The impact of the Tekay chromoviral elements on genome organisation and evolution of Anemone s.l. (Ranunculaceae)
Abstract
We studied the highly abundant chromoviral Tekay clade in species from three sister genera – Anemone, Pulsatilla and Hepatica (Ranunculaceae). With this clade, we performed a concomitant survey of its phylogenetic diversity, chromosomal organisation and transcriptional activity in Anemone s.l. in order to investigate dynamics of the Tekay elements at a finer scale than previously achieved in this or any other flowering clade. The phylogenetic tree built from Tekay sequences conformed to expected evolutionary relationships of the species; exceptions being A. nemorosa and A. sylvestris, which appeared more closely related that expected, and we invoke hybridisation events to explain the observed topology. The separation of elements into six clusters could be explained by episodic bursts of activity since divergence from a common ancestor at different points in their respective evolutionary histories. In Anemone s.l. the Tekay elements do not have a preferential position on chromosomes, i.e. they can have a: (i) centromeric/pericentromeric position; (ii) interstitial position in DAPI-positive AT-rich heterochromatic regions; can be (iii) dispersed throughout chromosomes; or even (iv) be absent from large heterochromatic blocks. Widespread transcriptional activity of the Tekay elements in Anemone s.l. taxa indicate that some copies of Tekay elements could still be active in this plant group, contributing to genome evolution and speciation within Anemone s.l. Identification of Tekay elements in Anemone s.l. provides valuable information for understanding how different localisation patterns might help to facilitate plant genome organisation in a structural and functional manner.
Introduction
Transposable elements (TEs) are DNA sequences that can change their position within the genome and insert at a new location within the genome of their host in a process called transposition. Retrotransposons are a special class of TE, which transpose via an RNA intermediate, transcribed from an existing element, which is reverse-transcribed prior to insertion. Such a ‘copy and paste’ mechanism of retrotransposon proliferation can rapidly increase the genome size (Hawkins et al. 2009). In contrast, retrotransposon removal through diverse recombination processes can slow down or reverse overall genome growth, contributing to the dynamic balance of host genome size. However, because of the mutagenic consequences of unchecked transposition, the transcriptional and/or transpositional activity of retroelements is repressed in most cases by the host genome through a combination of epigenetic mechanisms involving both transcriptional and post-transcriptional controls (Zilberman & Henikoff 2004; Slotkin & Martienssen 2007). Nevertheless, a low level of transcription is required to establish the transcriptionally silent state of heterochromatin through RNA interference (Matzke & Birchler 2005).
In plants, Long Terminal Repeat (LTR) retrotransposons are considered to be the largest order of TEs. In maize, LTR retrotransposons can make up more than 70% of the genome (Schnable et al. 2009). Their massive propagation and dynamic nature plays a central role in the organisation, function and evolution of plant genomes. The hallmark of LTR retrotransposons is the presence of direct LTRs that surround the internal domains (functional reverse transcriptase and/or other sequences). In plants, LTR retrotransposons have been classified into two superfamilies, Gypsy and Copia, based on domain arrangements of pol genes. The reverse transcriptase (RT) and RNAse H (RH) genes are located upstream of the integrase (IN) gene in the Ty3/Gypsy, and downstream in the Ty1/Copia superfamily. Chromoviruses represent the earliest diverging and most widespread lineage of the Gypsy superfamily. The most characteristic feature of chromoviruses is the presence of a chromodomain at the integrase C-terminus. The four chromoviral clades, CRM, Galadriel, Reina and Tekay, are widely distributed throughout gymnosperms and angiosperms (Kordis 2005). Tekay elements are considered the largest and most diverse group of chromoviruses, ranging in size from 7.7 to 16.7 kb (Gorinšek et al. 2004; Macas & Neumann 2007; Domingues et al. 2012; Weber et al. 2013).
The physical mapping of chromoviruses using fluorescence in situ hybridisation (FISH) yields abundant information on their genomic distribution. It was shown that chromoviruses accumulate in particular chromosomal regions, indicating different target specificities (Weber et al. 2013). Previous studies identified a role for the chromodomain in target site preference through recognition of characteristic chromatin modifications (Gao et al. 2008; Chatterjee et al. 2009). In general, CRM elements recognise a centromeric chromatin-specific marker, while Tekay, Reina and Galadriel elements recognise a marker specific to heterochromatin (Neumann et al. 2011). While the centromeric position is the norm for CRM elements, Tekay elements exhibit both centromeric (Gao et al. 2008; Hřibová et al. 2010) and dispersed localisation throughout the genome (Čermák et al. 2008; Neumann et al. 2011; Weber et al. 2013).
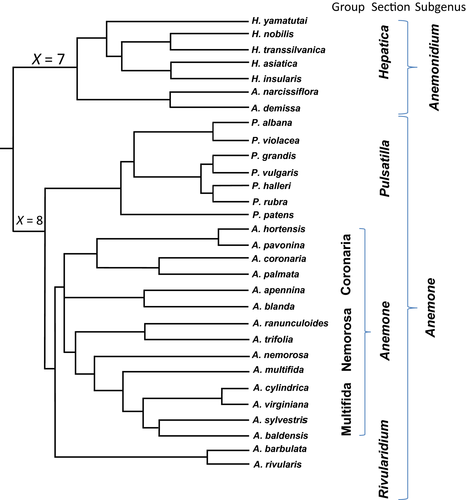
Anemone s.l. (Ranunculaceae) comprises six closely related genera: Anemone, Pulsatilla, Hepatica, Barneoudia, Oreithales and Knowltonia; of which Anemone, Pulsatilla and Hepatica are the subject of the present study. The Anemone genus consists of approximately 150 species (Tamura 1995), mainly distributed in the Northern Hemisphere. The Pulsatilla genus comprises ca. 38 species, which are distributed in northern temperate regions (Tamura 1995). The Hepatica genus is small, comprising about a dozen species disjunctly distributed in the temperate zone of the Northern Hemisphere. Although diploid members of Anemone s.l. contain 14 or 16 chromosomes, diploid nuclear content ranges four-fold, from an average 11.9 pg in A. parviflora (2n = 16) to 46.97 pg in A. narcissifolia (2n = 14) (J. Mlinarec, S. Šiljak-Yakovlev & V. Besendorfer, unpublished data). Large variations in genome size could be explained by differences in the amount of repetitive DNA in the genome of Anemone species. Fluorochrome staining revealed that the karyotypes of Anemone s.l. are characterised by large heterochromatic blocks located at centromeric, intercalary and terminal positions on the chromosomes (Weiss-Schneeweiss et al. 2007; Mlinarec et al. 2012a). The number and position of heterochromatic blocks varies from species in Mediterranean anemones of the Coronaria group, which have large amounts of heterochromatin, to New World anemones of the Multifida group, which have no detectable heterochromatic blocks (Mlinarec et al. 2012a).
The wide range in genome size, complex evolutionary history and well-established phylogeny make Anemone s.l. an excellent model for studying the evolutionary dynamics of the repetitive genome fraction (Mlinarec et al. 2009, 2012a,b). Despite a high proportion of repetitive DNA in Anemone (Rothfels et al. 1966), only a few repetitive DNA sequences have so far been isolated from this genus (Hagemann et al. 1993; Mlinarec et al. 2009). Previously, we isolated three repetitive DNA families from the Mediterranean A. hortensis L., of which the last, named repeat family AhDR, exhibits partial homology with the Ty3/gypsy-like retroelement (Mlinarec et al. 2009). FISH experiments have shown that the AhDR repeat family is dispersed over all chromosomes of A. hortensis, with local clustering in interstitial AT-rich DAPI-positive heterochromatic regions. No repetitive sequences or TEs have so far been isolated from the genomes of Hepatica and Pulsatilla genera.
Here, we focus on the highly repetitive chromoviral Tekay clade in Anemone, Pulsatilla and Hepatica genera of the Ranunculaceae. With this clade, we performed a concomitant survey of its phylogenetic diversity, chromosomal and genomic organisation, structure and transcriptional activity in Anemone s.l. in order to investigate dynamics of the Tekay elements at a finer scale than previously achieved in this or any other flowering clade. For this purpose, we analysed elements in 19 Anemone, five Hepatica and 15 Pulsatilla species to represent closest relatives or representatives of major Anemone s.l. clades. Earlier studies used a similar approach, but were usually based on either single species (Domingues et al. 2012) or a different group of elements (Gao et al. 2012). Data here provide a unique opportunity to obtain an insight into the diversity of TEs in Anemone s.l. Our results indicate that the same retrotransposon lineage could impact the genome in different ways at species level.
Material and Methods
Plant material
Information on all plant material used is given in Table 1. Plants were grown in pots in the Botanical Garden of the University of Zagreb. All species were identified from morphological and karyological characteristics. For karyological studies, actively growing root tip meristems were pre-treated with 0.05% colchicine (Sigma-Aldrich, Taufkirchen, Germany) for 4 h at room temperature, fixed in a solution of ethanol and acetic acid (3:1) for 24 h at −20 °C, and stored in 70% ethanol at −20 °C until use. For PCR amplification and cloning, high quality genomic DNA was isolated from young leaves using the Qiagen mini-kit (Hilden, Germany) according to the manufacturer's instructions.
| taxon | accession No. | origin and/or source | section | distribution | 2n |
|---|---|---|---|---|---|
| Anemone altaica Fisch. Ex C.A.Mey. | 4512B | Halle (Germany), Botanical Garden, University of Zagreb (Croatia) | Anemone | East Asia | 32 |
| Anemone apennina L. | 1446B | Dizdarica (Montenegro) | Anemone | Mediterranean region | 16 |
| Anemone baldensis L. | 698G | Vácrátót (Hungary) | Anemone | Northern Hemisphere | 48 |
| Anemone barbulata Turcz. | 1713 | Halle-Wittenberg (Germany) | Rivularidium | Himalayas | - |
| Anemone blanda Schott et Kotschy | 12418 | BG University of Vienna (Austria) | Anemone | Mediterranean region | 16 |
| Anemone coronaria L. | 1725H | Anecor, Esdraelo Plain, Tel Shiron, 25 km SE of Haifa (Israel) | Anemone | Mediterranean region | 16 |
| Anemone cylindrica A. Gray | 6559B | Chemnitz (Germany) | Anemone | Northern Hemisphere | 16 |
| Anemone demissa Hook & Thomson | 19910632 | Upper Mo Chu District (Bhutan), RBG Edinburgh (UK) | Homalocarpus | Northern Hemisphere | 14 |
| Anemone hortensis L. | 8216R | Island of Hvar (Croatia) | Anemone | Mediterranean region | 16 |
| Anemone multifida Poir. | 1247 | Chemnitz (Germany) | Anemone | America | 32 |
| Anemone narcissiflora L. | 1864L | Baden-Würtemburg, German Alps near Beuron (Germany), Botanical Garden, University of Zagreb (Croatia) | Homalocarpus | Northern Hemisphere | 14 |
| Anemone nemorosa L. | 558 | Dubravkin put, Zagreb (Croatia) | Anemone | Northern Hemisphere | 30 |
| Anemone palmata L. | 12434 | Morgion, Marseille (France) | Anemone | Mediterranean region | 16 |
| Anemone pavonina Lam. | 12724 | Bogdanci, Plajurci (Macedonia) | Anemone | Mediterranean region | 16 |
| Anemone ranunculoides L. | 289 | Delibatska peščara (Serbia) | Anemone | Northern Hemisphere | 32 |
| Anemone riparia Fern. | 314 | Otter Lake, Ottawa (Canada) | Anemone | Northern Hemisphere | 16 |
| Anemone rivularis Buch.-Ham. | 28070 | Himalaya (Nepal), RBG Kew (UK) | Rivularidium | Himalayas | 16 |
| Anemone sylvestris L. | 1451B | Čučerje, Medvednica (Croatia) | Anemone | Northern Hemisphere | 16 |
| Anemone trifolia L. | 562 | Botanical Garden, University of Zagreb (Croatia) | Anemone | Northern Hemisphere | 32 |
| Anemone virginiana L. | 11838B | Quebec, Country Deux-Montaignes (Canada) | Anemone | Northern Hemisphere | 16 |
| Clematis recta L. | 160A | Botanical Garden, University of Zagreb (Croatia) | Clematis | Europe | 16 |
| Hepatica asiatica Nakai | 17631C | Kyeungbuk Province (Korea) | Hepatica | Northern Hemisphere | 14 |
| Hepatica insularis Nakai | 17533D | Cheju Province (Korea) | Hepatica | Northern Hemisphere | 14 |
| Hepatica nobilis Schreber | 556 | Zagreb (Croatia) | Hepatica | Northern Hemisphere | 14 |
| Hepatica transsilvanica Fuss | Htr_HBV | BG University of Vienna (Austria) | Hepatica | Northern Hemisphere | 28 |
| Hepatica yamatutai Nakai | Sichuan Province (China) | Hepatica | Northern Hemisphere | 28 | |
| Pulsatilla albana Steven | 4510A | Vácrátót (Hungary) | Pulsatilloides | Northern Hemisphere | 16 |
| Pulsatilla ambigua (Turcz.) Juz. | 7461B | Oslo (Norway) | Pulsatilloides | Northern Hemisphere | 32 |
| Pulsatilla.armena Boi | 12731 | Kaunas (Lithuania) | Pulsatilloides | Northern Hemisphere | 32 |
| Pulsatilla dahurica (Fisch.) Spreng. | 12732 | Blagovešensk (Russland) | Pulsatilloides | Northern Hemisphere | 16 |
| Pulsatilla georgica Rupr. | 12733 | Tubingen (Germany) | Pulsatilloides | Northern Hemisphere | 16 |
| Pulsatilla grandis L. | 8438B | Veljun (Croatia) | Pulsatilloides | Northern Hemisphere | 32 |
| Pulsatilla grandis var.coccinea L. | 7950C | Sofia (Bulgaria) | Pulsatilloides | Northern Hemisphere | 32 |
| Pulsatilla halleri subsp.slavica Reuss | 11073A | Kaunas (Lithuania) | Pulsatilloides | Northern Hemisphere | 32 |
| Pulsatilla montana Hoppe | 1834N | Meyrinn (Germany) | Pulsatilloides | Northern Hemisphere | 32 |
| Pulsatilla patens (L.) Miller | 12734 | Tubingen (Germany) | Pulsatilloides | Northern Hemisphere | 16 |
| Pulsatilla rubra Lamarck | 4311N | Paris (France) | Pulsatilloides | Northern Hemisphere | 32 |
| Pulsatilla tenuiloba var. sukaczevii Juz. | 12735 | Jena (Germany) | Pulsatilloides | Northern Hemisphere | - |
| Pulsatilla turczaninovii Kryl. Et Serg. | 12736 | Göteborg (Sweden) | Pulsatilloides | Northern Hemisphere | 16 |
| Pulsatilla vernalis (L.) Miller | 8455L | Champex (France) | Pulsatilloides | Northern Hemisphere | 32 |
| Pulsatilla violacea Rupr. | 12589A | Oslo (Norway) | Pulsatilloides | Northern Hemisphere | 16 |
| Pulsatilla vulgaris Mill. | 4254A | Göttingen (Germany) | Pulsatilloides | Northern Hemisphere | 32 |
Amplification and cloning with PCR
The Ty3/gypsy fragments of ~2 kb, amplified as one PCR product, containing both reverse transcriptase (RT), RNase H (RH) and Integrase (IN) domains, were generated using genomic DNA of selected members of Anemone and Pulsatilla species with a degenerative primer pair RT (5′-(CTGGTTCGGCCCA) GTITAWYKTIGAYGAYRTIYTIRT-3′) and IN (5′-(CTCGCTCGCCCA) ICKYTCISWYTGICCRTCISTYTGIGG-3′) described in Suoniemi et al. (1998). Touchdown PCR with the following conditions was applied: after an initial denaturation step at 94 °C for 3 min, amplification was carried out in six cycles consisting of denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s and primer extension at 72 °C for 3 min, with annealing temperature reduced by 1.5 °C per cycle. This was followed by 31 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s and primer extension at 72 °C for 3 min, with final extension at 72 °C for 7 min (Suoniemi et al. 1998). Ty3/gypsy fragments of ~1 kb, amplified as one PCR product, containing RNase H (RH) and Integrase (IN) domains were generated using two primer combinations: (i) AhDR-1 (5′-GAAGGAGTTGAACATGAGACA-3′) and AhDR-2 (5′-GCTTCGTTCCCAAAGCCTTCT-3′) for amplification in Anemone and Hepatica and (ii) RH (5′-GTAATTGCCTATGCCTCTCGGC-3′) and IN (5′-(CTCGCTCGCCCA) ICKYTCISWYTGICCRTCISTYTGIGG-3′) for amplification in Pulsatilla. Primers AhDR-1 and AhDR-2 were designed based on the repetitive AhDR family, which showed similarity to RH and IN domains of Ty3/gypsy retrotransposon and was previously isolated from the genome of A. hortensis (Mlinarec et al. 2009). The primer RH was designed based on the RNaseH domain amplified in Pulsatilla with PCR using degenerative primer pair RT and IN, as described above. The PCR conditions were as follows: after an initial denaturation step at 94 °C for 3 min, amplification was carried out in 30 cycles consisting of denaturation at 94 °C for 35 s, annealing at 54 °C for 10 s and primer extension at 72 °C for 90 s, with final extension at 72 °C for 7 min. All PCR amplifications were carried out in 50 μl reaction mixture containing 10 ng template DNA, 0.4 μm of each primer, 200 μm dNTPs, 2.5 U GoTaq DNA polymerase and corresponding 1× (1.5 mm MgCl2) green reaction buffer (Promega, Madison, WI, USA). PCR products were separated on a 1% agarose gel (Sigma-Aldrich), after which individual DNA bands were purified using Qiaex II Gel extraction kit (Qiagen). When amplifying RT, RH and IN domains, bands ranged from 1.7 to 2.6 kb, while when amplifying only RH and IN domains the bands were approximately 1-kb long. Ligation and transformation procedures were carried out using the InsTAclone PCR Cloning Kit (Fermentas, Leon-Rot, Germany) or pGEM-T Easy Vector System (Promega) according to the manufacturer's instructions. Sequencing was carried out by Macrogen (Seoul, South Korea).
Phylogenetic analysis
All sequences obtained in this study result from cloning and their accession numbers are given in Tables S2 and S3. For translation of DNA sequences, the translate program was used at www.expasy.org/tools/dna.html. Retrotransposon sequences of all other species included in the analyses were retrieved from GeneBank and are listed in Table S1. Sequences were aligned with Clustal X version 1.81 (Thompson et al. 1997).
Two phylogenetic trees were constructed based on the PCR product; one region containing reverse transcriptase (RT), RNAse H (RH) and integrase (IN) regions, and the second containing RH and IN regions. For constructing the phylogenetic tree based on RT, RH and IN domains, we used the Ogre sequence of the Tat family, isolated from Pisum sativum, as outgroup (Neumann et al. 2003; Table S1), while for construction of the phylogenetic tree containing RH and IN sequences, as outgroup we employed the clone CrecRT_IN isolated from Clematis recta by PCR using degenerative prime pair RT and IN (Table S2). MP analysis was carried out using PAUP* 4.0b10 software (Swofford 2003), as an unweighted analysis, using heuristic searching, TBR branch-swapping and 100 random addition sequence replicates. Internal support for different nodes was estimated using non-parametric bootstrap searching (Felsenstein 1985), using 1000 bootstrap replicates with ten random addition sequence replicates each and SPR branch-swapping. The MP tree was obtained using the Subtree-Pruning-Regrafting (SPR) algorithm with search level 5, in which initial trees were obtained by random addition of sequences (100 replicates). The tree is drawn to scale; with branch lengths calculated using the average pathway and in units of number of changes over the whole sequence. All positions containing gaps and missing data were eliminated. Phylogroups corresponding to bootstrap percentages were interpreted as weak (<50%) moderate (50–75%) or high (>75%). The consistency index is 0.348562, retention index is 0.857934 and composite index is 0.331132 (0.299043) for all sites and parsimony-informative sites (in parentheses). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches.
Box plot analysis was done in order to determine sequence homology within each cluster. Calculations were made using Alcula box plot generator, based on estimates of evolutionary divergence between sequences. The number of base substitutions per site between sequences was used. Analyses were conducted using the Maximum Composite Likelihood model (Tamura et al. 2004). All positions containing gaps and missing data were eliminated. Evolutionary analyses were conducted in MEGA6 (Tamura et al. 2013).
Chromosome preparation and fluorescent in situ hybridisation
Chromosome preparations for FISH are described in Mlinarec et al. (2006). FISH experiments were done according to Mlinarec et al. (2012a). The clones Apav5RT_IN, Asyl2AhDR, Avir4AhDR, Abal19RT_IN and PrubRT_IN were used as probes. Probes were directly labelled with Cy3-dCTP (Amersham, GE Healthcare, Little Chalfont, UK) using a nick-translation kit according to the manufacturer's instructions (Roche, Mannheim, Germany). Probes were denatured at 75 °C for 15 min, followed by applying hybridisation mixture on slides and denaturation of slides at 72 °C for 5 min. The preparations were mounted in antifade buffer Vectashield (Vector Laboratories, Peterborough, UK) containing DAPI counterstain (2 μg·ml−1) and stored at 4 °C. Signals were visualised and photographed on an Olympus BX51 microscope, equipped with a highly sensitive Olympus DP70 digital camera. Images were uniformly processed using Adobe Photoshop for colour contrast and brightness. An average of ten well-spread metaphases was analysed for each individual. One to three individuals per taxon were analysed.
Southern hybridisation
To investigate organisation of the Tekay elements in Anemone s.l., Southern hybridisation against endonuclease restricted genomic DNAs was performed using Gene Images AlkPhos Direct Labelling and Detection System (Amersham). A total of 2 μg genomic DNA were digested overnight with restriction endonuclease HindIII or EcoRI (Fermentas). Restricted genomic DNA was size-fractionated in a 1% agarose gel (w/v), blotted onto a nitrocellulose membrane (Amersham) and hybridised with probes according to the manufacturer's instructions. Hybridisation and stringency washes were performed at 55 °C.
Extraction of RNA and reverse transcriptase (RT) PCR analysis
Leaf tissue was collected from plants grown in the Botanical Garden of the University of Zagreb. Total RNA was extracted using TRizol reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer's instructions. Total RNA was treated with DNAse I (New England BioLabs, Hitchin, UK) to remove any residual genomic DNA. An aliquot of 1 μg DNAse-treated RNA was used to generate first strand cDNA using SuperScript II Reverse Transcriptase (Invitrogen) or PrimeScript Reverse Transcriptase (Takara Bio, Tokyo, Japan) according to the manufacturers’ instructions. When using PrimeScript Reverse Transcriptase, 10 μl of reaction mixture I containing 1 μg RNA, 1 mm of each dNTP and 5 μm random hexameres (Roche) were placed in a 2720 thermal cycler (Applied Biosystems, Norwalk, CT, USA) and incubated at 65 °C for 5 min, followed by reverse transcription at 42 °C for 60 min after adding 10 μ; of reaction mixture II consisting of 20 U RNase inhibitor (Roche), 100 U PrimeScript Reverse Transcriptase (Takara) and the corresponding 1× prime script buffer. cDNA dilutions were used in PCR reactions as following: 2 μl cDNA, 10 pmol of each primer and 1× EmeraldAmp MAX PCR Master Mix (Takara) in a total volume of 25 μl. Primers AhDR-1 and AhDR-2 were used in assays of transcriptional activity of elements in A. hortensis, A. coronaria and A. baldensis, while primes RH and IN were used for P. georgica and P. vulgaris. Primers targeting the actin gene (forward and reverse) were used as positive control. To test for genomic DNA contamination, negative control reactions were performed by withholding the PrimeScript RT. Reactions were placed in a thermo-cycler under the following conditions: 95 °C for 2 min and 30 cycles at 94 °C for 1 min, 55 °C for 30 s and 72 °C for 1 min. All reactions were repeated at least twice.
The RT-PCR amplified cDNA fragment of Ty3/gypsy was cloned from A. hortensis using the InsTAcloneTM PCR Cloning Kit (Fermentas) and sequenced by Macrogen. The sequences have been deposited in GenBank, and accession numbers are given in Table S1.
Results
Identification of retrotransposon sequences of Anemone s.l
In order to isolate retrotransposon sequences from selected members of Anemone s.l., PCR amplification was applied using degenerative primer pairs matching reverse transcriptase (RT) and integrase (IN) domains. One to four clones per each species were taken for analysis. In total 30 sequences were isolated from 15 Anemone and four Pulsatilla species (Table S2). In Anemone the sequences ranged in size from 1751–2517 bp, while in Pulsatilla they ranged from 2258–2268 bp. BLAST search showed that all sequences exhibit similarity to reverse transcriptase (RT), RNAse H (RH) and integrase (IN) of the Ty3/gypsy retrotransposon. In order to classify elements, their putative amino acid sequences were aligned and used for phylogenetic analyses, together with the reference TEs (Neumann et al. 2011; Weber et al. 2013). In total, 59 reference elements representing six different Ty3/gypsy retrotransposon clades (Tekay, CRM, Reina, Galadriel, Tata and Athila), distributed across 31 plant species, belonging to 18 plant families and one fungus family were included in the phylogenetic analysis (Table S1). Ogre sequence of the Tat family isolated from Pisum sativum was used as outgroup (Neumann et al. 2003).
Supported by high bootstrap values, all Anemone retrotransposons are assigned to one of the four plant chromoviral clades: the Tekay clade. The other three chromoviral clades (CRM, Reina and Galadriel), as well as non-chromoviruses Athila and Tat, were placed on separate branches (Fig. 1). Within Anemone s.l. the Tekay elements fall into three clusters, named A, B and C (Fig. 1). Cluster A consists of elements isolated from Anemone and Pulsatilla genera, while clusters B and C consist of elements isolated from the genus Anemone. Cluster B consist of members of the Anemone subgenus (x = 8), while cluster C consist of the members of both subgenera Anemone (x = 8) and Anemonidium (x = 7). Cluster A is built from six elements; four elements are isolated from four Pulsatilla accessions (P. rubra, P. grandis, P. grandis forma coccinea and P. albana) while the other two elements are isolated from one member of the sister genus Pulsatilla: A. nemorosa. Cluster B is built from 19 elements isolated from 11 Anemone (x = 8) species and mainly reflects phylogeny (Fig. 3). The elements isolated from A. multifida associate with A. baldensis, whith whom it shares origin, while the Mediterranean anemones of the Coronaria group, A. pavonina, A. hortensis, A. palmata and A. apeninna, form a separate group within the cluster. However, some discrepancies exist within cluster B that do not reflect phylogeny. According to phylogeny, A. nemorosa and A. ranunculoides are sister species and are member of the Nemorosa group, while A. riparia is closely related to A. multifida and thus placed within the Multifida group (Hoot et al. 2012; Mlinarec et al. 2012a). In the phylogenetic tree based on Tekay elements, clones Anem4RT_IN and Anem5RT_IN isolated from A. nemorosa associate with the Rivularis (A. rivularis and A. barbulata) and Multifida (A. multifida and A. baldensis) groups (Fig. 1). Furthermore, clone Arip1RT_IN isolated from A. riparia associates with clone Aran17RT_IN isolated from A. ranunculoides. Finally, clone Apal1RT_IN isolated from A. palmata, a Mediterranean species and member of the Coronaria group, associates with the Multifida and Rivularis groups. Cluster C consists of five elements isolated from A. coronaria and A. palmata, members of the Anemone subgenus; and A. narcissifolia and A. demissa, with members of the Anemonidium subgenus. Cluster C associates with elements isolated from Lilium henryi (Del) and Vitis vinifera (VitV_ID214). All isolated Anemone s.l. elements display similar distances between the catalytic regions (reverse transcriptase/RNaseH/Integrase). However, the main difference lies in the size of the RNazeH. Cluster A lacks the last ten amino acid residues of the RNAseH domain at the C terminus.
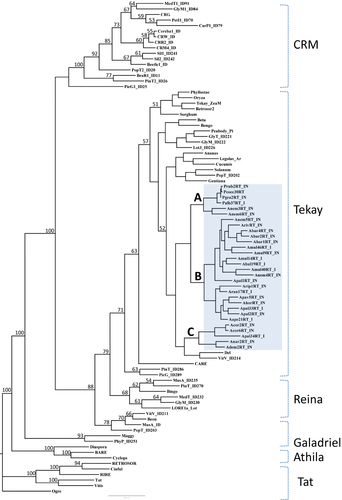
Phylogenetic reconstruction yields six distinct Tekay clusters in Anemone s.l
To provide more insight into sequence diversity and evolutionary relationships among the Tekay elements from Anemone s.l., a bootstrapped maximum parsimony (MP) tree was constructed from DNA sequences containing RNAse H (RH) and Integrase (IN) (Fig. 2). Amplification of the RT-RH-IN region (in total 30 sequences from 15 Anemone and four Pulsatilla species; Table S2) was the basis for design of specific primers for the RH-IN region of Tekay elements. In total, 107 Tekay elements were obtained from 19 Anemone, five Hepatica and 15 Pulsatilla species via PCR; the species are listed in Table 1. One to ten clones per each species were taken for analysis (Table S3). The sequences ranged in size from 833–938 bp. Only one clone isolated from A. ranunculoides was significantly shorter (663 bp). BLAST search showed that all sequences exhibit similarity to RNAse H (RH) and Integrase (IN) of the Ty3/gypsy retrotransposon. In order to classify elements at a finer scale, their DNA sequences were aligned and used for phylogenetic analyses. Longer RT_RH_IN amplicons were also included in construction of the phylogenetic tree. As outgroup we employed the clone CrecRT_IN isolated from Clematis recta, a member of the sister genus Clematis, with PCR using degenerative prime pair RT and IN.
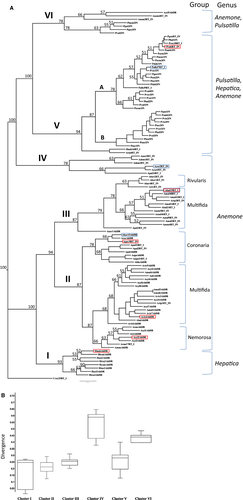
Phylogenetic reconstruction grouped the Tekay elements into six well-supported clusters (Fig. 2). Clusters I, II, III and IV contain members from single genus. Cluster I consists of members of the Hepatica genus, while clusters II, III and IV consist of members of the Anemone genus. Clusters V and VI consist of elements from three and two genera, respectively; cluster V consists of members of the Anemone, Pulsatilla, and Hepatica genera, while cluster VI consist of elements isolated from the members of the Pulsatilla and Anemone genera. Thus, among the six Tekay clusters, cluster V has the largest host range among the Anemone s.l. genomes examined here. Elements from one genome are grouped into multiple clusters. For instance, elements of A. ranunculoides, A. sylvestris, A. coronaria, A. baldensis, A. multifida, A. narcissifolia, P. vulgaris, P. montana, P. patens, P. georgica and H. nobilis fall into two clusters, while elements of A. palmata and A. nemorosa fall within three clusters.
Elements isolated from Anemone species are separated within five clusters (clusters II–VI). However, the majority of Tekay elements isolated from members of the Anemone genus are separated within clusters II (34 elements) and III (17 elements), while clusters IV, V and VI consist only of six, one and three elements, respectively, isolated from the Anemone species. Therefore, only the large clusters are correlated with phylogeny. Interestingly, the topology of these clusters agrees with the phylogeny of Anemone s.l. (Fig. 3). The Tekay elements of cluster II are separated within the Mediterranean Coronaria group, the North American Multifida group and the European Nemorosa group, while the elements of cluster III are separated within the Multifida and Rivularis groups of the Anemone section. However, there is a discrepancy, and it is related to the position A. sylvestris and A. nemorosa. Among six elements isolated from A. sylvestris, two (Asyl4AhDR, Asy6AhDR) associate with A. cylindica and A. virginiana, while three (Asyl1AhDR, Asyl2AhDR, Asyl3AhDR) associate with A. nemorosa, A. ranunculoides and A. trifolia. Furthermore, among five Tekay elements isolated from A. nemorosa, one element (AnemAhDR) associates with A. ranunculoides and A. trifolia, while the other four (Anem3AhDR, Anem4AhDR, Anem5AhDR and Anem6AhDR) associate with A. sylvestris and A. multifida.
Elements isolated from Pulsatilla are separated among closely related clusters V and VI. Cluster V is further subdivided into two subclusters – A and B exhibiting very short branch lengths present at the tips of multiple longer branches. Subclusters A and B have high sequence identity, 87% and 90%.
Within clades, pair-wise sequence diversity were calculated and displayed as a box plot (Fig. 2B; median indicated by a horizontal line, with upper and lower quartiles within the box, while whiskers include minimum and maximum values). Overall, mean divergence ranged from 0.203 to 0.501, being lowest in cluster I (0.203) and highest in cluster IV (0.501). Thus, box plot analysis showed that cluster IV is the most divergent, while cluster I has the lowest evolutionary divergence between the sequences.
Chromosomal localisation of the Tekay elements
For detailed cytogenetic analyses, representatives of Tekay cluster I (HnobAhDR), cluster II (Asyl2AhDR, Avir4AhDR and Apav5RT_IN), cluster III (Abal19RT_IN) and cluster V (PrubRT_IN) were chosen as probes in FISH experiments. Chromosomal distribution of Tekay elements was determined for seven Anemone, six Pusatilla and one Hepatica species (Figs 4 and 5). Among members of all three investigated sister genera, the Anemone species showed the most diverse pattern of retrotransposon distribution within the genome. The largest divergence in retrotransposon distribution was observed in the Mediterranean Coronaria group (Fig. 4A–F). In A. pavonina (Fig. 4A,B), the FISH image of probe Apav5RT_INT showed a pattern of widely dispersed signals along the length of all chromosome arms; however, there was increased hybridisation to intercalary heterochromatin regions (Fig. 4A,B; arrows). Strong signals were detected in both chromatids, indicating clusters most likely originating from a nested organisation. A similar FISH pattern was observed previously in the close relative A. hortensis (Mlinarec et al. 2009). Two other members of the Coronaria group showed a different pattern of retrotransposon distribution. In A. coronaria and A. palmata the hybridisation signal covered every chromosome almost uniformly, but was absent from major heterochromatic blocks (Fig. 4C–F, arrows). In the species that lack visible heterochromatic DAPI-positive AT-rich blocks, such as A. sylvestris and A. virginiana, FISH signals with the probes Asyl2AhDR and Avir4AhDR, respectively, were almost uniformly dispersed over entire chromosome lengths (Fig. 4G,H).
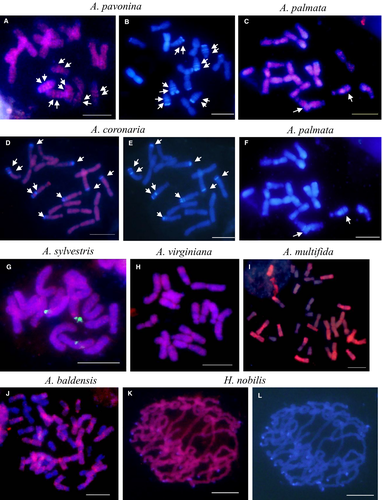
In allopolyploids, A. multifida (2n = 4x = 32; BBDD) and A. baldensis (2n = 6x = 48; AABBDD), FISH with the probe Abal19RT_IN showed a dispersed pattern of retrotransposon distribution in the A and B subgenomes, but no detectable FISH signals in the D subgenome (Fig. 4I,J). Absence of retroelements in one half and one-third of the chromosomes of hybrids A. multifida and A. baldensis, respectively, indicates that this element is absent or has substantially diverged in sequence between A, B and D subgenomes.
In H. nobilis signals with the probe HnobAhDR were uniformly dispersed over all chromosomes, although the signals were not visible in the subtelomeric/telomeric heterochromatic blocks (Fig. 4K,L).
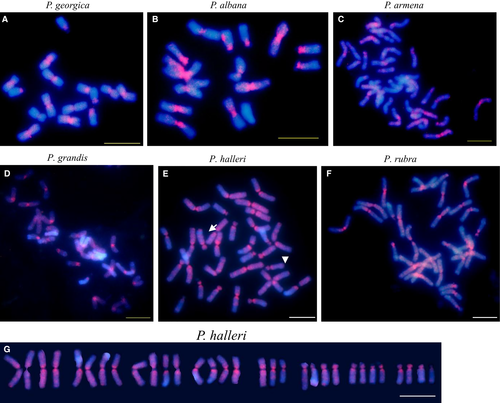
In order to determine the chromosomal position of Tekay elements in Pulsatilla, the probe PrubRT_IN was used for all six Pulsatilla species investigated here because sequence inspection as well as Southern experiments showed high similarity of Tekay elements among all investigated Pulsatilla species. Pulsatilla species showed a similar pattern of retrotransposon distribution (Fig. 5). FISH signals with probe PrubRT_IN were visible in the centromeric/pericentromeric regions on all chromosomes of diploids P. georgica and P. albana and polyploids P. armena, P. grandis, P. halleri and P. rubra (Fig. 5A–G), indicating that the Tekay elements preferentially integrate, or are retained, in the centromeric/pericentromeric heterochromatic regions. However, in Pulsatilla signals were also present interstitially on the arms of some chromosomes, although they were of lower intensity. In polyploids, the interstitial signals were stronger than in diploids (Fig. 5E,G). Among the four investigated Pulsatilla polyploids, the strongest interstitial signals were observed in P. halleri; however, in P. halleri the signals were not present in intercalary heterochromatic AT-rich blocks (Fig. 5E, arrows).
Genomic organisation of the Anemone s.l. Tekay elements
Comparative hybridisation to HindIII- or EcoRI-restricted genomic DNAs was performed to investigate genomic organisation and distribution of the chromoviral Tekay clade in Anemone and Pulsatilla genera (Fig. 6). The representatives of each Tekay cluster, Ahor15AhDR (cluster II), Abal19RT_IN (cluster III), Acor2RT_IN (cluster IV) and Palb37RT_IN (cluster V), were chosen as probes in Southern hybridisation experiments (Fig. 6). As expected, each probe gave the strongest signal with species from which the probe originated. Probe Ahor15AhDR, isolated from A. hortensis, gave the strongest signal with A. hortensis (Fig. 6A). The same probe also gave relatively strong signals with A. coronaria, the closest relative of A. hortensis. All other investigated Anemone species gave weak signals, suggesting high divergence of Tekay cluster II between Anemone species.
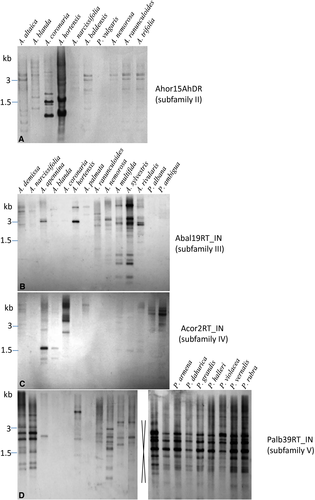
The probe Abal19RT_IN, isolated from the allohexaploid A. baldensis (2n = 6x = 48, AABBDD), gave the strongest signal with A. sylvestris, parental species and donor of the A subgenome of A. baldensis (Fig. 6B). Signals of probe Abal19RT_IN over a wide range of molecular weights were visible for members of the Nemorosa (A. ranunculoides and A. nemorosa) and Multifida groups (A. multifida and A. sylvestris). In members of the Coronaria (A. apennina, A. blanda, A. coronaria, A. hortensis and A. palmata) and Anemonidium (A. demissa and A. narcissifolia) groups one or two bands were observed, suggesting that Tekay cluster III is present in those species with a few copies, and at a single locus. Tekay clusters II and III were not detected in sister genus Pulsatilla, indicating that these clusters are genus-specific, or too diverged to be detected by Southern analysis in Pulsatilla species.
The probe Acor2RT_IN, isolated from A. coronaria, gave the strongest signal with A. coronaria (Fig. 6C). Strong signal was also observed in A. apennina, where one relatively strong band was detected. A band of the same size as those in A. apennina was detected in A. blanda, sister species of A. apennina, although the band was of significantly lower intensity. Interestingly, in A. palmata and A. hortensis, closest relatives of A. coronaria, the signal was very weak. Members of the Anemonidium (A. demissa and A. narcissifolia), Nemorosa (A. ranunculoides and A. nemorosa) and Multifida (A. multifida and A. sylvestris) groups showed no signal with this probe, suggesting high divergence of this Tekay cluster within Anemone.
The probe Palb37RT_IN, isolated from P. albana, gave a banding pattern similar in intensity and band sizes to all nine investigated Pulsatilla species (Fig. 6D). The presence of strongly hybridising fragments in all Pulsatilla species indicates high abundance of Tekay elements with conserved restriction sites present at multiple loci across all species of Pulsatilla. The signals of probe Palb37RT_IN were also observed in members of the Anemone genus, although the signals were of considerably lower intensity when compared with signals observed in Pulsatilla. The only exceptions are A. demissa and A. narcissifolia, where strongly hybridising fragments were observed. In A. blanda, A. coronaria and A. palmata, members of the Coronaria group, no signals were observed. These findings suggest that Tekay cluster V was probably present in the common ancestor of Anemone and Pulsatilla. The results of Southern experiments are consistent with the FISH experiments, which classified the Tekay family as a highly abundant component of the Pulsatilla genome.
Transcriptional activity of the chromoviral Tekay clade
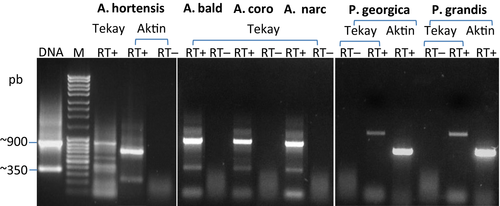
Transcriptional activity of the Tekay elements was assayed via RT-PCR for four Anemone (A. hortensis, A. coronaria, A. narcissifolia and A. baldensis) and two Pulsatilla (P. georgica and P. grandis) taxa. These assays were performed using the same primers used to amplify RNaseH and Integrase domains of Ty3/gypsy retrotransposon. DNA contamination was ruled out by negative control reactions in which the RT enzyme was withheld (Fig. 7). In the majority of investigated species (A. baldensis, A. coronaria, A. narcissifolia, P. georgica and P. grandis) one major band was observed. In Anemone the size of the band was ~900 bp, while in Pulsatilla it was ~1 kb. The band corresponded to PCR amplicons of genomic DNA, indicating transcriptional activity of the Tekay elements in leaf tissues of both diploid (A. hortensis, A. coronaria, and A. narcissifolia) and polyploid (A. baldensis, P. georgica, and P. grandis) Anemone and Pulsatilla taxa. In A. hortensis, additional bands were observed, which could be explained by differences in the enzyme and protocol used (see 2). In A. hortensis, the PCR amplicon was confirmed as a Ty3/gypsy transcript through cloning and sequencing a ~900 bp PCR product. In total, four clones were sequenced. The alignment of transcripts showed a high level of sequence identity (91–97%), as well as high similarity with clone Ahor15AhDR (92–97%). Two out of four clones were in open-reading frames, while the other two possessed one stop codon at the 5′ end. BLAST search of the genomic database showed that transcripts exhibited nucleotide similarity to partial reverse transcriptase, RNaseH and integrase, thus confirming transcriptional activity of the Tekay element in A. hortensis.
Discussion
Evolution of the Tekay clade in Anemone s.l
Phylogenetic reconstruction yielded six distinct Tekay clusters in Anemone s.l. In Anemone and Pulsatilla, the elements were arranged, respectively, into four and three clusters/subclusters (Fig. 2). Arrangement of the Tekay elements in several clusters suggests the existence of multiple ancient lineages of Tekay elements in Anemone s.l. and episodic bursts of activity at different points in their respective evolutionary histories. In Pulsatilla, very short branch lengths present at the tips of multiple longer branches of subclusters A and B suggest recent diversification of this genus. This is further supported by high sequence identity, 87% and 90%, of subclusters A and B, respectively, suggesting a sudden and recent burst of transposition, perhaps within the last one million years, preceded and followed by relative quiescence. Similar banding pattern in all investigated Pulsatilla species obtained with the Southern experiment is in accordance with the RT-IN tree, which provides further support for recent diversification of the Pulsatilla genus (Figs 2 and 6).
The topology of clusters II and III, consisting of elements isolated from 16 and eight Anemone species, respectively, agrees with the phylogeny of Anemone (Figs 2 and 3). Before this study, there was no information on phylogenetic affinity of A. barbulata based on DNA data. This study provides the first molecular evidence that A. barbulata is closely related to A. rivularis. However, there is a discrepancy related to the position of A. sylvestris and A. nemorosa. According to all previous phylogenic analyses (Meyer et al. 2010; Hoot et al. 2012; Mlinarec et al. 2012a), A. sylvestris is placed within the Multifida group and thus is closely related to North American species A. multifida, A. cylindrica and A. virginiana, as well as allopolyploid A. baldensis, with whom it shares an origin (Fig. 3). Furthermore, according to the same phylogeny, A. nemorosa is placed within the Nemorosa group and therefore is closely related to A. ranunculoides and A. trifolia (Fig. 3). In the Tekay tree, among six elements isolated from A. sylvestris, only two (Asyl4AhDR, Asy6AhDR) show the expected association with A. cylindica and A. virginiana, while three (Asyl1AhDR, Asyl2AhDR, Asyl3AhDR) associate with A. nemorosa, A. ranunculoides and A. trifolia. Furthermore, among five Tekay elements isolated from A. nemorosa, only one element (AnemAhDR) shows expected association with A. ranunculoides and A. trifolia, while the other four (Anem3AhDR, Anem4AhDR, Anem5AhDR and Anem6AhDR) associate with A. sylvestris and A. multifida, members of the Multifida group. Therefore, this study clearly shows that A. nemorosa and A. sylvestris are more closely related than previously shown on the basis of atpB-rbcL spacer region, NTS and ITS data (Meyer et al. 2010; Mlinarec et al. 2012a). A. nemorosa is hypoallotetraploid (2n = 4x = 30); to date, studies have failed to reveal the origin of this species. A. sylvestris is a diploid (2n = 16) and parental species of A. baldensis, a member of the Multifida group. One possible explanation for the close relationship between A. sylvestris and A. nemorosa is that in the past they formed hybrids that again backcrossed with their progenitors and/or other members of the Nemorosa and Multifida groups. A. sylvestris (member of the Multifida group) is widely distributed in Northern Europe and Asia, where it shares habitat with A. nemorosa, A. ranunculoides and A. trifolia, members of the Nemorosa group. A. nemorosa is widely distributed in temperate zones of the Northern Hemisphere, and in North America it shares habitats with A. multifida. Thus geographic proximity provides further evidence for hybridisation between A. nemorosa and A. sylvestris. An alternative hypothesis is that horizontal transfer occurred between A. nemorosa and A. sylvestris. Investigations of horizontal transfer (HT) for any of the identified transposable elements (TEs) requires detailed analysis of multiple intermediate species across a precisely chosen set of lineages, with demonstration of more conserved sequences of TEs between two distant relatives than for those TEs in close relatives (Roulin et al. 2009; Estep et al. 2013). Thus, phylogenetic incongruities, as showed here, provide evidence for HT of Tekay elements between members of the Nemorosa and Multifida groups. It is well known that geographic proximity of donor and host is a prerequisite for HT (Sharma & Presting 2014), as is the case with A. nemorosa and A. sylvestris. Several cases of HT involving retrotransposons have been documented (Cheng et al. 2009; Roulin et al. 2009). Exhaustive analysis of centromeric retrotransposons (CR) revealed horizontal transfer of CR elements between the oryzoid and panicoid grass lineages (Sharma & Presting 2014). The exact mechanism of HT is still unknown, as is its contribution to the retrotransposon repertoire and genome evolution in plants. It is suggested that a mechanism similar to viral infection might be involved in HT of retrotransposons between plants (Sharma & Presting 2014). However, an unequivocal statement regarding HT is only possible through analysis of the flanking LTR sequences. Thus, the hypothesis of horizontal transfer occurring between Anemone species should be considered with caution.
Chromosomal localisation of the Tekay elements
We carried out a comprehensive survey of the Tekay clade of chromoviruses in members of Anemone s.l., which provides insight into the genomic and chromosomal organisation, distribution, and evolution of this chromoviral clade. This is the first study providing data on the chromosomal position of Tekay elements among members of a large group of species. Previous studies focused on one (or rarely two) species within a genus showing diverse patterns of chromosomal distribution between different plant groups (Čermák et al. 2008; Gao et al. 2008; Hřibová et al. 2010; Neumann et al. 2011; Weber et al. 2013). The above studies showed that Arabidopsis thaliana (Gao et al. 2008) and Musa acuminata (Hřibová et al. 2010) exhibit a centromeric position of the Tekay elements, while in Pisum sativum (Neumann et al. 2011), Silene pendula and S. vulgaris (Čermák et al. 2008) and Beta vulgaris (Weber et al. 2013) the elements are uniformly dispersed over all chromosomes, including the intercalary and centromeric heterochromatin. In sugarcane, Tekay element Del1 hybridises to a broad region around and within the centromeric heterochromatic region (Domingues et al. 2012). Here, we have shown that a diverse pattern of chromosomal distribution of Tekay elements also exists within a plant group; namely in Anemone s.l. where Tekay elements do not have a preferential position on chromosomes, i.e. they can have: (i) a centromeric/pericentromeric position; (ii) an interstitial position in DAPI-positive AT-rich heterochromatic regions; or can be (iii) dispersed throughout chromosomes or even (iv) absent from large heterochromatic blocks (Fig. 4). The most striking differences in the chromosomal position of Tekay elements were observed between sister species of the Mediterranean Coronaria group. FISH with clone Apav5RT_IN as a probe (representative of Tekay cluster II) showed localisation of this particular element in the intercalary heterochromatic regions of A. hortensis and A. pavonina, while in A. palmata and A. coronaria the same group of elements is dispersed over all chromosomes but absent from the intercalary heterochromatic regions (Fig. 4). Previously we showed that large DAPI-positive heterochromatic blocks present in members of the Mediterranean Coronaria group are constituted of repetitive DNAs, such as satellite DNA and/or retrotransposons (Besendorfer & Mlinarec 2013). Therefore, one possible explanation is that large heterochromatic blocks of the Mediterranean anemones are constituted of different repetitive families. The heterochromatin of A. hortensis and A. pavonina is constituted of Tekay cluster II and satDNA family AhTR1 (this study; Besendorfer & Mlinarec 2013), while in A. coronaria and A. palmata other repetitive DNAs are amplified and become more abundant. It is well known that accumulation of transposable elements in heterochromatin is less deleterious as such insertions are less likely to result in gene disruption. However, the exact mechanism of the accumulation in heterochromatin is not well understood. It is unclear whether localisation of Tekay elements in heterochromatin is a result of heterochromatin targeting or some other mechanism. A previous study showed that in Arabidopsis thaliana the accumulation of retrotransposons in centromeres may be a result of not only targeting but also purifying selection from the centromere distal regions (Pereira 2004). However, not all chromoviruses have a preference for heterochromatin; e.g. the Reina element LORE1 does not appear to have a strong insertion preference for heterochromatin (Fukai et al. 2010).
The evolution of Tekay elements in polyploids
In the allopolyploids, A. multifida (BBDD, 2n = 4x = 32) and A. baldensis (AABBDD, 2n = 6x = 48), only half and two-thirds, respectively, of chromosomes were covered with strong FISH signals using clone Abal19RT_IN, a member of Tekay cluster III, as probe (Fig. 4I, J). These chromosomes belong to the B subgenome of A. multifida, and to the A and B subgenomes of A. baldensis. The chromosomes that showed none or very faint signals with this probe belong to the D subgenome, which is common to both polyploids. Therefore, the results of FISH suggest that the Tekay elements are confined to the A and B subgenomes, while absent or having diverged too much in the D subgenome. Previously, we showed that A. multifida (BBDD, 2n = 4x = 32) and A. baldensis (AABBDD, 2n = 6x = 48) originated from crosses of diploid members of the Multifida (donor of A and B subgenomes) and Baldensis (donor of D subgenome) groups. The A and B subgenomes are closely related to the genomes of A. sylvestris, A. virginiana and A. cylindrica, while the D subgenome is closely related to A. parviflora, indicating that these species, or their progeny, might be parental species of A. multifida and A. baldensis (Mlinarec et al. 2012b; unpublished data). Thus, the A and B subgenome specificity of the Tekay element, as determined with FISH, could be explained by the appearance of genome-specific elements in A. sylvestris, A. virginiana and/or A. cylindrica. The Southern hybridisation experiment, showing a similar banding pattern in lanes with A. multifida and A. sylvestris genomic DNA using clone Abal19RT_IN as probe, supports this hypothesis and suggests that the proliferation of Tekay elements could have mainly occurred in the diploid progenitor before it entered allopolyploidy (Fig. 6B). In A. parviflora, the donor of the D subgenome, other transposable elements might be dominant. The independent investigations of Peterson-Burch et al. (2004) and Zuccolo et al. (2007), showing that in smaller plant genomes the copia elements provide as much or more DNA than gypsy elements, support this hypothesis, as A. parviflora has the smallest genome size among the Anemone species (Soltis et al. 2006).
In this study we showed that in Pulsatilla, polyploids exhibit stronger FISH signals in comparison with diploids when using the Tekay element as a probe (Fig. 5). The most striking difference was in intensity of signals positioned on chromosome arms; namely, polyploids exhibited stronger FISH signals on chromosome arms in comparison with diploids. This finding suggests that in the Pulsatilla polyploids, Tekay elements are more abundant than in diploids. Subsequent to polyploidy, genomes undergo a process of diploidisation, whereby duplicate copies of genes may be lost and the chromosome number may increase (Renny-Byfield et al. 2013). Although global analyses of genome size in angiosperms reveal a trend towards DNA loss subsequent to polyploidy (genome downsizing), an increase in genome size is also known to occur. An increase in genome size is thought to arise via accumulation of repetitive DNA (Renny-Byfield et al. 2013). In Pusatilla, a genome increase subsequent to polyploidy could occur due to amplification of the Tekay elements. In the allopolyploid Nicotiana repanda, an increase in genome size occurred due to expansion of chromoviruses that were already inherited in high copy number from its parents (Renny-Byfield et al. 2013).
Transcriptional activity of Tekay elements
Transposable elements make up a substantial fraction of plant genomes and are often transcriptionally active (Domingues et al. 2012). In this research, RT-PCR experiments demonstrated that the Tekay elements are transcribed in leaf tissues of A. hortensis and very likely transcribed in other investigated Anemone and Pulsatilla species, suggesting that transcriptional activity might be a general feature of Anemone s.l. retrotransposons. Furthermore, it seems that the LTR retrotransposon lineage remains transcriptionally active in the hybrid species A. baldensis, suggesting that allopolyploidisation is likely to have neither positive nor negative effects on the proliferation of retrotransposons in Anemone hybrids. Transcriptional activity of Tekay elements has been detected in other plant species, such as wheat (Salina et al. 2011), sugarcane (Domingues et al. 2012) and beet (Weber et al. 2013). It is known that replication allows transposable elements (TEs) to survive as host parasites, but the higher the replication rate, the lower will be host fitness and, consequently, survival of the elements (Vukich et al. 2009; Domingues et al. 2012). Therefore, the activity of TEs is usually controlled by the host genome through the siRNA machinery. The proliferative nature of TEs enables both sense and anti-sense transcripts to be produced, generating dsRNA, and activating the siRNA system. Two main classes of siRNA, the 21-nt and 24-nt classes, operate at different levels. The 21-nt class regulates post-transcriptionally related mRNAs, while the 24-nt class is involved in heterochromatin maintenance and suppresses gene expression at transcriptional level (Baulcombe 2004). In sugarcane ‘24nt LTR’ pattern was observed for the Tekay element Del1 (Domingues et al. 2012), suggesting that in Anemone Tekay elements are probably regulated primarily at transcriptional level.
Centromeric Tekay elements in Pulsatilla species
The identification of a retroelement that localises to centromere regions of Pulsatilla species poses two intriguing questions for future work. First, is the element part of the functional centromere; and second, do the Tekay elements contribute to centromere function in Pulsatilla species? Additional studies, including high-resolution methods, such as fibre-FISH and ChIP, will be required to unambiguously determine whether the element is part of the functional centromere. As for the second question, we showed that in Pulsatilla, centromeric retrotransposons are probabaly transcriptionally active. The presence of transcripts suggests the capacity of Tekay elements for autonomous transposition. Several RT-PCR studies have identified transcriptional activity of chromoviruses, in particular of CRM clade members (Topp et al. 2004; Neumann et al. 2007, 2011; Weber & Schmidt 2009; Fukai et al. 2010). It is suggested that transcriptional activity of centromeric retrotransposons may be an important mechanism of centromeric chromatin assembly (Neumann et al. 2011). Thus, the chromodomain, as a key component of centromeric retrotransposons, facilitates the targeting process into the centromeric region, and might be therefore be responsible for generation of centromeric transcripts, which are involved in RNA interference-mediated centromere identity and function (Weber et al. 2013).
Conclusions
In our research, we were focused on a member of four chromoviral clades, the Tekay clade, to investigate the dynamics of this clade at a finer scale than previously achieved in this or any other flowering clade. The separation of elements into six clusters could be explained by episodic bursts of activity since divergence from a common ancestor, at different points in their respective evolutionary histories. Identification of Tekay elements in Anemone s.l. provides valuable information for understanding how different localisation patterns might help to facilitate plant genome organisation in a structural and functional manner.
Acknowledgements
We thank D. Mihelj for maintaining the plant material. This work was funded by the Ministry of Science, Education and Sport of the Republic of Croatia, grant no. 119-1191196-1201.




