Molecular characterisation of the broad-spectrum resistance to powdery mildew conferred by the Stpk-V gene from the wild species Haynaldia villosa
Abstract
- A key member of the Pm21 resistance gene locus, Stpk-V, derived from Haynaldia villosa, was shown to confer broad-spectrum resistance to wheat powdery mildew. The present study was planned to investigate the resistance mechanism mediated by Stpk-V.
- Transcriptome analysis was performed in Stpk-V transgenic plants and recipient Yangmai158 upon Bgt infection, and detailed histochemical observations were conducted. Chromosome location of Stpk-V orthologous genes in Triticeae species was conducted for evolutionary study and over-expression of Stpk-V both in barley and Arabidopsis was performed for functional study.
- The transcriptome results indicate, at the early infection stage, the ROS pathway, JA pathway and some PR proteins associated with the SA pathway were activated in both the resistant Stpk-V transgenic plants and susceptible Yangmai158. However, at the later infection stage, the genes up-regulated at the early stage were continuously held only in the transgenic plants, and a large number of new genes were also activated in the transgenic plants but not in Yangmai158.
- Results indicate that sustained activation of the early response genes combined with later-activated genes mediated by Stpk-V is critical for resistance in Stpk-V transgenic plants. Stpk-V orthologous genes in the representative grass species are all located on homologous group six chromosomes, indicating that Stpk-V is an ancient gene in the grasses. Over-expression of Stpk-V enhanced host resistance to powdery mildew in barley but not in Arabidopsis. Our results enable a better understanding of the resistance mechanism mediated by Stpk-V, and establish a solid foundation for its use in cereal breeding as a gene resource.
Introduction
Powdery mildew (Pm) disease, caused by Blumeria graminis f. sp. tritici (Bgt), is one of the most destructive diseases of wheat, and causes extensive yield losses worldwide (Bennett 1984). Breeding of wheat varieties with high Pm resistance is critical for disease control, and might be achieved by exploring new sources of resistance genes and through a better understanding of resistance mechanisms. In wheat, at least 60 Pm genes/alleles at 49 loci have been identified (McIntosh et al. 2014), however, most of the Pm genes incorporated into resistant lines are race-specific, leading to boom-and-bust cycles of the disease (Marone et al. 2013). Therefore, introducing cultivars with durable, broad-spectrum, Pm resistance genes is an important goal in agriculture. Among the identified Pm genes, several have been shown to confer broad-spectrum resistance to powdery mildew, such as Pm12, Pm16 and Pm21 (Reader & Miller 1991; Chen et al. 1995; Jia et al. 1996). Pm21 is located on chromosome 6VS of Haynaldia villosa (2n = 2x = 14, genome VV) and was transferred to wheat as a T6VS.6AL translocation (Chen et al. 1995). This translocation line and the later-developed interstitial and terminal translocations lines with small alien chromosome segments covering Pm21, NAU419 and NAU418, have been widely used as a key genetic resource in breeding for powdery mildew resistance (Chen et al. 2013).
Broad-spectrum resistance and race-specific resistance are two types of host response to pathogens that comprise a wide range of races/strains. In race-specific resistance, resistance (R) genes can recognise specific avirulence effectors produced by a pathogen to activate host defence responses (Burdon & Thrall 2009); however, this type of resistance genes is often overcome by new races of the pathogen that produce novel virulence effectors. Bgt is a species of biotrophic pathogen that undergoes rapid evolution, so the resistance conferred by race-specific powdery mildew R genes is ineffective against newly emerged virulent isolates when Pm genes are used on a large scale. For example, the resistance gene Pm8, located on chromosome arm 1RS of the wheat–rye T1BL.1RS translocation (Zeller 1973), was widely used worldwide and played an important role in controlling powdery mildew. However, newly emerged virulent isolates of Bgt caused large outbreaks of powdery mildew disease, accompanied by severe yield losses (Lutz et al. 1992). Broad-spectrum resistance genes are a class of genes that provide resistance to different pathogens or to different isolates/races/strains of the same pathogen. Broad-spectrum resistance cannot easily be overcome by new pathogen races, and, most importantly, this type of resistance is usually correlated with durability. Broader levels of protection and durability are properties that make broad-spectrum resistance genes highly valuable in plant breeding programmes. Molecular mechanisms underlying broad-spectrum resistance and genetic manipulation of broad-spectrum resistance genes have become important areas of considerable research focus.
Broad-spectrum resistance can be induced when pattern recognition receptors (PRRs), such as EFR, FLS2, CERK1 or CeBiP, recognise particular Pathogen-Associated Molecular Patterns (PAMPs) and initiate PAMP-triggered immunity (PTI) (Gomez-Gomez & Boller 2000; Jones & Dangl 2006; Miya et al. 2007; Zipfel 2008; Shimizu et al. 2010). The interaction of EFR with the prokaryotic elongation factor EF-Tu has been introduced into plant species to successfully induce broad-spectrum resistance to diverse bacteria (Lacombe et al. 2010). Broad-spectrum resistance can also be achieved by the interaction of avirulence effectors with the corresponding R gene. For example, resistance of rice Xa21 was initiated by recognising a 17–amino acid peptide (axYS22) derived from the N-terminal region of Ax21. AxYS22 is conserved in all analysed Xanthomonas species, so Xa21 confers broad-spectrum resistance to X. oryzae pv. oryzae (Lee et al. 2009). A third strategy to achieve broad-spectrum resistance is by mutating the susceptibility factors or negatively controlled genes in the resistance pathways. For example, Mlo is a negative regulator of powdery mildew resistance genes, but the corresponding recessive gene mlo confers broad-spectrum resistance to powdery mildew in barley (Büschges et al. 1997). Mutation of Xa5 and Xa13, two putative susceptibility factors, can also lead to broad-spectrum resistance against X. oryzae pv. oryzae in rice (Chu et al. 2006; Iyer-Pascuzzi & McCouch 2007). Broad-spectrum resistance often accompanies durable resistance, because the pathogen molecules recognised by broad-spectrum resistance genes are often critical to survival of the pathogen, and are thus rarely mutated (Kou & Wang 2010). Compared with race-specific resistance, broad-spectrum resistance is increasingly garnering more attention from plant breeders and plant pathologists.
In our previous studies, a serine-threonine protein kinase gene, Stpk-V, which is located at the Pm21 locus, was cloned and characterised using a strategy that combined molecular and cytogenetic techniques (Cao et al. 2011). The resistance spectrum test indicated that transgenic wheat over-expressing Stpk-V showed ‘0’ or ‘0;’ levels of resistance to 29 different isolates of Bgt, therefore, Stpk-V can be defined as a broad-spectrum resistance gene (Cao et al. 2011). Transgenic wheat plants expressing Stpk-V show a strong hypersensitive response to Bgt inoculation and a high concentration of H2O2 accumulation when analysed using histochemical methods. However, the molecular mechanism of resistance in the transgenic plants remains to be elucidated, which requires an alternative and powerful approach for investigation. Next generation sequencing (NGS) technology has emerged as a cutting-edge approach for high-throughput DNA sequence data production. NGS has improved the efficiency and speed of gene discovery and dramatically reduced costs (Shendure & Ji 2008). In this study, using Illumina NGS technology, digital gene expression (DGE) was used to compare gene expression profiles of the Stpk-V transgenic T5 line and the corresponding recipient Yangmai158 at three infection stages, 0, 6, and 24 h after inoculation (hai). The DGE results were confirmed by qRT-PCR for several selected signal pathway genes. From previous reports, salicylic acid (SA), jasmonic acid (JA) and ethylene (ET) signalling pathways play important roles in disease resistance in plants (Dong 1998), and have been reported as involved in Pm resistance in wheat (Li et al. 2011; Pal et al. 2013). PR (pathogenesis-related) proteins, which are induced in plants as a result of host–pathogen interactions, also play an important role in powdery mildew resistance. There is accumulating evidence to suggest that Ca2+ serves as a second messenger in host plant responses to pathogens (Diaz et al. 2002). Calmodulin (CaM), a key Ca2+ sensor in all eukaryotes, has been implicated in defence responses in plants (Heo et al. 1999) and CDPKs are Ca2+ sensor protein kinases critical for transcriptional reprogramming in plant innate immune signalling (Boudsocq et al. 2010). These presumed signal pathway response comparisons had been performed in this study and enabled us to better understand the molecular mechanism in wheat mediated by Stpk-V in response to Bgt infection. A previous study indicated that the percentage of H2O2 burst and cell death in the Bgt interaction cells in the Stpk-V transgenic lines was significantly higher than that in the recipient Yangmai158 (Cao et al. 2011). Hence, detailed histochemical observations were also conducted in both materials. We also investigated gene evolution of Stpk-V in several wheat relatives and also evaluated powdery mildew resistance of Stpk-V in barley and Arabidopsis. Our results facilitate the future use of Stpk-V in breeding wheat varieties with broad-spectrum resistance to powdery mildew.
Material and methods
Plant material and fungal isolate
Yangmai158 is a powdery mildew susceptible wheat variety that is preserved in the Cytogenetics Institute of Nanjing Agricultural University (CINAU). T5, the Stpk-V over-expressed transgenic wheat with high powdery mildew resistance, was produced by gene-gun bombardment in CINAU using cv. Yangmai158 as recipient and is a descendant of the T3-2 line used in Cao et al. (2011). Mixed isolates of Bgt were collected from Jiangpu Experimental Station, CINAU, and maintained on seedlings of susceptible wheat variety Sumai 3 in a spore-proof greenhouse. The mixed population of Bgh was collected from fields in the eastern provinces of China and maintained in the highly susceptible barley cultivar Hua30.
Secale cereale (2n = 14, RR), Aegilops longissima (2n = 14, SlSl), Hordeum vulgare (2n = 14, HH), Ae. geniculata (2n = 28, UgUgMgMg), Ae. peregrina (2n = 28, SSUU) and a series of alien chromosome addition lines were used. These addition lines used were: wheat–S. cereale DA1R, DA2R, DA3R, DA4R, DA6R and DA7R; wheat–Ae. longissima DA1S, DA2S, DA3S, DA4S, DA5S and DA6S and DA7S; wheat–H. vulgare DA2H, DA3H, DA4H, DA5H, DA6H and DA7H; wheat–Ae. peregrinum DA1U, DA2U, DA3U, DA4U, DA5U, DA6U and DA7U; wheat–E. elongatum DA1E, DA2E, DA3E, DA4E, DA5E, DA6E and DA7E; and wheat–Ae. geniculata DA1Mg, DA2Mg, DA3Mg, DA4Mg, DA5Mg, DA6Mg and DA7Mg in the background of wheat cv. Chinese Spring from the Wheat Genetic and Genomic Resources Center, Kansas State University, USA.
Sequencing of DGE with Illumina
Susceptible Yangmai158 and resistant T5 seedlings were inoculated with Bgt at the two-leaf stage, then leaves from about 20 seedlings were harvested at 0, 6 and 24 hai for RNA extraction using the Trizol reagent (Invitrogen, Waltham, MA, USA). Each sample of 6 μg of total mRNA was purified with Oligo (dT) magnetic bead adsorption. A DGE library was built and fixed onto the Illumina Sequencing Chip (flowcell) for sequencing. Six DGE libraries were constructed to study gene expression profiles in the Pm resistant Stpk-V transgenic T5 line and the susceptible recipient line Yangmai158. The libraries included non-inoculated Yangmai158 and T5 (designated Y-0 and T-0), Bgt-inoculated Yangmai158 and T5 at 6 and 24 hai (designated Y-6, Y-24, T-6 and T-24). Approximately 3.6 million raw tags were generated for each of the six samples, and after removing low quality reads, each library contained ~3.5 million clean reads. The number of mapped clean tags was calculated for each library and then mapped to the wheat unigene database. Then we performed cluster analysis of gene expression patterns with ‘cluster’ software (Eisen et al. 1998) and ‘Java Treeview’ software (Saldanha 2004) to construct the Hierarchical clustering (HCL) tree (using the Pearson correlation method with average linkage). To identify genes associated with resistance mediated by Stpk-V, powdery mildew-responsive genes in Bgt infected T5 and Yangmai158 were compared at 6 and 24 hai (DEG tag analysis data in Table S1).
The SYBR green real-time RT-PCR assay
All selected genes were used for SYBR green real-time RT-PCR, primers are listed in Table S2. A real-time RT-PCR reaction (20 μl) included 20 ng cDNA, 0.2 μm of each primer, 1× SYBR Premix ExTaq (TaKaRa, Tokyo, Japan). Reactions were performed on a Bio-Rad IQ single-colour Real-Time PCR detection System (Bio-Rad, Hercules, CA, USA) under the following conditions: 94 °C for 30 s, 40 cycles of 94 °C for 5 s, 60 °C for 15 s and 72 °C for 20 s to calculate cycle threshold (Ct) values, followed by 95 °C for 15 s, 60 °C for 1 min, then 95 °C for 15 s to obtain melt curves to ensure primer specificity.
Histochemical detection of fungal structures or host cells
Yangmai158 and T5 seedlings were treated with exogenous chemicals at the two-leaf stage: 10 mm dimethylthiourea (DMTU, H2O2 scavenger) and 7 mm H2O2 administered as a 0.05% Tween-20 solution, with 0.05% Tween-20 used as mock treatment (Garretón et al. 2002). The chemically treated leaves were then inoculated with Bgt and the first leaves harvested at the indicated time points. H2O2 production in the Bgt interaction host cells was detected using the DAB staining method (Thordal-Christensen et al. 1997). Cell death in the Bgt interaction sites were detected by in situ histochemical staining (Peterhänsel et al. 1997). After DAB or Trypan blue staining, the leaves were bleached in boiling 95% ethanol for 10 min then stored in 50% glycerol. Before observation, the stored leaves were stained in Coomassie blue [0.6% (w/v) Coomassie Brilliant Blue R 250 in methanol] for 30 s, rinsed in distilled water twice, and mounted on glass slides in 50% glycerol for microscopy (Olympus, Tokyo, Japan). For each time point, four leaf pieces and at least 200 infection sites on each leaf piece were observed. Standard deviations and t-test statistical analyses were performed using the SAS software and bars represent ±SD.
Homologous analysis
For the evolutionary study of Stpks in the grass family, all PCRs were performed using the primers Stpk-F (AGATCCAACACCAGTTCAAG) and Stpk-R (ATGTTAAGGAGGCTTGTGTC), covering the fifth and sixth exon, and amplification was performed at 94 °C for 3 min; 30 cycles of 94 °C for 30 s, 55–58 °C for 30 s, and 72 °C for 2 min; followed by 10 min at 72 °C.
Production and screening of Arabidopsis transgenic plants
To obtain the transgenic plasmid, the pAHC25 plasmid was digested with EcoRI and HindIII, then the transcript unit Ubi promoter::GUS::NOS was maintained and inserted into the pCAMBIA1301 digested with the same two restriction endonucleases. The Stpk-V gene was amplified with the forward SmaI-containing primer (5′-TCCCCCGGGATGTCAATTTCCAACATA-3′) and the reverse SacI-containing primer (5′-CGATCGCTCACTCACGCTCTGATATTGC-3′) from the clone vector p18MD-T- Stpk-V plasmid as template, then the PCR product and the plasmid pCAMBIA1301-AHC were digested with SmaI and SacI enzymes, following by a ligation reaction to construct the recombinant vector pCAMBIA1301-AHC-Stpk-V. The recombinant plasmid was introduced into Agrobacterium tumefaciens C58C1 for Stpk-V transformation in Arabidopsis as described previously (Xiang et al. 1999). The hygromycin B resistant plants were harvested and used to reproduce the T2 generation. Positive plants of the T2 generation were screened for hygromycin B resistance, X-gluc staining and target gene analysis. PCR and RT-PCR were performed to analyse the presence and expression level of Stpk-V transgenic Arabidopsis using the gene-specific primers Stpk-F (5′-GATCCAACACCAGTTCAAG-3′) and Stpk-R (5′-ATGTTATGGAGGCTTGTGTC-3′). The expression level of the Tubulin gene was used as control with the primers Tubulin-F: 5′-CTCATCACAGGCAAGGAAGAT-3′ and Tubulin-R: 5′-CAGAACCTCAGGGCAACG-3′. PCR and RT-PCR were then used to verify positive plants for the powdery mildew evaluation.
Single-cell transient expression assay
The single-cell transient expression assay was performed according to Shirasu et al. (1999). Reporter plasmids containing β-glucuronidase (GUS) genes and the plasmids pAHC:Stpk-V were mixed before coating the particles (molar ratio of 1:1; 1 μg total DNA). The Bgh susceptible cv. Hua 30 was used for the transient leaf assay. Bombarded barley leaves were transferred to 1% agar plates supplemented with 85 μm benzimidazole and incubated at 18 °C for 8 h before high-density inoculation with single Bgh spores. Leaves were stained for GUS to identify Stpk-V transformed cells at 48 h after spore inoculation. The haustorium index (percentage GUS-staining cells with haustoria in the total GUS-staining cells attacked by Bgh) is indicated from four independent experiments, each of at least 70 interactions.
Results
Proposed resistance mechanism of Stpk-V from analysis of DEGs in T5 and Yangmai158
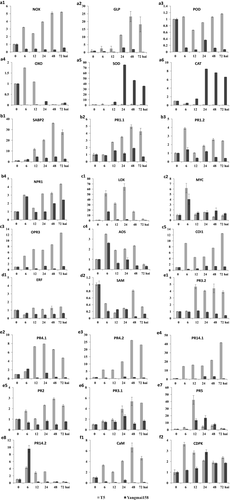
The Stpk-V was identified as a key member of the powdery mildew resistance gene Pm21. In the present study, we attempted to find the critical genes or pathways responsible for powdery mildew resistance mediated by Stpk-V through analysis of DEGs. Several disease-resistance-related genes involved in different signalling pathways were chosen to confirm the DGEs results with qRT-PCR (Fig. 1a–f).
Involvement of reactive oxygen species in Stpk-V-mediated resistance
In our previous study (Cao et al. 2011) transgenic Stpk-V plants showed accumulation of reactive oxygen species (ROS) and hypersensitive response cell death after Bgt infection, indicating that ROS production might be crucial in the resistance mediated by over-expression of Stpk-V. Among the unigenes in the six DGE libraries, we detected 12 unigenes related to the ROS pathway: two oxalate oxidases (OxO), three germin-like proteins (GLP), five peroxidases (POD) and two glutathione peroxidases (GPX) (Table S3). One NADPH oxidase (Nox) was additionally selected and identified as a marker gene for the ROS pathway. This transcriptome expression result was confirmed by qRT-PCR as shown in Fig. 1a1–a6. Similar to the DGEs sequencing results, the ROS-producing gene Nox (Ta#S52545338) was up-regulated after Bgt inoculation only in T5, and was unchanged in Yangmai158. The GLP gene (Ta#S52129023) was up-regulated after Bgt inoculation in both T5 and Yangmai158, however, the expression levels were higher in T5 than that in Yangmai158. POD (Ta#S26021080) and OxO (Ta#S12923252) genes were slight up-regulated in T5 after Bgt inoculation but down-regulated or unchanged in Yangmai158. Two other ROS-scavenging superoxide dismutase (SOD, Ta#S17986579) and catalase (CAT, Ta#S58904542) genes were up-regulated only in Yangmai158 after Bgt inoculation, and were unchanged in T5. These results indicate that ROS-generated genes were activated in both genotypes at an early stage, however, these genes were activated continuously only in T5. So, the Stpk-V mediated ROS burst in T5 was from both sustained expressed genes activated at an early stage and newly activated gens in the later stage, e.g. at 24 hai.
Salicylic acid (SA), jasmonic acid (JA) and ethylene (ET) pathways
To determine which hormone pathway was related to powdery mildew resistance mediated by Stpk-V, expression patterns of pathway-associated genes were analysed and compared in Yangmai158 and the T5 line.
Seven genes associated with the SA pathway were identified: one EDR1 gene, one SA binding protein and five PR1 genes (Table S3). The SA binding protein gene SBP2 (Ta#S52542789) and one of the PR1.2 genes (Ta#S18006174) were induced only in T5 after Bgt inoculation. Another PR1.1 gene (Ta#S58905285) and the NPR1 gene (Ta#S52545363) showed induced transcription in both genotypes, but the expression level of the gene was higher in the T5 line than that in Yangmai158 (Fig. 1b1–b4). This suggests that the SA pathway was activated in T5 and was involved in the defence response to Bgt infection, especially at late Bgt infection stage.
Fifteen genes related to the JA pathway were identified, 10 genes responsible for JA biosynthesis, including lipoxygenase (LOX), allene oxide synthase (AOS), allene oxide cyclase (AOC) and 12-oxo-phytodienoic acid reductase (OPR3), and five genes involved in JA signalling, including CORONATINE INSENSITIVE1 (COI1) and MYC (Table S3). Among these genes, one LOX gene (Ta#S52544755) and one MYC gene (Ta#S26028947) exhibited increased accumulation in both Yangmai158 and T5 at 6 hai; however, only the LOX gene was up-regulated continuously at 24 hai in T5. The OPR3 gene (Ta#S17889436), AOS gene (Ta#S16058046) and COI1 gene (Ta#S52543991) showed increased accumulation only in T5 after Bgt inoculation (Fig. 1c1–c5). These results indicate that the JA pathway is activated in both genotypes during early Bgt infection stage, but was only activated continuously in T5 through participation of Stpk-V at late Bgt infection stage.
Ten genes associated with the ET pathway were identified, four genes responsible for ET biosynthesis [including S-adenosylmethionine decarboxylase (SAM), ACC oxidase (ACO)] and six genes responsible for ET signalling [including ETHYLENE INSENSITIVE 3 (EIN3), ETHYLENE RESPONSE FACTOR1 (ERF)], however, expression of all these genes were unchanged or down-regulated in both genotypes. The results were confirmed by qRT-PCR for the ERF gene (Ta#s56463152) and SAM gene (Ta#S58902923) (Fig. 1d1, d2). This indicates that the ET pathway is perhaps not activated in the defence response to Bgt infection, especially at 6 or 24 hai.
Pathogenesis-related proteins
The 21 unigenes that encode PR proteins were identified in this study: five pathogenesis-related proteins 1.1 or 1.2 (PR1), two β-1-3-glucanases (PR2), eight chitinases (PR3), two vacuolar defence proteins (PR4), two thaumatin-like proteins (PR5) and three non-specific lipid transfer proteins (PR14) (Table S3). Among these, one PR3.2 gene (Ta#S17888136), two PR4 genes (Ta#S22379469 and Ta#S37933362) and one PR14.1 gene (Ta#S26027104) were only up-regulated in T5. One PR2 gene (Ta#S25791161), one PR3.1 gene (Ta#S12923205), one PR5 gene (Ta#S26028075) and one PR14.2 gene (Ta#S26025120) were up-regulated in both Yangmai158 and T5, but the expression level was higher in T5 than in Yangmai158 after Bgt inoculation over time (Fig. 1e1–e8). In total, 10 PR genes were up-regulated only in T5, among which nine were detected with high expression levels at 24 hai, indicated these genes were up-regulated by Stpk-V at the later stage.
Calcium signalling pathway
In our research, one calmodulin gene (Ta#S12923163) and one CDPK gene (Ta#S43837852) were differentially expressed in T5, and these genes showed up-regulation only after Bgt inoculation (Fig. 1f1, f2), indicating that the calcium signalling pathway is involved in the defence response to Bgt infection.
Analysis of ROS signal in resistance mediated by Stpk-V using histochemical methods
In this study, the Stpk-V transgenic T5 lines and Yangmai158 were treated with H2O2 and its antagonist DMTU, respectively, before Bgt inoculation to examine the influence of H2O2 on cell death and Bgt development. The percentages of H2O2 burst cells decreased in the DMTU-treated T5 and Yangmai158 at 6, 12 and 24 h after Bgt inoculation compared to the MOCK controls. Correspondingly, the percentages of H2O2 burst cells increased in the H2O2-treated samples compared to the MOCK controls (Fig. 2a–c). As to the percentage of cell death in the resistant T5, this decreased when treated with DMTU, but increased when treated with H2O2 (Fig. 2d–f). There was similar response trends in Yangmai158 and Stpk-V transgenic lines, however, the percentages of H2O2 burst cells and the percentages of cell death in Stpk-V transgenic lines were higher than in Yangmai158 at the three time points.
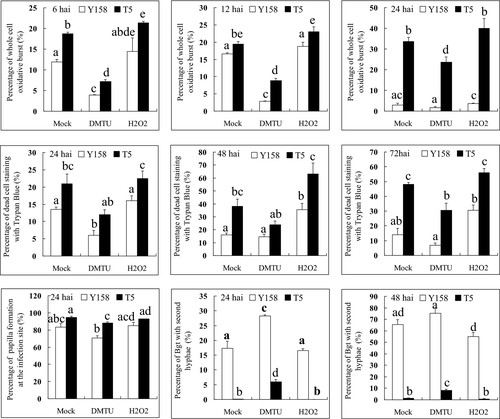
Papillae, built by the host cells surrounding the appresorium penetration pegs of Bgt, were considered important defence structures to suppress the attack of Bgt. In this study, a high percentage of papillae formation was found in both the susceptible Yangmai158 and resistant T5 line, indicating that papillae may be involved in basal resistance (Fig. 2d). In the resistant T5 line, slight changes in percentage of papillae formation were detected between the DMTU- and H2O2-treated samples after Bgt inoculation (Fig. 2g), suggesting that H2O2 might make a small contribution to papillae formation. However, the percentage of papillae formation was higher in the T5 line than in Yangmai158, indicating that another signal transduction pathway induced by Stpk-V, other than the H2O2, was responsible for the increased papillae formation in the T5 line.
The percentage of secondary hyphae formation was also compared between the DMTU-treated and MOCK controls of Yangmai158 and Stpk-V. As to the T5 line, the percentage of secondary hyphae formation at 24 and 48 hai was significant higher in the DMTU-treated samples than that in the MOCK controls and H2O2 treatment (Fig. 2h, i), which indicates that H2O2 had an important role in suppression of the normal development of Bgt. Also, there was a large difference between the H2O2-treated Yangmai158 and the T5 line, once again indicating that resistance of T5 was mediated through a complex network that included both H2O2 and other signals.
Evolution of the Stpk-V gene in the grass family
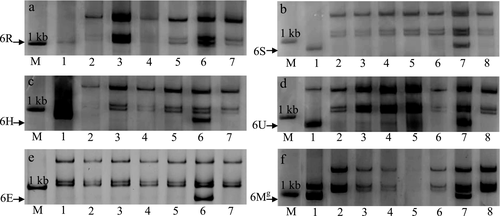
To investigate the conservation of Stpk-V in other grass species related to wheat, the partial sequence of Stpk were amplified from the disomic addition lines of the wheat variety ‘Chinese Spring’ and from corresponding wheat relatives. The tested wheat relatives were S. cereale, Ae. longissima, H. vulgare, Ae. geniculata, Aegilops peregrina. The PCR results showed that the same specific bands were amplified only from the wheat disomic addition lines containing the homologous group 6 alien chromosomes as that from the corresponding wheat relatives. This indicates that genes orthologous to Stpk-V are all located on homologous group 6 chromosomes (Fig. 3). Other genes orthologous to Stpk-V with high homology were also located to the syntenic chromosomal regions in rice, Brachypodium, sorghum and maize. Thus, Stpk is highly conserved in the grass family. However, homology of Stpk-V to genes in dicot species, such as Arabidopsis, was relatively low. Thus, Stpk is highly conserved in the grass family.
Resistance evaluation of Stpk-V to powdery mildew in barley and Arabidopsis
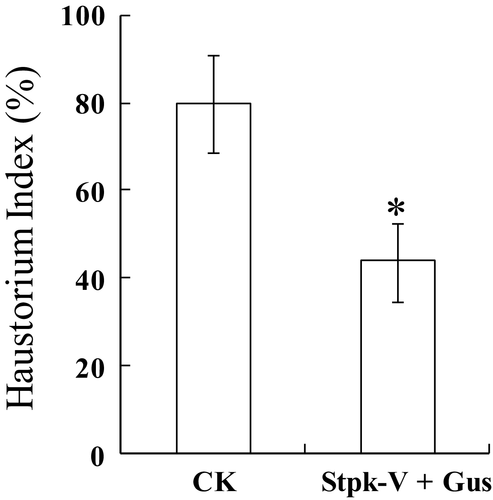
The Stpk-V confers broad spectrum resistance to the powdery mildew pathogen in wheat. To determine whether Stpk-V confers resistance against powdery mildew pathogens in other species, resistance was evaluated in the barley–Bgh and Arabidopsis–Golovinomyces orontii host–pathogen interaction systems. The successful formation of a mature haustorium, a structure for nutrient extraction during Bgh development, is usually considered as a compatible interaction between barley and Bgh (Bushnell et al. 1998). It was found that Stpk-V could prevent haustorium formation, so it mediated the before-haustorium formation resistance (Cao et al. 2011). In this study, a single-cell transient expression assay was conduced to evaluate the resistance effect of Stpk-V in the barley–Bgh system using the ‘haustorium index’ at 48 hai as parameter. The haustorium index was 79.7% in epidermal cells of the susceptible barley variety ‘Hua30’ transformed with the GUS gene. However, when the cells were co-transformed with GUS and Stpk-V, the haustorium index decreased to 42.7%, indicating that over-expression of Stpk-V could increase resistance of barley to Bgh (Fig. 4).
In the Arabidopsis–G. orontii host–pathogen system, we examined the effect of Stpk-V through stable transformation. Transgenic plants expressing Stpk-V were produced by introducing pCAMBIA1301:Stpk-V into the susceptible accession Col-0 via Agrobacterium-mediated transformation. Molecular screening and GUS staining in T1 and T2 generation plants were used to identify positive transgenic plants. Positive transgenic T3 generation plants were used to evaluate the resistance effect of Stpk-V to G. orontii infection. The phenotype of the leaf and growth of hyphae after pathogen inoculation were compared between the three homozygous transgenic lines and the wild-type Arabidopsis for powdery mildew resistance evaluation. Both wild-type Arabidopsis and the transgenic lines were totally covered in hyphae. To further confirm the results, we performed microscopy observations of the inoculated leaves, and no difference was detected in the development of spores between the wild-type Arabidopsis and the transgenic lines (Fig. 5). The results indicate that expression of Stpk-V in Arabidopsis could not improve its resistance to powdery mildew.

Discussion
Different effects of Stpk-V on powdery mildew resistance in monocots and dicots
The cereal powdery mildew fungus Blumeria graminis is classified into four formae speciales (f. sp.) based on their strict host specialisation: B. graminis f. sp. hordei on species of Hordeum, f. sp. tritici on Triticum, f. sp. secalis on Secale and f. sp. Avenae on Avena. Bgt and Bgh diverged about 10 MY ago, which was 2 MY later than the divergence of wheat and barley, based on analysis of the gene space region and transposons in the genomes of Bgt and Bgh (Oberhaensli et al. 2011). It has been proposed that there was originally a common, temporary Blumeria graminis formae that was able to infect both wheat and barley, which then evolved into forms specific to wheat or barley.
Due to the relatively close evolutionary relationship between the two hosts, wheat and barley, and between the two pathogens, Bgt and Bgh, there should be some common resistance pathways shared by the two hosts and activated by similar factors shared by the two pathogens. Broad-spectrum resistance can be obtained from genes that recognise conserved effectors produced by different isolates of the same pathogen (Dong 1998). The Stpk-V gene was shown to be located on chromosome arm 6VS, and the three orthologous genes Stpk-A, Stpk-B and Stpk-D were located to chromosomes 6AS, 6BS and 6DS of wheat, respectively (Cao et al. 2011). In this study, the orthologous genes of Stpk-V were located on the homoeologous group 6 chromosomes in many cereals, and the Stpk-V gene conferred resistance not only to different isolates of Bgt but also to the mixed population of Bgh. It is proposed that the Stpk-V gene can perceive the upstream signals induced by the conserved factors of Bgt and Bgh, and then activated downstream signals leading to the effective resistance to powdery mildew in the two hosts. It is also suggested that the resistance mechanism of the ancient gene Stpk-V to B. graminis established before the evolutionary divergence of Bgt and Bgh. It will be interesting to see whether the Stpk-V gene confers resistance to other formae speciales of B. graminis.
Golovinomyces orontii is a powdery mildew pathogen specific to Arabidopsis and other dicot species. The evolutionary relationship of Bgt and G. orontii is relatively more distant than that of Bgt and Bgh, and the genetic relationship of wheat and Arabidopsis is also far from that of wheat and barley. So, some factors shared by Bgt and Bgh might not be shared by G. orontii, and the resistance pathway activated by some genes in wheat and barley might not be established in Arabidopsis. It was found that overexpression of Stpk-V conferred no resistance to G. orontii in Arabidopsis. Stpk-V may be specific for monocot signalling or Blumeria resistance, based on results that the current construct (Maize Ubi Promoter:: Stpk-V) could not improve resistance of Arabidopsis.
Broad-spectrum resistance mediated by Stpk-V
The ROS pathway plays an important role in Stpk-V-mediated resistance
The ROS bursts occur at early stages after pathogen infection and later induce HR, resulting in rapid cell death in tissue immediately surrounding the infection sites to limit development of the pathogen (Torres et al. 2006). In our previous study, the most up-regulated gene in response to Bgt at 24 hai in Haynaldia villosa was an oxalate oxylase gene (OxO), a ROS-generating enzyme that can oxidise oxalate and thus generate H2O2. In this study, we also found that some ROS producing genes were activated continuously or specifically only in the T5 line. It was revealed from histochemical analysis and DGE information that the ROS burst was activated in the early basal resistance stage, both in susceptible Yangmai158 and resistant T5 transgenic plants; however, a high percentage of the H2O2 burst in the later specific resistance stage occurred only in resistant T5 plants. This implies that the early and transient ROS burst in the basal resistance stage made no long-term contribution to powdery mildew resistance in Yangmai158, while the continuous production of ROS is critical for regulation of HR leading to Pm resistance in Stpk-V transgenic plants, and the disease resistance conferred by Stpk-V may partially result from its constitutive regulation of the ROS-producing pathway (Fig. 6).
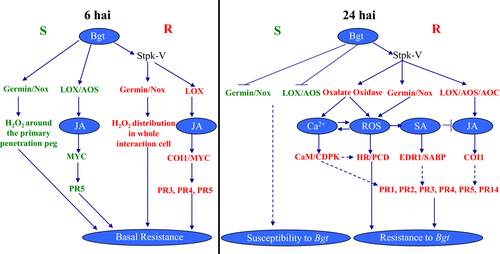
In our previous study, Stpk-V was characterised as a key member of the Pm21 gene, which could be induced by Bgt and exogenous H2O2, but also mediated an increase of endogenous H2O2, leading to cell death and powdery mildew resistance, when the plant was attacked by Bgt (Cao et al. 2011). Therefore, we examined whether ROS pathway was blocked by DMTU or activated directly by H2O2 in the wild-type transgenic recipient Yangmai158 and the Stpk-V transgenic lines, whether the ROS response or resistance to Bgt changed and how the related pathway gene acts. The results help us to further understand the relationship between the Stpk-V-mediated resistance and the H2O2-mediated ROS pathway. The results showed that there were similar response trends in Yangmai158 and Stpk-V transgenic lines, however, the percentages of H2O2 burst cells and the percentage of cell death in Stpk-V transgenic lines was higher than that in Yangmai158, which implied that Stpk-V was a critical gene for H2O2 accumulation and took part in regulation of the hypersensitive responses.
Both SA and JA play important roles in the resistance pathway
Our study suggests the importance of both JA and SA pathways in Pm resistance mediated by Stpk-V in wheat. For the JA signalling pathway, the DGE data analysis showed that JA biosynthesis and signal pathway-related genes were activated in both T5 and Yangmai158 at 6 hai, however, it was activated continuously only in T5 not in Yangmai158 at 24 hai. This was confirmed by qRT-PCR for five genes that are involved in JA biosynthesis and the signalling pathway (Fig. 1c). Hence, the continuous activation of the JA pathway may be critical to powdery mildew resistance mediated by Stpk-V. For the SA pathway, unlike the JA pathway, the signalling gene SABP2 and downstream marker PR genes PR1, PR2, PR3, PR4 and PR14 (Table S3) were activated, but the biosynthesis of SA was not activated in the T5 line (Fig. 1b).
It has been suggested that the activation of important signal transduction factors in the SA pathway, but not the initiation of SA synthesis, can also play an important role in disease resistance (Lin et al. 2004; Lu et al. 2013). In this study, we found that overexpression of Stpk-V does not result in the increased expression of any known genes responsible for biosynthesis of SA (Fig. 6). However, SABP2, a critical gene responsible for converting the biologically inactive MeSA produced in the plastid to active SA in the cytoplasm during SAR (Kumar 2014), was up-regulated through Stpk-V. Transformation of a SABP2-like gene directly induced resistance in plants, and knockdown of this gene made transgenic tobacco plants less resistant and defective in mounting a robust SAR response, suggesting an important role for SABP2 in disease resistance (Kumar & Klessig 2003).
Perhaps Stpk-V is activated by specific factors in wheat after Bgt inoculation
Overexpression of some resistance genes or the key factors involved in the signalling pathway sometimes result in automatic expression of quantitative downstream genes, even without pathogen inoculation, and, unfortunately, the plants usually showed some unfavourable phenotypes as a by-product of the increased resistance. In this study, the hypersensitive response occurred rapidly on leaves after Bgt inoculation in the Stpk-V transgenic wheat, however, no such changes were observed from the microscopy examination without Bgt infection, and no abnormal phenotypic changes in the plants were detected in the greenhouse or in the field. DGE analysis showed that only 180 genes were differentially expressed between T5 and Yangmai158 in the absence of Bgt infection, however, 1,768 genes were differentially expressed between the T5 and Yangmai158 lines 24 h after Bgt inoculation when the hypersensitive response in leaves had become obvious. So, the result of bioinformatics analysis corresponded to the results of histochemical and phenotypic observations. Through a backcross using the Stpk-V transgenic plant as donor parent, the Stpk-V gene was transferred to seven Pm susceptible varieties with different genetic backgrounds that are widely cultivated in China. Comprehensive evaluation of the high generation of backcross plants implied that the resistance was successfully introduced to each variety without any adverse changes in any of the combinations, which suggested that Stpk-V has huge potential for genetic engineering.
Pathogen-associated molecular pattern-triggered immunity (PTI) could be induced both in resistant and susceptible material. In the susceptible plants, PTI was blocked by the effector-triggered susceptibility, but weak ETI was triggered. However, in the resistant plant, genes involved in the PTI were found in the effector-triggered immunity (ETI) stage (Jones & Dangl 2006). In this study, most of the genes (82%) activated in the early stage were still up-regulated in the later infection stage in the resistant Stpk-V transgenic plant, while only 16% of the genes induced in the early stage were still up-regulated in the later infection stage in the susceptible Yangmai158. It was proposed that the transformed Stpk-V gene in wheat could not automatically induce expression of the downstream genes; however, Stpk-V appeared to activate the resistance pathway, after detection of conserved factors from pathogen Bgt or guard genes in wheat, when inactive Stpk-V protein changed into an active structure that not only induced a large amount of new gene expression at the later infection stage, but also maintained gene expression induced in the early infection stage, leading to increased resistance (Fig. 6). A yeast two-hybrid library of Haynaldia villosa inoculated with Bgt was constructed and several interacting proteins of Stpk-V screened. The proteins modified by or modifying Stpk-V are now being studied.
Acknowledgements
The research was supported by the Natural Science Foundation of China (31171540, 31471489, 31671685), Fundamental Research Funds for the National Central Universities (KYZ201601, KYYJ201602, KYZ201401), Important National Science & Technology Specific Projects of Transgenic Research (2013ZX08002001-007, 2014ZX0800202B-002), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and Jiangsu Collaborative Innovation Center for Modern Crop Production (JCIC-MCP). Aizhong Cao, Peidu Chen, Xiue Wang and Chen Qian participated in design of the experiment. Chen Qian, Chaofan Cui and Jin Xiao took part in statistical analysis. Chen Qian, Liping Xing, Chaofan Cui, Xiaojuan Wang, Chuanyu Zhou and Meina Li performed gene expression analysis, gene transient assay, gene transformation and powdery mildew evaluation. Ping Hu and Ruochen Li conducted the histochemical observation. Chen Qian, Liping Xing and Aizhong Cao wrote the manuscript. All authors have read and approved the final manuscript.




