Integration of genetic and seed fitness data to the conservation of isolated subpopulations of the Mediterranean plant Malcolmia littorea
Abstract
- Plant autoecology and population genetics provide a perspective on the likelihood of natural regeneration, which is critical when designing conservation strategies for endangered species. The threatened coastal plant Malcolmia littorea (Brassicaceae) was sampled across its European distribution and studied for genetic diversity and seed fitness, with the aim of providing information for the conservation of isolated and declining populations.
- Nine microsatellite markers (five chloroplast and four nuclear) were analysed to assess population genetic diversity and structure and to conduct a spatial analysis using the software DIVA-GIS. Germination percentages and rates were assessed by incubating the seeds under eight constant temperatures (0–27 °C).
- The genetic diversity was found to be similar among subpopulations (chloroplast H = 0.04–0.17; nuclear Ho = 0.20–0.37), with no correlation between subpopulation diversity and the area of occupancy (AOO). The subpopulations were found to be clustered in three genetic groups, and three of them were identified as conservation priorities due to their unique genetic composition. The germination study revealed a significant influence of the maternal environment and AOO on seed germination, with the smaller subpopulations showing lower germination percentages (P < 0.05).
- These results highlight the importance of obtaining information on isolated subpopulations through different experimental approaches (e.g. seed germination plus population genetics) to enable planning of effective conservation actions. For M. littorea, seed collection for both in situ and ex situ conservation should take into account the local adaptation of the subpopulation and the genetic structure of the species.
Introduction
Mediterranean sandy coastal habitats are considered to be among the most endangered environments in Europe (Feola et al. 2011) from stressors that have localised effects (e.g. trampling; Fenu et al. 2013a) of global significance (e.g. sea level rise due to global climate change; Defeo et al. 2009). In the last two centuries, these habitats have been strongly altered by direct human activity (Defeo et al. 2009; Fenu et al. 2013b), becoming more severely fragmented or destroyed primarily as a result of urbanisation and industrialisation (Acosta et al. 2000). Coast-bound tourism, in particular, which became a mass phenomenon from the 1950s onwards, is now considered the principal cause of degradation of coastal dunes (Acosta et al. 2000). As a result, many plant species have become increasingly fragmented and affected in their structure (Thompson 1999), forced into small and isolated populations (Oostermeijer et al. 1994) with the associated risk of local extinction (Carranza et al. 2010).
Genetic diversity is usually low in isolated populations as a consequence of genetic drift, inbreeding, bottlenecks and the founder effect (Lammi et al. 1999). Isolated populations nevertheless can have a unique genetic composition or other unique features compared to those in the central part of their range (Petit et al. 1998; Papuga et al. 2015). Understanding the extent and distribution of genetic diversity of fragmented wild populations of threatened species is critical to the design of strategies for their effective and appropriate conservation (Frankham 2010). Investigating the genetic diversity within the target species using molecular markers can clarify the impact of evolutionary forces such as drift and migration, and provide, with adequate experimental design, basic information on the biology of the species (Muller et al. 2009). In the last 20 years, microsatellite markers (or SSR – single sequence repeat) have been used effectively and efficiently in population genetics studies (Clauss et al. 2002; Edh et al. 2007; Muller et al. 2009) due to their reproducibility and polymorphism (Provan et al. 2001). The combination of chloroplast and nuclear microsatellite markers provides a more comprehensive method to study population structure and to generate information useful for the implementation of a conservation strategy, including the restoration of populations based on recent and ancient evolutionary processes (Petit et al. 2005). While nuclear microsatellite loci are usually highly polymorphic, codominant and inherited in a Mendelian mode, chloroplast microsatellites are uniparentally inherited and linked because of the non-recombinant nature of the chloroplast chromosome (Navascués & Emerson 2005). Furthermore, chloroplast microsatellites show levels of polymorphism quite variable across loci and across species, and some loci have been found to be monomorphic in all the studied species (Navascués & Emerson 2005).
To estimate the future conservation status of an endangered species and plan effective conservation activities, it is also critical to measure population fitness, especially for the smallest and isolated populations that may be more susceptible to inbreeding depression, genetic drift and the accumulation of deleterious mutations than larger populations (Barrett & Kohn 1991; Oostermeijer et al. 1994; Fischer & Matthies 1998). Fitness components that are subjected to selection, such as seed germination, may reduce with decreasing population size (Menges 1991; Heschel & Paige 1995; Fischer & Matthies 1998), while being crucial for the persistence of small and isolated plant populations (Lammi et al. 1999). Monitoring seed fitness in small and isolated populations may be key to understand their regeneration potential. Furthermore, understanding if seed fitness is maintained after a period of long-term conservation, measured by the ability to germination efficiently, is important for predicting the likelihood of successful laboratory propagation for reintroduction purposes. In most plant species, seeds vary in their degree of germinability among and within populations (Ouborg & Van Treuren 1995; Gutterman 2000; Jacquemyn et al. 2001) and these variations can be correlated to a fitness disparity, exposed early in the life of the sporophyte plant (i.e. seed germination; Menges 1991) and to maternal environmental conditions (e.g. day length, light quality, water stress, temperature or mineral nutrition; Gutterman 2000). Moreover, by comparing the germination requirements among populations of varying sizes and different locations across the distribution range, it is possible to elucidate the relationships between population size and plant fitness (Menges 1991; Heschel & Paige 1995; Jacquemyn et al. 2001). Such relationships can be useful in predicting how a species can adapt to a changing environment, and how its germination response differs in the range of environments occurring across its distribution area (Wagmann et al. 2012). Having available data on both genetic variation and seed fitness, such as germination performance, it is then possible to test their inter-relation (Oostermeijer et al. 1994; Lammi et al. 1999; Luijten et al. 2000; Hensen & Wesche 2006).
This study aimed to obtain data on both population genetics and germination performance of subpopulations of the Mediterranean threatened species Malcolmia littorea (L.) R. Br. across its European distribution, especially focussing on sub-regions where isolated subpopulations occur, so as to provide recommendations for their practical conservation and management. No previous studies on this species have considered its genetics and seed fitness at a wide geographic scale. In contrast, two studies have investigated M. littorea seed ecology at local scale. In Italy, De Vitis et al. (2014), detected the optimal conditions (light, temperature, soil burial depth) for seed germination, while in Spain, Novoa et al. (2012) investigated the effect of moisture, pH and salinity on seed germination.
We hypothesised that M. littorea populations occurring in south central Spain (Valencia), France and Italy may represent distinct genetic groups due to their geographic isolation from each other and from other populations; that seed germination performance could vary with population size and this might relate to the genetic diversity. Specifically, the present study had the objectives of: (i) quantifying the genetic variation within a set of European subpopulations and inferring the pattern of differentiation among them; (ii) evaluating and comparing their seed fitness by means of germination response to temperature and testing its correlation with neutral genetic diversity; (iii) assessing the effect of subpopulation area of occupancy on both genetic diversity and germination performance; and (iv) identifying priority actions for the conservation of M. littorea in Europe.
Material and Methods
Study species
Malcolmia littorea (L.) R. Br. is a suffruticous chamaephyte belonging to the Brassicaceae, typical of the coastal dune habitats of the southwest Mediterranean (Greuter et al. 1986). In Europe, it is characterised by a disjointed distribution and is considered under extinction risk in several regions due to the human-derived threats such as habitat modification and fragmentation, urbanisation, tourism and trampling; for example, in the Spanish autonomous communities of Asturias (Fernández Prieto et al. 2014) and Valencia (Serra Laliga 2007), and Italy (Del Vecchio et al. 2012a). Its conservation status has only been evaluated at a local level rather than at a global or European level. In the Spanish province of Alicante, it is considered as Vulnerable (Serra Laliga 2007); in the Spanish community of Asturias, it is considered under extinction risk (Fernández Prieto et al. 2014); in Italy, the species is considered to be Critically Endangered (CR) according to the IUCN category and criteria (Rossi et al. 2013). Furthermore, along the French coasts, a 30% decline in the species distribution in the last 30 years has been noticed, although it has not yet been included in the French Red List (source: http://flore.silene.eu).
In this species, the length of siliquas ranges from 13 to 79 mm with an average value of 47 ± 9 mm; each fruit contains a number of seeds comprised between seven and 117 with an average of 60.42 ± 15.70 (data obtained across 14 European subpopulations; De Vitis, data not published), and the dispersal is assumed to be barochory, as seeds have no dispersal structures (De Vitis, personal observation).
Population sampling
This study focused on the European portion of M. littorea (L.) R. Br. global population (sensu IUCN 2012). Fresh leaves and ripe siliquas were collected from a total of 15 coastal sites, during July–August 2013 (Fig. 1), all occurring in the Mediterranean bioclimate (Rivas-Martínez et al. 2011). The following subpopulations were considered: two in Portugal (one northern and one southern), five in the Spanish region of Valencia, five in France and three in Italy (Table 1). In particular, the Spanish, French and Italian subpopulations represent three isolated groups, while the Portuguese occurred in the core of the species European distribution (Fig. 1).
| country | code | TGB code | locality (province) | AOO (ha) | altitude (m a.s.l.) | genetic analysis | germination analysis |
|---|---|---|---|---|---|---|---|
| Portugal | PT-1 | A27013 | Vila do Conde (Oporto) | ~7.50 | 3 | ✓ | ✓ |
| Portugal | PT-2 | A27113 | Praia dos Salgados (Albufeira) | ~62.06 | 3 | ✓ | ✓ |
| Spain | ES-1 | A26513 | Playa de Moncófar (Castellón) | ~0.08 | 1 | ✓ | ✓ |
| Spain | ES-2 | A26613 | Puerto de Sagunto (Valencia) | ~2.54 | 0 | ✗ | ✓ |
| Spain | ES-3 | A26713 | Playa de Tavernes de Valldigna (Valencia) | ~15.49 | 1 | ✗ | ✓ |
| Spain | ES-4 | A26813 | Albufera Natural Park (Valencia) | ~171.12 | 2 | ✓ | ✓ |
| Spain | ES-5 | A26913 | Oliva (Valencia) | ~9.51 | 4 | ✓ | ✓ |
| France | FR-1 | A26013 | Fos-sur-Mer | ~1.09 | 2 | ✓ | ✓ |
| France | FR-2 | A26113 | Beauduc (Camargue Regional Nature Park, Arles) | ~180.00 | 0 | ✗ | ✓ |
| France | FR-3 | A26213 | Portiragnes | ~8.57 | 4 | ✓ | ✓ |
| France | FR-4 | A26313 | Plage des Aresquiers (Frontignan) | ~2.07 | 0 | ✓ | ✓ |
| France | FR-5 | A26413 | Plage du Grand Travers (La Grande-Motte) | ~13.34 | 2 | ✓ | ✓ |
| Italy | IT-1 | A25813 | Torre Olevola (Latina) | ~0.02 | 3 | ✓ | ✓ |
| Italy | IT-2 | Terracina (Latina) | ~0.30 | 5 | ✓ | ✗ | |
| Italy | IT-3 | A25913 | Sabaudia (Latina) | ~0.01 | 13 | ✓ | ✓ |
For the genetic analysis, 12 subpopulations, representative of the sampling sub-regions, were selected. Leaves were collected from 20 to 25 adult plants per subpopulation and were individually stored in bags of breathable fabric, which in turn were placed in plastic bags containing silica gel for desiccation. In the laboratory, DNA was extracted from 50 to 100 mg of leaf tissue, using the DNeasy™ Plant Mini Kit (Qiagen, Valencia, CA, USA) and following the manufacturer's protocol. The DNA was used to first conduct a marker screening and then a population genetic analysis (see below Primer selection, PCR conditions and microsatellite analysis).
For the germination experiments, seeds were collected from 14 out of 15 subpopulations, leaving out IT-2 due to poor fruiting in 2013. Ripe siliquas were harvested from ten to 50 adult plants per subpopulation, according to their area of occupancy (AOO), and stored in paper bags until they were cleaned in the laboratory. Seeds were stored for 1 month in a dry room at 15 °C and 15% RH (relative humidity) and then transferred to −20 °C. The experiments started after 1 year and 2 months of cold, dry storage which is known to maintain seed quality (Genebank Standards 1994; Linington 2003).
For each subpopulation, AOO was assessed by polygon calculation based on GPS coordinates (see Table 1), applying one of the IUCN methods to estimate species AOO (IUCN 2016). For each sampling locality, average temperature (minimum, mean and maximum) and precipitation values over the period 1950–2000 were extrapolated from WorldClim climate grids (Hijmans et al. 2005) using the software DIVA-GIS 7.5 (Hijmans et al. 2012), while temperature and relative humidity mean values of the year 2013 for the meteorological stations closest to each site were obtained from the National Climatic Data Center (NCDC) of the National Oceanic and Atmospheric Administration (NOAA, Washington, DC, USA).
Primer selection, PCR conditions and microsatellite analysis
A total of 46 primers were screened on M. littorea DNA, and five chloroplast and four nuclear microsatellite primers used (Table S1). These are already described in the literature (Provan 2000; Clauss et al. 2002; Ceplitis et al. 2005) and produced strong and reproducible amplification. All forward primers were given a tail with the M13 sequence (5′-CACGACGTTGTAAAACGAC-3′) (Schuelke 2000) and used in combination with reverse primer and a third M13-primer dyed with FAM, NED or VIC (Thermo Fisher Scientific, Waltham, MA, USA). For all microsatellite primers, PCR amplification was carried out in 10 μl reaction mix, with 3 μl DNA, 1 μl Buffer 10×, 0.3 μl MgCl2, 2 μl dNTP, 1 μl F primer, 0.5 μl R primer and M13-primer, and 0.04 μl polymerase DNA Taq, in sterile water. Conditions of the PCR amplification were as follows: 94 °C (5 min), then 30 cycles at 94 °C (30 s)/56 °C (45 s)/72 °C (45 s), followed by eight cycles at 94 °C (30 s)/53 °C (45 s)/72 °C (45 s), and a final extension at 72 °C for 10 min (Schuelke 2000). Amplification products (1 μl) were added to 9.8 μl formamide and 0.2 μl Genescan-500 LIZ. The samples were run on ABI PRISM 3130 DNA sequencer. The resulting row data were collected applying GeneMapper v. 5.0 (Applied Biosystems, Foster City, CA, USA), which was also used to size and score the microsatellite profiles.
Seed traits and germination tests
Following cold storage at −20 °C, seeds were warmed in a dry room (15 °C, 15% RH) for 24 h. Seed mass was calculated for each subpopulation by weighing five replicates of 50 seeds each (fresh weight: FW). Seed moisture content (MC), expressed on a fresh weight basis, was calculated by drying the same replicates at 103 °C for 17 h and reweighing to obtain the dry weight (DW; ISTA 2015).
Germination tests followed the methods of De Vitis et al. (2014). Seeds of each population were incubated in the dark at eight constant temperatures (0, 5, 10, 15, 20, 23, 25, and 27 °C) and scored every day under green safelight (light density of 14.0 W·m−2). For each subpopulation, five replicates of 20 seeds were sown on the surface of 1% agar-water, which provided a solid, non-sterile medium for germination, in 90 mm plastic Petri dishes. The dark was achieved by putting the dishes inside light-proof aluminium foil bags. Germination was defined as visible radicle emergence. Experiments lasted for a maximum of 30 days, at which time no further germination was observed. At the end of the germination tests, a cut-test was carried out to determine the viability of the remaining seeds (soft or firm), and the final germination percentage was calculated on the basis of the total number of firm seeds as the mean of the five replicates ± SE.
Data analysis
Population genetics
Within each subpopulation, for both chloroplast and nuclear genomes, a set of genetic statistics was calculated using the program GENALEX 6.5 (Peakall & Smouse 2012): the average (Nacp and Nanuc for chloroplast and nuclear markers, respectively) and effective (Necp and Nenuc for chloroplast and nuclear markers, respectively) number of alleles per locus, the chloroplast gene diversity (H), and the observed and expected nuclear heterozygosity (Ho and He, respectively). One-way anova (for normally distributed data) or Kruskal–Wallis test (for data not normally distributed or when the assumption of homoscedasticity was rejected) were used to compare the estimated genetic parameters among the 12 subpopulations. To estimate the hierarchical partitioning of genetic variation within and among subpopulations and groups, analyses of molecular variance (AMOVA; Excoffier et al. 1992) was performed with 999 permutations. The global and pair-wise differentiation indices (ΦPT for haploid data; FST for diploid data) were calculated and principal coordinates analyses (PCoA) were performed to visualise the patterns of genetic differentiation in both genomes. Based on the nuclear data, the overall and subpopulation-specific coefficients of inbreeding (FIS) were computed and their statistical significance was tested using a non-parametric approach described by Excoffier et al. (1992) with 999 permutations. Furthermore, using both the chloroplast and nuclear data, the overall diversity (Hcp and Hnuc for chloroplast and nuclear markers, respectively) and the mean haploid number of migrants (Nmcp and Nmnuc for chloroplast and nuclear markers, respectively) were calculated, and the correlations between the AOO and the indices of genetic variability (H, Ho, He, AMOVA sum of squares) were tested.
The spatial pattern of distribution of genetic diversity was studied by means of a Mantel test (Sokal & Rohlf 1995), which estimates the correlation between the matrices of geographic distances and genetic distances between individuals, using 999 random permutations. The pair-wise, individual-by-individual linear geographic distances matrix was generated from X and Y coordinates, while the calculation of pair-wise genetic distances followed the method of Huff et al. (1993).
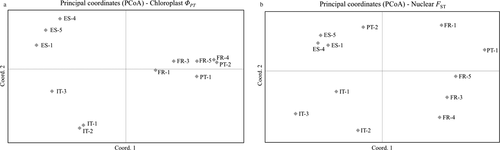
Based solely on the nuclear microsatellite data, a spatial diversity analysis was performed using the DIVA-GIS 7.5 software (Hijmans et al. 2012). First, all the subpopulations were georeferenced through the GPS coordinates. An allelic richness analysis was performed using the rarefaction method (Petit et al. 1998; Leberg 2002), which recalculates the diversity measured at each subunit of the study area to a standardised identical number of samples. Then, the geographic distribution and the frequency of individual alleles were estimated in order to identify the locally common alleles. Finally, to assist in defining priority conservation areas for M. littorea in Europe, Reserve Selection procedure (Rebelo & Siegfried 1992) was performed, which uses an optimisation algorithm and combines the information on molecular diversity to define the minimum number of geographic units needed to conserve all species genetic diversity, and to identify the geographic units that should be prioritised for conservation.
Seed traits and germination tests
Seed dry weight values were compared among the subpopulations with a Kruskal–Wallis test (since data were not normally distributed), followed by a post-hoc test (Mann–Whitney pair-wise comparison). Moisture content values and final germination data expressed as a proportion were analysed for each subpopulation using Generalised Linear Models (GLM) with binomial distribution and logit link function. For moisture content, subpopulations were included as fixed factors while for germination data, subpopulation, temperatures and their interaction were included as fixed factors. For germination data, after model fitting, to assess the significance of main effects and interactions, the Wald's χ2 was calculated, and the mean and SE of each treatment were estimated with GLM based on actual data.
Germination rate (GR) was calculated as the inverse of the number of days (days−1) to achieve 25%, 50% and 75% of the maximum germination in one replicate (GR25 = 1/t25, GR50 = 1/t50 and GR75 = 1/t75) for each subpopulation (Garcia-Huidobro et al. 1982). The germination rates in the sub-optimal temperature range were then plotted as a function of temperature and regressed using an ordinary least squares linear model, to estimate the minimum or base temperature (Tb) as the intercept on the x-axis. The theoretical Tb, at which germination rate is equal to zero, was calculated for each subpopulation as the mean of the three sub-subpopulations (25%, 50% and 75%). Regression lines calculated on the basis of GR50 were also tested statistically for homogeneity of slopes (Huitema 2011). Analysis of covariance (ancova) was used to test differences of germination rates in the response to constant temperature among the 14 subpopulations, setting the temperature as the independent variable, or covariate (x), and the germination rate as the dependent variable (y). Thermal time (θ50) estimates for each subpopulation were also calculated as the inverse of the sub-optimal regression equations of GR50 (Garcia-Huidobro et al. 1982; Covell et al. 1986), indicating the thermal time units (°Cd) accumulated to germinate for the 50% of each subpopulation.
Germination proportion data at each constant temperature were tested for correlation with the log-transformed AOO and with several climatic parameters, by the means of an ordinary least square regression. In particular, environmental minimum (Tmin), mean (Tmean) and maximum (Tmax) temperature and precipitations (Prec.) and the relative humidity (RH) of the months in which the mother plants usually fruit (May, June) were considered, to evaluate whether the specific conditions of the maternal environment are correlated with the germination behaviour of the European subpopulations. These analyses were conducted with the average climatic values over 50-years, from 1950 to 2000, and records for the single year 2013. When a significant correlation was found, the appropriateness of the linear regression model was assessed examining the residual plots.
Finally, association between genetic parameters (H, Ho, He, Fst) and germination performance (germination proportion data at eight tested temperatures) was analysed by the means of a multivariate multiple linear regression. For these analyses, software Past 2.13 (Hammer et al. 2001) was used, except for GLM analysis, for which GenStat release 12.1 (VSN International, Oxford, UK) was used.
Results
Population genetics
Only two of the five examined SSRs chloroplast loci were polymorphic, both showing four alleles, while all the four nuclear loci investigated were polymorphic, with the number of alleles per locus ranging from two to six (Table S1). The average (Nacp and Nanuc) and effective (Necp and Nenuc) number of alleles per locus, the chloroplast gene diversity (H), the observed and expected nuclear heterozygosity (Ho and He), and the coefficient of inbreeding (FIS) are reported in Table 2 for each subpopulation. No significant differences were found comparing the mean values of H, Ho and He among the subpopulations (Kruskal-Wallis test: P > 0.05).
| Pop. | Nacp | Necp | Nanuc | Nenuc | H | H o | H e | F IS |
|---|---|---|---|---|---|---|---|---|
| PT-1 | 1.60 | 1.12 | 2.25 | 1.58 | 0.07 ± 0.07 | 0.27 ± 0.09 | 0.32 ± 0.11 | 0.19 |
| PT-2 | 1.40 | 1.06 | 2.00 | 1.62 | 0.05 ± 0.05 | 0.37 ± 0.13 | 0.33 ± 0.12 | −0.06 |
| ES-1 | 1.40 | 1.05 | 2.75 | 1.86 | 0.04 ± 0.04 | 0.41 ± 0.18 | 0.35 ± 0.15 | −0.15 |
| ES-4 | 1.60 | 1.34 | 2.25 | 1.85 | 0.13 ± 0.13 | 0.40 ± 0.17 | 0.33 ± 0.15 | −0.16 |
| ES-5 | 1.40 | 1.26 | 2.75 | 1.69 | 0.11 ± 0.11 | 0.36 ± 0.15 | 0.33 ± 0.14 | −0.09 |
| FR-1 | 1.40 | 1.25 | 2.00 | 1.69 | 0.15 ± 0.10 | 0.36 ± 0.13 | 0.36 ± 0.12 | 0.01 |
| FR-3 | 1.60 | 1.32 | 2.00 | 1.66 | 0.17 ± 0.11 | 0.29 ± 0.13 | 0.34 ± 0.12 | 0.18 |
| FR-4 | 1.20 | 1.09 | 3.00 | 1.64 | 0.06 ± 0.06 | 0.20 ± 0.12 | 0.34 ± 0.12 | 0.46*** |
| FR-5 | 1.60 | 1.22 | 2.00 | 1.73 | 0.13 ± 0.09 | 0.45 ± 0.16 | 0.37 ± 0.12 | −0.19 |
| IT-1 | 1.60 | 1.26 | 2.75 | 1.87 | 0.14 ± 0.10 | 0.30 ± 0.15 | 0.33 ± 0.15 | 0.10 |
| IT-2 | 1.40 | 1.22 | 2.25 | 1.69 | 0.14 ± 0.09 | 0.25 ± 0.13 | 0.28 ± 0.16 | 0.13 |
| IT-3 | 1.40 | 1.16 | 1.75 | 1.43 | 0.10 ± 0.08 | 0.26 ± 0.15 | 0.23 ± 0.13 | −0.13 |
| P-value | >0.05 | >0.05 | >0.05 |
For the chloroplast genome, the overall genetic diversity Hcp was 0.11 and the AMOVA indicated that 44% of the variation was found among subpopulations, whereas 56% of the variation was within subpopulations. The overall value of ΦPT was 0.44 (P = 0.001) and the average Nmcp was estimated to be 0.63.
Considering the nuclear loci, the overall genetic diversity Hnuc was 0.33. The AMOVA showed that 12.78% of the variation occurred among subpopulations, 0.98% among individuals within subpopulations, and 86.24% within individuals. According to these results, the overall FIS and FST were 0.01 (P > 0.05) and 0.13 (P < 0.0001), respectively. The average Nmnuc was 1.71.
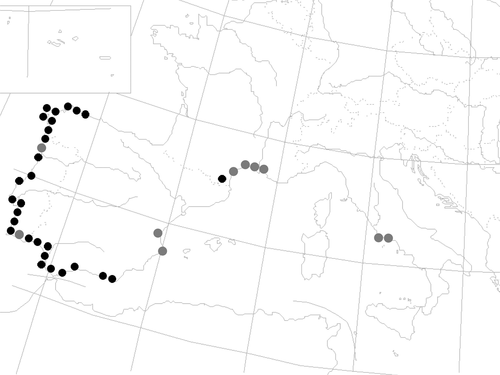
The PCoAs, based on the ΦPT and FST pair-wise matrixes, highlighted a similar differentiation of the subpopulations into three groups: the French–Portuguese, the Italian and the Spanish groups (Fig. 2a, and b). The first and second axes of the PCoAs explained 57.91% and 21.64% variability in the chloroplast genome (Fig. 2a), and 64.84% and 20.61% of the variability in the nuclear genome (Fig. 2b), respectively. The distance values, derived from the pair-wise comparison, ranged between 0.000 (FR-3 – FR-5, FR-4 – FR-5, and IT-1 – IT-2) and 0.813 (PT-2 – ES-1) for the chloroplast genome and between 0.000 (ES-1 – ES-4, ES-4 – ES-5, and IT-1 – IT-2) and 0.404 (PT-1 – IT-3) for the nuclear genome.
The AOO was not significantly correlated with any index of molecular variability. The correlation between geographic and genetic distance was positive, but very weak for both the chloroplast (R2 = 0.02; Rxy = 0.13; P = 0.001) and nuclear (R2 = 0.01, Rxy = 0.09, P = 0.001) loci.
The richness analysis conducted with DIVA-GIS detected four level of allelic richness, with FR-4 showing the highest value and FR-1 and IT-3 showing the lowest (Fig. 3). Through the analysis of geographic distribution of individual alleles, AthGAPAb-196 and AthGAPAb-198 were identified as locally common alleles, while three subpopulations (FR-4, ES-1 and IT-1) were identified as priorities for conservation in the Reserve Selection procedure (Fig. 3).
Seed traits and germination tests
Among the 14 subpopulations, the seed dry weight (DW) ranged between 0.09 ± 0.00 and 0.16 ± 0.00 mg, while the post-handling moisture content ranged between 3.3 ± 0.4% and 5.6 ± 0.3% (mean ± SD; Table S2). For dry seed weight, the Kruskal-Wallis test detected a significant difference among the subpopulations (P < 0.0001) and similarly, the GLM analysis showed a significant effect of subpopulation on moisture content (P < 0.001). Highly significant effects (P < 0.001) on final germination percentages were found for both factors (subpopulation and temperature), and for their interaction, with the Wald's χ2 being 361.6. Despite the differences in the final germination estimates (Table S3), similar dependencies of total germination on temperature were observed among the subpopulations with the highest germination percentages at 10 and 15 °C and the lowest at 27 °C for most subpopulations, and in some cases at 0 °C (Table S3).
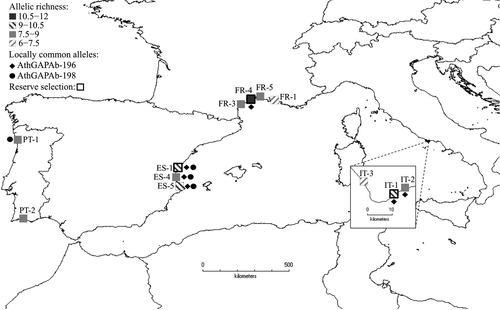
The relationship between the log-transformed AOO and the total germination was found to be positive by means of the correlation tests. In particular, they were significantly and positively correlated at all the tested temperatures, except 25 °C, with a Pearson's r coefficient ranging between 0.68 and 0.72 (P < 0.01) for 5, 10 and 15 °C, and between 0.58 and 0.64 (P < 0.05) for 0, 20, 23 and 27 °C. The test of homogeneity of slopes (parallelism) showed that the slopes of the regression lines were not significantly different (F = 0.08, P = 0.99), justifying application of ancova, which showed a significant difference among the seven regression lines (F = 16.59, P < 0.001), underlying the significant effect of temperature on germination (Fig. 4).
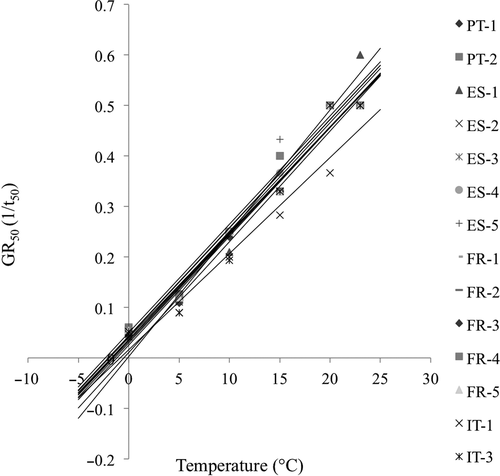
Regarding germination rate (GR), the values were very similar under the tested temperatures, and slightly differed among subpopulations only in a few specific cases. Overall, the regression equations relative to GR50 of the 14 subpopulations met the assumption of homogeneity of slopes (F = 0.43, P = 0.95), and the ancova did not show a significant difference among them (F = 1.38, P = 0.19; Fig. 5). Also, the mean Tb of the 14 subpopulations were not significantly different (P = 0.08), ranging from −1.7 ± 0.5 to 0.0 ± 0.1 °C (mean ± SE; Fig. 5), and the thermal times to reach 50% germination (θ50) ranged between 40.91 °Cd (ES-1) and 52.52 °Cd (IT-1).
For the climatic dataset for the period 1950–2000, only the precipitation values for June were found to be correlated with germination proportion data at 5 (P < 0.05), 10 (P < 0.01) and 20 °C (P < 0.05). However, more climatic variables in 2013 were significantly correlated with the germination proportions. In particular, May Tmin was correlated with germination values under 5 (P < 0.05) and 10 °C (P < 0.01), May Tmean with germination results under 5, 10 (P < 0.01), 15, 20 and 23 °C (P < 0.05), and June Tmean with germination values at 5, 10 and 15 °C (P < 0.05).
The correlation between genetic parameters (H, Ho, He, Fst) and germination proportions was not significant in any of the cases (P > 0.05).
Discussion
Revealing the genetic diversity and priority for conservation across Europe
In M. littorea, three of the five chloroplast microsatellite loci examined were found to be monomorphic. This finding supports the theory that organelle genomes are associated with relatively low mutation rate and a high degree of sequence conservation (Provan et al. 2001; Provan & Campanella 2003). These three loci were previously found to be monomorphic in other Brassicaceae species, such as Capsella bursa-pastoris (Ceplitis et al. 2005) and four Brassica spp. (Edh et al. 2007; Zamani-Nour et al. 2013).
The overall Hcp (0.11) signifies a good level of chloroplast diversity in respect to the geographic distribution of the species (Pironon et al. 2017). In comparison, a higher value was found for a worldwide species (0.19 in Capsella bursa-pastoris; Ceplitis et al. 2005) and a lower value for an island species (0.00 in Brassica cretica; Edh et al. 2007) with similar life history. Likewise, the overall Hnuc (0.33) was similar (Clauss et al. 2002; Gaudeul et al. 2007) or higher than (Edh et al. 2007) to other perennial Brassicaceae species. As for chloroplast loci, the within-subpopulation nuclear variability (Ho, He) did not significantly differ among the investigated subpopulations, even if one of the smallest subpopulations (IT-3) in an isolated group, showed the lowest He value, indicating the likely action of genetic drift. The only significant positive value of FIS was found in FR-4, which also showed high genetic diversity and allelic richness values. All these characteristics indicate the long-term isolation of this subpopulation from the other sites of the species distribution, presumably resulting from the unique geography of Plage des Aresquiers, being a narrow strip of coastal habitat between the sea and a system of lagoons.
Considerable genetic diversity is retained among the subpopulations without any difference in diversity between isolated and central populations (Pironon et al. 2017). Similarly, Cires et al. (2011), studying the genetic structure of peripheral European populations of Cochlearia pyrenaica (Brassicaceae), found that these peripheral populations have a similar level of diversity as other Central European populations. On the other hand, in M. littorea the population genetics of the species is not strongly correlated with the AOO. A recent decline in size of some subpopulations may be the reason for the similar level of genetic diversity among large and small subpopulations, as populations that remain small for long periods are likely to be considerably less diverse than those that have only recently become small (Barrett & Kohn 1991). For the Italian subpopulations, the recent history is known and it validates the hypothesis of a recent decline of the small subpopulations of M. littorea. Thus, the suitable area for M. littorea in the Latium region has declined considerably during the last 20 years, where a well-developed coastal dune system has been levelled out with the removal of the vegetation, to make way for human settlements (Del Vecchio et al. 2012a). This emphasises the importance of taking population history into account when interpreting genetic diversity results (e.g. Ouborg & Van Treuren 1995).
Regarding subpopulation differentiation, the overall value for chloroplast genome (ΦPT = 0.44) was higher than the nuclear genome (FST = 0.13), and is presumably due to the more limited dispersal ability of seeds compared to pollen, since chloroplast genome is maternally inherited in most plant species (Petit et al. 2005). A comparable population structure was observed using both chloroplast and nuclear data, and the hypothesis of the three isolated groups representing different genetic clusters (French, Spanish and Italian clusters) was confirmed. In M. littorea, the differentiation values (ΦPT and FST) were found to be lower than in other perennial Brassicaceae species showing a strong population differentiation (Edh et al. 2007; Gaudeul et al. 2007; Cires et al. 2011), while, conversely, the average migration rates (Nmcp = 0.63, Nmnuc = 1.71) were higher than other species of the same family, justifying the lack of a strong differentiation. The occurrence of a discreet level of gene flow in M. littorea is likely due to long-distance pollen flow by pollinators such as the migratory butterfly Colias croceus (personal observation), which is known to move across southwest Europe and as far as Britain (Sparks et al. 2007). Bensusan et al. (2014) suggested that this butterfly migrates between Africa and Europe, and uses stopover sites in Southern Europe, such as Gibraltar, to refuel prior to reaching more northerly sites. Despite the long distance among the study sub-regions, a non-significant isolation-by-distance effect was detected by the Mantel test, which is likely explained by the similarity between distant subpopulations (e.g. Portuguese and French). This in turn explains the result of both the gene flow and the historical distribution of the species.
One of the detected locally common alleles (AthGAPAb-196) was shared by all the isolated groups (Spanish, French and Italian), but not by the Portuguese one, making it a possible indicator of isolation. Locally common alleles are repeatedly observed in a small geographic area and the probability of their occurrence is much lower than the probability of observing single alleles only once; they can indicate adaptation to local conditions but they are vulnerable to losses due to their restricted distribution. For these reasons, they are considered more important than both private (van Zonneveld et al. 2012) and broadly distributed (Frankel et al. 1995) alleles for conservation purposes.
A relevant finding for M. littorea conservation was the identification of three priority subpopulations (FR-4, ES-1 and IT-1), unique for their genetic attributes, each one belonging to a different genetic cluster. Conservation efforts should focus on these subpopulations, as they represent critical sources of genetic diversity.
Major influences on seed germination responses
In the 14 subpopulations of M. littorea, the mean seed DW differed significantly, although the range of variation was slight (under twofold). Under eight constant temperatures, the germination pattern of the subpopulations was similar, without considering the absolute values. Similarly, the germination requirements of three European populations of the coastal Crucianella maritima had the same pattern of response to temperature, but a lower overall level of seed germination was detected for the Mallorca population, probably related to the smaller seed mass of that particular seed lot (Del Vecchio et al. 2012b). Considering the final germination values, most subpopulations occurring in the isolated groups (Spain, France and Italy) did not show any difference in germination performance when compared with each other or with central subpopulations. Likewise, previous studies (Lammi et al. 1999; Abeli et al. 2009) found that the reproductive performance of peripheral isolated plant populations is similar to that of central range populations.
Germination proportions data were associated with AOO, and other studies have similarly reported an effect of population size on germination (Silene regia, Caryophyllaceae, Menges 1991; Ipomopsis aggregata, Polemoniaceae, Heschel & Paige 1995; Gentianella germanica, Gentianaceae, Fischer & Matthies 1998), which reinforces the view that genetic diversity is not the only factor influencing reproduction (Hensen & Wesche 2006). Differences in seed quality and germination can be largely influenced by non-genetic causes such as the particular demographic history of the species (Ouborg & Van Treuren 1995), deterioration in environmental conditions which the maternal plants in small populations encounter (Oostermeijer et al. 1994), or low pollen availability (Byers 1995). When a plant population becomes too small, pollinator visits decrease and/or the remaining pollinators are less effective (Ouborg & Van Treuren 1995; Fischer & Matthies 1998; Jacquemyn et al. 2001). As most pollinator movements occur within rather than between plants, a greater proportion of seeds will be produced by within-plant crosses (Menges 1991). Moreover, in small populations it is also more likely that asynchronous flowering of compatible mates occurs than in large populations, in which temporal density of flowering plants is generally higher (Luijten et al. 2000).
The germination proportions of the 14 M. littorea subpopulations also showed a stronger correlation with the climatic parameters of 2013, the year in which the seeds used in the experiment were produced, than with the average climatic parameters of the period 1950–2000. In particular, the mean temperatures of May and June 2013, recorded at the 14 European localities, were significantly correlated with the germination levels of the respective subpopulations, highlighting the important influence of the annual maternal environment on seed germination. The role of the maternal environment during seed development, as a factor that can influence germination behaviour of M. littorea, has already been reported (De Vitis et al. 2014). It was shown that after one generation of ex situ plant cultivation under different conditions than the natural environment, seed germination performance was altered, compared to the seeds produced by the native population (De Vitis et al. 2014).
The timing of germination is highly responsive to environmental conditions (Donohue 2002). Several studies have found that germination rates and Tb change with environmental conditions, such as along an altitudinal (Orrù et al. 2012) or latitudinal (Daws et al. 2004) gradient. In the present study, the 14 European subpopulations of M. littorea showed similar thermal responses for germination, which presumably reflects the environmental similarity with respect to having a shared climate of the Mediterranean coastal region (sensu Rivas-Martínez et al. 2011) within small altitudinal (0–13 m) and latitudinal (37.09–43.56°) ranges.
In M. littorea, no association between genetic diversity and germination percentages was evident, similar to many other species (Oostermeijer et al. 1994; Lammi et al. 1999; Luijten et al. 2000; Hensen & Wesche 2006). Where the association between genetic diversity, population size and traits related to fitness, such as seed germination, has been investigated, these correlations appeared to vary according to species (Lammi et al. 1999). The general perception is that there is no strong relationship between decreasing germination with greater inbreeding coefficient, or between increasing germination and higher population genetic diversity (Baskin & Baskin 2015).
These results demonstrated that growing in an isolated group is not a factor limiting the germination performance in M. littorea European subpopulations. On the contrary, factors such as the subpopulation size and the yearly environmental conditions may be stronger drivers of seed fitness component.
Implications for Malcolmia littorea conservation in Europe
Isolated plant populations are particularly important genetically and ecologically, and more attention should be focused on their conservation (Abeli et al. 2009; Cires et al. 2011) and on understanding the differences between them and central populations (Lammi et al. 1999).
The present study highlights the importance of integrating different approaches to investigate the conservation status of isolated subpopulations of endangered species and of considering subpopulations as independent sampling units, to understand biological and ecological processes at a finer scale. In fact, even if small subpopulations were found to retain a good level of genetic variability, lower seed quality, as judged by the germination phenotype, suggests that they may be in an early phase of genetic erosion (Ouborg & Van Treuren 1995 and literature therein).
In the most updated version of the European Strategy for Plant Conservation 2008/2014 (ESPC, target 8.1), Planta Europa (2008) suggested conservation measures for populations at the border of their distribution area. In this context, we suggest implementing both in situ and ex situ conservation actions on M. littorea to all isolated subpopulations. However, if economic resources are limited, conservation measures can be focussed only on a few subpopulations. It is essential, therefore, to identify subpopulations and areas that show high values of genetic diversity and divergence and that merit the most attention in terms of conservation priority (Mattioni et al. 2017). In M. littorea, such priority units have been identified and the first conservation action to be implemented should be greater physical protection of the sand dune systems where they occur or other in situ measures, such as translocations. In fact, only one of these subpopulations (Plage des Aresquiers, Frontignan; FR-4) occurred in a coastal site already physically protected, while the others do not. Specifically, at Playa de Moncófar (Spain; ES-1), a micro-reserve was established through a LIFE project (93/NAT/E/000766) but it is evident that this action of assigning a conservation status to an area without physically protecting it, does not ensure its effective conservation. The Italian subpopulation occurring at Torre Olevola (IT-1) is in peril as its small and degraded habitat is surrounded by private constructions without any chance to expand and colonise other areas. This subpopulation requires reintroduction or translocation into the closest protected area. For such conservation actions the germplasm to be used for plant propagation or direct sowing should be collected from the closest subpopulations to ensure adaptation of the reintroduced material to the local conditions, as the local maternal environment is an important modulator of the germination response. Alternatively, germplasm should be collected from subpopulations which belong to the same genetic cluster, to maintain or re-establish the natural gene flow and to avoid outbreeding depression, likely expressed as a reduced fitness of the subsequent generations due to the disruption of gene complexes or of the local adaptation (Vander Mijnsbrugge et al. 2010; Baskin & Baskin 2015).
Seed sampling for ex situ conservation is a key strategy for the preservation of wild species germplasm (Li & Pritchard 2009), including M. littorea in Europe. We have already committed to the ex situ conservation of 14 European subpopulations by storing seeds in two seed banks (Tuscia Germplasm Bank, Italy and the Royal Botanic Gardens, Kew's Millennium Seed Bank, UK) and established that seed quality had been maintained. Whilst the benefits of long-term banking of germplasm of endangered genotypes is recognised, the importance of fully characterising the germination phenotype is less pursued. Such work clearly provides a framework for understanding species ecology, including natural regeneration. These combined approaches point clearly towards the implementation of urgent conservation measures for those subpopulations that showed both unique genetic composition and reduced germination percentages.
Acknowledgements
The authors are grateful to C. Silva Neto who collected the Portuguese samples; to O. Argagnon, F. Médail, E. Estrelles, P.P. Ferrer Gallego, and F. Collado who helped the first author in the selection and sampling of French and Spanish populations; and to F. Chiocchini and M. Ciolfi for their help with the spatial analysis. The support of the Seed Conservation Department, Royal Botanic Gardens, Kew is gratefully acknowledged. This work was partly supported by the Circeo National Park within the project “In situ conservation of the critically endangered Italian population of Malcolmia littorea (L.) R. Br.”.




