Does water availability regulate biomass partitioning between trunk and branches?
Abstract
- The extent to which a vertical trunk is differentiated from its branches is a key trait for the architecture of trees and may affect interspecific relationships.
- In this study, we analysed the effect of soil water availability on biomass partitioning for Nothofagus pumilio by means of a nursery experiment. Juvenile trees were subject to three irrigation conditions: no irrigation, intermediate irrigation and high irrigation. Irrigation conditions emulated the mean precipitation of the most representative environments inhabited by N. pumilio.
- Changes in soil water availability modified the biomass partitioning patterns of trees. In comparison to the other two conditions, high irrigation caused: (i) a higher ratio of biomass partitioning to stems than roots; (ii) more trunk growth in relation to its branches; and (iii) more photosynthetic organs relative to the aboveground biomass. Trunk size relative to that of its most recent branches was not increased by water availability.
- Water availability may play a significant role in the capacity of N. pumilio for space occupation due to the effects on axis differentiation.
Introduction
One of the features that define the architecture of a plant is the extent of differentiation among the axes that build up the plant's branching system. Such differentiation is a key determinant of the ecological role of the plant in its environment (Poorter et al. 2006; Charles-Dominique et al. 2015). In the case of trees, the differentiation of the trunk, or vertical axis, from its branches is the main determinant of their architecture (Hallé et al. 1978) and modulates the dominance–suppression relationships between individuals (Charles-Dominique et al. 2012; Puntieri et al. 2013). Trees with a clear differentiation between trunk and branches may show faster height growth and would thus be able to reach the forest canopy earlier than trees of similar biomass but with a poorly differentiated vertical axis (Stecconi et al. 2010; Hérault et al. 2011). Such differentiation may be numerically evaluated through the relationship between the size of branches and that of their bearing trunk, using biomass or other related variables such as stem diameter (Puntieri et al. 2013).
It has been shown that tree architecture may exhibit intraspecific variations between individuals growing under different environmental conditions (Hallé et al. 1978; Passo et al. 2002; Vennetier et al. 2013; Magnin et al. 2014). However, scientific evidence concerning the actual factors involved in architectural plasticity is lacking. Better knowledge on those factors involved in intraspecific architectural plasticity may improve our understanding of the effects of climate variations on plant structure, as well as plant–plant interactions. Because of the importance of water as a factor frequently limiting plant growth (e.g., Singer et al. 2012; Brunner et al. 2015), water availability could be considered as one important environmental factor modulating architectural traits in trees. Plant plasticity in response to changes in water availability is a central issue in ecological and physiological studies (Reynolds et al. 2004; Martínez Pastur et al. 2007; Lambers et al. 2008; Padilla et al. 2009; Peri et al. 2009), but information concerning the effects of water availability on the architectural plasticity of woody plants is scarce.
One of the perspectives employed in the scientific evaluation of the effects of resource availability on plant growth is that of the functional partitioning theory, which proposes that plants should allocate resources to increase their uptake of the resource that is most limiting to growth (Poorter et al. 2012). In environments where the dry period of the year matches the growing season, between-year changes in soil water availability due to variations in precipitation can have significant effects on resource partitioning (Reynolds et al. 2004; Padilla et al. 2009; Martínez Pastur et al. 2011). Plants would respond to variations in water availability by shifting the allocation of resources to different organs so as to maximise growth rate (Poorter & Nagel 2000 and references therein), which influences carbon sequestration and cycling (Binkley et al. 2004). However, most of the studies in support of this view have focused on annual plants and grasses (Sher et al. 2004; Maestre & Reynolds 2007). There is limited information about the effects of different water availability on the resource partitioning of woody plants beyond the seedling stage and under natural growth conditions. In woody plants, water resources can affect compartmentalisation and biomass partitioning not only between aboveground and belowground organs, but also among the axis categories that make up aboveground and belowground branching systems (e.g., Singer et al. 2012; Brunner et al. 2015), thus playing a role in axis differentiation. Such differential partitioning of resources among axis categories, would allow a plant to adapt to new environmental conditions and modulate dominance–suppression relationships among plants (Charles-Dominique et al. 2012). For instance, those individuals that grow with higher soil water availability could dominate the tree canopy faster, as they would proportionally allocate more biomass to the trunk with respect to the branches.
In the present study we experimentally evaluated the effect of soil water availability on the biomass partitioning between stem and roots, and the differentiation of the trunk relative to branches for Nothofagus pumilio (Poepp. et Endl.) Krasser (known as ‘lenga’), a tree species widely distributed in the temperate Andean forests of southern South America. Through controlled irrigation, we emulated the variations in water supply that normally occur in the most representative environments inhabited by N. pumilio (dry, mesic and moderately wet sites). Based on previous studies from temperate and mesic regions, we may predict that N. pumilio trees would allocate more biomass to stems relative to roots as water availability increases (Poorter & Nagel 2000; Lencinas et al. 2007; Padilla et al. 2009). Regarding axis differentiation, there is no experimental background on how water availability affects the architecture of N. pumilio trees. A previous study on N. pumilio indicates that trunk length and volume are higher in wetter areas such as valley bottoms or sites with abundant precipitation than in drier and more exposed areas (González et al. 2006; Stecconi 2006). It has also been pointed out that trunk forking through the development of co-dominant distal branches, which is related to the loss of trunk differentiation, is promoted in N. pumilio trees under drier or deeply shaded conditions (Stecconi 2006; Stecconi et al. 2010). We hypothesise that water availability plays a significant role in the partitioning of resources among axes in this species, increasing the size of the trunk relative to that of its branches.
Material And Methods
Study species
Nothofagus pumilio grows in the Patagonian Andes between 35°35′ S and 55°31′ S along diverse environmental conditions (González et al. 2006). At the southern end of its distribution, mean annual temperature is around 6 °C and precipitation is evenly distributed throughout the year; in more northern regions of its range, rainfall is concentrated in autumn–winter and mean annual temperature is close to 12 °C at lower elevations (Villalba et al. 1997; Conti 1998). The present study was carried out at 41° S where the total annual precipitation decreases along a west-to-east gradient, reaching 4,000 mm in western, Chilean areas, and around 500 mm in the eastern border limiting the Patagonian steppe (Conti 1998). However, at this latitude most N. pumilio is distributed between 800 and 1,800 mm isohyets (De Fina 1972; González et al. 2006). The altitudinal range of N. pumilio at this latitude (between 1,000 and 1,800 m a.s.l.) encompasses significant variations in temperature and precipitation (Rusch 1993; Villalba et al. 1997). N. pumilio grows on different soil types, but predominantly on those of volcanic origin (trumaos; Gerding & Thiers 2002). The morpho-architectural variations of this species could be related to phenotypic (Barrera et al. 2000; Stecconi et al. 2010) and/or genetic factors (Premoli et al. 2004, 2007). Spontaneous regeneration of N. pumilio is commonly observed in canopy gaps (González et al. 2006).
Experimental design
The study was conducted on 5-year-old N. pumilio plants derived from seeds collected from different natural populations in the Chubut Province of Argentina (42°50′–44°53′ S, 71°10′–71°40′ W). These specimens, about 90 cm tall and 12 mm in diameter (Table 1), had been raised in pots at the tree nursery of the Instituto Nacional de Tecnología Agropecuaria, INTA, Bariloche (41°07′ S, 71°14′ W, 790 m a.s.l.). This site is located near the eastern limit (dry) of the natural range of this species. We selected an area of 13 m2 located under the canopy of 7-m tall juvenile and adult N. nervosa and N. obliqua trees that were at least about 5 m from one another. This canopy provided an intermediate level of shading (canopy opening between 44% and 46% for all treatments was determined using hemispherical photographs analysed with the GLA program), in an attempt to emulate those canopy conditions under which N. pumilio regenerates in native forests. Precipitation in the area is similar to that recorded for areas at the same latitude where natural regeneration of N. pumilio occurs (Heinemann et al. 2000). Six 1.2 m × 1.8 m plots were established in a row. The plots were separated from each other and from the surrounding soil with phenolic panels that were buried 80 cm in the ground. Each panel was placed protruding 10 cm above ground level to prevent water flow between plots. Soil characteristics in the plots were similar to those derived from volcanic ash deposition (N: 0.24%; Organic matter: 5.7%; P: 10 mg kg−1; K: 244 mg kg−1; Electric conductivity: 0.6 ds m−1; pH-H2O: 6.2; pH-sodium fluoride: 9.7; Ferrari 2010). Ninety specimens of N. pumilio without obvious signs of damage were selected and planted in the plots in winter 2009. In each plot, 15 randomly selected individuals of N. pumilio were planted in five rows with three individuals each (minimum distance between trees = 30 cm).
| treatment | height (cm) | diameter (mm) |
|---|---|---|
| no irrigation | 94.8 ± 5.8 | 12.1 ± 0.5 |
| intermediate irrigation | 94.2 ± 7.6 | 11.9 ± 0.5 |
| high irrigation | 91.1 ± 6.6 | 12.1 ± 0.6 |
In October 2010 three water supply regimes were established. Each condition was assigned to two non-contiguous plots (i.e., 30 plants per treatment). These conditions were as follows: (I) no irrigation (or control); water supply depended exclusively on precipitation; (II) intermediate irrigation, precipitation during the experiment supplemented by an amount of water equivalent to the mean local precipitation; and (III) high irrigation, precipitation during the experiment supplemented by twice the mean local precipitation (Fig. 1). Conditions II and III emulated, respectively, precipitation in mesic and moderately wet sites during the growing period of N. pumilio (October–March). To estimate the amount of water to be added in conditions II and III, we used the mean precipitation record from the weather station at INTA-Bariloche over the last 30 years (~800 mm annual precipitation, i.e., a relatively dry site for N. pumilio). From 2010 to 2012, plants were irrigated through the growing season (6 months every year) at 14- and 7-day intervals for conditions II and III, respectively, so that, by the end of the growing season, they had received approximately 200 and 400 mm more water than plants in condition I (Fig. 1). To check whether the water supply treatments were actually modifying water availability, predawn leaf water potentials (obtained with a pressure chamber, PMS 1000; PMS Instruments, Corvallis, OR, USA) were measured for the three treatments in the period of greatest drought in the growing season. These water potentials (no irrigation: −1.8 MPa, intermediate irrigation: −0.96 MPa, high irrigation: −0.57 MPa), agreed with bibliographic records for the same species under different water regimes (Peri et al. 2009).
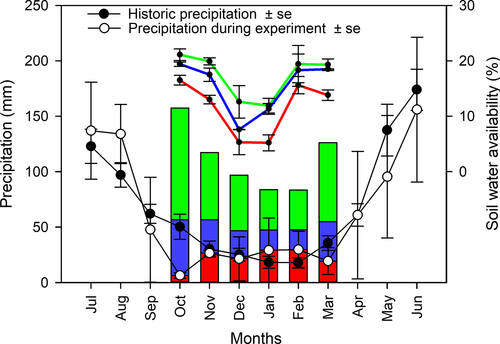
Measurements
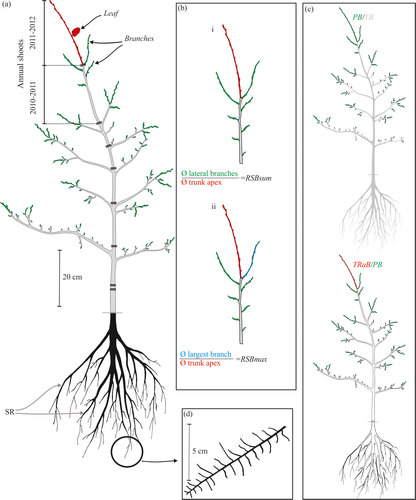
In May 2012 (after complete leaf shedding), two growth seasons after establishing the irrigation experiment, all plants were harvested. The roots were dug up carefully with spades, rakes and brooms, so as to minimise damage to fine roots. The annual shoots that were developed on the trunk of each plant in the 2010–2011 and 2011–2012 growth seasons were identified using morphological markers widely used in Nothofagus spp.: cataphyll scars and main branch positions (Barthélémy et al. 1999). For the trunk annual shoot developed in 2010–2011, the number of leaves (counted based on scars left by the leaf petiole on the stem after leaf abscission) and the axillary production (presence or absence of branch) at each node were recorded (Fig. 2a). Basal stem diameters were measured for the most distal shoot of the trunk and each of the most recently developed branches (those extended in 2011–2012) using digital calipers (Fig. 2a). For the belowground structure, we measured lengths and basal diameters of at least ten secondary roots (i.e., those derived directly from the major root; Fig. 2a); each major root had 14.5 ± 0.1 (mean ± SE) secondary roots. In two secondary roots per plant, we measured the number of tertiary roots on a 10-cm long distal segment (Fig. 2d).
To obtain a proxy for soil water availability, we took one sample per plot every week during the experimental period (about 10 cm below ground level). For each sample, we calculated the gravimetric water content (%) corresponding to each treatment by measuring the weight of a soil sample before each watering, and re-measuring its weight after drying the sample at 105 °C for 48 h.
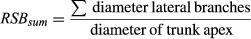
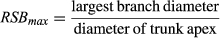
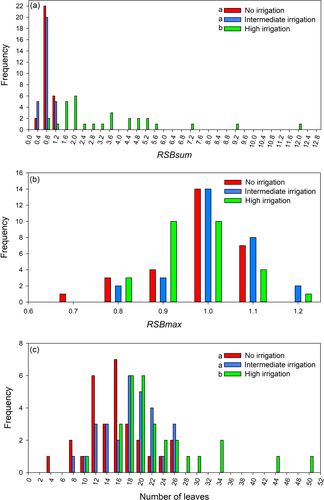
These two variables would provide different perspectives on trunk differentiation, despite the fact that high values of both RSBsum and RSBmax would indicate low differentiation of the trunk relative to its branches. High RSBsum values would indicate the development of a large branching system in relation to the trunk apex (i.e., the trunk's annual shoot), whereas high values of RSBmax would, unlike RSBsum, reflect a tendency towards the formation of a branch, which, judging by its size would compete with the trunk apex for dominance.
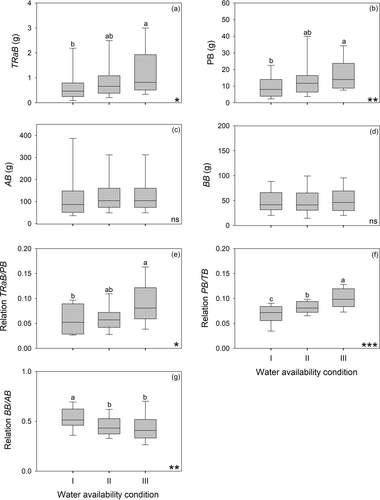
Each plant was divided in the following compartments: (i) annual shoot of the trunk developed in the 2011–2012 growth season, (ii) all annual shoots of the 2011–2012 growth season excluding that of the trunk, (iii) all aboveground axes excluding shoots extended in the 2011–2012 growth season, and (iv) roots (Fig. 2). Each compartment was dried at 60 °C to constant weight. We obtained the following dry biomass compartments: TRaB = trunk apex biomass (i.e., biomass of annual shoot developed in 2011–2012), PB = primary growth biomass, which included all shoots developed in the 2011–2012 season, AB = aboveground plant biomass, BB = belowground biomass (root biomass), and TB = total plant biomass. In this study ‘compartmentalisation of the dehydrated biomass’ is used with reference to the absolute amount of dry biomass in each type of organ, and ‘partitioning’ to the amount of biomass present in one or more organs in relation to the total biomass of the plant or the biomass in all other compartments. The following ratios were computed: BB/AB: biomass partitioning to roots relative to the stem; PB/TB: biomass partitioning to primary growth in 1 year relative to total plant biomass; TRaB/PB: biomass partitioning to the trunk primary growth in 2011–2012 relative to the biomass assigned to the entire primary growth in the same period (Fig. 2c).
Statistical analyses
The RSBsum, RSBmax, root, compartmentalisation and biomass partitioning variables were compared between the three irrigation conditions using one-way ANOVA (with water availability as fixed factor), followed by a posteriori comparisons with Holm-Sidak tests. Due to non-homoscedasticity of the data, RSBsum was evaluated using the varIdent function from the nlme package in R (R Development Core Team 2014), followed by Tukey tests. The relationships BB/AB, PB/TB and TRaB/PB were log-transformed to adjust data to normal distributions. A 5% error probability was adopted in all comparisons.
Results
The RSBsum was significantly higher for plants in condition III than for those in conditions I and II (F = 17.3, P < 0.001); the frequency distribution of this variable was notably right-skewed for condition III, but not for the other two conditions (Fig. 3a). RSBmax was similar between conditions in both mean and distribution outline (F = 2.5, P = 0.09; Fig. 3b). The most vigorous branch was similar to or slightly smaller than the trunk apical shoot, which resulted in high frequencies of RSBmax of between 0.8 and 1.1. The number of leaves in the trunk apical shoot was lower for individuals in conditions I and II than for those in condition III. The frequency distribution for the number of leaves per trunk shoot was more right-skewed in plants under condition III than under conditions I and II (Fig. 3c).
The biomass of shoots developed in the 2011–2012 growth season (i.e., primary growth) increased with water supply, both for the trunk (TRaB) and for the whole tree (PB; Fig. 4a,b). In contrast, total aboveground biomass (AB) and belowground biomass (BB) were not affected by water supply (Fig. 4c,d). The TRaB/PB ratio was higher for individuals under high than low water supply (Fig. 4e). The PB/TB ratio increased with water supply (Fig. 4f). BB/AB was higher for plants in condition I than in conditions II and III (Fig. 4g).
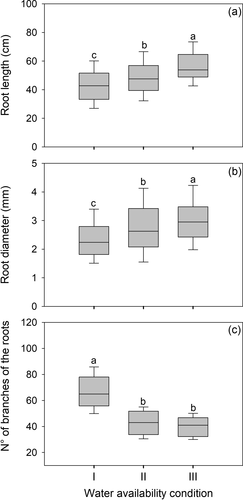
The length and diameter of secondary roots were maximum in plants of condition III, intermediate in plants of condition II, and minimum in those of condition I (Fig. 5a,b). Secondary roots of non-irrigated plants developed more tertiary branches at their distal end than those of plants that received additional water supplies (Fig. 5c).
Discussion
Resource partitioning and trunk differentiation in woody plants
Morphological differentiation between axes is a key determinant of tree architecture and affects use of the aerial space by the crown (Hallé et al. 1978; Barthélémy & Caraglio 2007). In particular, the development of a clearly differentiated vertical axis or trunk in many tree species contributes to establish size hierarchies among trees in a population or community (Stevens & Perkins 1992). Axis differentiation is considered as linked to variations in resource allocation to the structural units that make up plant axes (Charles-Dominique et al. 2012). However, there is no general agreement as to how axis differentiation should be numerically evaluated. In this study, we employed two approaches for such evaluation. The first one focused on the compartmentalisation of primary growth in different types of axes at the whole plant level, whereas the second focused on the size of the distal branches relative to the size of their bearing trunk shoot. In turn, for the latter approach we proposed two quantitative relationships by considering either the summed size of all branches arising from the trunk, or only the size of the largest branch developed from the trunk.
Our analysis based on primary growth at a whole plant level supports the idea that a larger water supply during the growth season increases the size of the trunk relative to that of all other tree axes. As different water conditions did not affect tree biomass, the effect of water supply on trunk differentiation at the whole plant level may not be linked to a possible allometric relationship between trunk size and plant size. Similar observations have been recorded in other species subject to different water availability regimes within the same (Lencinas et al. 2007) and other habitats (Padilla et al. 2009; Pizarro & Bisigato 2010).
The results of the present study based on the distal part of the trunk show that the summed size of the distal branches of the trunk relative to the size of the distal trunk shoot (i.e., high value of RSBsum) was higher in plants under the highest water supply than in those under low or intermediate water supply. It is noteworthy that the dispersion of RSBsum was also higher in the former than in the other two conditions. Such differences in RSBsum among conditions would have a morphogenetic basis. In N. pumilio, each newly extended shoot includes a set of leaves that had remained as leaf primordia in winter buds until spring, i.e., preformed leaves (on average 16 leaves for the trunk of juvenile trees; Souza et al. 2000). Sometimes, simultaneous differentiation and extension of additional leaves – known as neoformed leaves – take place after preformation extension in late spring or early summer (Barthélémy et al. 1999). As the number of preformed leaves in N. pumilio winter buds is defined in the autumn preceding bud break (Souza et al. 2000), and the watering treatments started at the beginning of the 2010–2011 growth season, in spring, plants under all three water conditions must have had similar average numbers of preformed leaves. Consequently, differences in number of leaves of the distal trunk shoot and RSBsum linked to the level of water availability should be linked to variations in the development of neoformed organs, both in the distal trunk shoot and in the distal trunk branches. Further support for this idea is provided by the higher degree of skewness of the number of leaves of trunk shoots and RSBsum in plants under high water supply, as the number of neoformed organs developed by annual shoots of woody species usually exhibits a more skewed and scattered distribution than the number of preformed organs, as neoformation proceeds in a step-wise fashion (Guédon et al. 2006). It is interesting to notice that both the RSBsum and the number of leaves of the distal trunk shoot were similar between plants with intermediate and low water availability, which suggests that a threshold level of water availability should be reached for the neoformation process to be triggered. The development of neoformed leaves at the distal end of the trunk (including the distal trunk shoot and distal branches) could be beneficial in terms of light interception. Thus, our results provide support for the long-standing but little supported idea that neoformation is an opportunistic strategy to take advantage of unforeseen favourable conditions for growth (Sabatier et al. 1999; Guédon et al. 2006).
Contrary to our expectations, trunk forking (measured by RSBmax) did not decrease with increasing water supply. Apparently, more water supply favours, to a similar extent, the development of the shoot engaged in trunk length growth, and that of the most vigorous distal branch, so that the co-dominance between these two axis types is kept constant. It has been observed that trunk forking in N. pumilio, as in other Nothofagus species, increases with tree age (Barthélémy et al. 1999) and also depends on light conditions (Stecconi et al. 2010); for instance, trunk forking is more frequent in understorey than in gap-dwelling trees (Puntieri et al. 1999; Stecconi et al. 2010). A similar pattern has been observed for other tree species (Delagrange et al. 2006; Charles-Dominique et al. 2012; Puntieri et al. 2013). It may be proposed that light would be a key factor in the differentiation between the trunk and its most distal branches. Other environmental factors positively correlated with light availability, e.g., exposure to wind, snow accumulation and soil erosion, may have a counteracting effect on trunk differentiation (Villalba et al. 1997; Wardle 1998); the genetic composition of trees that establish under different sets of environmental conditions could also play a role in intraspecific architectural variations (Premoli et al. 2004, 2007).
Resource partitioning and water supply
In woody species growing under markedly seasonal climates, such as N. pumilio, it is possible to differentiate the annual shoots that are formed in a particular year as a result of primary growth (Passo et al. 2002; Magnin et al. 2014). In N. pumilio, as in other deciduous species, primary growth in one growth season provides the origin for all the leaves present in the plant in that period. Consequently, the dry weight of shoots developed in the most recent growth season may be used as an estimate of the photosynthetic activity in that season, and the PB/TB ratio can be interpreted as a measure of the relative biomass partitioning to leaves. This is supported by a number of studies showing that the allometric relationship between leaf mass and stem + root mass does not change with plant size (Poorter & Nagel 2000). In this sense, the high correlation between TRaB and number of leaves per trunk shoot found here (r = 0.70, P < 0.001) provides support for this idea. Following these assumptions, our results would indicate that low water availability limits the development of leaves, and therefore the evaporative area of N. pumilio trees. However, measurements of leaf area and biomass in plants under different water treatments would be necessary in order to provide robust evidence for this idea. Thus, the PB/TB ratio supports the functional equilibrium theory proposed by Poorter & Nagel (2000), which predicts larger relative biomass allocation to the photosynthetic system when water is not the limiting factor.
In our study, neither above- nor belowground biomass in N. pumilio trees was affected by water availability, which could be related to the inertial effect of similar growth conditions for the 5-year period prior to the experiment. However, and despite this inertial effect, biomass partitioning to roots relative to stems (BB/AB) was larger in plants growing with lower water availability, in agreement with many previous studies (Poorter & Nagel 2000; Ammer 2003; Padilla et al. 2009; Poorter et al. 2012; Brunner et al. 2015). For other species of Nothofagus from Patagonia it has been observed that plants allocated more biomass to roots than to shoots when site quality was low in terms of water (Gargaglione et al. 2010) and nutrient availability (Peri et al. 2006; Agüero et al. 2014). On the other hand, the interaction between water and light availability, may impose some limitations to the resource partitioning of N. pumilio (Lencinas et al. 2007). Seedlings of N. pumilio may increase their photosynthesis rates with canopy opening as long as water is not limiting. When light increases and soil moisture becomes a limiting factor (predawn leaf water potential less than –1.7 MPa), the photosynthetic response of N. pumilio seedlings is reduced (Peri et al. 2009). In this sense, it could be expected that biomass partitioning to shoots with respect to roots, and to the trunk apex with respect to other axes, increases with light availability as long as water is not a limiting factor.
In the present study secondary roots of N. pumilio were shorter and thinner, but had more branches in plants subject to lower water supply. Shorter and more branched roots would increase the absorption capacity of water and nutrients in the soil volume that is already occupied by the plant, with a reduced cost in terms of root mass per unit area (Chapin et al. 1987; Wright & Westoby 1999; Lambers et al. 2008). This may indicate that the response of N. pumilio to water shortages involves more intensive soil exploitation rather than extensive soil exploration.
Conclusions
Our results show significant architectural changes in N. pumilio trees after 2 years of experimental conditions with different water availability. Soil water levels as high as those in N. pumilio wet forests contribute to increase: (i) biomass partitioning to stems relative to roots, (ii) primary growth of the trunk relative to other axes, and (iii) primary growth of all stems relative to total biomass. Although the level of differentiation of the trunk apex relative to its distal branches seems to be rather reduced when water availability is high, the development of larger shoots at the distal end of the trunk would provide better conditions for future growth in a competitive environment.
Acknowledgements
We thank M. Stecconi for support in measuring length growth and the Instituto Nacional de Tecnología Agropecuaria, INTA, Argentina, for providing the experimental area and study plants. This study was partially funded by Universidad Nacional del Comahue (B 164) and CONICET (PIP 112-2008010-1026 and PIP 112-2011010-0809).




