Hydration status influences seed fire tolerance in temperate European herbaceous species
Abstract
Prescribed burning is an important management tool in many parts of the world. While natural fires generally occur during the driest and warmest period of the year, prescribed burning is often timed out-of-season, when there is higher soil moisture and lower biomass combustibility. However, fire season may influence seedling recruitment after fire, e.g. through the effect of seed hydration status on fire tolerance. In non-fire-prone temperate regions, anthropogenic fire may occur exclusively in periods outside the growing season with higher soil moisture, which may have negative consequences on seedling recruitment. Fire tolerance of moist and dry seeds of 16 temperate European herbaceous species belonging to four families was assessed using heat treatment of 100 °C for 5 min and subsequent germination trials. Moist seeds of Asteraceae, Poaceae and Brassicaceae had a predominantly negative reaction to the heat treatment, while those of Fabaceae tolerated it or germination was even enhanced. The reaction of dry seeds was completely different, with positive responses in three species of the Fabaceae and fire tolerance in species of other families. Our results point out that hydration status may significantly influence the post-fire germination of seeds. Dry seeds were found to tolerate high heat, while moist seeds were harmed in more than half of the species. This implies that if prescribed burning is applied in temperate grasslands of Europe, it should be timed to dry periods of the dormant season in order to protect seeds from negative effects of fire.
Introduction
Prescribed burning is an important management tool in many parts of the world. It helps to sustain higher biodiversity, as well as populations of threatened and rare plant species (Collins et al. 1998; Pendergrass et al. 1999); it generates post-fire succession, promotes landscape heterogeneity (e.g. Mallik & Gimingham 1983; Fuhlendorf et al. 2009) and controls invasive species (e.g. Cummings et al. 2007; but see Keeley 2006). Fire is also used to decrease fuel levels to control the incidence, size and intensity of natural fires, and so reduce fire hazard (Fernandes & Botelho 2003; Agee & Skinner 2005).
Timing of prescribed burns is an important question for conservation managers, since it influences the survival and post-fire regeneration of plant species, and thus the structure and composition of plant communities (Trabaud & Lepart 1981; Hobbs & Atkins 1990; Howe 1995; Pyke et al. 2010; Céspedes et al. 2014). In fire-prone ecosystems, most natural fires occur during the driest and warmest season, when plant biomass is almost completely dry and, due to its high combustibility, it can serve as fuel for fire. Thus, in the Mediterranean Basin for example, fire peaks in summer, whereas in southern California it peaks in summer or autumn (Keeley et al. 2012). However, many practical, safety and seemingly nature conservation reasons are in favour of timing prescribed burns to the wet season, i.e. early spring or winter, instead of summer or autumn. During such out-of-season burns, the soil is often moist and the air temperature is lower, and there is a lower fuel combustibility, which results in a lower fire intensity. Compared to more severe summer fires, a low-intensity spring or winter fire decreases nutrient loss, prevents or decreases degradation of soil physical properties, reduces damage to micro- and macrofauna and alterations in microbial populations (Neary et al. 1999; Pyke et al. 2010). In addition, the risk of fire spread into other, non-target areas, is also lower. Furthermore, the wet period is the most appropriate for regeneration from seed, since the moisture and temperature conditions are favourable for establishment of seedlings (Baskin & Baskin 2001). If a prescribed burn is timed to the wet period, these favourable climatic conditions overlap with beneficial circumstances induced by fire, e.g. higher resource availability and lower competition, which together may multiply chances of successful seedling recruitment.
However, in fire-prone ecosystems plant species and whole communities are adapted to a particular fire regime: season, intensity and recurrence frequency (Keeley et al. 2012). Alterations of the natural fire regime may have serious consequences on the phenology, survival and regeneration of plant species. In a Mediterranean heathland, Céspedes et al. (2014) found that while fire season did not influence resprouting, it affected seedling recruitment. Early season fire resulted in higher seedling recruitment than late season fire. Based on experimental results on species of the Californian chaparral, Le Fer & Parker (2005) also pointed out that fire season may affect the success of plant recruitment from seeds, since soil moisture content may greatly influence the reaction of seeds to fire. Seeds of 61.5% of the species studied by these authors had lower germination after heat treatments when the seeds were moist, while seeds of only 38.5% of the species suffered from heat treatment if seeds were dry. They concluded that prescribed burns should be timed to the dry season in order not to decrease diversity due to reduced germination and seed survival of certain species (Le Fer & Parker 2005).
In non-fire-prone ecosystems, such as temperate grasslands of Europe, prescribed burning is being increasingly proposed as an alternative to traditional management in situations when sustaining mowing or grazing is no longer feasible (Page & Goldammer 2004; Goldammer 2013; Deák et al. 2014; Valkó et al. 2014). Moreover, uncontrolled (accidental or deliberate) early spring or autumn burning is widespread in Central, Southern-and Eastern Europe, despite the prohibition by law on vegetation burning (Valkó et al. 2014). Under a temperate continental climate, precipitation is most abundant during summer, and plants remain green throughout the growing season. Thus, planned or unplanned anthropogenic burns may occur exclusively in periods outside the growing season but without snow: early spring or late autumn. However, in these periods, soil is commonly moist and soil-stored seeds are hydrated. In our former study of moist, cold-wet stratified seeds of 37 temperate European herbaceous species, we found a very high representation (75.7%) of species with seeds not tolerating fire (Ruprecht et al. in press). Based on the results of Le Fer & Parker (2005) described above, hydrated seeds may negatively react to high heat created by fire. Thus, we hypothesised that the low fire tolerance of seeds that we observed in species of non-fire-prone temperate grasslands may be due not only to a lack of seed adaptations to fire but also to circumstances where seeds are hydrated at the time of vegetation burning.
The goal of this study was to examine the impact of anthropogenic fire occurring during spring or autumn on seed germination of temperate European herbaceous species. The more specific objective was to determine the role of seed hydration status (moist versus dry) on the response of seeds to a heat treatment simulating fire. Dry or moist seeds of 16 temperate European species of grassland and ruderal habitats were heat-treated for 5 min at 100 °C, and their germination then tested. Species of four common families, Asteraceae, Poaceae, Brassicaceae and Fabaceae, were used. Our hypothesis was that, on average, moist seeds are less heat-tolerant than dry seeds. If this is true, a risk exists that spring or autumn burns, which are proposed for land management in temperate grasslands of Europe, may negatively influence the recruitment from seed of many plant species. Understanding the effect of soil moisture at the time of fire can help to predict the response of seeds to prescribed burning.
Material and methods
Species selection and seed collection
We selected 16 herbaceous plant species that are typical constituents of grassland and ruderal habitats of Transylvania, Romania. The species belonged to four common plant families of the region's open habitats (Table 1). Diaspores (seeds or one-seeded, indehiscent fruits) were collected in bulk from autochthonous populations in Transylvania in 2013. Collections included at least 30 different plant individuals from one population each. Seeds or one-seeded fruits, cleaned of appendages, were subsequently dry-stored in darkness at room temperature (ca. 20 °C) until the beginning of the experiment.
| Species | Family | Seed mass (mg) | Water content (%) | Hydration period |
|---|---|---|---|---|
| Artemisia vulgaris | Asteraceae | 0.12 | 57.02 | 1 day |
| Anthemis arvensis | 0.29 | 107.17 | 1 day | |
| Centaurea apiculata subsp. spinulosa | 3.49 | 107.29 | 1 day | |
| Onopordum acanthium | 11.14 | 54.88 | 3 days | |
| Poa annua | Poaceae | 0.27 | 66.67 | 2 days |
| Dactylis glomerata | 0.60 | 45.42 | 1 day | |
| Setaria pumila | 3.87 | 50.26 | 3 days | |
| Hordeum murinum | 4.81 | 42.07 | 7 h | |
| Descurainia sophia | Brassicaceae | 0.09 | 125.64 | 1 day |
| Lepidium ruderale | 0.21 | 246.35 | 7 h | |
| Lepidium campestre | 2.45 | 860.68 | 1 day | |
| Sinapis arvensis | 2.10 | 86.34 | 1 day | |
| Trifolium arvense | Fabaceae | 0.43 | 4.83 | 1 day |
| Melilotus officinalis | 1.84 | 21.49 | 1 day | |
| Astragalus cicer | 2.99 | 6.68 | 1 day | |
| Vicia cracca | 11.01 | 37.41 | 3 days |
Germination experiment
First, we divided the seeds of each species in two groups. The first batch of seeds was hydrated and the second was kept dry. In order to determine the necessary period for hydration of seeds, we put 100 seeds of each species into Petri dishes on moistened filter paper and hourly, later daily, measured the mass of the seed batches and checked germination of the seeds. We calculated the hydration period of seeds for each species as the time elapsed from the beginning of hydration until the seeds reached their ‘maximum’ hydration (mass of 100 hydrated seeds did not change between two consecutive measurements) or the first seed germinated from the 100 examined. Hydration took from 7 h to 3 days, depending on the species (Table 1). This time period was considered sufficient for the seeds to imbibe water (to become hydrated) and be able to germinate. We determined the water content of seeds at ‘maximum’ hydration by subtracting dry seed mass of 100 seeds from the mass of these 100 seeds while hydrated, and calculated the water content ((moist seed mass – dry seed mass) × dry seed mass−1).
In order to find out how seeds react to intense heat during fire, dry and hydrated seeds were subjected to a heat treatment of 100 °C for 5 min in a temperature-controlled oven. We chose this heat treatment because, from several heat treatments (80 °C for 1 min, 100 °C for 1 min, 100 °C for 5 min and 120 °C for 1 min) we applied to seeds of 37 temperate European herbaceous species in our previous study, seed responses to the 100 °C for 5 min treatment proved to correlate with their response to experimental fire (Ruprecht et al. in press). The experimental fire applied by us in our previous study simulated a grassland fire with moderate fuel load (365 g m−2), which leads to low to moderate heat dosage (Ruprecht et al. 2013). After the heat treatment, seeds were sown into 1-l pots (ca. 10 cm × 10 cm × 10 cm) filled with a 2:1 mixture by weight of commercial potting soil and sand. Each species × seed treatment (heat treated dry or hydrated) had six replicates, and each pot contained 40 seeds. As a control for both treatments, we installed pots with 40 seeds each in six replicates per species with untreated (not subjected to heat treatment) seeds.
The pots were placed in an open-air facility in the University Botanical Garden of Cluj-Napoca, arranged in three blocks. Blocks were represented by wooden frames covered with a transparent polyethylene sheet, and surrounded by a fine mesh to protect against animals. Pots were watered regularly. Germinated seeds (radicle protruding from the seed coat) and appearing seedlings were counted and removed once a week from the end of March (first germination events) until late November 2014 (no further germination). At the end of the experiment we calculated the cumulative germination of seeds at pot level. We did not check the viability of those seeds that failed to germinate, thus we do not know whether these seeds were damaged by heat or remained dormant.
Data analysis
We used linear mixed model to analyse the effect of experimental treatments, plant family and species identity on germination percentage of seeds. We included plant family, species nested within family, treatments and the interactions of plant family and species with treatments as fixed factors. Block was included as a random term in the model. Germination percentage was arcsin square root-transformed prior to the analysis. We followed the protocol of Zuur et al. (2009) to find the minimum adequate model: first the random part, and then the fixed effects were simplified, excluding non-significant terms based on ML test. Finally, a model containing only the significant fixed effects was used to estimate parameters. Significant differences between treatments across all species, within families and for each species separately were examined with Tukey's HSD post-hoc tests. All analyses were performed using the R statistical environment, version 3.0.3, using the lme function in the ‘nlme’ package and glht function in the ‘multcomp’ package (R Core Team 2014).
Results
Hydrated seeds prepared for the experiment differed greatly in their water content, with values ranging from 4.83% to 860.68% (Table 1). We found the lowest water content in species of the Fabaceae, and highest values in species of the Brassicaceae (Table 1).
Our treatments had a significant effect on seed germination of the tested species (Table 2). Across all species, the germination of moist seeds decreased on average by 24.79% with the 100 °C heat treatment for 5 min, compared to the control (Tukey's post-hoc test; control versus moist heat: diff = 0.33, P < 0.0001), while the germination percentage of heat-treated dry seeds did not differ from that of control seeds (control versus dry heat: diff = 0.01, P = 0.828). The germination percentage of moist seeds was significantly lower (on average by 23.44%) than that of dry seeds (moist versus dry heat: diff = 0.32, P < 0.0001) after the heat treatment.
| Source of variation | df | MS | F | P |
|---|---|---|---|---|
| Species (Family) | 12 | 13.27 | 1.11 | <0.0001 |
| Family | 3 | 10.34 | 3.45 | <0.0001 |
| Treatment | 2 | 6.73 | 3.37 | <0.0001 |
| S (Fa) × T | 24 | 10.13 | 0.42 | <0.0001 |
| Fa × T | 6 | 4.29 | 0.72 | <0.0001 |
| Residuals | 240 | 2.97 | 0.01 |
There was a significant interaction between treatment and family, which means that plant families responded differently to applied treatments (Table 2). Moist seeds of both Asteraceae and Poaceae species responded negatively to heat, while their dry seeds tolerated the heat treatment (Tukey's post-hoc test; Asteraceae, control versus moist heat: diff = 0.57, P < 0.0001; control versus dry heat: diff = 0.06, P = 0.753; Poaceae, control versus moist heat: diff = 0.55, P < 0.0001; control versus dry heat: diff = 0.03, P = 1.000). The germination of both moist and dry seeds of Brassicaceae species was reduced by the heat treatment, but moist seeds suffered more from the heat (Tukey's post-hoc test; control versus moist heat: diff = 0.28, P < 0.0001; control versus dry heat: diff = 0.15, P = 0.001). Fabaceae was the only family where we found positive responses of seeds to the heat treatment. While moist seeds did not respond to the treatment (Tukey's post-hoc test; control versus moist heat: diff = 0.08, P = 0.265), the germination of dry seeds was stimulated by the 100 °C heat treatment applied for 5 min (control versus dry heat: diff = 0.14, P = 0.001).
Together with a strong family effect in the reaction of seeds to the heat treatments, species-specific responses emerged within each family (Tables 2, 3). However, the response of species to the heat treatments varied, there were five main response types (Fig. 1). Dry seeds of 50% of the species tolerated heat, but the germination of their moist seeds was decreased by heat treatment and, interestingly, there was no species with an opposite response (Table 3, Fig. 1). Both dry and moist seeds of three species of the 16 (19%) tolerated the heat treatment (Fig. 1b), and there was only one species, Lepidium campestre, in which both moist and dry seeds responded negatively to the heat treatment (Table 3, Fig. 1). Positive responses occurred exclusively in species of the Fabaceae, with seeds of three of the four species being stimulated by the heat treatment when seeds were dry (Fig. 1d), and in the case of one species, Trifolium arvense, the germination of moist seeds was higher after the heat treatment compared to the control (Table 3, Fig. 1). It is important to note that there was only one species, L. campestre, in which dry seeds had a significantly lower germination percentage after the heat treatment, while germination of moist seeds of 56.25% of the species was negatively affected by the 100 °C heat treatment for 5 min, simulating a grassland fire (Table 3).
| Species | Family | Heat-treated dry seeds | Heat-treated moist seeds |
|---|---|---|---|
| Artemisia vulgaris | Asteraceae | no | – – |
| Anthemis arvensis | no | – – | |
| Centaurea apiculata subsp. spinulosa | no | − | |
| Onopordum acanthium | no | no | |
| Poa annua | Poaceae | no | – – |
| Dactylis glomerata | no | – – | |
| Setaria pumila | no | no | |
| Hordeum murinum | no | − | |
| Descurainia sophia | Brassicaceae | no | – – |
| Lepidium ruderale | no | − | |
| Lepidium campestre | − | − | |
| Sinapis arvensis | no | no | |
| Trifolium arvense | Fabaceae | no | + |
| Melilotus officinalis | + | no | |
| Astragalus cicer | + | no | |
| Vicia cracca | + | no |
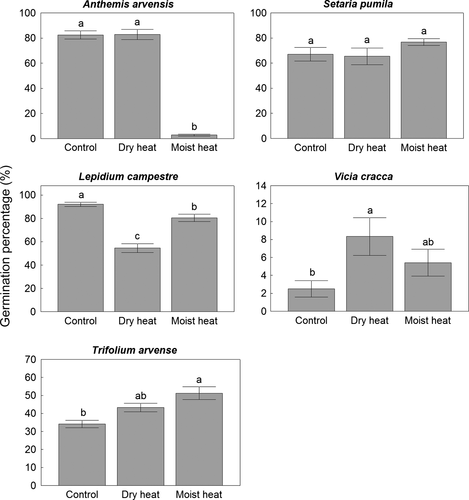
Discussion
In a previous study of 37 temperate European herbaceous species, we found that the effect of a 100 °C heat treatment for 5 min on seed germination correlated with that of an experimental fire (Ruprecht et al. in press). The experimental fire in our previous study, and thus the heat treatment applied in the current experiment, simulated the effect of a grassland fire with a moderate fuel load (365 g m−2) on seeds at the soil surface. Heat treatment of moist seeds caused a significant reduction in germination percentage of more than half of the studied species, while dry seeds of almost all species tolerated the heat treatment (Table 3). Our results are in line with the findings of Le Fer & Parker (2005), who also found that moist seeds of the majority of species of various families from Californian chaparral were harmed by heat treatments, while heat caused a reduction in germination of dry seeds in only five out of the 13 species. Based on the results of these studies, it appears that hydration status has a similar influence on the fire tolerance of seeds in fire-prone ecosystems and in areas without natural fire regimes.
Seeds of species of Asteraceae, Poaceae and Brassicaceae successfully imbibed water during the hydration period (water content of seeds at ‘maximum’ hydration >42% in all species), probably due to their water-permeable seed coat. The highest seed water content was measured in species of the Brassicaceae, with remarkable values such as 246.35% in the case of Lepidium ruderale or 860.68% in the case of L. campestre. This may be surprising, however, as seeds of species in this family produce a pectinaceous mucilaginous layer around the seed, which is hydrophilous, they rapidly absorb a large quantity of water (Yang et al. 2012). Species of these three families showed prolific germination in control conditions (>50% germination), except for Onopordum acanthium (16.25%) and Sinapis arvensis (30%). After heat treatment, moist seeds of small-seeded species of these families germinated to a very low percentage (Tables 1, 3). The reaction of moist seeds of larger-seeded species of these families to the heat treatment was more varied: some species tolerated it while others were harmed (Tables 1, 3). It seems that water content influenced the reaction of larger seeds to heat, since e.g. the seeds of L. campestre (Brassicaceae) and Centaurea apiculata (Asteraceae) contained a much higher quantity of water at maximum hydration and thus reacted negatively to the heat treatment, compared to the confamilial S. arvensis (Brassicaceae) and O. acanthium (Asteraceae), which tolerated heat.
In support of this pattern, seeds of all Fabaceae species absorbed a small quantity of water (4.8–37.4% of dry mass) compared to seeds of other families, and did not exhibit a negative response to heat treatment while moist. These results are in concert with the findings of Martin et al. (1975) on legumes from the southeastern USA. Low hydration of seeds in the Fabaceae may be related to the hard and often water-impermeable seed coat (Keeley & Fotheringham 2000; Baskin & Baskin 2001; Moreira et al. 2010). Moreover, in three of the four species, germination of seeds was stimulated by heat when seeds were dry, thus they had a higher germination percentage after the heat treatment in relation to the control (Table 3). In one species, T. arvense, heat treatment of moist seeds enhanced germination. This means that there was a difference in dormancy release as a result of heat treatment between dry and moist seeds in species of Fabaceae (see also Hu et al. 2009; van Klinken & Goulier 2013). In fire-prone ecosystems, many species of the Fabaceae are known to require a heat shock in order to break physical seed dormancy and thus, usually, they have prolific germination after fire (Auld & O'Connell 1991; Keeley & Fotheringham 2000; Moreira et al. 2010).
Our results referring to species of Fabaceae are surprising, since no one would expect heat-stimulated germination in species inhabiting non-fire-prone ecosystems (but see Granström & Schimmel 1993; Hanley 2009; Ruprecht et al. 2013). In his study of NW European Genisteae, Hanley (2009) proposed that his studied species might be pre-adapted to the high temperatures associated with fire because of their evolutionary origins in fire-prone ecosystems of the Mediterranean Basin. However, Fabaceae species studied by us have a predominantly Central and Eastern European distribution (Tutin et al. 1964), and they do not originate in fire-prone areas. On the other hand, it is very probable that seeds of Fabaceae tested in our experiment would not react positively to higher temperatures than that applied by us. Vicia cracca, in which dry seeds were stimulated by the heat treatment of 100 °C for 5 min, was not found to be a fire follower in a Quercus suber forest in NE Spain (Pausas 1997). Similarly, germination of moist seeds of the annual T. arvense was enhanced by the heat treatment, while this species usually lacks a positive fire response in fire-prone ecosystems (Trabaud et al. 1997; Valbuena & Trabaud 2001; but see Kazanis & Arianoutsou 2004). This last species was used in one of our former studies, where we found a positive effect of the 100 °C for 5 min heat treatment on germination of cold-wet stratified seeds, while seeds were not stimulated by a higher heat shock of 120 °C for 1 min (Ruprecht et al. 2013). All these data confirm that in the case of hard-seeded species, there is a particular temperature or temperature interval that serves as germination trigger, thus fire intensity is a very important determinant of regeneration success (e.g. Auld & O'Connell 1991; Auld & Bradstock 1996).
Contrary to the predominantly negative reaction of moist seeds to heat, dry seeds of all but one species (L. campestre) tolerated the heat treatment simulating fire, or the heat treatment even enhanced seed germination compared to the control. This implies that high soil moisture during burns negatively affects the germination of many plant species that would otherwise survive a fire if their seeds were dry. When seeds are dry, they are likely to be in a ‘glassy’ state, which means that the cytoplasm has extremely high viscosity and low molecular mobility (Murthy et al. 2003). It has been demonstrated that in the ‘glassy’ state, certain deteriorative reactions that occur during seed storage, such as protein denaturation and the formation of free radicals and reducing sugars, are significantly inhibited (Sun & Leopold 1997; Sun et al. 1998). As seed moisture content increases, the seed will undergo a ‘glass-to-liquid stage’ transition, resulting in an increase in molecular mobility. Thus, it is plausible to assume that dry seeds are more tolerant to heat, or any other stress, than hydrated seeds because damaging physiological processes related to high heat stress are slowed or completely blocked when a seed is in the ‘glassy’ state.
Our findings have implications for conservation management planning, and suggest that seeds of temperate European herbaceous species may, in general, tolerate high temperatures on the soil surface during grassland burns if such burns are timed to dry periods of the year. However, dry soil may amplify soil surface temperature during fire, thus seeds would face higher heat dosages than that applied by us, and this would have a larger impact on seed survival of dry seeds. In addition, finding longer dry periods during the dormant season to allow seeds to dry (hydration is a fast process while dehydration probably needs a much longer time) would require fine-tuned planning of prescribed burns in temperate regions having frequent rainfall. It seems that protecting seeds during prescribed burns raises serious difficulties during management planning in temperate grasslands of Europe. In fire-prone ecosystems with a longer dormant season and dry soil, proper timing of prescribed burns in order not to negatively affect recruitment from seed is more feasible.
Acknowledgements
We are very grateful to J. Geréd, R. Kiss, B. Lózer, O. Pál, D. Sándor and C. Szabó for assistance with preparing and accomplishing the germination experiment. We thank the Alexandru Borza Botanical Garden of Cluj-Napoca for ensuring the necessary infrastructure during the experiment. During writing of the manuscript, ER was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences.




