A transcriptome-wide study on the microRNA- and the Argonaute 1-enriched small RNA-mediated regulatory networks involved in plant leaf senescence
Abstract
Leaf senescence is an important physiological process during the plant life cycle. However, systemic studies on the impact of microRNAs (miRNAs) on the expression of senescence-associated genes (SAGs) are lacking. Besides, whether other Argonaute 1 (AGO1)-enriched small RNAs (sRNAs) play regulatory roles in leaf senescence remains unclear. In this study, a total of 5,123 and 1,399 AGO1-enriched sRNAs, excluding miRNAs, were identified in Arabidopsis thaliana and rice (Oryza sativa), respectively. After retrieving SAGs from the Leaf Senescence Database, all of the AGO1-enriched sRNAs and the miRBase-registered miRNAs of these two plants were included for target identification. Supported by degradome signatures, 200 regulatory pairs involving 120 AGO1-enriched sRNAs and 40 SAGs, and 266 regulatory pairs involving 64 miRNAs and 42 SAGs were discovered in Arabidopsis. Moreover, 13 genes predicted to interact with some of the above-identified target genes at protein level were validated as regulated by 17 AGO1-enriched sRNAs and ten miRNAs in Arabidopsis. In rice, only one SAG was targeted by three AGO1-enriched sRNAs, and one SAG was targeted by miR395. However, five AGO1-enriched sRNAs were conserved between Arabidopsis and rice. Target genes conserved between the two plants were identified for three of the above five sRNAs, pointing to the conserved roles of these regulatory pairs in leaf senescence or other developmental procedures. Novel targets were discovered for three of the five AGO1-enriched sRNAs in rice, indicating species-specific functions of these sRNA–target pairs. These results could advance our understanding of the sRNA-involved molecular processes modulating leaf senescence.
Introduction
Plant microRNAs (miRNAs), mostly associated with Argonaute 1 (AGO1), play key roles in various essential biological processes through cleavage-based regulation of the target genes (Jones-Rhoades et al. 2006; Voinnet 2009). In addition to the miRNAs, the AGO1-associated silencing complexes also recruit other 5′ U-started, 21-nt-long small RNAs (sRNAs) as molecular guiders to perform cleavages on specific target transcripts (Baumberger & Baulcombe 2005; Mi et al. 2008; Vaucheret 2008). Leaf senescence is the final stage of leaf development, and is an important phase during the plant life cycle. The molecular mechanisms underlying the processes of leaf senescence have been extensively investigated (Lim et al. 2003, 2007; Yoshida 2003). Internal factors, such as hormones, external cues, such as nutrient limitation and pathogen attack, transcription factors, such as NAC and WRKY families, and the senescence-associated genes (SAGs) together contribute to a highly complex regulatory network involved in leaf senescence. However, to date, systemic studies on the biological roles of the miRNAs in leaf senescence are limited. Moreover, knowledge on whether other AGO1-enriched sRNAs are implicated in this process through cleavage-based regulation of the SAGs is lacking.
In this study, we intended to uncover the sRNA regulators with potential regulatory impact on leaf senescence. A total of 5123 and 1399 AGO1-enriched sRNAs, excluding miRNAs, were identified in Arabidopsis thaliana and rice (Oryza sativa). In both plants, a large portion of the AGO1-enriched sRNAs are 21 nt in length, and start with 5′ U, which correlates well with the previous report of Mi et al. (2008). After retrieving the cDNAs of the SAGs from Leaf Senescence Database (LSD 2.0; Liu et al. 2011; Li et al. 2014), all of the AGO1-enriched sRNAs and the miRBase-registered miRNAs (release 20; Griffiths-Jones et al. 2008) of the two plants were included for target identification. Based on degradome sequencing data, 200 regulatory pairs involving 120 AGO1-enriched sRNAs and 53 transcripts encoded by 40 SAGs, and 266 regulatory pairs involving 64 miRNAs and 54 transcripts encoded by 42 SAGs were discovered in Arabidopsis. Moreover, 13 genes predicted to interact with some of the above-identified target genes at protein level were validated as regulated by 17 AGO1-enriched sRNAs and ten miRNAs in Arabidopsis. In rice, only one SAG was targeted by three AGO1-enriched sRNAs, and one SAG was targeted by miR395. However, five AGO1-enriched sRNAs were conserved between Arabidopsis and rice. Target genes conserved between the two plants were identified for three of the above five sRNAs, indicating conserved roles of these regulatory pairs in leaf senescence. Novel targets were discovered for three of the five AGO1-enriched sRNAs in rice, pointing to species-specific functions of these sRNA–target pairs. In summary, a comprehensive study on the involvement of miRNAs and other AGO1-enriched sRNAs in the regulation of SAGs was conducted. The above results could advance our understanding of the sRNA-involved molecular processes modulating plant leaf senescence, and could serve as a basis for further experimental investigations.
Material and methods
Data sources
The cDNA sequences of SAGs in Arabidopsis and rice were retrieved from the FTP site (ftp://ftp.cbi.pku.edu.cn/pub/database/LSD_2.0/) of the Leaf Senescence Database (LSD 2.0; Liu et al. 2011; Li et al. 2014). The mature miRNA sequences of the two plant species were obtained from miRBase (release 20; http://www.mirbase.org/; Griffiths-Jones et al. 2008). The gene annotations were retrieved from TAIR (The Arabidopsis Information Resource; release 10; Huala et al. 2001) and MSU Rice Genome Annotation Project (release 7; Yuan et al. 2003). The high-throughput sequencing (HTS) datasets utilised to search for AGO1-enriched sRNAs were downloaded from GEO (Gene Expression Omnibus; http://www.ncbi.nlm.nih.gov/geo/; Barrett et al. 2009). Briefly, in Arabidopsis, the HTS datasets were divided into six groups: (i) GSM149079 (sRNAs from whole extracts) and GSM149080 (sRNA from AGO1 complexes); (ii) GSM642335 (AGO1-associated sRNAs_control) and GSM642336 (AGO1-associated sRNAs); (iii) GSM707678 (flower_control) and GSM707682 (flower_AGO1-associated sRNAs_two-step purification); (iv) GSM707679 (leaf_control) and GSM707683 (leaf_AGO1-associated sRNAs_two-step purification); (iv) GSM707680 (root_control) and GSM707684 (root_AGO1-associated sRNAs_two-step purification); (vi) GSM707681 (seedling_control) and GSM707685 (seedling_AGO1-associated sRNAs_two-step purification). In rice, there are three groups: (i) GSM455965 (seedling_total extracts) and GSM455962 (seedling_AGO1a-associated sRNAs); (ii) GSM455965 (seedling_total extracts) and GSM455963 (seedling_AGO1b-associated sRNAs); (iii) GSM455965 (seedling_total extracts) and GSM455964 (seedling_AGO1c-associated sRNAs). For expression analysis of the Arabidopsis regulatory miRNAs and sRNAs in rice, GSM278532, GSM278534, GSM278535, GSM409318, GSM722128 and GSM943194 were retrieved from GEO, and ShCn2D, ShK2D, ShKNa1I, ShKNa2D, ShN2D, ShP2D and ShS2D were downloaded from Next-Gen Sequence Databases (http://mpss.udel.edu/common/web/library_info.php?SITE=rice_sRNA2&showAll=true; Nakano et al. 2006).
Prediction and validation of the sRNA targets
Target prediction was performed using the miRU algorithm (Zhang 2005; Dai & Zhao 2011) with default parameters. The degradome sequencing data were utilised to validate the predicted sRNA–target pairs. First, in order to allow cross-library comparison, the normalised read count (in RPM, reads per million) of a short sequence from a specific degradome library was calculated by dividing the raw count of this sequence by the total counts of the library, and then multiplied by 106. Second, all the degradome signatures were mapped onto the predicted target transcripts. Then, the previously proposed criteria (Shao et al. 2013) were applied to extract the potential cleavage sites. In summary, (i) ‘Average_Read count_Cleavage site’ is the averaged read count (in RPM) of all the degradome signatures (belonging to one library) with their 5′-ends mapped to a potential cleavage site; ‘Average_Read count_Surrounding’ is the averaged read count of all the degradome signatures (also belonging to this library) that mapped to the regions surrounding the cleavage site; ‘Average_Read count_Cleavage site’ should be five times or more than ‘Average_Read count_Surrounding’. (ii) Also for this degradome library, among the degradome signatures mapped to a potential cleavage site, the most abundant tag should be amongst the top 12 most-abundant degradome signatures that perfectly mapped to the corresponding transcript. (iii) The cleavage site should reside within the 9th to 10th, 10th to 11th or 11th to 12th nucleotide region of the regulatory sRNA. For any degradome library, if the three rules were fulfilled, the potential slicing sites were retained. Finally, both global and local target plots were drawn to perform manual screening, referring to our previous study (Meng et al. 2011). Only the transcripts with cleavage signals easily recognised were extracted as potential sRNA–target pairs.
Results and discussion
Search for the AGO1-enriched sRNAs in Arabidopsis and rice
To do a comprehensive search for the AGO1-enriched sRNAs, HTS data prepared from the AGO1-associated sRNAs were downloaded from GEO (Barrett et al. 2009). Then, the HTS datasets were classified into six groups in Arabidopsis and three groups in rice (Fig. 1; see also 2 for details). We adopted the following criterion to extract the AGO1-enriched sRNAs: for each group, the accumulation levels of an AGO1-enriched sRNA should be ten RPM or higher in the ‘AGO1’ dataset, and should be undetectable in the ‘control’ dataset. As a result, 1267, 62, 516, 171, 3175 and 157 AGO1-enriched sRNAs were discovered in the six groups of Arabidopsis, and 77, 830 and 545 AGO1-enriched sRNAs were identified in the three groups of rice. Then, for each plant, the AGO1-enriched sRNAs extracted from different groups were combined. The redundant ones and the miRBase-registered miRNAs (release 20; Griffiths-Jones et al. 2008) were removed. For the following analysis, sRNAs shorter than 31 nt were retained (four sRNAs were removed in Arabidopsis and none in rice). As a result, a total of 5,123 and 1,399 AGO1-enriched sRNAs were identified in Arabidopsis and rice, respectively (Data S1 and S2).
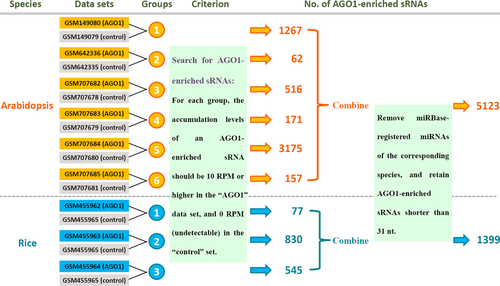
Next, the sequence characteristics of the 5123 and 1399 AGO1-enriched sRNAs were analysed separately. Quite consistently, in both Arabidopsis and rice, large portions of the sRNAs are 21 nt in length [1243 (24.26%) in Arabidopsis and 655 (46.82%) in rice], and start with 5′ U [2250 (43.92%) in Arabidopsis and 914 (65.33%) in rice; Fig. 2). This observation correlates well with the previous report on the sequence features of the sRNAs enriched in the AGO1-associated silencing complexes (Mi et al. 2008). To some extent, it also indicates that the criterion for the identification of AGO1-enriched sRNAs is effective and reliable.

Several SAGs are targeted by AGO1-enriched sRNAs in addition to miRNAs in Arabidopsis
To address the question whether the miRNAs and the above-identified AGO1-enriched sRNAs are implicated in leaf senescence, the cDNAs of the SAGs were obtained from LSD 2.0 (Liu et al. 2011; Li et al. 2014). Then, all of the miRNAs and the AGO1-enriched sRNAs were included for target prediction using the miRU algorithm with default parameters (Zhang 2005; Dai & Zhao 2011). The predicted targets were validated using degradome sequencing data according to the previously reported approach (Meng et al. 2011; Shao et al. 2013). As a result, 200 regulatory pairs involving 120 AGO1-enriched sRNAs and 53 transcripts encoded by 40 SAGs, and 266 regulatory pairs involving 64 miRNAs and 54 transcripts belonging to 42 SAGs were discovered in Arabidopsis (a total of 61 SAGs were targeted by the AGO1-enriched sRNAs or miRNAs, or both). Notably, evident cleavage signals resided within the 9th to 11th nucleotide of the sRNAs or the miRNAs were detected for most of the regulatory pairs (Fig. 3A, B, D; Figures S1 and S2). However, in rice, only one transcript (LOC_Os03g53230.1) was targeted by miR395, and one transcript (LOC_Os06g12870.1) targeted by three AGO1-enriched sRNAs (OS_AGO1_sRNA302, OS_AGO1_sRNA304 and OS_AGO1_sRNA495; Fig. 3C, E; Figures S3 and S4).
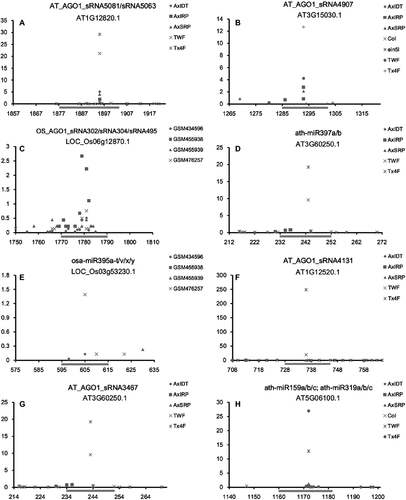
Although the SAGs retrieved from LSD 2.0 were considered as involved in leaf senescence according to reports from Luo's group (Liu et al. 2011; Li et al. 2014), we performed a GO (Gene Ontology) enrichment analysis to interrogate the functional correlation of the target genes with leaf senescence in Arabidopsis. A web toolkit agriGO (Du et al. 2010) was employed for this analysis by using the following parameters: (i) ‘Singular Enrichment Analysis (SEA)’ was selected; (ii) the species was A. thaliana; and (iii) using suggested background ‘Arabidopsis gene model (TAIR9)’. The 53 transcripts targeted by the 120 AGO1-enriched sRNAs and the 54 transcripts targeted by 64 miRNAs were analysed separately. The results showed that for both groups of targets, the GO terms ‘organ development’, ‘shoot development’ and ‘leaf development’ were highly enriched compared to the background (Figures S5 and S6). Detailed annotations of the 61 SAGs were obtained from TAIR (release 10; Huala et al. 2001) for further investigation. In support, 23 out of the 61 SAGs are annotated as involved in ‘leaf development/morphogenesis/differentiation/senescence’ (AT2G31070, AT3G15030, AT4G18390, AT5G05700, AT5G39610 and AT5G62000), ‘shoot apical meristem formation’ (AT1G56010 and AT2G45160), ‘programmed cell death’ (AT3G11440 and AT5G48380), ‘anthocyanin-containing compound biosynthetic process’ (AT5G06510), or located/expressed in ‘chloroplast’ (AT2G41220, AT3G11960, AT3G59950, AT4G13400, AT5G01600, AT5G03560, AT5G04360, AT5G18100 and AT5G41620) or ‘guard cell’ (AT1G15125, AT3G01080, AT4G13400, AT4G27310, AT5G06510; Table 1). For the two rice SAGs targeted by miR395 and three AGO1-enriched sRNAs, LOC_Os06 g12870 encodes a leaf senescence-related protein according to the annotation of MSU Rice Genome Annotation Project (release 7; Yuan et al. 2003). Besides, a previous study indicated that the other SAG (LOC_Os03 g53230: bifunctional 3-phosphoadenosine 5-phosphosulfate synthetase) might be involved in the early senescence process of rice flag leaves (Liu et al. 2008). Taken together, our functional analyses demonstrate that the target genes, at least a large portion of them, are strong SAG candidates, and the corresponding regulatory miRNAs and AGO1-enriched sRNAs play potential roles in leaf senescence or other related developmental processes.
| locus identifier | gene model name | functional annotation |
|---|---|---|
| AT1G04140 | AT1G04140.1 | Transducin family protein/WD-40 repeat family protein. Located in chloroplast |
| AT1G04140.2 | ||
| AT1G12520 | AT1G12520.1 | Copper-zinc superoxide dismutase copper chaperone. Localized to the chloroplast. Expressed in roots and shoots. Up-regulated in response to copper and senescence |
| AT1G12520.2 | ||
| AT1G12520.3 | ||
| AT1G15125 | AT1G15125.1 | S-adenosyl-L-methionine-dependent methyltransferases superfamily protein. Expressed in guard cell |
| AT1G56010 | AT1G56010.1 | Encodes a transcription factor involved in shoot apical meristem formation and auxin-mediated lateral root formation |
| AT1G56010.2 | ||
| AT2G28190 | AT2G28190.1 | Encodes a chloroplastic copper/zinc superoxide dismutase CSD2 that can detoxify superoxide radicals |
| AT2G31070 | AT2G31070.1 | TCP family protein involved in heterochronic regulation of leaf differentiation |
| AT2G41220 | AT2G41220.1 | Encodes a gene whose sequence is similar to ferredoxin dependent glutamate synthase. Located in chloroplast, chloroplast envelope, and plastid |
| AT2G45160 | AT2G45160.1 | LOM1 (LOST MERISTEMS 1). Promotes cell differentiation at the periphery of shoot meristems and helps to maintain their polar organization |
| AT3G01080 | AT3G01080.1 | Member of WRKY transcription factor. Expressed in guard cell and stem |
| AT3G11440 | AT3G11440.1 | Member of the R2R3-MYB gene family. Involved in positive regulation of programmed cell death |
| AT3G11960 | AT3G11960.1 | Cleavage and polyadenylation specificity factor (CPSF) A subunit protein. Located in nucleus and chloroplast |
| AT3G15030 | AT3G15030.1 | TCP family transcription factor. Involved in heterochronic regulation of leaf differentiation |
| AT3G15030.2 | ||
| AT3G59950 | AT3G59950.3 | Peptidase family C54 protein. Located in chloroplast and cytoplasm |
| AT4G13400 | AT4G13400.1 | 2-oxoglutarate and Fe(II)-dependent oxygenase superfamily protein. Located in chloroplast. Expressed in guard cell |
| AT4G18390 | AT4G18390.1 | TEOSINTE BRANCHED 1, cycloidea and PCF transcription factor 2 (TCP2). Involved in cell differentiation, leaf development and leaf morphogenesis |
| AT4G18390.2 | ||
| AT4G27310 | AT4G27310.1 | B-box type zinc finger family protein. Expressed in guard cell |
| AT5G01600 | AT5G01600.1 | Encodes a ferretin protein that is targeted to the chloroplast |
| AT5G03560 | AT5G03560.2 | Tetratricopeptide repeat (TPR)-like superfamily protein. Located in chloroplast |
| AT5G04360 | AT5G04360.1 | Encodes an enzyme thought to be involved in the hydrolysis of the α-1,6 linkages during starch degradation in seed endosperm. Involved in carbohydrate metabolic process, and chlorophyll catabolic process. Located in chloroplast, chloroplast stroma |
| AT5G05700 | AT5G05700.1 | Encodes an arginyl-tRNA:protein transferase (ATE1). Mutants of ATE1 display delayed leaf senescence |
| AT5G06100 | AT5G06100.1 | Encodes a member of the myb family of transcription factors (MYB33). Involved in positive regulation of programmed cell death |
| AT5G06100.2 | ||
| AT5G06100.3 | ||
| AT5G06510 | AT5G06510.1 | Nuclear Factor Y, subunit A10 (NF-YA10). Involved in anthocyanin-containing compound biosynthetic process. Expressed in collective leaf structure and guard cell |
| AT5G06510.2 | ||
| AT5G06510.3 | ||
| AT5G18100 | AT5G18100.1 | A putative peroxisomal CuZnSOD inducible by a high-light pulse. Located in chloroplast |
| AT5G18100.2 | ||
| AT5G21930 | AT5G21930.1 | P-type ATPase, mediates copper transport to chloroplast thylakoid lumen. Required for accumulation of copper-containing plastocyanin in the thylakoid lumen and for effective photosynthetic electron transport |
| AT5G21930.2 | ||
| AT5G21930.3 | ||
| AT5G39610 | AT5G39610.1 | Encodes a NAC-domain transcription factor. Positively regulates aging-induced cell death and senescence in leaves |
| AT5G41620 | AT5G41620.1 | Located in chloroplast and plasma membrane |
| AT5G48380 | AT5G48380.1 | Encodes a BAK1-interacting receptor-like kinase named BIR1. Involved in negative regulation of cell death |
| AT5G53480 | AT5G53480.1 | ARM repeat superfamily protein. Located in nucleus, chloroplast, nuclear pore, and cytoplasm |
| AT5G62000 | AT5G62000.1 | Auxin Response Factor. Mutants have many defects including enlarged rosette leaves, reduced fertility, later senescence, hypocotyl elongation defects, enlarged seeds and enlarged cotyledons |
| AT5G62000.2 | ||
| AT5G62000.3 |
- The functional keywords indicating the potential involvement of the 29 target genes in leaf senescence or development in Arabidopsis are highlighted in grey background colour. The target genes identified through protein interaction prediction followed by degradome-based target identification are bold italicised.
Construction of AGO1-enriched sRNA-mediated networks involving target interactions at the protein level
Based on the sRNA/miRNA–target list identified above, a regulatory network involved in leaf senescence could be constructed in Arabidopsis (Figure S7). However, we recognised that many factors had not been included in this network, suggesting a far more a complex network in planta. One of the important factors not considered is protein–protein interactions within the network. First, for the 61 target genes identified in Arabidopsis, are there any candidates that could interact with them at the protein level? Are these interacting candidates targeted by the miRNAs or the other AGO1-enriched sRNAs in Arabidopsis? To address these questions, we set out to predict candidate genes interacting with the 61 target genes at the protein level. PAIR (Predicted Arabidopsis Interactome Resource) was used for this prediction (Lin et al. 2011). As a result, a total of 459 interacting candidates was discovered (Data S3). Then, target identification was performed to investigate whether these candidate genes could be targeted by the miRNAs or the AGO1-enriched sRNAs in Arabidopsis. Fortunately, based on the degradome sequencing data, 16 transcripts encoded by eight interacting candidate genes were validated as regulated by 17 AGO1-enriched sRNAs in Arabidopsis (Figure S8), and 11 transcripts of five interacting candidate genes were targeted by ten miRNAs in Arabidopsis (Figure S9; a total of ten interacting candidate genes were targeted by the AGO1-enriched sRNAs or the miRNAs, or both). Notably, according to the TAIR annotations, six out of the ten target genes are either located in ‘chloroplast’ (AT1G04140, AT1G12520, AT2G28190, AT5G21930 and AT5G53480) or involved in ‘programmed cell death’ (AT5G06100) (Table 1). In this regard, the ten interacting candidate genes, along with the regulatory miRNAs and the AGO1-enriched sRNAs, were integrated into the leaf senescence-associated network (Figure S7).
Search for conserved sub-networks in rice
If the regulatory network identified in Arabidopsis is indeed important for the modulation of leaf senescence-associated processes, we speculated that some of the sub-networks should be highly conserved among diverse plant species. In this respect, all of the regulatory miRNAs and the AGO1-enriched sRNAs within the above established network of Arabidopsis were included to search for conserved sRNAs in rice. For the regulatory miRNAs of Arabidopsis, the families miR156, miR159, miR160, miR164, miR169, miR171, miR319, miR393, miR396, miR397 and miR414 are conserved in rice. Notably, most of these families are widespread among diverse plant species according to the miRBase registries (release 20; Griffiths-Jones et al. 2008), indicating the indispensible roles of these miRNAs in leaf senescence, which needs further experimental validation. For the remaining miRNA families including miR157, miR163, miR170, miR782, miR854, miR858, miR5021, miR5643 and miR5658, no conserved partner is reported in rice. Although not registered in the current release of miRBase, we observed that the sRNA sequence identical to miR157a, miR157b and miR157c of Arabidopsis was highly accumulated in the tricellular pollen of rice. Moreover, the sRNA sequence identical to ath-miR163 was abundant in the grains and roots of rice (Table 2). Both sRNAs were weakly expressed in the leaf of rice. Thus, whether these rice sRNAs possess species-specific roles, such as reproduction and root development, needs further investigation.
| sRNA ID | sRNA sequence | GSM278532 Grain | GSM278534 Root | GSM278535 Shoot | GSM409318 Shoot apex | GSM455962 AGO1a | GSM455964 AGO1c | GSM455965 Control | GSM722128 Tricellular pollen | GSM943194 Leaf | ShCn2D Shoot | ShK2D Shoot | ShKNa1I Shoot | ShKNa2D Shoot | ShN2D Shoot | ShP2D Shoot | ShS2D Shoot |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AT_AGO1_sRNA3814 (conserved in rice) | UGGAGAAGCAGGGCACGUG | 0 | 0 | 0 | 0 | 5.8 | 81.97 | 1.49 | 1.15 | 7.16 | 0 | 0.74 | 0 | 0.33 | 0 | 0 | 0 |
| AT_AGO1_sRNA3923 (conserved in rice) | UUGAGCCGUGCCAAUAUCA | 23.46 | 0 | 89.09 | 0 | 0 | 12.33 | 0.99 | 0 | 0 | 2.23 | 0 | 0 | 0 | 0 | 0 | 1.02 |
| AT_AGO1_sRNA4033 (conserved in rice) | UUCCACAGCUUUCUUGAAC | 0 | 0 | 0 | 0 | 13.19 | 6.68 | 5.21 | 0 | 2.58 | 16.87 | 9.13 | 6.85 | 5.68 | 10.91 | 16.06 | 5.44 |
| AT_AGO1_sRNA5067 (conserved in rice) | UCCACAGCUUUCUUGAACU | 0 | 0 | 0 | 0 | 5.54 | 7.19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AT_AGO1_sRNA5074 (conserved in rice) | UGCCUGGCUCCCUGUAUGC | 0 | 0 | 0 | 17.53 | 0 | 7.71 | 0.25 | 0 | 0 | 0.64 | 0 | 0 | 0 | 0 | 0 | 0 |
| ath-miR157a | UUGACAGAAGAUAGAGAGCAC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100.73 | 0.57 | 0 | 0 | 0 | 0 | 0.31 | 0.33 | 0 |
| ath-miR157b | |||||||||||||||||
| ath-miR157c | |||||||||||||||||
| ath-miR163 | UUGAAGAGGACUUGGAACUUCGAU | 23.46 | 158.96 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
- The expression evidence supporting AGO1-enriched sRNAs in rice is highlighted in grey background colour; the high accumulation levels of specific sRNAs in specific tissues are shown in bold.
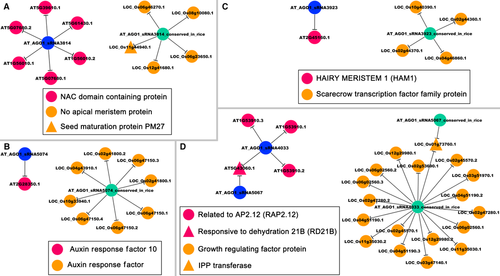
Five sRNAs whose sequences were identical to AT_AGO1_sRNA3814, AT_AGO1_sRNA3923, AT_AGO1_sRNA4033, AT_AGO1_sRNA5067 and AT_AGO1_sRNA5074 were identified as highly abundant in the AGO1-associated silencing complexes of rice. Thus, the five sRNAs were named AT_AGO1_sRNA3814_conserved_in_rice (81.97 RPM in AGO1c versus 1.49 RPM in control set), AT_AGO1_sRNA3923_conserved_in_rice (12.33 RPM in AGO1c versus 0.99 RPM in control), AT_AGO1_sRNA4033_conserved_in_rice (13.19 RPM in AGO1a versus 5.21 RPM in control), AT_AGO1_sRNA5067_conserved_in_rice (7.19 RPM in AGO1c versus 0 RPM in control) and AT_AGO1_sRNA5074_conserved_in_rice (7.71 RPM in AGO1c versus 0.25 RPM in control), respectively. More interestingly, four out of the five sRNAs are highly accumulated (> 5 RPM) in the leaves or shoots of rice. This expression-based evidence points to the possibility that the five sRNAs might have leaf senescence-associated functions through cleavage-based regulation of certain downstream targets. In this consideration, degradome sequencing databased identification of the target genes was performed. As a result, five transcripts encoded by five genes were targeted by AT_AGO1_sRNA3814_conserved_in_rice, four transcripts encoded by four genes were targeted by AT_AGO1_sRNA3923_conserved_in_rice, 18 transcripts encoded by ten genes were targeted by AT_AGO1_sRNA4033_conserved_in_rice, one transcript LOC_Os01g73760.1 was regulated by AT_AGO1_sRNA5067_conserved_in_rice, and eight transcripts encoded by four genes were regulated by AT_AGO1_sRNA5074_conserved_in_rice (Fig. 4; Figure S10). Based on annotations from the MSU Rice Genome Annotation Project (release 7; Yuan et al. 2003), certain sRNA–target pairs were identified as conserved between Arabidopsis and rice. For examples, in Arabidopsis, AT_AGO1_sRNA3814 regulates AT1G56010, AT5G07680, AT5G39610 and AT5G61430 encoding NAC domain-containing proteins. AT_AGO1_sRNA3814_conserved_in_rice also targets LOC_Os06g23650, LOC_Os06g46270, LOC_Os08g10080 and LOC_Os12g41680 encoding no apical meristem proteins belonging to the NAC family (Fig. 4A). Based on previous studies, the NAC gene family members play highly conserved roles in modulating the biological processes of leaf senescence in diverse plant species, such as Arabidopsis (Lin & Wu 2004; Guo & Gan 2006; Kim et al. 2009; Balazadeh et al. 2010; Yang et al. 2011; Lee et al. 2012; Zhang & Gan 2012; Hickman et al. 2013), rice (Liang et al. 2014), cotton (Gossypium hirsutum) (Fan et al. 2015) and wheat (Triticum aestivum) (Zhao et al. 2015). In Arabidopsis, it has been reported that miR164-mediated regulation of ORE1, a NAC transcription factor, is essential for age-dependent cell death during leaf senescence (Kim et al. 2009). In our study, AGO1_sRNA3814 was conserved in Arabidopsis and rice, and had the conserved target genes belonging to the NAC families in both plants. Thus, in addition to miR164, whether AGO1_sRNA3814 or some other sRNAs is indeed involved in leaf senescence, and whether such regulation is widespread across diverse plant species need to be further investigated. AT_AGO1_sRNA5074 targets AT2G28350 encoding an Auxin Response Factor (ARF), and AT_AGO1_sRNA5074_conserved_in_rice regulates LOC_Os02g41800, LOC_Os04g43910, LOC_Os06g47150 and LOC_Os10g33940 also encoding ARFs (Fig. 4B). According to previous reports, ARF1 and ARF2 positively regulate leaf senescence in Arabidopsis (Lim et al. 2010; Ellis et al. 2005). In rice, a genome-wide analysis of miRNAs and the target genes related to leaf senescence revealed that several ARF genes targeted by miR160 or miR167 were potentially implicated in miRNA-mediated leaf senescence (Xu et al. 2014). Here, we identified that AGO1_sRNA5074 also targeted ARF genes, and was related to leaf senescence in both Arabidopsis and rice. Thus, it will be interesting to thoroughly clarify the miRNA- and AGO1-enriched sRNA-mediated auxin signalling pathways related to plant leaf senescence. AT_AGO1_sRNA3923_conserved_in_rice targets LOC_Os02g44360, LOC_Os02g44370, LOC_Os04g46860 and LOC_Os10g40390 encoding SCARECROW family proteins, and AT_AGO1_sRNA3923 targets AT2G45160 encoding HAIRY MERISTEM 1 (HAM1) homologous to SCARECROW proteins (Fig. 4C). Some reports pointed to involvement of SCARECROW genes in leaf or shoot development (Wysocka-Diller et al. 2000; Dhondt et al. 2010); however, to our knowledge, the SCARECROW gene family has not been clearly related to leaf senescence. In this regard, whether the AGO1_sRNA3923–SCARECROW regulatory pathway plays an important role in leaf senescence needs to be investigated. Taken together, these conserved sub-networks mediated by the three sRNAs (AGO1_sRNA3814, AGO1_sRNA3923 and AGO1_sRNA5074; Fig. 4A, C) might play conserved roles in leaf senescence in Arabidopsis and rice. Notably, some novel target genes were identified in rice. In addition to the NAC genes, AT_AGO1_sRNA3814_conserved_in_rice also regulates LOC_Os11 g44940 encoding a seed maturation protein, PM27 (Fig. 4A), indicating its involvement in seed development in rice. In Arabidopsis, AT_AGO1_sRNA4033 targets AT1G53910 [Related to AP2.12 (RAP2.12)], and AT5G43060 [Responsive to Dehydration 21B (RD21B)] is targeted by AT_AGO1_sRNA4033 and AT_AGO1_sRNA5067. However, in rice, AT_AGO1_sRNA4033_conserved_in_rice targets nine genes encoding growth-regulating factors, and LOC_Os01g73760 (IPP transferase) is targeted by AT_AGO1_sRNA4033_conserved_in_rice and AT_AGO1_sRNA5067_conserved_in_rice (Fig. 4D). These regulatory pairs indicate that divergent biological roles of the three sRNA sequences (AGO1_sRNA3814, AGO1_sRNA4033 and AGO1_sRNA5067) might exist between Arabidopsis and rice.
In summary, a comprehensive study of the involvement of miRNAs and other AGO1-enriched sRNAs in the regulation of SAGs was conducted. The obtained results could advance our understanding of the sRNA-mediated molecular processes modulating leaf senescence, and could serve as a basis for further experimental investigations.
Acknowledgements
We would like to thank all the publicly available datasets and the scientists behind them. This work is financially supported by the National Natural Science Foundation of China (31100937), Zhejiang Provincial Natural Science Foundation of China (Y15C060021), China Postdoctoral Science Foundation (2013M540887), the Starting Grant funded by Hunan Agricultural University (14RCPT05), and the Starting Grant funded by Hangzhou Normal University to Yijun Meng (2011QDL60).




