Enhanced expression and interaction of GmRDR1 and GmSGS3 proteins in resistant soybean cultivars synergistically regulate antiviral defense against mungbean yellow mosaic India virus
Abstract
- Mungbean yellow mosaic India virus (MYMIV) causes significant losses to soybean productivity in India. Resistance to MYMIV is reported to be linked with two QTLs. It was hypothesized that within these QTLs, two RNA silencing-related genes, RNA-DEPENDENT RNA POLYMERASE-1 (GmRDR1) and SUPPRESSOR OF GENE SILENCING (GmSGS3), may have a role in governing resistance.
- In this study, coding regions of the above genes were sequenced from resistant (SL 1074) and susceptible (JS 335) soybean cultivars. While GmRDR1 had identical sequences in both cultivars, two synonymous SNPs in GmSGS3 were identified. Based on one of these SNPs, a CAPS marker was developed, which differentiates resistant and susceptible genotypes.
- In silico docking and yeast two-hybrid assays confirmed the interaction between GmRDR1 and GmSGS3. Gene expression analysis showed that resistant genotypes expressed higher levels of these transcripts after MYMIV inoculation. Additionally, reducing expression of either gene via RNA interference increased viral accumulation, indicating reduced resistance.
- This study highlights the critical role of GmRDR1 and GmSGS3 in soybean resistance to MYMIV, suggesting that their enhanced expression and interaction facilitate antiviral defense. Future research should explore molecular pathways involved, which could improve breeding strategies for MYMIV resistance in soybean.
INTRODUCTION
Soybean (Glycine max L. Merr.) is an important edible oilseed crop, with a distinct chemical composition, comprising ca. 20% oil and 40% protein. This unique profile underscores its potential to address dietary protein needs of the global vegetarian population. Soybean is cultivated during the kharif season (July–October) in the rainfed regions of central and peninsular India. This crop is crucial for the livelihoods of millions of Indian farmers and plays a significant role in the country's agribusiness sector, being utilized in livestock feed, oil production, and industrial applications (Ghosh et al. 2020; Rani et al. 2021). Furthermore, soybean promotes soil fertility through nitrogen fixation, making it an essential element in sustainable farming practices that aligns with India's broader objectives for agricultural sustainability and productivity (Singh et al. 2022). The rising domestic and international demand for soy-based products – ranging from tofu and soy milk to protein supplements – further highlights the crop's importance in national and global markets (Kumar et al. 2019). While India accounts for roughly 10% of global soybean cultivation, its contribution to overall production is just 4%. This gap points to India's low productivity, with an average yield of 1.1 ton ha−1 compared to the global average of 2.2 ton ha−1, emphasizing the challenges in fully harnessing the crop's potential (Agarwal et al. 2013).
One of the most significant challenges in soybean cultivation in India is yellow mosaic disease (YMD). This disease is characterized by yellowing and mosaic-like patterns on leaves of soybean plants. When the disease manifests early, the affected plants become stunted with low pod setting, leading to 15%–75% yield losses (Sharma et al. 2014). This, in turn, adversely affects farmers' income and increases reliance on imported edible oils (Patil et al. 2021; Raj et al. 2022). In India, YMD of soybean is caused by an isolate of mungbean yellow mosaic India virus (Usharani et al. 2005) in Begomovirus vignaradiataindiaense (Begomovirus; Geminiviridae). MYMIV is a bipartite, circular ssDNA virus transmitted by whitefly (Bemisia tabaci). Besides soybean, MYMIV also causes similar yellow mosaic disease in other legume crops like mungbean (Vigna radiata), blackgram (V. mungo), cowpea (V. unguiculata), and pigeon pea (Cajanus cajan) (Malathi et al. 2017). The annual losses from this disease for soybean, mungbean, and black gram crops exceed $300 million (Varma & Malathi 2003).
Conventional management strategies for YMD, such as insecticides to control whitefly vector populations, have limited effectiveness, often leading to persistent pest problems and increased agricultural costs. As a result, developing soybean varieties resistant to MYMIV offers a more environmentally sustainable and economically viable solution (Bhanu et al. 2019). Although significant advances have been made in breeding MYMIV-resistant soybean crops, with development of many resistant varieties, debate continues regarding inheritance patterns of YMD resistance in soybean. This may be because many results were based solely on the F2 generation (Talukdar 2013) or F2/F3 generations (Bhattacharyya et al. 1999; Rani et al., 2017). There are two known sources of YMD resistance in soybean: one originates from the wild progenitor, Glycine soja (Singh et al. 1974), while the other comes from the cultivated gene pool (Singh & Mallick 1978; Bhattacharyya et al. 1999). Reports indicate that resistance derived from G. soja might be controlled by either a single dominant gene (Bhattacharyya et al. 1999) or by two duplicate dominant genes (Rani et al. 2018). In contrast, resistance to YMD in Glycine max is governed by either a single dominant gene (Talukdar 2013; Nichal et al. 2018) or one or two recessive genes (Singh & Mallick 1978; Rani et al. 2017). Additionally, researchers have identified quantitative trait loci (QTLs) associated with YMD resistance on chromosomes 6, 8, 14, and 17 using association mapping or bulk segregant analysis (BSA) (Kumar et al. 2015; Rani et al. 2018). Recently, composite interval mapping using F1, F2, F2:3, BC1F1, and BC1F2 (backcrossing with both parents) revealed that inheritance of resistance to MYMIV is governed by inhibitory gene action in conjunction with two QTLs on chromosomes 2 and 6 (Khosla et al. 2021). Furthermore, two potential candidate genes—RNA-DEPENDENT RNA POLYMERASE 1 (GmRDR1) and SUPPRESSOR OF GENE SILENCING 3 (GmSGS3) – within the QTLs on chromosomes 2 and 6, respectively, could be integral to the resistance mechanism (Khosla et al. 2021). However, no further research has been conducted to determine the role of these genes in soybean cultivars with resistance to MYMIV.
The present study characterizes GmRDR1 and GmSGS3 genes from both resistant and susceptible soybean cultivars. It investigates the interactions of proteins encoded by these genes, as well as expression profiles under virus-inoculated conditions, to gain a better understanding of the synergistic roles these two genes in conferring resistance to MYMIV in soybean. Additionally, the study explores the impact of gene knockdown in resistant cultivars on virus accumulation. The results will contribute to elucidating mechanisms through which GmRDR1 and GmSGS3 regulate antiviral defense against MYMIV in soybean.
MATERIAL AND METHODS
Plant material
Eight soybean (G. max L. Merr.) genotypes were used, among which five (SL 958, SL 1074, DS 3105, DS 3106, and SL 955) were resistant, and three (JS 335, AMS 19-01, and JS 25-08) were susceptible to yellow mosaic disease. The resistant and susceptible nature of the soybean cultivars used was established through prior field screening as well as whitefly-mediated and agroinoculation-based artificial challenge (Chavan et al. 2025). Seeds were procured from the Division of Genetics at the Indian Agricultural Research Institute, New Delhi. Plants were grown in an environmentally controlled growth chamber maintained at 24 ± 2°C, with average humidity of 65%, and a light/dark cycle of 16 h/8 h.
Amplification and cloning of complete CDS of GmRDR1 and GmSGS3
Following the standard protocol, total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA) from both resistant (SL 1074) and susceptible (JS 335) soybean cultivars. RNA samples were treated with DNase I (Ambion) to eliminate residual genomic DNA. An aliquot of 1 μg DNA-free RNA was used for cDNA synthesis. The 20 μL reaction mix for cDNA synthesis included 1× buffer, 1 mM dNTPs, 25 ng μL−1 Oligo dT primer, 1 U μL−1 RiboLock RNase Inhibitor and 10 U μL−1 RevertAid reverse transcriptase (Thermo Fisher Scientific, Waltham, MA, USA). The complete CDSs of GmRDR1 and GmSGS3 were amplified from the cDNA using GmRDR1 F/GmRDR1 R and GmSGS3 F/GmSGS3 R, respectively (Table S1). PCR amplification used ~200 ng cDNA with 0.02 U μL−1 Phusion high-fidelity polymerase (Thermo Fisher Scientific) with 1x HF buffer, 0.2 mM dNTPs, and 0.5 μM primers (forward and reverse). The PCR amplicons were purified using Promega Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA) and ligated into the pUC18 vector following restriction enzyme-based cloning, where SalI and SacI were used for GmRDR1 and BamHI and EcoRI were used for GmSGS3. The ligated products were then used to transform E. coli DH5α cells. Recombinant colonies were screened through colony PCR and restriction digestion of recombinant plasmids. Confirmed clones for GmRDR1 and GmSGS3 were sequenced through outsourcing (Central Instrumentation Facility (CIF), University of Delhi South Campus, New Delhi, India).
Analysis of GmRDR1 and GmSGS3 sequences
The GmRDR1 and GmSGS3 CDS sequences were subjected to NCBI BLASTn to identify their homologs. SNPs were identified through pairwise sequence alignment. The amino acid sequences of GmRDR1 and GmSGS3 proteins were deduced using the ExPasy Server (https://www.expasy.org/). BioEdit (Hall 1999), and MEGA X (Kumar et al. 2018) software were used for multiple sequence alignment using the amino acid sequences of RDR1 and SGS3 proteins obtained from resistant and susceptible soybean genotypes and related species, selected based on relevance to the study. Phylogenetic analysis was conducted using the neighbor-joining method with 1000 bootstrap replications.
Development and validation of SNP-based CAPS marker
Based on sequence analysis, SNPs were identified in GmSGS3 CDS between resistant and susceptible genotypes. Consistency of SNPs was confirmed through sequencing a fragment of GmSGS3 from four more resistant and two more susceptible genotypes. Using one SNP in GmSGS3 CDS, a CAPS marker was developed, where XhoI was the enzyme that distinguished resistant and susceptible genotypes. To validate the CAPS marker, the complete CDS was initially amplified and digested with XhoI from two resistant (SL 1074, SL 958) and two susceptible (JS 335, AMS 19-01) soybean genotypes. For validation of SNPs from genomic DNA, this was extracted from leaf tissues of resistant (SL 1074, SL 958, DS 3106, DS 3105, and SL 955) and susceptible (JS 335, AMS 19-01, and JS 25-08) soybean genotypes using the CTAB method (Doyle & Doyle 1987). DNA quality and concentration were confirmed using a NanoDrop spectrophotometer (Genetix, India). A fragment of the GmSGS3 gene containing two SNPs was amplified using the primer pair SmSGS3F and SmSGS3R (Table 1). The amplified product was directly digested with the XhoI enzyme (Thermo Scientific) to generate genotype-specific SNP fragment pattern.
| Sl No | primer name | sequence | amplicon size | Tm | target gene | organism | purpose | reference |
|---|---|---|---|---|---|---|---|---|
| 1 | SmSGS3 F | ATCTTCGGGAAATCCCTGGCAGAC | 433 bp | 59 | GmSGS3 | Glycine max | Amplification of a small fragment | Designed in this study |
| 2 | SmSGS3 R | TGGACAATGCCACTGTCTTTCT |
Analysis of amino acid sequences of GmRDR1 and GmSGS3 proteins and molecular docking
Physicochemical properties, including molecular weight, isoelectric point (pI), and amino acid composition, of GmRDR1 and GmSGS3 proteins were analyzed using the ProtParam tool (https://web.expasy.org/protparam/) (Swiss Institute of Bioinformatics, ExPASy). Conserved domains and functional motifs within these proteins were identified using InterPro-Pfam (https://www.ebi.ac.uk/interpro/) (Mitchell et al. 2019) and MOTIF Search (https://www.genome.jp/tools/motif/) (Pagni et al. 2007) servers, providing insights into functional regions. Homology models for both GmRDR1 and GmSGS3 proteins were generated using the SWISS-MODEL server (https://swissmodel.expasy.org/) (Guex et al. 2009). These models were validated through the SAVES server (https://saves.mbi.ucla.edu/), with PROCHECK used for Ramachandran plot analysis (Laskowski et al. 1993), ERRAT for assessing overall model quality (Colovos & Yeates 1993), and ProSA-web to predict Z-scores, ensuring structural reliability (Wiederstein & Sippl 2007). To study the protein–protein interactions between GmRDR1 and GmSGS3, molecular docking was performed using the Gramm docking server (https://gramm.compbio.ku.edu/) (Singh et al. 2024), and further validated using the ClusPro server (https://cluspro.org/) (Kozakov et al. 2017). Active site predictions were obtained using the PrankWeb server (https://prankweb.cz/) (Jendele et al. 2019) to highlight potential interaction regions. Both homology models were submitted for docking analysis, and the resultant complex evaluated based on clustering and energy scores. The optimal docking model was selected for detailed structural and interaction analysis, displaying most favorable interaction and stability. The PDBsum server (https://www.ebi.ac.uk/thornton-srv/databases/pdbsum/) (Laskowski et al. 2005) was used to predict interacting amino acids of GmRDR1 and GmSGS3 proteins using the docked file generated by the Gramm server.
Yeast two-hybrid (Y2H) assay
The yeast two-hybrid (Y2H) assay was employed to investigate the interaction between GmSGS3 and GmRDR1 proteins of soybean, following the protocol described by Peng & Neff (2020). The coding sequences of GmRDR1 and GmSGS3 were amplified for cloning into yeast vectors using pGAD-dRDR-F/ pGAD-dRDR-R and pGBK-dSGS3-F/ pGBK-dSGS3-R, respectively (Table S1). The amplified GmRDR1 and GmSGS3 CDS were subsequently cloned into the pGADT7-AD (activation domain) and pGBKT7-BD (binding domain) vectors, respectively, using the NEBuilder® HiFi DNA Assembly Cloning Kit, which ensures high efficiency and specificity of gene insertion. The pGADT7-GmRDR1 (prey) and pGBKT7-GmSGS3 (bait) constructs were used to transform the yeast strains Y187 and Y2HGold (Takara Bio Inc.), respectively, using the lithium acetate transformation method according to the manufacturer's instructions. Successful transformants were selected on synthetic dropout (SD) media lacking leucine (SD/-Leu) for pGADT7-AD, or lacking tryptophan (SD/-Trp) for pGBKT7-BD constructs. An empty pGBKT7-BD or pGADT7-AD vector was used as a negative control to confirm specificity of the interactions. For interaction analysis, the Y2HGold cells harboring pGBKT7-GmSGS3 were mated with Y187 cells harboring pGADT7-GmRDR1 on SD medium lacking leucine and tryptophan (SD/-Leu/-Trp). The resulting diploids were subsequently selected on triple dropout (SD/-Leu/-Trp/-His) medium then plated on quadruple dropout medium (SD/-Leu/-Trp/-His/-Ade), which lacks leucine, tryptophan, histidine, and adenine, to confirm positive protein–protein interactions. Colony growth was monitored for 5 days at 30°C to assess interaction viability. To further confirm stringency of the interaction, colonies from four dropout media were plated on a quadruple dropout medium supplemented with X-α-gal and Aureobasidin A, following the manufacturer's protocol (Clontech Laboratories). Yeast cells grown on selective SD media were then analyzed for blue color development, indicating a positive interaction. Standard autoactivation analysis were performed using empty vector as one partner.
Gene expression analysis using real-time PCR
To quantify expression of GmRDR1 and GmSGS3, cDNA was synthesized from total RNA extracted from resistant (SL 1074 and SL 958) and susceptible (JS 335 and AMS 19-01) soybean genotypes at 15, 30, and 45 days post-inoculation (dpi), following the standard protocol described above. Quantitative reverse transcriptase PCR (qRT-PCR) was performed using the Bio-Rad CFX96 Touch Real-Time PCR Detection System, with GmRDR1 primers (GmRDR1 RT F/GmRDR1 RT R) and GmSGS3 primers (GmSGS3 RT F/GmSGS3 RT R), while for the housekeeping gene GmACT, primers used were GmACT F/GmACT R (Table S1). Each analysis was performed using three independent biological replicates, each with three technical replicates. Relative gene expression levels were calculated using the 2−ΔΔCT method (Livak & Schmittgen 2001), with the soybean actin gene as internal control. Statistical significance between treatment and control conditions was evaluated using a two-tailed Student's t-test, assuming equal variances with significance at P < 0.05, 0.01, and 0.001. The analysis was performed and graphs generated in Microsoft Excel. Data were visually inspected for consistency and approximate normality through residuals and distribution plots. Error bars represent standard errors of the mean (SEM) unless otherwise noted.
Development of hairpin RNAi constructs
siDirect v. 2.1 (http://sidirect2.rnai.jp/) was used to identify siRNA hotspot regions within GmRDR1 and GmSGS3 CDS, leading to design and validation of hairpin RNAi constructs, hpRDR1 and hpSGS3, targeting these genes. The hairpin RNAi constructs were developed by amplifying target regions through PCR using GmRDR1 hairpin primers (RDR1-GG F/RDR1-R) and GmSGS3 hairpin primers (SGS3-GG F/SGS3-GG R) (Table S1). The amplified products were then cloned into the pRNAi-GG vector (obtained from ABRC, Ohio State University) using the Golden Gate assembly method (Yan et al. 2012). Recombinant clones were confirmed through amplification with P21, P22, and gene-specific reverse primers (Table S1). The confirmed hairpin RNAi constructs were introduced into Agrobacterium tumefaciens strain EHA 105 using the freeze–thaw method, and transformed cultures used for plant inoculation. Agrobacterium cultures of the hairpin RNAi constructs were prepared in MES buffer (10 mM MES, pH 5.7, 10 mM MgCl₂, 150 μM acetosyringone) and adjusted to OD₆₀₀ 0.5.
Agroinoculation of hairpin constructs in resistant genotypes followed by challenge inoculation with agroinfectious clones of MYMIV
To assess effects of GmRDR1 and GmSGS3 knockdown on MYMIV resistance, seedlings of the resistant genotypes SL 1074 and SL 958 at the two-leaf stage were infiltrated with agrocultures containing hairpin constructs of these genes. For each genotype, 10 plants were inoculated with each hairpin construct. Five days following infiltration of the hairpin constructs, agroinfectious clones of DNA-A (accession no. OR050594) and DNA-B (accession no. OR050595) of MYMIV, previously developed in our laboratory, were introduced to the hairpin-primed plants. Differential expression of GmRDR1 and GmSGS3 transcripts was analyzed at 15, 30, and 45 dpi using qRT-PCR, as described above. Additionally, MYMIV accumulation in GmRDR1 and GmSGS3 knockdown plants was evaluated after isolating total DNA, followed by qPCR using BV1 F/BV1R primers (Table S1).
RESULTS
GmRDR1 shows no nucleotide sequence variation, while GmSGS3 has two synonymous SNPs that differentiate resistant and susceptible genotypes
Amplicons of ca. 3.4 kb and ca. 1.9 kb from cDNAs of GmRDR1 and GmSGS3, respectively, were successfully obtained from both resistant (SL 1074) and susceptible (JS 335) soybean genotypes (Fig. S1A) using primers GmRDR1 F/GmRDR1 R and GmSGS3 F/GmSGS3 R (Table S1). The recombinant plasmids containing GmRDR1 cDNA were further validated through digestion with SalI and SacI restriction enzymes, which released the expected ca. 3.4 kb insert from the vector backbone (2.7 kb) (Fig. S1B). Similarly, digestion of the recombinant plasmid containing GmSGS3 cDNA with BamHI and EcoRI yielded an anticipated insert of ca. 1.9 kb (Fig. S1C).
Sequencing of GmRDR1 and GmSGS3 coding sequence (CDS) revealed that GmRDR1 CDS is 3378 bp, while GmSGS3 is 1917 bp. The GmRDR1 CDS from SL 1074 and JS 335 were deposited in GenBank (accession nos PP663024, and PP601412, respectively). Similarly, GmSGS3 CDS from SL 1074 and JS 335 were deposited in GenBank (accession nos PP601410 and PP601411, respectively). Sequence analysis revealed no variation in the GmRDR1 CDS between SL 1074 and JS 335. However, in GmSGS3 CDS, two single nucleotide polymorphisms (SNPs) at CDS coordinates 441 and 543 were identified between resistant (SL 1074) and susceptible (JS 335) genotypes. At position 441, cytosine (C) was found in JS 335, whereas thymine (T) was present in SL 1074. Similarly, at position 543, JS 335 had thymine (T), while SL 1074 contained cytosine (C) (Fig. 1A). Notably, these SNPs do not alter amino acid sequence, indicating they are synonymous SNPs. To determine whether these SNPs were the result of sequencing errors, two additional clones were sequenced, and the same SNPs were obtained. Furthermore, to explore the association of these SNPs with the resistant trait, a fragment of GmSGS3 CDS containing both SNPs was amplified using the primers smSGS3 F and smSGS3 R (Table 1), and amplicons sequenced from four more resistant and two more susceptible genotypes. Interestingly, both SNPs (Fig. 1B) consistently and clearly distinguished GmSGS3 CDS between resistant and susceptible genotypes.
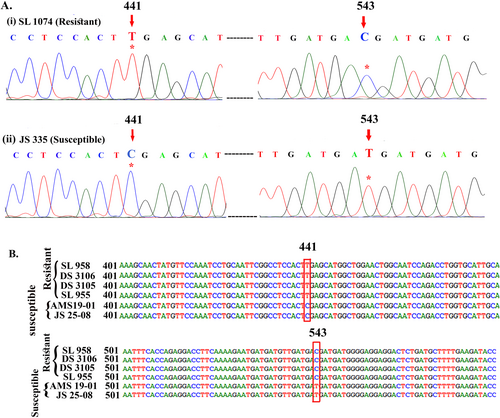
A CAPS marker involving SNP 441 of GmSGS3 distinguishes resistant and susceptible soybean genotypes
The SNP position 441 in the GmSGS3 coding sequence creates an XhoI restriction endonuclease site in susceptible soybean genotypes, making it a promising candidate for Cleaved Amplified Polymorphic Sequences (CAPS) markers to differentiate between resistant and susceptible genotypes (Fig. 2A). To verify this, initially, the complete GmSGS3 CDS from two resistant genotypes (SL 1074 and SL 958) and two susceptible genotypes (JS 335 and AMS 19-01) were amplified. The resulting PCR amplicons were then digested with XhoI. As anticipated, the amplicon from the susceptible genotypes yielded two fragments of 1474 bp and 439 bp, while the amplicon from the resistant genotypes remained intact, producing a single fragment of 1917 bp (Fig. 2B).
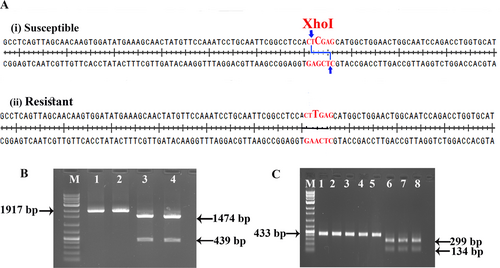
To further validate efficacy of the CAPS marker in distinguishing resistant and susceptible genotypes at DNA level, a fragment of the GmSGS3 gene was amplified using primers smSGS3 F and smSGS3 R (Table 1) from five resistant genotypes (SL 1074, SL 958, DS 3106, DS 3105, and SL 955) and three susceptible genotypes (JS 335, AMS 19-01, and JS 25-08). All genotypes produced an amplicon of 433 bp, which was then subjected to XhoI digestion. As expected, the three susceptible genotypes yielded two digestion products of 299 bp and 134 bp, while the five resistant genotypes retained an undigested 433 bp band. These results confirm efficacy of the SNP-based CAPS marker in differentiating between resistant and susceptible genotypes (Fig. 2C).
Amino acid sequence of GmRDR1 and GmSGS3 contains important catalytic motifs and domains with a close phylogenetic relationship within the Leguminosae
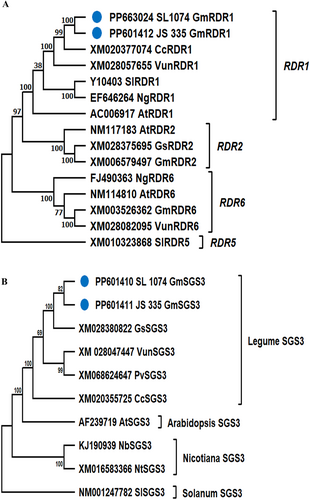
The GmRDR1 protein consists of 1125 amino acids with a molecular weight of 129,182.74 Da. The ProtParam tool revealed GmRDR1 protein had a theoretical isoelectric point (pI) of 8.14, indicating a slightly basic nature. It contains 139 negatively charged residues (Asp + Glu) and 144 positively charged residues (Arg + Lys), resulting in a net positive charge at physiological pH. Phylogenetic analysis using amino acid sequences of GmRDR1 from resistant genotype SL 1074 and susceptible genotype JS 335 placed them in the RDR1 clade, clearly distinct from other RDR clades, such as RDR2 and RDR6 (Fig. 3A). GmRDR1 shared high amino acid sequence identity with Cajanus cajan (CcRDR1, 85%) and Vigna unguiculata (VunRDR1, 80%), reflecting the close evolutionary relationships among legume crops (Table S2). In contrast, GmRDR1 had lower amino acid sequence identity with other RDR1 proteins reported from Arabidopsis thaliana (AtRDR1, 63%), Nicotiana glutinosa (NgRDR1, 62%), and Solanum lycopersicum (SlRDR1, 62%), showing moderate divergence in non-legume species (Table S2). As expected, GmRDR1 had significantly lower amino acid sequence identity with RDR2 (38.2%, 38.1%, and 36% with G. soja, A. thaliana, and G. max, respectively) and RDR6 (32%, 32%, 31%, and 30.5% with G. max, V. unguiculata, N. glutinosa, and A. thaliana, respectively) proteins (Table S2).
The InterPro-Pfam and Motif Search server predicted domain architecture of GmRDR1, which consisted of an RNA recognition motif 3–60 amino acids (aa) essential for interacting with single-stranded RNA, contributing to RNA processing, RNA-dependent RNA polymerase domain (375–945 aa), which is essential for RNA replication, and RNA binding domain (565–596 aa), likely having a role in regulating RNA transcription and translation (Table 2). Amino acid sequence-based multiple alignment of different RDR sequences revealed the presence of a DLDGD catalytic motif, a hallmark of RDRα clade proteins and essential for RNA-dependent RNA polymerase (RdRP) activity, is conserved across all RDR1, RDR2, and RDR6 aa sequences (Fig. S2). However, there was a notable variation in RDR5 from Solanum lycopersicum, where the motif was DFDGD, reflecting phylogenetic divergence. Besides this conserved stretch of aa, all RDR1 sequences had six other conserved aa sequence stretches (NVSNRV, NRQLITLLSTLGV, FRASKLL, MMGCLDE, NYIVN, IANAH) within the RNA-dependent RNA polymerase domain (Fig. S2). Additionally, two other conserved aa sequence stretches (KLGNLM, YGIKTEAEIL) were present at the C-terminus of all RDR1 proteins (Fig. S2).
The GmSGS3, with 638 amino acids and MW 73,529.77 Da, has a pI of 6.00, indicating slight acidity. It contains 109 negatively charged (Asp + Glu) and 96 positively charged (Arg + Lys) residues, giving it a net negative charge at physiological pH, favoring interactions with positively charged molecules under neutral to mildly acidic conditions. Phylogenetic analysis of GmSGS3 aa sequences revealed GmSGS3 clustered within the legume SGS3 clade, indicating minimal genetic divergence among legumes (Fig. 3B). GmSGS3 shared 100% identity with GsSGS3 (Glycine soja), highlighting the close evolutionary relationship within Glycine species (Table S3). Besides Glycine species, GmSGS3 shared high amino acid sequence identity with other legume species, displaying 88.7% identity with VunSGS3 (V. unguiculata) and 87.8% identity with PvSGS3 (Phaseolus vulgaris) (Table S3). In contrast, GmSGS3 had less amino acid sequence identity with SGS3 homologs from non-legume species such as N. tabacum (55%), N. benthamiana (53.7%), A. thaliana (54.1%), and S. lycopersicum (52.2%) (Table S3).
Like GmRDR1, InterPro-Pfam, and Motif Search server predicted GmSGS3 had XS domain (316–430 aa) and the zinc finger XS (zf-XS) domain (245–283 aa), both of which are crucial for RNA binding and stabilization in gene silencing processes (Table 2).
| protein | motifs/domains | position (aa) | function | InterPro ID/Pfam ID |
|---|---|---|---|---|
| GmRDR1 | RNA recognition motif (RRM) | 3–60 | RNA binding; interacts with single-stranded RNA, involved in RNA processing | cd00590 |
| GmRDR1 | RNA-dependent RNA polymerase domain | 375–945 | RNA replication processes | PF05183 |
| GmRDR1 | RNA binding domain (RBD) | 565–596 | RNA binding, likely involved in RNA transcription and translation regulation | IPR035979 |
| GmSGS3 | zf-XS | 245–283 | Gene silencing and RNA processing, forming and stabilizing double-stranded RNA for RNAi pathways | IPR005380, PF03468 |
| GmSGS3 | XS | 316–430 | RNA binding and possibly protein–protein interactions, suggesting a role in RNA metabolism or processing | IPR005381, PF03470 |
GmRDR1 protein interacts with GmSGS3 protein
The predicted 3-D structure of GmRDR1 and GmSGS3 proteins was developed through homology modeling using the SWISS-MODEL server (Fig. S3Ai,Bi). The structural integrity of these models was evaluated using SAVES server by analyzing ERRAT scores and Ramachandran plot, confirming quality and accuracy. Ramachandran plot showed that 94.8% and 81.3% of amino acid residues of GmRDR1, and GmSGS3, respectively, were in the favoured regions, indicating good structural integrity (Fig. S3Aii,Bii). Additionally, ERRAT scores provided further validation, with GmRDR1 and GmSGS3 achieving 94.38% and 98.54%, respectively, affirming high reliability of both models (Fig S3Aiii,Biii). The ProSA-web Z-scores aligned with expectations for well-constructed protein structures, with scores of −14.31 and −6.41 for GmRDR1 and GmSGS3, respectively, further confirming their structural quality and suitability for subsequent analyses (Fig. S3Aiv,Biv).
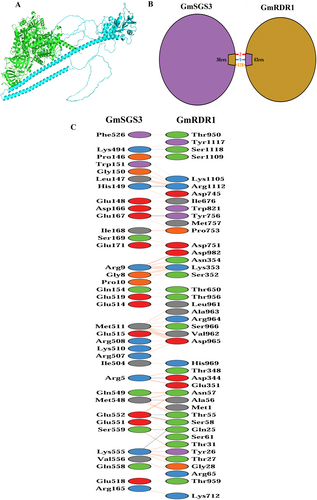
Molecular docking analysis using ClusPro and Gramm servers predicted stable protein–protein interactions between GmRDR1 and GmSGS3 proteins (Fig. 4A). ClusPro server-generated top-ranked docking complex from Cluster 0 yielded a center-weighted score of −1309.2 and lowest energy score of −1493.2 Kcal mol−1 (Table S4), indicating strong binding affinity and stable interaction. The interacting residues between GmSGS3 and GmRDR1 proteins were analyzed using PDBsum. This analysis revealed that they interact through a substantial interface area, with GmSGS3 covering 2527 Å2 and GmRDR1 covering 2509 Å2. Within this interface, 36 residues from GmSGS3 and 43 residues from GmRDR1 were involved (Fig. 4C). The interaction includes formation of two salt bridges and nine hydrogen bonds, together with 428 non-bonded contacts (Fig. 4B), highlighting key interactions that likely contribute to the stability and specificity of the GmSGS3–GmRDR1 complex.
The yeast two-hybrid (Y2H) assay was used to validate the predicted docking result between the GmSGS3 and GmRDR1 proteins (Fig. 5). The pGADT7-RDR1 (prey) and pGBKT7-SGS3 (bait) constructs were successfully transformed into yeast strains Y187 and Y2HGold, respectively, as confirmed by growth of transformants on SD selective media lacking leucine (SD/-Leu) for pGADT7 and lacking tryptophan (SD/-Trp) for pGBKT7 constructs. There was no growth for negative controls containing empty vectors, demonstrating specificity of transformation and vector insertion. Following mating, diploid yeast cells containing both bait and prey constructs were selected on SD/-Leu/-Trp media, confirming successful mating. Diploids were subsequently plated on SD/-Leu/-Trp/-His medium to assess the potential interaction between GmSGS3 and GmRDR1 proteins. Colony growth on the SD/-Leu/-Trp/-His medium indicated a positive interaction between GmSGS3 and GmRDR1, as evidenced by colony formation, compared to negative controls. To confirm and further evaluate the strength of the interaction, diploid colonies were plated on SD/-Leu/-Trp/-His/-Ade medium. Colony growth on the quadruple dropout medium lacking leucine, tryptophan, histidine, and adenine provided further evidence of the specific interaction between GmRDR1 and GmSGS3 proteins. The negative controls had no colony growth under these stringent selection conditions, while significant colony growth was observed in experimental setups containing both bait and prey constructs. For additional confirmation, diploid yeast cells were plated on SD/-Leu/-Trp/-His/-Ade medium supplemented with X-α-gal and Aureobasidin A. Yeast diploid colonies harboring both GmRDR1 and GmSGS3 constructs turned blue in the presence of X-α-gal, demonstrating β-galactosidase activity indicative of protein–protein interaction. In contrast, no blue color development was observed in the negative controls. Moreover, colony growth in the presence of Aureobasidin A further supported the specificity and robustness of the GmRDR1-GmSGS3 interaction.

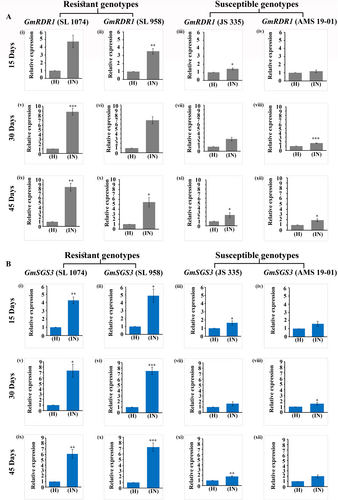
GmRDR1 and GmSGS3 expression upregulated in resistant soybean genotypes after MYMIV inoculation
The transcript dynamics of GmRDR1 and GmSGS3 were analyzed using qRT-PCR in two resistant soybean genotypes, SL 1074 and SL 958, and two susceptible soybean genotypes, JS 335 and AMS 19-01, at 15, 30, and 45 days post-inoculation (dpi) with MYMIV. Corresponding non-inoculated (healthy) plants for these time points were used as controls. For GmRDR1, SL 1074 markedly increased expression at 15 dpi, with a 4.76-fold change relative to its healthy control (Fig. 6Ai). SL 958 also exhibited a substantial response, with a 3.55-fold change at 15 dpi (Fig. 6Aii), while JS 335 (Fig. 6Aiii) and AMS 19-01 (Fig. 6Aiv) showed lower fold changes of 1.43 and 1.19, respectively, indicating weaker upregulation of GmRDR1. At 30 dpi, GmRDR1 expression was 8.83- and 6.80-fold for SL 1074 (Fig. 6Av) and SL 958 (Fig. 6Avi), respectively. Conversely, JS 335 (Fig. 6Avii) and AMS 19-01 (Fig. 6Aviii) showed only modest increases with fold changes of 2.66 and 1.70, respectively, after 30 dpi. Results at 45 dpi were also consistent for both groups. At 45 dpi, relative fold changes of GmRDR1 expression were 8.40 and 5.44 for SL 1074 (Fig. 6Aix) and SL 958 (Fig. 6Ax), respectively, whereas for JS 335 (Fig. 6Axi) and AMS 19-01 (Fig. 6Axii) values were 2.35 and 1.89, respectively.
For GmSGS3, a similar trend was observed, with resistant genotypes displaying notably higher expression post-inoculation of MYMIV. At 15 dpi, SL 1074 (Fig. 6Bi) and SL 958 (Fig. 6Bii) exhibited fold changes of 4.25 and 4.907, respectively, relative to their healthy controls. In contrast, JS 335 (Fig. 6Biii) and AMS 19-01 (Fig. 6Biv) had minimal increases, with fold changes of 1.66 and 1.584, respectively. At 30 dpi, SL 1074 (Fig. 6Bv) and SL 958 (Fig. 6Bvi) had further elevated GmSGS3 expression levels, reaching fold changes of 7.36 and 7.49, respectively, while JS 335 (Fig. 6Bvii) and AMS 19-01 (Fig. 6Bviii) remained low at 1.606 and 1.53, respectively. By 45 dpi, high expression levels were sustained in SL 1074 (Fig. 6Bix) and SL 958 (Fig. 6Bx), with fold changes of 6.08 and 7.21, respectively, while JS 335 (Fig. 6Bxi) and AMS 19-01 (Fig. 6Bxii) showed minimal increases of 1.77 and 1.97, respectively.
Transient knockdown of GmRDR1 and GmSGS3 transcripts in resistant cultivars of soybean result in increased MYMIV accumulation
Bioinformatic analysis using siDirect software was employed to identify siRNA hotspot regions within the GmSGS3 and GmRDR1 of resistant varieties SL 958 and SL 1074. A sequence of 632 bp in GmSGS3 and 601 bp in GmRDR1 exhibited a high density of potential siRNA targets with minimal off-target effects, making them optimal candidates for subsequent siRNA-mediated gene silencing studies. Targeted 632 bp of GmSGS3 and 601 bp of GmRDR1 were amplified using primers RDR1-GG F/RDR1-GG R and SGS3-GG F/SGS3-GG R, respectively (Fig. S4A). These amplicons were cloned in the pRNAi-GG vector to generate hairpin RNAi constructs hpSGS3 and hpRDR1. The recombinant colonies were confirmed through colony PCR using primers P21 and gene-specific reverse primers (Table S1). Colony PCR yielded expected amplicons of 899 bp for hpSGS3 and 868 bp for hpRDR1 (excess size due to addition of vector backbone sequence using p21 primer) (Fig. S4B). Amplification of the recombinant plasmids using P21, P22, and gene-specific reverse primers showed 899 and 632 bp amplicons for hpSGS3 and 868 bp and 601 bp amplicons for hpRDR1, respectively, validating cloning of the amplicons in sense and antisense orientations (Fig. S4C).
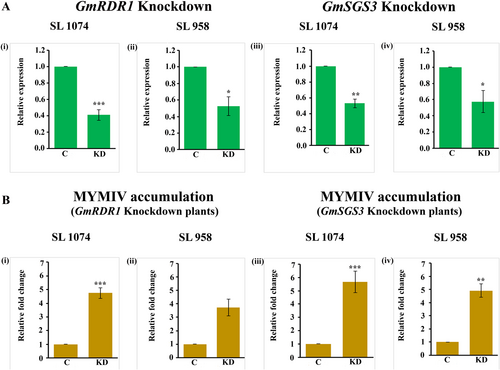
The hpRDR1 and hpSGS3 constructs were mobilized to Agrobacterium tumefaciens strain EHA 105, and the Agrobacterium cultures containing both constructs were infiltrated into seedlings of the resistant cultivars SL 1074 and SL 958; followed by agroinfiltration of a partial tandem repeat construct of MYMIV. The effectiveness of gene silencing and virus accumulation was assessed by real-time PCR. In SL 1074, hpRDR1 led to a 2.44-fold reduction in GmRDR1 gene expression compared to the control (Fig. 7Ai). This downregulation corresponded with a 4.74-fold increase in MYMIV accumulation (Fig. 7Bi). Similarly, in genotype, SL 958, GmRDR1 expression decreased 1.91-fold (Fig. 7Aii), which was associated with a 3.72-fold increase in MYMIV viral load (Fig. 7Bii). For GmSGS3, silencing in SL 1074 resulted in a 1.89-fold decrease in its expression (Fig. 7Aiii), leading to a 5.67-fold increase in MYMIV accumulation (Fig. 7Biii). In SL 958, GmSGS3 expression was reduced by 1.75-fold (Fig. 7Aiv) due to hpSGS3 treatment, resulting in a 4.91-fold increase in viral load (Fig. 7Biv).
DISCUSSION
This study aimed to elucidate the roles of GmRDR1 and GmSGS3, two genes associated with the RNA-silencing pathway, in determining resistance to MYMIV in soybean. The silencing mechanism plays a crucial role in plant defense against viral infections, with RDRs and SGS3 serving as essential factors that initiate biogenesis of viral-derived small interfering RNAs (vsiRNAs) (Lopez-Gomollon et al. 2022). Plants possess six distinct RNA-dependent RNA polymerases (RDRs); among these, RDR2 and RDR6 have been implicated in endogenous transcript silencing, whereas RDR1 has a distinct role primarily associated with antiviral defense (Dalmay et al. 2000; Mourrain et al. 2000; Xie et al. 2004; Cao et al. 2014). SGS3 is another central protein in RNA silencing, required for post-transcriptional gene silencing and trans-acting small interfering RNA (siRNA) production (Mourrain et al. 2000; Peragine et al. 2004). SGS3 is believed to bind single-stranded RNA, protecting it before its conversion into double-stranded RNA by an RDR (Yoshikawa et al. 2005).
In the present study, analysis of the GmRDR1 coding sequence revealed no nucleotide sequence variation between resistant and susceptible soybean genotypes. However, two SNPs were identified in the GmSGS3 coding sequence during comparison of these genotypes. Although these SNPs do not result in any changes to the protein sequence, their consistent presence across all tested resistant and susceptible genotypes facilitated development of an SNP-based CAPS marker that effectively distinguishes between resistant and susceptible soybean genotypes. The development of gene-specific molecular markers has significantly transformed plant breeding, particularly in identification of sources resistant to viral pathogens. CAPS markers are invaluable in differentiating between resistant and susceptible genotypes in crops, thereby enabling timely selections in breeding programs (Sorri et al. 1999; Kasai et al. 2000; Ramkumar et al. 2010, 2011). Previous research highlighted the effectiveness of CAPS markers, including ADG2/BbvI, which can serve as diagnostic tools for selecting potato virus Y (PVY) resistant genotypes (Ottoman et al. 2009). This underscores the importance of marker-assisted selection (MAS) in addressing plant viral diseases (Chen et al. 2013). The GmSGS3-derived CAPS marker developed in this study is essential for identifying resistant sources and selecting breeding lines of soybean capable of withstanding MYMIV pressure, ultimately contributing to sustainable soybean breeding programs aimed at enhancing disease resilience.
Amino acid-based phylogenetic analysis indicated that GmRDR1 and GmSGS3 were highly conserved within the Leguminosae. Such family-specific conservation of RDR1 proteins has been reported in plants in the Cucurbitaceae and Solanaceae (Liu et al. 2009; Leibman et al. 2022). As in earlier studies with RDR1, a conserved motif DLDGD was found in GmRDR1. This DLDGD motif, shared among RDR1, RDR2, and RDR6, aligns with its function in RNA synthesis, where it likely contributes to nucleotidyl transferase activity by coordinating a divalent cation (Iyer et al. 2003; Wassenegger & Krczal 2006). Notably, DFDGD replaces DLDGD in RDR3, RDR4, and RDR5, a variation that distinguishes these RDRs and may relate to their unique functions (Wassenegger & Krczal 2006). Besides the DLDGD motif, GmRDR1 also had 10 other short amino acid stretches reported to be conserved within all the plant RDR1 (Wassenegger & Krczal 2006; Liu et al. 2009). The presence of these conserved regions, including the DLDGD signature in GmRDR1, underscores its functional similarity to other plant RDR1s, and likely positions GmRDR1 as a key player in soybean antiviral defense mechanisms.
Within GmSGS3, we identified the conserved XS domain, characteristic of SGS3 proteins, which has a unique RNA-binding function through an RNA recognition motif (RRM) (Zhang & Trudeau 2008). This XS domain, along with an adjacent putative nucleic acid-binding zinc finger domain at the N-terminus, supports the involvement of GmSGS3 in stabilizing RNA intermediates and safeguarding them from degradation prior to RNA silencing. Additionally, in some SGS3 proteins, a C-terminal XH domain accompanies the XS domain, further reinforcing its RNA-binding capabilities and contributing to formation of stable complexes for effective gene silencing (Zhang & Trudeau 2008).
In Arabidopsis, SGS3 interacts with RDR6, forming a complex involved in RNA silencing by stabilizing miRNA cleavage products and facilitating secondary siRNA production (Kumakura et al. 2009). The coordinated role of SGS3 and RDR6 has also been documented in N. benthamiana for triggering defense against begomovirus (Li et al. 2017). Additionally, studies indicate that RDR1 and SGS3 regulate cuticular wax biosynthesis, highlighting the underlying interaction, which could significantly improve breeding strategies to enhance MYMIV resistance in soybean (Lam et al. 2012). However, there were no reports indicating that RDR1 also interacts with SGS3 to govern antiviral defense. In the present study, we predicted interaction behavior between GmRDR1 and GmSGS3 through protein–protein docking, and further confirmed their interaction using a yeast two-hybrid (Y2H) assay. This study thus underscores that GmSGS3 may enhance antiviral defense in soybean by interacting with GmRDR1, promoting siRNA amplification and aiding in degradation of viral RNAs to govern MYMIV resistance in soybean.
Upon virus inoculation, resistant soybean genotypes showed significantly higher expression of GmRDR1 and GmSGS3 compared to susceptible genotypes. These results align with earlier reports where upregulation of RDR1 orthologs upon virus inoculation was reported from Arabidopsis, Nicotiana spp., Medicago sp., maize, and rice (Satoh et al. 2003; Yang et al. 2004; Alamillo et al. 2006; He et al. 2010). The similar expression patterns and antiviral role of RDR1 in various crops thus emphasize the conserved function of RDR1 in enhancing resistance across plant species. Likewise, the higher expression of SGS3 in soybean appears consistent with its antiviral functions, as documented in Nicotiana and other species, where it assists in producing double-stranded RNA (dsRNA) and trans-acting siRNAs, essential for post-transcriptional gene silencing (PTGS) during viral infection (Li et al. 2017).
To further confirm whether increased expression of GmRDR1 and GmSGS3 under post-virus inoculation conditions in resistant plants governs resistance, we used RNAi to knockdown these genes in resistant genotypes. This knockdown led to a notable increase in MYMIV accumulation, indicating that both genes are crucial for effective viral silencing. Previous research has similarly shown increased virus accumulation when RDR1 was silenced in Arabidopsis, tomato, and tobacco (Yu et al. 2003; Alamillo et al. 2006; Rakhshandehroo et al. 2009; Liao et al. 2015). RDR1 knockdown enhances viral replication by impeding dsRNA amplification, which is essential for PTGS. In Nicotiana sp., loss of functional SGS3 increased geminivirus infection, leading to a higher viral load, further supporting the role of SGS3 in PTGS (Mourrain et al. 2000; Li et al. 2017). Collectively, these findings confirm that GmRDR1 and GmSGS3 genes are integral to soybean defense against MYMIV, restricting viral replication and spread, thus underscoring their importance for enhancing resistance in breeding programs. These findings underscore the importance of enhanced expression and interaction of GmRDR1 and GmSGS3 in the defense against MYMIV. Future investigations are warranted to elucidate the molecular mechanisms underlying this interaction, which could significantly improve breeding strategies to enhance MYMIV resistance in soybean.
AUTHOR CONTRIBUTIONS
Conceptualization and design, AR; methodology, DDC, MS and FM; validation, DDC, AM and YMB; interpretation of data, DDC, MS, FM, BM, SKL, BM and AR; original draft preparation, DDC and AR; revised draft manuscript, AR, BM and RK.
ACKNOWLEDGEMENTS
First author is grateful to the Indian Council of Agricultural Research (ICAR), New Delhi, India, for a Senior Research Fellowship. Authors would like to acknowledge the Director and Joint Director (R) of ICAR-Indian Agricultural Research Institute, New Delhi-110012, for their constant support and guidance.
FUNDING INFORMATION
This research was funded by IARI In-house Project code: CRSCIARISIL20210030315; ICAR-Consortium Research Platform Project on Vaccines and Diagnostics.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article and its supplementary information files.




