impact on wheat production, and one of the most important diseases of wheat worldwide is the wheat stripe rust, caused by Puccinia striiformis f. sp. tritici (Pst) (Zheng et al. 2013). Allison and Isenbeck (1930) were the first to establish the existence of races in Pst based on differential susceptibility of wheat cultivars. China is the largest epidemic region in the world (Stubbs 1988, Asad et al. 2012); the most destructive epidemics of stripe rust occurred in 1950, 1964 and 1990, caused yield losses of 29.3, 13.3 and 1.8% of the national total production, respectively (Li and Zeng 2000).
Pst is a biotrophic fungus that infects living cells and takes up water and nutrients from the living host. Infection can occur from the seedling to maturity stage. The fungus forms yellow to orange colored pustules (uredinia), and each uredinium contains thousands of urediospores, which spread from the pustules by wind. Infection requires high humidity for 4–6 h at 10–15°C. Compared to other rust fungi, Pst prefers a lower temperature for development, which limits this fungus as a major disease in many areas of the world (Stubbs 1985, Wellings and McIntosh 1990).
Reactive oxygen species (ROS) are toxic by-products of many aerobic metabolic processes. Moreover, various stress conditions cause increases in ROS generation, leading to oxidative damage of lipids, proteins and DNA (Apel and Hirt 2004). Under biotic stress, ROS act as signaling molecules to activate pathogenesis-related proteins and systemic acquired resistance in cells adjacent to the infection site to prevent further pathogen spread (Draper 1997). ROS serve as signaling molecules at low levels, but can also induce cell death at high levels (Sharma et al. 2012). The production of ROS is normally counter-acted by an enzymatic anti-oxidative system [catalase (CAT); ascorbate peroxidase (APX); glutathione peroxidase (GPX); and superoxide dismutase (SOD)], and by a non-enzymatic anti-oxidative system [ascorbic acid (AA); glutathione (GSH); tocopherols (TOCs); and phenolic compounds] to protect plant cells against ROS toxicity. The monodehydroascorbate reductase (MDAR) enzyme is involved in the ascorbate–glutathione cycle, and plays an important role in directly reducing monodehydroascorbate (oxidized ascorbate) to ascorbate using NAD(P)H as an electron donor (Apel and Hirt 2004).
The roles of MDAR have been extensively reported under abiotic stress such as ozone, salt, polyethylene glycol (PEG) (Sharma and Davis 1997, Eltayeb et al. 2007) and drought (Sharma and Dubey 2005), which showed that MDAR genes are regulated by abiotic stresses to reduce oxidative damages through maintaining redox status (Eltelib et al. 2011). Therefore, MDAR genes have been used as indicators of plant resistance to several abiotic stresses (Ali et al. 2005, Sharma and Dubey 2005). For example, in Brassica campestris, transcript levels of BcMdhar were strongly upregulated in response to oxidative stress (Yoon et al. 2004). In Physcomitrella patens, PpMDHAR1 and PpMDHAR3 were induced during salt stress and osmotic stress (Lunde et al. 2006). In contrast, few studies have examined the roles of MDAR under biotic stress, particularly during the wheat–Pst interactions. In our previous studies, we identified two members encoding MDAR from wheat (TaMDHAR4 and TaMDHAR), which demonstrated that the suppression of TaMDHAR4 and TaMDHAR enhances wheat resistance to Pst (Feng et al. 2014a). Besides, TaMDHAR is regulated by miRNA PN-2013 during Pst infection (Feng et al. 2014b). TaMDHAR is a cytoplasmic protein. In addition, TaMDHAR4 has been considered a peroxisomal protein (Feng et al. 2014a,2014b). By contrast, TaMDAR6 is a chloroplastic protein. Plant chloroplasts are the most significant generators of ROS (Ishikawa and Shigeoka 2008, Maruta et al. 2012), and are important elements in the regulation of programmed cell death (PCD), which is an essential component of plant defense to pathogen attacks. Thus, the sub-cellular localization of TaMDAR6 in plant chloroplasts opens the possibility of the involvement of TaMDAR6 in the regulation of PCD. Therefore, TaMDAR6 was specially investigated. Our findings demonstrated that TaMDAR6 acts as a negative regulator of PCD. In addition, we reported for the first time the involvement of TaMDAR6 gene in stomatal regulation and substomatal vesicles formation during the wheat–Pst interaction. Therefore, this study provides new insights into the role of TaMDAR in the wheat–Pst interaction.
Materials and methods
Plant material and inoculation
Compatible and incompatible interactions were established using the wheat cultivar Suwon 11 and two Chinese races of Pst (CYR23 and CYR31). Suwon 11 shows high susceptibility to CYR31 and high resistance to CYR23 (Wang et al. 2010). Plant cultivation and inoculation with Pst were performed according to Kang et al. (2003). Inoculated leaves were sampled, quickly frozen in liquid nitrogen and stored at −80°C until used for RNA isolation.
RNA extraction and first strand cDNA synthesis
Total RNA (3 µg) was extracted using the BIOZOL Total RNA Extraction Reagent (Bioer Technology Company Limited, Binjiang, China) following the manufacturer's protocol. Any DNA contamination was removed by treatment with DNase1 before synthesis of cDNA. Extracted RNA was reversely transcribed into cDNA using the i-SCRIPT Kit (Bio-Rad, Hercules, CA) according to the manufacturer's instructions.
Cloning and sequencing
The full-length sequence was obtained by screening of our cDNA database of wheat–Pst interactions (Ma et al. 2009) and the wheat EST database in the National Center for Biotechnology Information (NCBI). Homologous sequences were assembled using the CAP3 (http://pbil.univ-lyon1.fr/cap3.php/) and multiple sequence alignments were performed using the dnaman 6.0 software (Lynnon BioSoft, Pointe-Claire, Canada). Specific primers were designed according to the assembled sequence using the Primer 5.0 software (Table S1, Supporting Information). The full length open reading frame (ORF) was amplified from cDNA synthesis using RNA isolated from wheat cv. Suwon 11 leaves after inoculation with Pst CYR23 (incompatible interaction) or CYR31 (compatible interaction). Amplification was conducted in a DNA thermocycler (Bio-Rad) using the following program: 95°C for 3 min; 35 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 70 s; and 72°C for 10 min. The amplified ORF was ligated into the pGEM T-easy vector (Promega, Madison, WI) following the manufacturer's protocol and transformed into competent Escherichia coli (JM109) by the CaCl2 procedure (Ausubel et al. 1997). Sequencing was performed using an ABI PRISM 3130XL Genetic Analyzer (Applied BioSystems, Foster City, CA). The amino acid sequence and conserved domains were analyzed using NCBI (http://www.ncbi.nlh.nih.gov/gorf/gorf.html), InterProScan (http://www.ebi.ac.uk/cgi-bin/iprscan/), Compute pI/MW (http://web.expasy.org/compute_pi/) and ProtParam (http://www.expasy.org/tools/pi_tool.html). The prediction of chromosomal location of the corresponding gene was performed using the International Wheat Genome Sequencing Consortium database (http://wheat-urgi.versailles.inra.fr/Seq-Repository/BLAST). The sequence is deposited at GenBank under accession number KP201873.
Quantitative real-time PCR
TaMDAR6 transcript levels were analyzed by qRT-PCR (7500 Real-Time PCR System, Applied Biosystems) using synthesized cDNA, as previously explained, at different time points after inoculation (12, 18, 24, 48 and 120 hpi) and primer pairs (Table 1) specific to the gene of interest. Previous time points were chosen according to our previous studies of the interactions between Chinase Pst races (CYR31, CYR23) and wheat cv. Suwon 11 (Wang et al. 2007). The RT-PCR (real-time PCR) System was programmed as follows: 95°C for 1 min; 40 cycles at 95°C for 10 s, 60°C for 20 s, and 72°C for 40 s; 1 cycle at 95°C for 15 s, 60°C for 1 min and 95°C for 15 s; and finally 60°C for 15 s. The relative expression of the investigated gene was normalized using the reference gene TaEF-1 (GenBank accession number: Q03033), and relative expression was estimated using the 2–ΔΔCT method (Livak and Schmittgen 2001). Data are the means of three independent experiments.
| Constructs | Symptoms | Necrosis activityb | Necrotic area %c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 dpi | 6 dpi | 7 dpi | 8 dpi | 5 dpi | 6 dpi | 7 dpi | 8 dpi | 5 dpi | 6 dpi | 7 dpi | 8 dpi | |
| PVX:EV & PVX:EV (co-infiltration, negative ck) | No visible | No visible | No visible | No visible | - | - | - | - | 0 | 0 | 0 | 0 |
| PVX:BAX & PVX: EV (co-infiltration, positive ck) | Necrosis | Necrosis | Necrosis | Necrosis | ++++ | ++++ | ++++ | ++++ | 100 | 100 | 100 | 100 |
| PVX:TaMDAR & PVX:EV (co-infiltration) | No visible | No visible | No visible | No visible | - | - | - | - | 0 | 0 | 0 | 0 |
| PVX:BAX & PVX:TaMDAR (co-infiltration)a | Very small necrotic lesions | Small necrotic lesions | Large necrotic lesions | Necrosis | + | ++ | +++ | ++++ | 10 | 30 ± 5 | 80 ± 5 | 100 |
Sub-cellular localization of TaMDAR6 in Nicotiana benthamiana
To construct the fusion vector pCaMV35S:TaMDAR-GFP, the complete ORF of TaMDAR6 was amplified using specific primers that have restriction sites for SpeI and AvrII (Table 1). The digested amplicon was ligated into the 5′ end of the green fluorescent protein (GFP) coding region of pCaMV35S:GFP. The newly constructed plasmid was introduced into Agrobacterium tumefaciens GV3101 through electroporation (Wise et al. 2006). Transformed cells were infiltrated into Nicotiana benthamiana leaves (Van der Hoorn et al. 2000). GFP signals were detected using an Olympus BX-51 fluorescence microscope (Olympus Corp., Tokyo, Japan). The experiment was performed twice and with three replicates each time.
Agrobacterium-mediated transient gene expression
PVX:BAX, which was kindly provided by Dr D. Dou (Purdue University, West Lafayette, IN), was digested with ClaI and XmaI. The BAX fragment was replaced with GFP to construct PVX:GFP or with TaMDAR6 to construct the PVX:TaMDAR (Fig. S3). Agrobacteria carrying respective plasmids were cultured in 5 ml of LB medium, supplemented with kanamycin, rifampicin and gentamycin. Cells were harvested at an OD600 of 0.6 to 1.2 and resuspended in 10 mM MgCl2. Bacterial suspensions were adjusted to a final density of 0.2–0.5 at OD600. Transient expression assays were performed using 4- to 5-week-old N. benthamiana plants. Suspensions were infiltrated into N. benthamiana leaves (abaxial surface) using a 2-ml disposable syringe without a needle (Van der Hoorn et al. 2000). At least six leaves on different plants were used in infiltration experiments; each leaf was divided into four sites then infiltrated with A. tumefaciens cell suspensions as follows; first and second sites were infiltrated with A. tumefaciens carrying PVX:00 (empty vector), third and fourth sites were infiltrated with A. tumefaciens carrying (PVX:TaMDAR). Then the infiltrated leaf was incubated at 25°C. After 2 days, the same areas were infiltrated again as follows; first and third sites were infiltrated with A. tumefaciens carrying (PVX:00), second and fourth sites were infiltrated with A. tumefaciens carrying (PVX:BAX). A. tumefaciens strains carrying PVX:GFP were used to confirm the efficiency of our experiments. Pictures were taken 5–8 days after the last infiltration. The experiment was repeated three times.
Virus-induced gene silencing (VIGS)
VIGS was mediated by the barley stripe mosaic virus (BSMV). BSMV vectors (α, β, γ and BSMV:PDS4as) were provided by Dr Scofield (Purdue University). A139 bp fragment was chosen and amplified from a plasmid containing the TaMDAR6 gene using specific primers with restriction sites for NotI and PacI (Table 1, Fig. S6). The PCR product was cloned into the pGEM T-simple vector. Extracted plasmids were digested with NotI and PacI, followed by replacement of the TaPDS coding sequence in BSMV:TaPDS to construct plasmid BSMV:TaMDARas for gene silencing according to Holzberg et al. (2002). Linearized plasmids were transcribed into RNA using the mMessage T7 in vitro transcription kit (Ambion, Austin, TX) following the manufacturer's instructions.
The second leaves of wheat seedlings were gently inoculated by rubbing the leaf surfaces from base to tip two times using a mixture of each transcriptional reaction. In total, 30 plants were used for each treatment (5 plants/pot), and another 15 plants were inoculated with 1 × FES buffer (0.1 M glycine, 0.06 M K2HPO4, 1% w/v tetrasodium pyrophosphate, 1% w/v bentonite and 1% w/v celite, pH 8.5) as control. Post 9 days virus inoculation, the fourth leaf was inoculated with urediospores of Pst CYR23 or CYR31. Infection types of Pst were recorded 15 dpi. The fourth leaves were also sampled for RNA isolation and histological observation. Relative transcript levels of TaMDAR6, pathogenesis-related protein genes (TaPR2, DQ090946; TaPR5, FG618781) and reactive oxygen species-related genes (TaCAT, catalase, X94352; TaPOD, class III peroxidase, TC303653) were assessed using qRT-PCR as described above. The experiment was repeated three times.
Histological analysis
Samples were stained as described (Wang et al. 2007). According to Parlevliet (1986), aborted substomatal vesicles were considered aborted penetration attempts. Therefore, only infection sites where substomatal vesicles had formed were considered. Fungal development and host responses for each treatment (a minimum of 50 infection sites for each time point) were observed under an Olympus BX-51 microscope (Olympus Corp., Tokyo, Japan) using bright field and UV light. Auto-fluorescence of mesophyll cells in infected leaves was measured as necrotic area using epifluorescence microscopy (excitation filter, 485 nm; dichromic mirror, 510 nm and barrier filter, 520 nm). The percentage of stomata that remained open was determined from records of at least 100 stomata observed on four independent leaves. Hyphal length, hyphal branches and necrotic area were calculated using dp-bsw software. Statistical analyses were performed using spss software.
Results
Cloning and sequence analyses of TaMDAR6
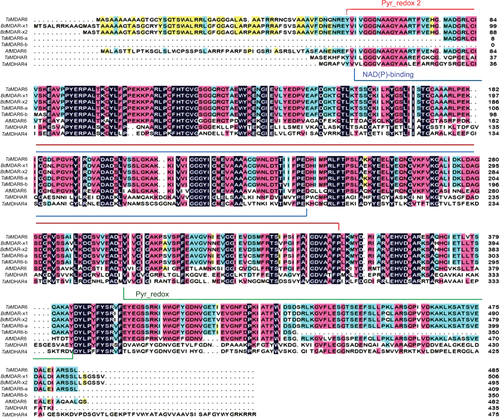
Specific primers were designed to amplify the cDNA fragments of the corresponding gene. The full-length cDNA sequence was isolated from cDNA of wheat cv. Suwon 11, which is highly susceptible to virulent Pst race CYR31 and highly resistant to avirulent Pst race CYR23 (Wang et al. 2010). The predicted ORF of TaMDAR6 spans 1458 bp and encodes a protein of 485 amino acids with a predicted molecular mass of 52.04 kDa. The prediction of protein domains using InterProScan and NCBI databases indicated that TaMDAR6 protein contains pyridine nucleotide-disulfide oxidoreductase domains (pyr redox 2, pyr redox and NAD(P)-binding Rossmann-like domain) (Fig. 1, Fig. S1). In addition, the corresponding gene is a homolog of AtMDAR6, which encodes an MDAR protein localized in chloroplasts of Arabidopsis. Hence, the corresponding gene was designated as TaMDAR6. The prediction of chromosomal location using the International Wheat Genome Sequencing Consortium database (http://wheat-urgi.versailles.inra.fr/Seq-Repository/BLAST) indicated that there were three copies in the wheat genome, located on chromosomes 7AL, 7BL and 7DL, respectively, indicating that the TaMDAR6 gene family may be located in the homologous group 7 chromosomes. In addition, phylogenetic analysis and cDNA multiple sequences alignment both revealed that our corresponding gene of this study is located on chromosome 7DL (Fig. 2, Fig. S2). Moreover, phylogenetic analysis (Fig. 2, Table S2) revealed that TaMDAR6 is most similar to TaMDAR6-a, BdMDAR chloroplastic isoforms (X1 and X2) from Brachypodium distachyon, and TaMDAR6-b, respectively, but showed the lowest similarity with other wheat monodehydroascorbate reductase (TaMDHAR4 and TaMDHAR).
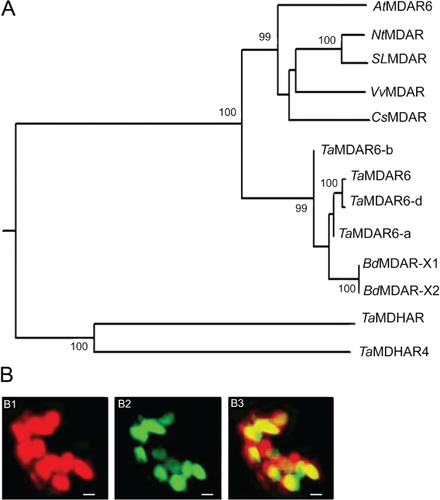
TaMDAR6 is localized in chloroplasts of N. benthamiana
Agrobacteria carrying pCaMV35S:TaMDAR-GFP were infiltrated into N. benthamiana leaves to determine the sub-cellular localization of TaMDAR6. Fluorescence microscopic analysis revealed that green fluorescence of TaMDAR-GFP was only detected in chloroplast, totally matched with the red autofluorescence of chlorophyll. This result indicated that TaMDAR-GFP is localized in the chloroplast of N. benthamiana (Fig. 2B).
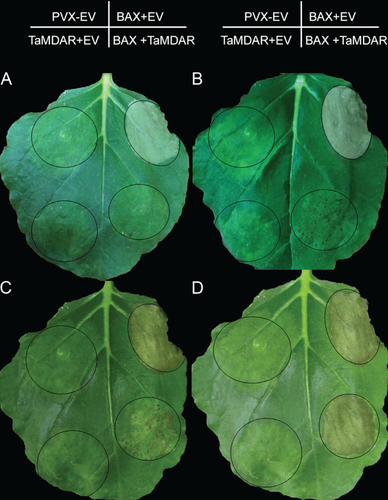
TaMDAR6 has a negative effect on programmed cell death (PCD) induced by the Bax gene
To improve our understanding concerning TaMDAR6 function in PCD at the molecular level, TaMDAR6 transient expression was performed through the infiltration of A. tumefaciens into N. benthamiana leaves. To evaluate the efficiency of our approach, the transient expression of the GFP gene was investigated. N. benthamiana leaves were infiltrated with A. tumefaciens carrying PVX:GFP. Fluorescence microscopic analysis revealed that green fluorescence of PVX:GFP was detected in N. benthamiana cells. These results indicated that GFP was successfully expressed, which demonstrated that our transient expression system was functional (Fig. S4).
N. benthamiana leaves were infiltrated with A. tumefaciens carrying PVX:00 empty vector + PVX:00 empty vector, or PVX:BAX + PVX:00 empty vector, or PVX:TaMDAR + PVX:00 empty vector, or PVX:Bax + PVX:TaMDAR. As shown in Fig. 3 and in Table 1, no necrotic area occurred at areas infiltrated with PVX:00 + PVX:00, or PVX:TaMDAR + PVX:00. By contrast, areas infiltrated with A. tumefaciens carrying PVX:Bax + PVX:00 became completely necrotic within 5 dpi. To determine whether TaMDAR6 enhances or suppresses PCD triggered by Bax, A. tumefaciens cultures carrying PVX:Bax and PVX:TaMDAR vectors were infiltrated into N. benthamiana leaves at the same site. The percentage of infiltrated leaf area that had become necrotic at 5, 6, 7 and 8 dpi increased over time, however, this increase was much slower than observed for the positive control (PVX:Bax + PVX:00) (Table 1). Only extremely small necrotic lesions were observed 6 dpi at the infiltration area with PVX:Bax + PVX:TaMDAR, and these lesions constantly evolved to include all of the infiltrated area after 8 dpi. This result indicates that the area infiltrated with PVX:Bax + PVX:TaMDAR became completely necrotic after 8 dpi, which was much slower than the necrosis that occurred with PVX:Bax + PVX:00 (Fig. 3).
Transcriptional responses of TaMDAR6 to Pst infection
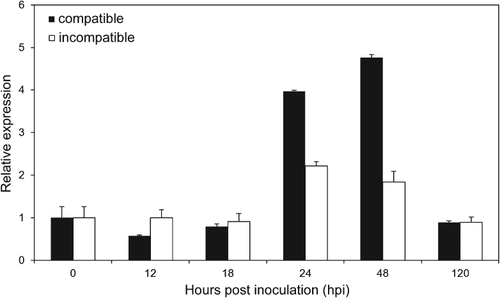
In the incompatible interaction, transcript levels of TaMDAR6 remained at the control level from 12 to 18 hpi, a transcript peak was observed at 24 hpi, with an approximately 2.2-fold increase, and subsequently a return to control levels at 120 hpi (Fig. 4). In the compatible interaction, transcript levels of TaMDAR6 were changed a little at 12 to 18 hpi. However, t ranscript levels were strongly upregulated at 24 and 48 hpi, by approximately 3.9- and 4.7-fold, respectively. At 120 hpi, the control level was reached again (Fig. 4). Therefore, the relative transcript level of TaMDAR6 in the compatible interaction was much higher than that in the incompatible interaction, at least at 24 and 48 hpi (Fig. 4).
Suppression of TaMDAR6 enhances wheat resistance to Pst
A virus-induced gene silencing (VIGS) system established in cv. Suwon 11 was used to evaluate the function of TaMDAR6 gene during the interaction between wheat and Pst (Fig. S5). To evaluate the efficiency of our VIGS system, we tested the silencing of the wheat phytoene desaturase gene (PDS). The second leaves of a two-leaf wheat seedling was inoculated with BSMV:TaPDSas or BSMV:γ (empty vector) as positive control, or FAS buffer as negative control. As shown in Fig. 5A, mild virus symptoms occurred on the new leaves at 15 dpi. Meanwhile, BSMV:TaPDSas inoculated plants showed large photo-bleached areas on the fourth leaf, while there were no such symptoms on plants treated with FAS buffer, which showed that our VIGS system was functional.
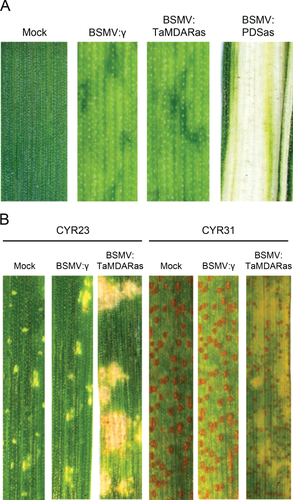
To make the silencing specific to TaMDAR6 (7DL), a 139 bp fragment was chosen and amplified using specific primers (Table 1, Fig. S6). The fragment located at the 5′ untranslated region (UTR) and the origin of the ORF. Rigorous efforts were made to choose the VIGS fragment to get a specific silencing construct. Nucleotide sequences of wheat MDAR genes were downloaded from NCBI and alignment analysis was conducted. Alignment analysis indicated no homology between the TaMDAR6 gene and other wheat MDAR genes in the VIGS fragment (Fig. S6). Al-Attala et al. (2014) and Holzberg et al. (2002) supported the view that a sequence with 81% identity or less does not cause targeted gene silencing. Therefore, the second leaves of a two-leaf tested plant were inoculated with BSMV:TaMDARas or with BSMV:γ (empty vector as control). All BSMV-infected plants displayed mild virus symptoms. The fourth leaves were inoculated with avirulent race CYR23 for the incompatible interaction or with virulent race CYR31 for the compatible interaction.
To clarify whether TaMDAR6 was successfully silenced, transcript levels of all three TaMDAR6 copies (7AL, 7BL, 7DL) were examined using quantitative RT-PCR (qRT-PCR). In comparison to BSMV:γ-infected plants, the expression level of TaMDAR6 (7DL) was decreased in the fourth leaves of BSMV:TaMDARas-infected plants at 0, 24, 48 and 120 hpi with Pst by approximately 80, 88, 90.5 and 80% for the incompatible interaction and by 80, 89.7, 92.2 and 80% for the compatible interaction, respectively (Fig. S7). These results indicated that TaMDAR6 was successfully silenced. By contrast, transcript levels of other two copies TaMDAR6-a (7AL) and TaMDAR6-b (7BL) were not affected (Fig. S7), indicating that the VIGS construct was very specific to TaMDAR6 (7DL).
Disease development was scored at 15 dpi with Pst races to evaluate the effect of gene silencing on the wheat–Pst interaction. BSMV:γ-infected plants (empty vector) had no changes in their resistance or susceptibility against CYR31 or CYR23 (Fig. 5B). In the incompatible interaction, both treatments (BSMV:TaMDARas and BSMV:γ) had no changes in their resistance (infection type 1) according to the 0–9 scale of McNeal et al. (1971), HR areas were elicited in both treatments with BSMV:TaMDARas and BSMV:γ, and no uredosori formed (Table 2, Fig. 5B). In the compatible interaction, although heavy sporulation was observed in both treatments (BSMV:TaMDARas and BSMV:γ), BSMV:TaMDARas-infected plants showed a higher degree of resistance (infection type 8) than control plants (infection type 9) according to the 0–9 scale of McNeal et al. (1971). Moreover, PCD areas were elicited around the infection sites in the BSMV:TaMDARas-infected plants, but no such cell death areas were observed in the control (Table 3, Fig. 5B).
| Treatmentsa | Hyphal length (µm)c | Hyphal branchesd | Haustorial mother celle | H2O2 area (µm2)f | Necrotic area (µm2)g48 hpi | Successful infection site %h | Open stomata %i | Stoma size (µm)j | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 hpi | 48 hpi | 24 hpi | 48 hpi | 24 hpi | 48 hpi | 24 hpi | 48 hpi | 24 hpi | 48 hpi | 24 hpi | 48 hpi | 24 hpi | 48 hpi | ||
| BSMV:γ/CYR23 | 25.93 ±2.00 | 28.71 ± 2.39 | 2.07 ± 0.53 | 2.00 ±0.50 | 2.03 ±0.50 | 2.05± 0.60 | 734 ±196 | 822±191 | 531± 192 | 79 | 81 | 73 | 73 | 69.45 ± 4.27 × 4.14 ± 0.92 | 55.69 ± 4.62 × 6.59 ± 1.23 |
| BSMV:TaMDAR/CYR23 | 21.88 ± 2.50b | 22.91 ± 2.00b | 1.60 ± 0.50b | 1.54 ± 0.50b | 1.71 ± 0.52b | 0.85 ± 0.20b | 1054 ± 201b | 2398 ± 192b | 1206 ± 190b | 80 | 25 | 63 | 20 | 70.41 ± 4.25 × 3.90 ± 0.67 | 123.93 ± 9.93 × 5.39 ±1.52b |
| Treatmentsa | Hyphal length (µm)c | Hyphal branchesd | Haustorial mother celle | Haustoriaf48 hpi | H2O2 area (µm2)g48 hpi | Necrotic area (µm2)h120 hpi | Successful infection site %i | Open stomata %j | Stoma size (µm)k | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 hpi | 48 hpi | 24 hpi | 48 hpi | 24 hpi | 48 hpi | 24 hpi | 48 hpi | 24 hpi | 48 hpi | 24 hpi | 48 hpi | ||||
| BSMV:γ/CYR31 | 27.73 ± 2.50 | 32.82 ± 2.10 | 1.82 ± 0.50 | 2.97 ± 0.50 | 1.87 ± 0.50 | 2.70 ± 0.55 | 1.83 ± 0.20 | — | — | 85 | 97 | 92 | 95 | 72.17 ± 4.25 × 5.59 ± 1.00 | 72.59 ± 4.52 × 5.43 ± 1.00 |
| BSMV:TaMDAR/CYR31 | 21.75 ± 2.50b | 25.94 ± 2.00b | 1.50 ± 0.50 | 1.77 ± 0.53b | 1.50 ± 0.49 | 1.72 ± 0.55b | 0.45 ± 0.20b | 183 ± 51 | 503 ± 192 | 82 | 95 | 90 | 94 | 72.33 ± 4.27 × 5.17 ± 1.03 | 72.56 ± 4.67 × 5.02 ± 1.10 |
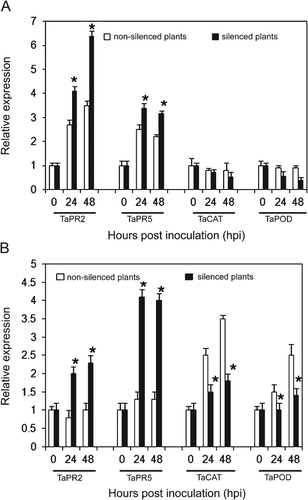
To demonstrate that the increases of PCD was involved in the resistance response; the transcriptional responses of some defense-related genes were examined, because those genes have been used as indicators of HR and are necessary for plant resistance (Schaffrath et al. 1997; Van Loon and Van Strien 2002). The transcriptional responses of defense-related genes (TaPR2, TaPR5, TaCAT and TaPOD) to TaMDAR6 suppression were determined by qRT-PCR during incompatible and compatible interactions at different time points 0, 24 and 48 hpi. Transcript levels of PR genes were significantly upregulated during compatible and incompatible interactions at all time points (Fig. 6). By contrast, transcript levels of catalase (TaCAT) and class III peroxidase (TaPOD) were significantly reduced in the compatible interaction (Fig. 6). In addition, there was no significant change in the transcript levels of TaCAT and TaPOD (reduced a little) in the incompatible interaction (Fig. 6). These results indicated that the hypersensitive and resistance responses of wheat to Pst were enhanced. Therefore, the increases of PCD may be involved in the resistance responses of wheat to Pst.
Histological observation of interactions between Pst and TaMDAR6 knocked-down plants
To improve our understanding concerning the wheat–Pst interaction, TaMDAR6 knocked-down plants were infected by CYR31 (virulent Pst race) or CYR23 (avirulent Pst race), then microscopically examined. In the incompatible interaction, H2O2 production at the interaction sites gradually increased in the TaMDAR6 knocked-down plants at 24 and 48 hpi. In addition, hypersensitive cell death seemed to be enhanced in the TaMDAR6 knocked-down plants at 48 hpi (Table 2, Fig. S8). Growth of hyphae was significantly reduced at both time points (Table 2). Numbers of hyphal branches and of haustorial mother cells were also significantly reduced in the TaMDAR6 knocked-down plants at 24 and 48 hpi (Table 2, Fig. S8).
In the compatible interaction, hyphal growth was also significantly shorter than that observed in the BSMV:γ-infected plants at 24 and 48 hpi (Table 3). Numbers of hyphal branches, haustorial mother and haustoria cells were significantly reduced in the TaMDAR6 knocked-down plants at 48 hpi (Table 3, Fig. S8). Moreover, H2O2 production at the site of interaction was induced in the TaMDAR6 knocked-down plants at 48 hpi (Table 3, Fig. S8). PCD was induced in the TaMDAR6 knocked-down plants at 120 hpi. However, at 120 hpi, H2O2 production areas were too large, leading to difficulties in taking other measurements (hyphal growth, numbers of hyphal branches and haustorial mother cells).
In the incompatible interaction, urediospores germinated well in BSMV:γ (empty vector as control) and BSMV:TaMDARas plants at all time points. In control plants, Pst germ tubes reached the stomata, with successful penetration at 24 and 48 hpi. In the TaMDAR6 knocked-down plants, Pst germ tubes also reached stomata, however, with successful penetration only at 24 hpi. At 48 hpi, there were two observations: (1) germ tubes did not end over stomata, and (2) germ tubes grew over stomata, in both cases not penetrating the stoma (Fig. S9). Moreover, in comparison with control plants, the percentage of stomata that remained open in BSMV:TaMDARas-infected plants decreased at 24 and 48 hpi by approximately 10 and 53%, respectively (Table 2). Meanwhile, a significant change in stomatal shape (length × width) of BSMV:TaMDARas-infected plants could be observed at 48 hpi (Table 2, Fig. 7). However, no significant difference in stomatal shape was observed between BSMV:TaMDARas-infected and BSMV:γ-infected leaves at 24 hpi (Table 2, Fig. 7). Besides, the percentage of the infection sites where substomatal vesicles had formed underneath the stomata (successful penetration) in the TaMDAR6 silenced plants was lower than that in control plants at 48 hpi by approximately 56% (Table 2). In the compatible interaction, there was no significant change in stomatal behavior or in percentage of infection sites (Table 3).
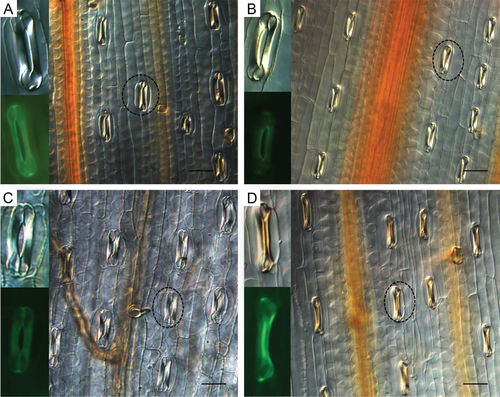
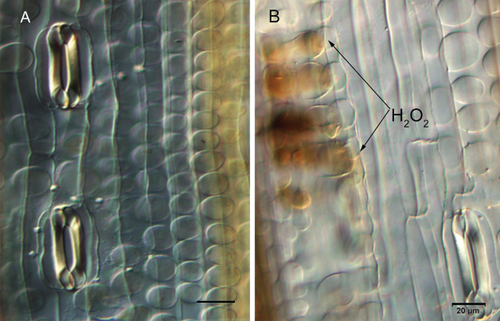
Suppression of TaMDAR6 induces H2O2 accumulation without Pst attack
To demonstrate that the suppression of TaMDAR6 induces ROS accumulation, wheat leaves were infected with BSMV:TaMDARas or with BSMV:γ without Pst inoculation. In comparison to BSMV:γ-infected leaves, H2O2 production was induced in the fourth leaves of BSMV:TaMDARas-infected plants (Table S3, Fig. 8). In addition, there also was no significant change in stomatal shape in the fourth leaves of BSMV:TaMDARas-infected plants (Table S3).
Discussion
TaMDAR6 functions as a negative regulator of PCD
PCD is an important element for plant immunity against biotic and abiotic stress as well as for plant development and proliferation (Gadjev et al. 2008), including vacuolar cell death, necrosis and hypersensitive cell death (Van Doorn et al. 2011). In fact, plant hypersensitive cell death occurs during plant infection by hemibiotrophic and biotrophic pathogens, and displays the same microscopic features of both necrosis and vacuolar cell death (Van Doorn et al. 2011). In addition, ROS have been considered as key factors of the induction and modulation of the PCD during plant–pathogen interaction (Desikan et al. 1998, Gadjev et al. 2008). The lifetime of ROS within the cellular environment is determined by the antioxidative system, which provides crucial protection against oxidative damage (Apel and Hirt 2004). As mentioned, MDAR proteins also play important roles in ROS accumulation. It is worth mentioning that plant chloroplasts are considered as the most important sources of ROS (Ishikawa and Shigeoka 2008, Maruta et al. 2012). In addition, the role of TaMDAR6 encoding chloroplastic TaMDAR protein in the wheat–Pst interaction and in PCD has not been reported. Therefore, it is tempting to speculate that the chloroplastic TaMDAR protein may participate in PCD regulation. To study this hypothesis, we isolated the TaMDAR6 gene encoding chloroplastic TaMDAR from wheat, then studied its role in PCD triggered by a mammalian Bax gene and by Pst.
The mammalian Bax gene is an animal pro-apoptotic protein, absent in plants, which induces PCD (HR-like cell death) primarily through the accumulation of ROS similar to plant hypersensitive cell death (Cai and Jones 1998, Fujita et al. 1998, Aravind et al. 1999, Kawai-Yamada et al. 2001). The co-infiltration of TaMDAR6 together with Bax resulted in a slower cell death than that induced by Bax alone. This finding is consistent with most reports that have indicated plant antioxidants as a negative regulator of Bax-induced PCD (Kampranis et al. 2000, Moon et al. 2002 and Chen et al. 2004). In these studies, the over-expression of plant antioxidants (ascorbate peroxidase; glutathione-S-transferase/peroxidase, and phospholipid hydroperoxide glutathione peroxidase) reduces ROS accumulation and suppresses Bax expression in N. benthamiana and in yeast. The authors conclude that plant antioxidants suppress Bax-induced PCD through decreasing ROS generation. Taken together, we suggest that the differences observed in the necrotic development are caused by TaMDAR6 expression, which in turn functions as a negative regulator of PCD triggered by Bax.
In this study, a VIGS approach was used to determine the role of TaMDAR6 (located on chromosome 7DL) in pathogen-induced cell death. Macroscopic and microscopic analyses showed that the average necrotic area was significantly increased in the TaMDAR6 knocked-down plants upon Pst infection. El-Zahaby et al. (1995) stated that plant antioxidants suppress the formation of necrotic symptoms during barley–Bgh interactions. Therefore, we conclude that silencing of TaMDAR6 is able to increase PCD during the wheat–Pst interactions. Meanwhile, H2O2 accumulation was significantly increased in the TaMDAR6 knocked-down plants over time, suggesting that increase in necrotic area may be linked to an increase in H2O2 accumulation, indicating that TaMDAR6 silencing increases PCD through increased H2O2 accumulation. Thus, TaMDAR6 has a negative effect on PCD through reducing H2O2 accumulation. Therefore, we conclude that TaMDAR6 is involved in PCD during wheat–Pst interactions.
TaMDAR6 has a negative role in Pst recognition
Our previous histological studies stated that an oxidative burst was triggered following the recognition of elicitor(s), released from haustoria of an avirulent race by a receptor of the host cell (Wang et al. 2007, 2010). From this study, we detected H2O2 accumulation at the infection sites after TaMDAR6 was knocked-down in the compatible interaction, indicating that the wheat cells became able to recognize the virulent race of Pst. Thus, the interaction between pathogen elicitors and host receptors probably activates a signal transduction cascade that involves H2O2 accumulation. Therefore TaMDAR6 has a negative role in pathogen recognition during the wheat–Pst interaction. Furthermore, hyphal growth, the number of hyphal branches, and the number of haustoria and haustorial mother cells were significantly reduced in the TaMDAR6 knocked-down plants for both interactions. These results open the possibility that increased H2O2 production reduces initial growth of Pst, consistent with the findings of Feng et al. (2014a, 2014b) in wheat against Pst during suppression of TaMDHAR4 and TaMDHAR genes. Ellingboe (1972) reported that development of hyphae is a good indicator for establishment of a compatible interaction. Therefore, we conclude that the knocking down of TaMDAR6 enhances plant resistance to Pst.
TaMDAR6 negatively regulates ROS accumulation
Transcript levels of TaMDAR6 were upregulated upon Pst infection in compatible and incompatible interactions. Therefore, we suggest that TaMDAR6 is involved in wheat–Pst interactions. Moreover, transcript levels of TaMDAR6 were higher in a compatible interaction than that in incompatible interaction. Burhenne and Gregersen (2000) and Vanacker et al. (1998), stated that the inoculation of barley leaves with Blumeria graminis (Bgh) causes significant increases in the expression levels and activities of antioxidant enzymes such as ascorbate peroxidase in compatible interactions more than incompatible interactions, suggesting that antioxidant enzymes may contribute to plant sensitivity against Bgh infection. Feng et al. (2014a) described that transcript levels of TaMDHAR4 gene were downregulated at 12–18 hpi during incompatible interaction then upregulated at 48 hpi in both interactions. The authors suggested that suppression of TaMDHAR4 might be important to the incompatible interaction. In contrast, transcript levels of TaMDAR6 remained at the control level from 12 to 18 hpi then upregulated at 24 and 48 hpi in both interactions. Therefore, it is tempting to speculate that there may be a required level of TaMDAR6 expression in wheat plants to show incompatible interaction against Pst, and that an increase in that level may contribute to plant sensitivity against Pst.
In this study, we studied for the first time the relationship between TaMDAR6 gene and substomatal vesicles formation and stomata regulation during the Pst–wheat interaction. The knocking down of TaMDAR6 during wheat–Pst interactions reduced disease symptoms and induced HR, suggesting that the suppression of TaMDAR6 increases wheat resistance against Pst. Besides, histological observations of the Pst inoculated plants indicate that urediospores germinated successfully in both wheat–Pst interactions. However, the percentage of the infection sites where substomatal vesicles formed (successful penetration) was influenced by the knocking down of TaMDAR6 in the incompatible interaction. Meanwhile, H2O2 accumulation and the expression level of genes encoding pathogenesis-related proteins significantly increased over time in the TaMDAR6 knocked-down plants. Dangl and Jones (2001) stated that H2O2 accumulation could activate many plant defenses to eliminate the pathogen. In addition, Broers and López-Atilano (1996) during a study of the effects of wheat resistance on the development of Pst, suggested that some of the formed substomatal vesicles disintegrated later by PR proteins that were activated after pathogen colonization. Therefore, we conclude that the decrease in substomatal vesicle formation might be linked to an increase in H2O2 accumulation, which in turn may trigger other resistance mechanisms that have a negative effect on the formed substomatal vesicles.
Furthermore, some of the germ tubes of Pst in the incompatible interaction did not reach the stomata or grew over them without penetration, suggesting that the germ tubes became unable to recognize stomata on the TaMDAR6 knocked-down plants. In addition, non-recognition of stomata by the germ tubes of Pst was associated with changes in the shape of the stomata and with decreases in the percentage of stomata that remained open in the incompatible interaction. This suggests that the suppression of TaMDAR6 induced changes in stomatal morphology and enhanced stomatal closure on the TaMDAR6 knocked-down plants in the incompatible interaction. In addition, the inability of Pst germ tubes to recognize stomata may be triggered by changes in morphological features of the stomata. Moreover, changes of stomatal shape in the TaMDAR6 knocked-down plants might be triggered by an increase in H2O2 accumulation in the incompatible interaction. Allen et al. (2000) and McAinsh et al. (1996) stated that H2O2 has a marked effect on stomatal behavior, induces changes in guard cells and promotes stomatal closure. In addition, TaMDAR6 localizes to the chloroplast of N. benthamiana. Maruta et al. (2012) stated that plant chloroplasts are a major source of H2O2. Thus, TaMDAR6 plays important role in H2O2 regulation. Therefore, we conclude that TaMDAR6 participates indirectly in stomatal regulation through its effects on H2O2 accumulation during the wheat–Pst incompatible interaction. According to these results, the knocking down of TaMDAR6 enhances plant responses to avoid further penetration, reducing Pst infection efficiency through reducing the probability of Pst germ tubes to locate or recognize the stomata during incompatible interaction.
In contrast, there was no significant change in stomatal behavior in silenced plants for either compatible interaction or non-inoculated plants even though there was an increase of the H2O2 accumulation in both cases. Bhattacharjee (2012) and Melillo et al. (2006), stated that ROS may induce different types of responses during plant infection, depending on their level. Therefore, these results open the possibility that H2O2 levels in both cases were not sufficient to induce significant changes in stomatal behavior or to reduce the percentage of the infection sites in the compatible interaction. Therefore, we conclude that change in stomatal behavior depends on the level of ROS.
Collectively, the results of this study provide strong evidence that TaMDAR6 functions as a negative regulator of PCD. In addition, the knocking down of TaMDAR6 induces ROS accumulation, which triggers other resistance mechanisms, depending on their level, such as changes in stomatal behavior. Thus, the knocking down of the TaMDAR6 gene enhances the resistance of wheat to Pst.
Author contributions
M. A. A. A., X. W. and Z. K. performed conception, design and interpretation of data; M. A. A. A., M. N. A. A., Q. X.; G. Z. performed the experiments; M. A. A. A. and X. W. analyzed the data; M. A. A. A., X. W. drafted the article or revised; Z. K. contributed the final approval of the version to be published.
Acknowledgements
This study was supported by the National Basic Research Program of China (No. 2013CB127700), the Key Grant Project of the Chinese Ministry of Education (313048), the National Natural Science Foundation of China (No. 31271990), the Young star of science and technology, Shaan'xi (2012KJXX-15) and by the 111 Project from the Ministry of Education of China (B07049). We thank Nagi Abou-zeid of the Plant Pathology Research Institute, ARC, Egypt for his advice and continuous guidance; S. R. Scofield of the Department of Agronomy, Purdue University, West Lafayette, USA for providing BSMV vectors; and D. Dou for providing transient expression vectors.




