Drivers of tree fecundity in pedunculate oak (Quercus robur) refugial populations at the species’ southwestern range margin
Abstract
- The current low latitudinal range margins of many extra-tropical plant species consist of small and scattered populations that persist locally in microrefugia. It remains poorly understood how their refugial distribution affects mating patterns and reproductive success. Here we examine flower and acorn production and their determinants in refugial populations of the widespread European forest tree pedunculate oak (Quercus robur).
- We monitored male flower, female flower and acorn production in 159 adult trees from 12 oak stands over 2 years. We related these and derived parameters to a series of ecological and genetic predictor variables extrinsic (stand size, density and isolation as well as elevation, topography and forest cover) or intrinsic (size, phenology and several genotypic measures) to the target tree.
- Tree fertility was unrelated to extrinsic factors but determined by tree size, although we detected size-independent variation in reproductive investment. Female flower number accurately predicted acorn crop size. Fruit set differed between years, evidencing the existence of pollen limitation at the landscape but not at the local scale. Fruit set also tended to increase with the number of mates of the target tree. We detected no other evidence for genetic constraints on mating.
- Reproduction was triggered by a combination of small-scale and landscape-scale drivers. Although short-distance mating prevailed, limited pollen flow did not appear to significantly constrain reproductive success. The high intrinsic ability of populations to maintain their reproductive capacity may help explain their successful long-term persistence in an adverse broader environment.
Introduction
The current low latitudinal range limits of many temperate and boreal tree species consist of populations that have persisted roughly in place through the multiple glacial-interglacial cycles of the Quaternary (Gavin et al. 2014). Today, these populations typically are restricted to scattered islands of favourable habitat within heterogeneous landscapes, termed microrefugia (Dobrowski 2010). Long-term refugial populations are most often small and so isolated that local extinction events cannot easily be buffered by regional metapopulation dynamics. Their performance and viability thus depends heavily on inherent population characteristics (e.g. effective size, genetic diversity) as well as on constraints imposed by the surrounding landscape (e.g. triggering their spatial distribution or gene flow; Sexton et al. 2009; Hampe & Jump 2011; Levin 2011). Long-term refugial populations are important conservation targets and excellent models for investigating how species can successfully persist over extended periods close to their environmental tolerance limit (Hampe & Petit 2005; Levin 2011; Woolbright et al. 2014). While a rapidly growing number of studies have assessed their relationships with spatial variation in the current climate (Keppel et al. 2012; Hylander et al. 2015), the intrinsic dynamics that have enabled them to persist locally under the constraints of their climate-driven confinement remain poorly understood. Thus, we still have a limited understanding of how patterns of mating and gene flow can influence key components for long-term population persistence in refugia, such as effective population size, genetic diversity and adaptive potential. And we ignore how landscape complexity combines with individual tree traits to result in the distinct fecundity patterns of relictual scenarios (Bacles & Jump 2011; Hampe & Jump 2011).
Long-term refugial tree populations share many features with younger small and isolated populations, such as pioneer stands at the leading range margin or those resulting from anthropogenic fragmentation (Kramer et al. 2008; Bacles & Jump 2011; Hampe et al. 2013). But they also share some specific characteristics that could render them particularly prone to experiencing reduced levels of effective mating and pollen flow, and hence to depending on a favourable fine-scale mating environment for successful reproduction. First, they typically grow many kilometres away from large pollen sources such as extensive populations in the core distribution range. Moreover, their particular refugial habitats usually are located in topographic settings that help maintain a certain minimum humidity (Hampe & Jump 2011), such as shady valleys, gorges or ravines; these habitats often occur in rugged terrain and contain dense vegetation that tends to represent an obstacle to long-distance pollen flow (Damschen et al. 2014; Shohami & Nathan 2014). Finally, their continued persistence at relatively low population size implies that refugial populations probably have undergone genetic bottlenecks and extensive drift. These processes may imply a significant loss of alleles at self-incompatibility (S) loci (Levin 2011) as well as increased post-zygotic inbreeding depression (especially relevant for tree species; Petit & Hampe 2006). Hence, long-term refugial tree populations could experience stronger pollen limitation and less long-distance gene flow than other tree populations that have experienced fragmentation and isolation more recently as a consequence of human activity (Kramer et al. 2008; Bacles & Jump 2011). Detailed knowledge of the ecological drivers that influence tree fecundity, mating success and ultimately fitness therefore can provide key information on the environmental constraints and conservation challenges that such populations face in their current refugial context.
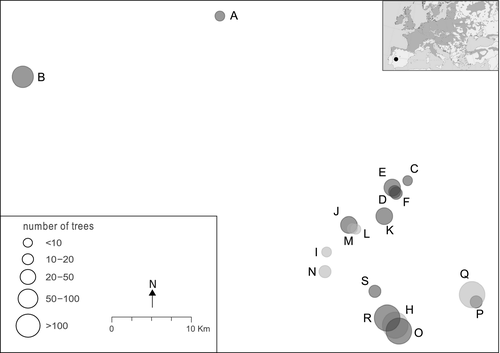
Here we perform a detailed analysis of the patterns as well as the ecological and genetic correlates of tree fecundity and reproductive success in a set of 12 refugial stands of pedunculate oak (Quercus robur) situated at the southwestern periphery of its range (Fig. 1). Pedunculate oak is one of the major European forest tree species and a model organism for tree genetic and molecular ecological studies (e.g. Petit & Kremer 2002; Petit et al. 2004; Plomion et al. 2015). A recent molecular study of our target stands (Moracho et al. 2016) showed that they are remarkably distinct, indicating that they have experienced little effective gene exchange over an extended period of time. Contemporary pollen flow between stands was likewise infrequent while hybridization with the locally abundant sister species Pyrenean oak (Q. pyrenaica) was rare (Moracho et al. 2016). In accordance, the stands’ genetic diversity was relatively low. The present study combines field and molecular data in order to identify intrinsic (tree size, phenology and genotypic measures) and extrinsic (stand size and isolation, neighbourhood density, elevation, topography and forest cover) determinants of tree mating and reproductive success in our study system. For this, we assess patterns of flowering and acorn production at both the stand and the individual tree level. Moreover, we investigate the phenotypes of acorn families from our target trees to assess eventual effects of the small-scale pollination environment (or ‘pollen cloud’) on offspring fitness. Our analysis addresses the following research questions: (i) what are the principal determinants of acorn production, fruit set, functional gender and acorn size (a proxy for offspring fitness) in our study system; (ii) at what spatial scale do these determinants act; and (iii) what is the relative importance of intrinsic versus extrinsic determinants? The ultimate goal of this study, with its tight integration of ecological and genetic perspectives, is to broaden our understanding of the ecological and evolutionary determinants of tree reproductive success and resulting population viability in the particular context of long-term refugia.
Methods
Study system
The southwestern range limit of Q. robur is located in the mountain ranges of central Spain. A comprehensive survey across the north of the autonomous region Extremadura (W Spain, ca. 20,000 km2) identified a total of 18 refugial Q. robur forest stands (Pulido et al. 2007). Large and continuous populations of the species occur in north and northwest Spain. The present study was conducted across 12 of the detected stands, including six from the ten investigated by Moracho et al. (2016). They occur in locally humid environments where the summer dry regional Mediterranean climate is mitigated, most typically along streams where Q. robur coexists with other mesic tree species such as Celtis australis, Castanea sativa and Alnus glutinosa among others. The landscape surrounding this habitat is largely covered with broadleaved forests dominated by Q. pyrenaica where it has not been transformed for horticulture or livestock exploitation. The investigated Q. robur stands vary considerably in size (n = three to 340 adult trees) and level of geographic isolation (Fig. 1).
Quercus robur is a strongly outcrossing, wind-pollinated species with inter-annual variation in seed production that can range from alternating to classical masting. In the study area, the species flowers between March and early April, shortly before bud burst, and acorns ripen in late September and early October. Oak populations exhibit a great ability for long-distance pollen flow (e.g. Craft & Ashley 2007; Buschbom et al. 2011; Hampe et al. 2013). However, different lines of evidence also indicate that pollen limitation could be a widespread phenomenon in oak populations (Knapp et al. 2001; Koenig et al. 2012; Lagache et al. 2013; Pearse et al. 2015).
Field sampling
Female flower production
We selected a total of 159 adult trees from across the 12 stands for our field survey of flower and acorn production (Fig. 1). This sample included 65% of all the adult trees growing in small stands (i.e. with up to 35 individuals) and a minimum of 20 trees in each of the larger stands. Counts were performed in the first week of November 2012 and 2013, when virtually all female flower structures had fallen to the ground but remained mostly intact. The number of female flowers produced by a given target tree was estimated by counting the number of aborted and of fully developed acorn cupules in 0.5 m × 0.5 m quadrats randomly placed beneath the tree canopy. The number of quadrats sampled was roughly proportional to the total crown projection of the target tree (range: 2–8 quadrats per tree) and their summed surface spanned at least 15% of the projected area. Total flower production per tree was then computed by extrapolating the number of cupules counted in quadrats to the full crown projection. We inferred the overall production of female flowers as the sum of aborted and fully developed acorn cupules, while fruit set was estimated as the proportion of the overall female flower production that arrived to produce fully developed acorns. Note that our fruit set estimates could be somewhat inflated due to the difficulty in accurately detecting the earliest flower abortions (even though we spent much effort in identifying them, a task that was facilitated by the conspicuous peduncules of Q. robur).
Male flower production
In early April 2013 (i.e. near peak flowering period), we counted the number of catkins on two randomly selected branches from each of the 159 trees also used for the female flower survey. Counts were performed using binoculars and extrapolated to the total crown surface. The validity of this sampling procedure had previously been tested and corroborated by directly counting the total number of catkins on 37 trees that covered the entire range of flower production in the area. Besides, we collected ten catkins from 12 trees in order to determine the average number of male flowers per catkin (18.5 ± 3.5, mean ± SD) and computed the number of male flowers per tree as the product of the number of catkins and the average number of flowers per catkin.
Floral sex ratio
We used the 2013 field data on female and male flower production to derive the functional gender (Gi) of individual trees. This was done through the function Gi = gi/(gi + ai × E), where gi is the number of female flowers produced by i tree, ai is the number of male flowers, and E = ∑gi/∑ai. E is an equivalence factor that equates the probability of androecial units and gynoecial units contributing genes to the next generation (Lloyd 1979, 1980). Using this procedure, Gi characterises the functional ‘femaleness’ of an individual plant.
Acorn mass
We randomly collected 11 full-sized, mature acorns from each tree in 2013. Their fresh mass was determined by weighing them to the nearest 0.1 mg.
Tree size and flowering phenology
We measured the diameter at breast height (DBH) of all the trees sampled and characterised their flowering phenology on two dates somewhat before and after the peak of the flowering period 2013 (5 and 23 April). Four phenological stages of male flowers were distinguished during each survey: (i) swelling buds (score 0); (ii) emerging and immature catkins (score 1); (iii) mature catkins (score 2); and (iv) old dried catkins (score 3). The phenological stage of the upper and the lower part of each tree was recorded separately and averaged later. Repeating the survey in the following year (2014) allowed us to corroborate that the phenological ranking of trees within stands remains reasonably consistent between years (Spearman rank correlation, rs = 0.38, P = 0.004; see also Bacilieri et al. 1995).
Ecological and genetic correlates of flowering and acorn production
For each of our 12 target stands, we obtained the following variables: (i) size, calculated as the number of adult trees belonging to the stand, and (ii) geographic isolation, measured as the average distance to the other 18 stands. Within stands, we measured the following variables for each target tree: (i) elevation, according to a digital elevation model with a resolution of 5 m (Instituto Geográfico Nacional, distributed by Centro Nacional de Información Geográfica CNIG; http://centrodedescargas.cnig.es); (ii) topographic index (TPI), computed as the difference between the elevation of a focal tree and the mean value of the surrounding landscape (25-m radius) as implemented in package spatialEco function tpi (R Development Core Team 2014). This measure describes whether a tree is located on a top (TPI > 0) or in a hollow (TPI < 0); (iii) percentage of forest cover within a 25-m radius around each focal tree (with the two classes <50% and >50%); and (iv) population density, measured as the number of conspecific trees within the same radius.
All target trees had been genotyped using 20 nuclear microsatellite markers and the procedures are described in detail in Moracho et al. (2016). Based on the trees’ multilocus genotypes, we computed the mean genetic relatedness of each target tree with its conspecific neighbours growing within a 25-m radius using the coefficient of Queller & Goodnight (1989). In addition, we used the individual genotypes of the acorn families from 47 trees reported in Moracho et al. (2016) to calculate, for each mother tree's progeny, the following variables: (i) gene diversity (HE), (ii) mean kinship (computed using the co-ancestry coefficient of Loiselle et al. 1995), (iii) inbreeding coefficient (FIS) and (iv) number of pollen donors (computed as described in Moracho et al. 2016). These variables can be considered to characterise the ‘pollen cloud’ of individual mother trees. All genetic parameters were obtained with SPAGeDi 1.4 (Hardy & Vekemans 2002) except for the number of pollen donors that was based on paternity analyses conducted in CERVUS 3.0 (Kalinowski et al. 2007).
Statistical analyses
Flowering and acorn production
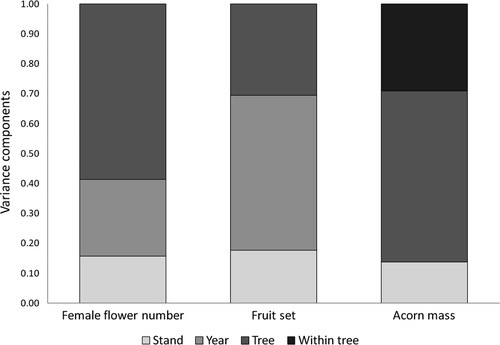
We first tested for correlations between our different response variables (numbers of male and of female flowers, acorn number, fruit set, acorn mass, floral sex ratio). Then we estimated variance components of the female fecundity parameters (female flower number, fruit set and acorn mass) considering four levels: among stands, among individuals within stands, within individuals, and between years. Finally, we used linear mixed models considering a hierarchical nested structure of random effects to assess the statistical support of our variance components analysis. No fixed terms where included in these models. These analyses were performed in R version 3.0.2 using the packages ‘ape’ and ‘lme’.
Ecological and genetic correlates of flowering and acorn production
We attempted to identify both extrinsic and intrinsic determinants of among-tree variation in the three response variables, female flower number, fruit set and acorn mass. Two analyses focused on extrinsic determinants. The first model examined among-stand variation, considering stand size and geographic isolation as predictor variables. The second model examined within-stand variation with elevation, topographic index, forest cover and population density as predictors. Two further analyses focused on trees’ intrinsic determinants (again for the three response variables female flower number, fruit set and acorn mass). The first model included DBH, flowering phenology and mean genetic relatedness with conspecific neighbours as predictor variables. The second model used only the subset of trees for which we had analysed acorn progeny and included the (progeny-based) variables gene diversity (HE), mean progeny kinship, inbreeding coefficient and number of pollen donors.
Finally, we constructed two more models with the floral sex ratio as response variable. The first model considered the extrinsic variables elevation, topographic index, forest cover and population density as predictors, while the second model included the intrinsic variables DBH, phenology and relatedness.
We employed general linear mixed models (GLMMs) in a maximum likelihood framework (Burnham & Anderson 2002) using the lme function (package ‘nlme’) to specify Gaussian errors and the glmer function (package ‘lme4’) to specify Poisson errors when estimating models with the variable female flower number. We considered the stand as a random factor and also the individual tree when fecundity data were available for 2 years (e.g. for female flower number; see Table 1). All predictor variables of the six models were considered as fixed factors.
| 2012 | 2013 | |
|---|---|---|
| Male flower number | – | 3,387,400 ± 3,106,100 |
| Female flower number | 17,000 ± 23,900 | 10,100 ± 15,700 |
| Floral sex ratio | – | 0.455 ± 0.232 |
| Acorn number | 7,400 ± 11,600 | 3,700 ± 7,500 |
| Fruit set (%) | 42.6 ± 18.9 | 33.5 ± 15.6 |
| Acorn mass (g) | 5.9 ± 1.7 | – |
Results
Patterns of flowering and acorn production
Average values for flowering and acorn production are summarised in Table 1. Male flower number was positively related to both female flower number (Spearman rs = 0.68, n = 156, P < 0.0001) and acorn number (rs = 0.64, n = 155, P < 0.0001). Even after accounting for tree size, male and female flower number retained a clear positive relationship (Spearman rs = 0.62, n = 156, P < 0.001). Female flower number closely matched acorn production in both years (2012: rs = 0.92, n = 157, P < 0.001; 2013: rs = 0.93, n = 157, P < 0.001) and floral sex ratio (measured as ‘femaleness’: rs = 0.37, n = 156, P < 0.001) showed a similar albeit weaker trend. Finally, fruit set was unrelated to female flower number (rs = −0.02, n = 157, P > 0.05), male flower number (rs = 0.13, n = 154, P > 0.05) or floral sex ratio (rs = 0.03, n = 154, P > 0.05).
Despite marked between-year variation in the number of female flowers, the fertility ranking of individual trees in 2012 and 2013 remained highly consistent (Spearman rs = 0.84, n = 156, P < 0.001). Accordingly, the variance component analysis revealed that the main source of overall variation in female flower number were differences among trees (CV = 0.59; Fig. 2). Fruit set also varied substantially between the 2 years of study (CV = 0.52), being higher in the year of more abundant female flower production (2012). The ranking of individual trees regarding fruit set was rather consistent between years (Pearson r = 0.60, n = 155, P < 0.001), albeit less than female flower number. Acorn weight varied primarily among trees (Fig. 2). Among-stand variation was low (although statistically significant) for all fecundity parameters (Fig. 2). AIC-based tests indicated that including all these sources of variation as random effects in the models of ecological and genetic correlates significantly improved their fit with respect to the null model.
Determinants of flowering and acorn production
Extrinsic determinants
Stand characteristics influenced the fruit set (Table 2), which increased with increasing stand size and decreased with stronger isolation. Stand size also had a marginal effect on female flower number (P = 0.07), with trees in larger stands producing more flowers. Acorn mass was unaffected by stand identity.
| female flower number | fruit set | acorn mass | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | β | SE | P | |
| Extrinsic among-stand | |||||||||
| Stand size | 0.32 | 0.18 | – | 0.06 | 0.00 | ** | −0.45 | 0.35 | ns |
| Isolation | 0.01 | 0.18 | ns | −0.05 | 0.07 | * | −0.05 | 0.34 | ns |
| Random effects | 1.30/0.39 | 0.10/0.03 | −/0.83 | ||||||
| Sample size (n) | 318 | 318 | 159 | ||||||
| Extrinsic within-stand | |||||||||
| Elevation | −0.29 | 0.19 | ns | −0.01 | 0.02 | ns | 0.23 | 0.26 | ns |
| Topographic index | 0.01 | 0.12 | ns | 0.00 | 0.01 | ns | −0.06 | 0.15 | ns |
| Forest cover | −0.39 | 0.13 | ** | 0.00 | 0.01 | ns | −0.26 | 0.15 | |
| Population density | −0.10 | 0.13 | ns | 0.01 | 0.01 | ns | 0.12 | 0.16 | ns |
| Random effects | 1.27/0.49 | 0.10/0.08 | −/0.74 | ||||||
| Sample size (n) | 220 | 220 | 110 | ||||||
| Intrinsic tree-based | |||||||||
| DBH | 1.00 | 0.13 | *** | −0.01 | 0.02 | ns | 0.62 | 0.18 | *** |
| Phenology | 0.11 | 0.14 | ns | 0.01 | 0.02 | ns | 0.29 | 0.20 | ns |
| Relatedness | −0.08 | 0.11 | ns | 0.01 | 0.01 | ns | 0.02 | 0.16 | ns |
| Random effects | 1.03/0.59 | 0.08/0.06 | −/0.30 | ||||||
| Sample size (n) | 220 | 220 | 110 | ||||||
| Intrinsic progeny-based | |||||||||
| Kinship of progeny | 0.12 | 0.22 | ns | 0.02 | 0.02 | ns | −0.51 | 0.50 | ns |
| Pollen donors | 0.06 | 0.22 | ns | 0.04 | 0.02 | −0.33 | 0.52 | ns | |
| Inbreeding | 0.10 | 0.20 | ns | −0.02 | 0.02 | ns | 0.50 | 0.48 | ns |
| Random effects (SD) | 1.17/0.62 | 0.01/0.04 | −/1.06 | ||||||
| Sample size (n) | 94 | 94 | 47 | ||||||
- Results of GLMM are shown: – P < 0.1, *P < 0.05, **P < 0.01, ***P < 0.001.
Within stands, forest cover was the only relevant variable in predicting female flower number, with more fecund trees occurring in less forested vicinities. We detected no effects on fruit set, while a tendency towards larger acorns occurring in less forested sites was only marginally significant (P = 0.1). Finally, floral sex ratio variation was affected by the elevation of trees’ growing sites, although the slope of the relationship was virtually horizontal (slope = 0.001; Table 3).
| β | SE | P | |
|---|---|---|---|
| Extrinsic | |||
| Elevation | 0.001 | 0.000 | ** |
| Topographic index | −0.001 | 0.009 | ns |
| Forest cover | −0.063 | 0.040 | ns |
| Population density | −0.003 | 0.006 | ns |
| Random effect (SD) | 0.092 | ||
| Intrinsic | |||
| DBH | 0.000 | 0.001 | ns |
| Phenology | −0.068 | 0.039 | – |
| Relatedness | 0.101 | 0.080 | ns |
| Random effect (SD) | 0.135 | ||
- Results of GLMM are shown: – P < 0.1, **P < 0.01.
Intrinsic determinants
Unsurprisingly, female flower number was strongly predicted by the DBH of target trees (Table 2). The DBH was moderately but significantly related to female flower number (rs 2012 = 0.35, rs 2013 = 0.34), male flower number (rs = 0.32) and acorn number (rs 2012 = 0.31, rs 2013 = 0.26; P < 0.01 for all variables). Acorn mass also increased with the DBH, whereas fruit set was unaffected by any of the tested predictor variables (Table 2). Our analysis of the trees with available progeny data revealed instead that fruit set tended to be predicted by the number of pollen donors (P = 0.06; Table 2). In contrast, neither female flower number nor acorn mass showed any relationship with the genetic properties of the progeny. Finally, the floral sex ratio was marginally affected by trees’ flowering phenology (P = 0.08; Table 3).
Discussion
Patterns of flowering and acorn production
We observed ample variation in flower and acorn production as well as in acorn mass. The principal source of variation were differences among individual trees, whereas differences among stands were throughout very minor. Both flower and acorn numbers also differed markedly between the 2 years of study. These patterns of variation largely correspond to those reported from various other oak species in similar environments (e.g. Sork et al. 1993; Perez-Ramos et al. 2015; Pesendorfer et al. 2016). At the level of individual trees, the initial number of female flowers was a powerful predictor of the resulting acorn crop (rs = 0.92–0.93), implying that fruit set in a given year was remarkably consistent among trees (Fig. 2; see also Feret et al. 1982; Sork et al. 1993). At the level of the entire population, however, fruit set was about a third higher in the year with a higher initial abundance of female flowers (2012), indicating considerably more frequent pollination and resulting in a twofold acorn crop. Although based on only 2 years of data, our observation is fully in line with reports indicating that the mating environment largely triggers inter-annual variation in oak acorn crops (‘wind pollination hypothesis’: Koenig et al. 1994; see also García-Mozo et al. 2007; Koenig et al. 2015; Pearse et al. 2015; Pesendorfer et al. 2016). Meteorological conditions may have further supported the observed trend (Bell & Clark 2016) as the accumulated precipitation during the acorn maturation period was three times higher in 2012 than in 2013 (Agencia Estatal de Meteorología; www.aemet.es). Finally, the strong effect of female flower number on acorn crop size probably also triggered the observed (weaker) relationship between floral sex ratio and acorn production.
Determinants of flowering and acorn production
Unsurprisingly, the production of flowers and acorns was consistently related to tree size (estimated via the DBH). This relationship remained virtually unaltered by the observed between-year variation in flowering and fruiting, suggesting that trees of different sizes tended to behave similarly across years (Sork et al. 1993; but see Hirayama et al. 2008). We also observed larger female flower numbers in less forested sites; however, this trend is most probably just a secondary effect that arises from a tendency of larger trees to grow in open sites (E. Moracho, unpublished data).
Male and female flower number retained a remarkably strong positive relationship (rs = 0.62) even after accounting for tree size, indicating that some trees invested systematically more in reproductive structures than others. This phenomenon has repeatedly been shown in oaks and has been related to small-scale variation in growing site productivity (Knops & Koenig 2012; Pérez-Ramos et al. 2014) or microclimate (Koenig et al. 2015). It is in line with the notion that, for long-lived organisms, a plastic resource-tracking response to environmental fluctuations may be more adaptive than directly linking life-history traits through trade-offs such as well-defined functional genders (Knops & Koenig 2012; see also Petit & Hampe 2006). Fruit set stood out from the other tree fecundity measures as the only parameter that was primarily determined by stand-level characteristics. Both the size and the geographic isolation of stands affected fruit set in a way that points to the existence of pollen limitation at a landscape scale (cf. Fig. 1). Fruit set hence appears to underlie the same constraints as previously shown for the rate of between-stand pollen flow (Moracho et al. 2016). In contrast, we found very little evidence for pollen limitation at finer spatial scales despite using several ecological and genetic indicators (population density, tree phenology, genetic relatedness, number of mates, progeny kinship and inbreeding). The only eventual and very indirect indication consists in the fact that fruit set showed a marginally significant trend (P = 0.06) of being higher in those trees that received pollen from a larger pool of mates. Our inability to detect local-scale pollen limitation is at odds with several other studies in oaks (Knapp et al. 2001; Koenig et al. 2012; Lagache et al. 2013; Pearse et al. 2015) that have led to increasing awareness of the phenomenon (Koenig & Ashley 2003; Friedman & Barrett 2009). The absence of local-scale pollen limitation in our system could also explain why we failed to detect any influence of phenology on fruit set, whereas Koenig et al. (2012) reported clear evidence for stabilising selection on flowering time that arises from pollen limitation of very early or late flowering individuals.
Acorn size also was positively related to tree size, a phenomenon that has previously been reported in detail by Koenig et al. (2009). This relationship could be of particular relevance in our refugial context because larger acorns are known to produce better performing offspring (Gómez 2004; Sage et al. 2011), an important fitness advantage in Mediterranean-type environments where early germination and rapid growth can greatly enhance seedling survival with summer drought. Hence large trees would have a double fitness advantage in our context, given that their reproductive success exceeds that of smaller conspecifics, both in terms of offspring quantity and quality.
Consequences for tree reproductive success in a refugial context
Overall, the reproductive success of our refugial oak stands appears to be triggered by a combination of local-scale and landscape-scale drivers. Flower and acorn production depend basically on individual tree size, whereas mating success in terms of fruit set is primarily determined by the size and geographic isolation of the stand. The fact that pollen limitation became evident at the landscape scale but not at the local stand scale allows two inferences: (i) most pollen dispersal appears to occur over relatively short distances (Moracho et al. 2016; see also Knapp et al. 2001; Sork et al. 2002) and (ii) the current population structure with adult trees growing at relatively high density within their refugial habitats still provides sufficient opportunities for effective mating. If these inferences are correct, then our study implies that conservation measures should pay particular attention to securing the inner functioning of local stands (e.g. through securing a sufficient number and density of actively reproducing individuals). Moracho et al. (2016) showed that, at least in years of abundant acorn crops, by far most mating events occur within local stands. This finding, together with the remarkably strong genetic subdivision of stands, indicates that the fecundity of our long-term refugial populations suffers unusually little from incompatibility and inbreeding, which are an otherwise widespread phenomenon in small and isolated tree populations (Petit & Hampe 2006). This intrinsic ability to maintain their reproductive capacity may help explain the successful long-term persistence of refugial tree populations in an adverse larger environment (Hampe & Jump 2011).
Acknowledgements
We thank Gerardo Moreno, Juan Carlos Benito and Cristina Rigueiro for valuable help with the field and laboratory work, and Francisco Rodríguez for statistical advice. This study was funded by an FPI predoctoral fellowship to EM and the project PERSLIM (CGL2010-18381) from the Spanish Ministerio de Economía y Competitividad, as well as a Severo Ochoa Excellence Award (SEV-2012-0262), a Junta de Andalucia Excellence Grant (RNM-5731) and the EU ERA-NET project TipTree (BiodivERsA2012-15).




