The Impact of Reduced Immunosuppression on Alloimmunity: A Retrospective Study of Pediatric Kidney Transplant Recipients
Funding: Grants from the University of Southern Denmark, The Danish Patient Kidney Association, and the Augustinus Foundation supported the work.
ABSTRACT
Background
Kidney transplantation is the best treatment for end-stage kidney disease but requires immunosuppressive medications, which have significant side effects. Many pediatric recipients experience these side effects, leading to dosage reductions, which potentially increase the risk of alloimmunity. We aimed to describe the alteration in immunosuppressive medication, explore the reasons for the reductions, and assess the potential impact on alloimmunity.
Method
Data from 49 pediatric kidney transplant recipients receiving an allograft from 2009 to 2020 were retrospectively studied. The recipients were screened for HLA antibodies after the transplantation.
Results
The median age of recipients was 11 years (IQR 8), with a median follow-up of 5 years (IQR 5). Eighty percent of the transplantations were corticosteroid-free. During follow-up, 11% developed de novo donor-specific antibodies (dnDSA), and 60% had detectable HLA antibodies. The 1-year rejection rate was 4%. Immunosuppressive medication was altered substantially in most recipients, resulting in 72% being on mono- or dual therapy with a reduced mycophenolate mofetil (MMF) dosage by the end of the first posttransplant year. The median MMF dose was nearly half of the intended. Tacrolimus levels were maintained close to the target of 5 ng/mL. No association was found between reduced immunosuppression and dnDSA or rejections. Reductions were primarily due to MMF-related side effects: leukopenia in 77%, gastrointestinal issues in 34%, and infections with Epstein–Barr virus, cytomegalovirus, and BK polyomavirus in 49%.
Conclusions
Reduced MMF with a sufficient trough tacrolimus level in a population of mainly corticosteroid-free pediatric kidney transplant recipients did not lead to unacceptable alloimmunity.
Abbreviations
-
- ABOi
-
- ABO incompatible
-
- AUC
-
- area under the curve
-
- BK
-
- BK polyomavirus
-
- BSA
-
- body surface area
-
- CAKUT
-
- congenital anomalies of the kidney and urinary tract
-
- CI
-
- confidence interval
-
- CMV
-
- cytomegalovirus
-
- CsA
-
- cyclosporine A
-
- DNA
-
- deoxyribonucleic acid
-
- dnDSA
-
- de novo donor-specific antibodies
-
- DSA
-
- donor-specific antibodies
-
- EBV
-
- Epstein–Barr virus
-
- eGFR
-
- estimated glomerular filtration rate
-
- HLA
-
- human leukocyte antigen
-
- IQR
-
- interquartile range
-
- KT
-
- kidney transplantation
-
- MFI
-
- mean fluorescence intensity
-
- MMF
-
- mycophenolate mofetil
-
- MPA
-
- mycophenolic acid
-
- PCR
-
- polymerase chain reaction
-
- RCT
-
- randomized controlled trial
-
- TDM
-
- therapeutic drug monitoring
-
- UTI
-
- urinary tract infection
1 Introduction
Kidney transplantation (KT) is the preferred treatment in children with end-stage kidney disease, as it improves survival, growth, cognitive development, and quality of life compared to other renal replacement therapies [1, 2].
Randomized controlled trials (RCT) of immunosuppressive medication in pediatric transplantations are sparse. Thus, evidence primarily comes from observational studies and extrapolation from adult studies [3-5]. This has resulted in a wide range of immunosuppressive protocols. In Europe, there has been an increasing trend toward using maintenance therapy with tacrolimus, mycophenolate mofetil (MMF), and corticosteroids the first 30 days after KT, without induction therapy in approximately 70% of cases [5]. In the United States of America, induction therapy is used in the majority (94%) of pediatric KT, and most commonly triple immunosuppression with tacrolimus, MMF, and corticosteroids is used (54%), followed by dual therapy with tacrolimus and MMF (37%) [6].
Despite the diversity of immunosuppressive protocols, there is a consensus that tacrolimus and MMF are the most effective drugs for maintenance therapy. This is reflected in the guidelines of the Kidney Disease Improving Global Outcomes (KDIGO) [7] and the National Institute for Health and Care Excellence (NICE) [8].
MMF was introduced in the 1990s, replacing azathioprine as the preferred antiproliferative agent. Observational studies in pediatric kidney transplant recipients comparing cyclosporine (CsA), MMF, and corticosteroids to historical cohorts receiving azathioprine showed lower rates of acute rejections [9], while no RCTs comparing MMF and azathioprine have been conducted. Furthermore, evidence of MMF in a tacrolimus-based regimen is limited [3, 4].
The appropriate MMF dosage in pediatric KT has been evaluated based on analyses of the area under the curve (AUC) of mycophenolic acid (MPA), with the target AUC of 30–60 mg h/L extrapolated from the adult population [10-12]. The pharmacokinetics of MPA varies considerably both between subjects and within subjects, and since MPA trough levels do not correlate to the AUC, therapeutic drug monitoring (TDM) with multiple measurements is more accurate but time and cost-demanding [12]. In pediatric recipients, dosing based on body surface area (BSA) is more precise than weight [9]. CsA augments MMF clearance compared to tacrolimus, and dose reduction for tacrolimus MMF combination is advised [13] with a recommended MMF dosage with concomitant use of tacrolimus of 900–1200 mg/m2/day [3, 4, 8].
Tacrolimus was approved in the 1990s and has replaced CsA as the preferred calcineurin inhibitor. A single RCT comparing CsA and tacrolimus in pediatric KT with concomitant azathioprine and corticosteroids demonstrated improved graft survival and fewer rejections with tacrolimus [14].
Several RCTs conducted in pediatric KT have focused on corticosteroid-free or -withdrawal regimens, with corticosteroid-induced growth retardation as a particular concern. A meta-analysis of five RCTs concluded that corticosteroid-free and -withdrawal regimens are safe regarding rejection rates and improve prepubertal growth [15]. Additionally, improved lipid profiles, decreased incidence of posttransplant diabetes mellitus, and improved blood pressure control have been demonstrated [3, 16].
Due to side effects and infections, reduction of the predefined standard immunosuppressive medication is often necessary in pediatric recipients [10, 17, 18]. Reducing immunosuppression increases the risk of rejection and sensitization with human leukocyte antigen (HLA) antibodies. De novo donor-specific (dnDSA) HLA antibodies constitute a significant concern, as they are associated with reduced graft survival [19], increased risk of rejection [20, 21], and complicated retransplantation [22].
Since 1990, we have performed corticosteroid-free pediatric KT at Odense University Hospital [23-25] and have extensive experience in corticosteroid-free KT in adults [26, 27]. Our standard protocol for immunologically uncomplicated transplantations consists of induction with basiliximab and maintenance with MMF and tacrolimus.
However, we have observed a significant need for reducing immunosuppressive therapy due to side effects, raising concerns regarding potential increased alloimmunity.
We, therefore, aimed to describe the alteration in immunosuppressive medication, explore the reasons for the reductions, and assess the potential impact on alloimmunity in a cohort of pediatric kidney transplant recipients.
2 Methods and Material
2.1 Study Design and Population
Retrospective study of pediatric kidney transplant recipients below 18 years transplanted at Odense University Hospital between January 1, 2009 and December 31, 2020. Data were attained from electronic patient files, and the local data protection committee and the National Committee on Health Research Ethics approved the study (ID: 21/23234, ID: S-20190179).
Fifty pediatric patients received a kidney transplant during this period, but data were not attainable for one recipient; therefore, 49 were included in the study.
2.2 Immunosuppressive and Antiviral Medication
2.2.1 Baseline Immunosuppressive Medication
Immunological low-risk (HLA and ABO compatible) kidney transplant recipients received induction with anti-thymocyte globulin from day zero to four until November 2009, when basiliximab on days zero and four replaced anti-thymocyte globulin. Standard maintenance therapy consisted of tacrolimus and MMF. Tacrolimus trough level was aimed at 12–15 ng/mL the first 4 weeks, 10–12 ng/mL weeks 4–8, 8–10 ng/mL weeks 8–12, and 5–8 ng/mL onward. From 2015, the aim of tacrolimus trough level was 15 ng/mL during the first 4 weeks, 10 ng/mL during weeks 4–8, and 5 ng/mL afterward. MMF dose was targeted at 900 mg/m2/day at transplantation and reduced to 600 mg/m2/day after week 12. Target marginally lower than current recommendations [3, 8, 28].
ABO-incompatible (ABOi) recipients and recipients with preformed donor-specific HLA antibodies (DSA) were treated with rituximab, intravenous immunoglobulins, and plasmapheresis/immunoadsorption according to the strength of antibodies. Induction consisted of prednisolone and either basiliximab (ABOi) or anti-thymocyte globulin (DSA). Prednisolone was added to the maintenance therapy and tapered from 60 mg/m2 to either hold or 3 mg/m2 according to the individual immunological risk and complications.
2.2.2 Anti-Rejection Therapy
T-cell mediated rejections were treated with intravenous methyl-prednisolone 10 mg/kg the first day followed by 5 mg/kg for 3 days, succeeded by oral prednisolone tapered to hold after 6 months. Anti-thymocyte globulin 1.5 mg/kg for 5 days was applied in case of no response or if the Banff classification exceeded IIA. No antibody-mediated rejections were diagnosed.
2.2.3 Infection Prophylaxis
All recipients received antiviral prophylaxis independent of serological status for 3 months. Aciclovir was applied until February 2014, when it was replaced with valganciclovir. Recipients receiving increased immunosuppressive medication had additional prophylaxis with sulfamethoxazole/trimethoprim or trimethoprim for 3 months.
2.3 Analytical Methods
2.3.1 HLA Antibodies Detection With Solid Phase Assay
HLA antibodies in patient sera were examined using Labscreen Mixed and Labscreen Single Antigen (One Lamda Inc.), following the manufacturer's instructions. The beads are analyzed on the Luminex platform, generating a semiquantitative output—mean fluorescent intensity (MFI). Antibodies with an MFI above 1000 were considered positive.
2.3.2 EBV, CMV, and BK Polyomavirus PCR
Statens Serums Institut quantified BK polyomavirus until 2012. After 2012, a laboratory-developed test was used. A laboratory-developed test quantified cytomegalovirus (CMV) and Epstein–Barr virus (EBV) for the whole study period. In brief, DNA was extracted from ethylenediamine tetraacetic acid- anticoagulated plasma, and a quantitative polymerase chain reaction determined viral load. The quantification cycle (cq) was quantified with external standard curves. Results below 1000 copies/mL were reported as < 1000 copies/mL. A quality check of the performance of the assays was performed twice a year [29].
2.4 Definitions
2.4.1 HLA Antibodies
Routine pretransplant and yearly posttransplant screening with solid phase assays were introduced in 2011, with recipients below 18 years protocolled for screening. The recipients were considered sensitized if a DSA was detected or if an HLA antibody was consistently present, and MFI ≥ 1000. DSA were characterized as de novo (dnDSA) when appearing after transplantation. In the analysis regarding dnDSA, recipients with DSA but no pretransplant solid phase assay (n = 3) and recipients without posttransplant solid phase assay (n = 2) are excluded. All recipients with posttransplant solid phase assays (n-47) are included in the sensitization analysis.
2.4.2 Rejection
All biopsies were indication biopsies. Rejection was diagnosed from biopsy or if strong clinical suspicion led to the initiation of anti-rejection therapy after excluding other reasons for graft dysfunction. Rejections were classified according to the Banff classification applied at the time of the rejection.
2.4.3 Graft Loss
Retransplantation or initiation of dialysis.
2.4.4 Infections
Infections were recorded as (1) infections requiring hospitalization and (2) the overall number of infections. The infections handled in the outpatient clinic were registered when requiring antibiotics, antiviral treatment, change in immunosuppressive medication, or being symptomatic combined with fever or an increase in C-reactive protein.
CMV and EBV were registered as infections if viral replication was confirmed by two measurements, if antiviral therapy or reduction in immunosuppressive medication were necessary, or if causing symptoms. CMV and EBV screening was performed weekly during the first month, every second week during the second and third months, and every 3–4 months continually.
BK Polyomavirus screening was only performed in recipients receiving prednisolone in maintenance therapy or on indication, and screening was performed every 6 weeks for the first 2 years. BK viruria was recorded if a reduction in immunosuppressive medication was required, while BK viremia was always recorded.
2.4.5 Hospitalizations
A hospitalization was recorded if the recipient stayed in the hospital for at least one night.
2.4.6 Estimated Glomerular Filtration Rate (eGFR)
eGFR was calculated by the CKID25 formula without cystatin C [30].
2.5 Statistics
Statistical analyses were conducted using Stata 17.0 (TX, US: StataCorp) and GraphPad Prism 9.2.0 (San Diego, US: Graph Pad Software). Two-sided p values less than 0.05 were considered statistically significant.
Data were presented as frequencies, mean, or median depending on normal distribution evaluated with a QQ plot. Groups compared with Wilcoxon Mann–Whitney test or linear regression (assessed by plots of residuals on fitted values and assuming a normal distribution of residuals). Graft loss was described with Kaplan Meier survival analysis censoring death. Only one recipient died during the study; therefore, censoring death did not affect the survival analysis results. Groups were compared by log-rank test. Time to dnDSA was described with Kaplan–Meier survival analysis. Rejection-free survival was described by the cumulative incidence function considering graft loss and death as competing risks. Groups compared with Cox regression (proportional hazards assessed). A linear mixed-effect model was applied to evaluate changes and differences in eGFR and immunosuppression (normal distribution of residuals assessed).
3 Results
3.1 Description of the Study Population (Table 1)
| ALL recipients (n = 49) | DnDSA negative (n = 42) | DnDSA positive (n = 5) | |
|---|---|---|---|
| Follow-up (years), median (IQR, range) | 5 (3–8; 0–13) | 5 (3–8; 0.02–13) | 5 (3–6; 2–6) |
| Age at transplantation, median (IQR, range) | 11 (6–14; 1–16) | 10 (6–13; 1–16) | 13 (2–16; 1–16) |
| < 6 years | 12/49 (25%) | ||
| 6–11 years | 15/49 (31%) | ||
| > 12 years | 22/49 (45%) | ||
| Weight (kg), median (IQR, range) | 27.5 (18–44; 10–77) | 27.3 (18–43; 10–27) | 34.5 (13–48; 12–54) |
| Body surface area (m2), median (IQR, range) | 0.98 (0.57–1.21; 0.46–1.86) | 0.98 (0.76–1.44; 0.47–1.49) | 1.12 (0.53–1.45; 0.46–1.86) |
| Male, n (%) | 29/49 (59%) | 25/42 (60%) | 3/5 (60%) |
| Graftloss, n (%) | 6/49 (12%) | 4/42 (10%) | 2/5 (40%) |
| Rejection, n (%) | 4/49 (14%) | 5/42 (12%) | 1/5 (20%) |
| Cause of end-stage kidney disease, n (%) | |||
| CAKUT | 18/49 (37%) | 14/42 (33%) | 4/5 (80%) |
| GN/Vasculitis | 6/49 (12%) | 5/42 (12%) | 1/5 (20%) |
| GN genetica | 2/49 (4%) | 2/42 (5%) | 0 |
| Geneticb | 17/49 (35%) | 16/42 (47%) | 0 |
| Other | 2/49 (4%) | 1/42 (2%) | 0 |
| Unknown | 4/49 (8%) | 4/42 (10%) | 0 |
| Pretransplant status, n (%) | |||
| Pre-emptive | 14/49 (29%) | 13/42 (31%) | 1/5 (20%) |
| Hemodialysis | 18/49 (37%) | 15/42 (36%) | 3/5 (60%) |
| Peritoneal dialysis | 15/49 (31%) | 12/42 (29%) | 1/5 (20%) |
| Combined HD and PD | 2/49 (4%) | 2/42 (5%) | 0/5 |
| Type of transplantation, n (%) | |||
| Living donor | 25/49 (51%) | 24/42 (57%) | 1/5 (20%) |
| Deceased donor | 24/49 (49%) | 18/42 (43%) | 4/5 (80%) |
| HLA and ABO compatible | 41/49 (84%) | 34/42 (91%) | 5/5 (100%) |
| Pretransplant DSA | 6/49 (12%) | 36/42 (86%) | 0/5 |
| ABOi | 2/49 (4%) | 2/42 (5%) | 0/5 |
| Retransplantation | 8/49 (16%) | 7/42 (17%) | 1/5 (20%) |
| HLA incompatibilities | |||
| HLA-A, -B, -DR incompat, median (IQR; range) | 3 (2.4–4; 0–5) | 3 (2–4; 0–5) | 3 (3–3.5; 3–4) |
| HLA antigen incompat (HLA-A, -B, -Cw, -DRB1, -DQB1), median (IQR; range) | 5 (4–6; 0–8) | 4.5 (4–6; 0–8) | 5 (5–6; 5–6) |
| HLA allele incompat (HLA-A, -B, -C, -DRB1, -DQA1, -DQB1, -DPB1, -DPA1, -DRB3/4/5), median (IQR; range) | 7 (6–9; 2–12) | 7 (5–7; 2–12) | 8 (7–8; 7–8) |
| Immunosuppressive medication, n (%) | |||
| Induction | |||
| Anti-thymocyte globulin | 9/49 (18%) | 8/42 (19%) | 0 |
| Basiliximab | 40/49 (82%) | 34/42 (81%) | 5/5 |
| Rituximab | 6/49 (12%) | 6/42 (14%) | 0/5 |
| Maintenance | |||
| Tac, MMF | 39/49 (80%) | 32/42 (76%) | 5/5 |
| Tac, MMF, prednisolone | 10/49 (20%) | 10/42 (24%) | 0 |
- Abbreviations: AB0i, AB0 incompatible transplantation; CAKUT, congenital anomalies of the kidney and urinary tract; DnDSA, de novo donor-specific antibodies; DSA, donor-specific antibodies; GN, glomerulonephritis; HD, hemodialysis; HLA, human leukocyte antigen; MMF, mycophenolate mofetil; PD, peritoneal dialysis; Tac, tacrolimus; TGL, antithymocyte globulin.
- a GN genetic—C3 glomerulonephritis with mutation in CFH, Dense Deposit Disease with CFH mutation.
- b Genetic diseases: Conorenal syndrome, mitochondrial cytopathy, HNF1B MODY, Renal coloboma syndrome, Podocin mutation, primary hyperoxaluria I, ARPKD, Congenital nephrotic syndrome (Finnish type), Nephronophtisis, Schimke immuno-osseous dysplasia, Branchio-oto-renal syndrome, VACTERL syndrome.
Congenital anomalies of the kidney and urinary tract (CAKUT) were the most common reason for end-stage kidney disease, closely followed by a heterogeneous group with different genetic diseases.
Six recipients had performed DSA, two KT were ABOi, and eight were retransplantations. The median age of the retransplanted recipients was 13 years, with an average graft survival of the former graft of 5 years.
Forty-five percent of the cohort had a 2:1 HLA–A, -B, -DRB1 mismatch. The HLA allele mismatch was significantly higher in recipients receiving a graft from a deceased donor compared to a living (9 [IQR 2.5] vs. 7 [IQR 2] [p < 0.001]).
3.2 Alterations in the Immunosuppressive Medication Over Time
Most recipients (78%) received standard low-immunological risk immunosuppression. Eight KT had DSA pretransplant or were ABOi KT and intensified immunosuppression was applied (Table 1). The majority of recipients followed the standard medication protocols, but five had personalized medication based on specific considerations.
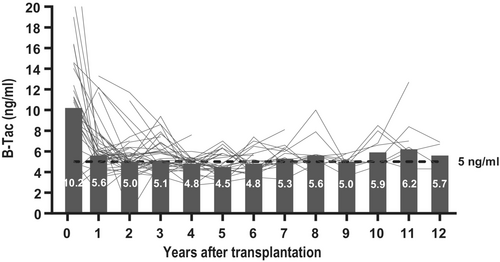
All recipients were initially treated with tacrolimus (Table 2). Two ceased tacrolimus during the first year due to EBV, one developed posttransplant lymphoproliferative disorder. After the first year, the median trough level of B-tacrolimus was at the desired target—5.6 ng/mL, and tacrolimus continued to be well-regulated according to the target level of 5 ng/mL in the subsequent years (Figure 1).
| Maintenance therapy | Transplantation | Year 1 |
|---|---|---|
| Tacrolimus, n (%) | 49/49 (100%) | 45/47 (95.7%) |
| B-Tacrolimus level (ng/mL), median (IQR; range) |
13.7 (5.5; 6.5–33.4) (Day30) |
5.6 (1.9; 2–13.3) |
| MMF, n (%) | 49/49 (100%) | 37/47 (78.7%) |
| Dose/BSA (mg/m2/day), median (IQR; range) | 846.4 (124; 336–2164) | 353.9 (419; 50–1007) |
| Reduced dose or no MMF, n (%) | 4/46a (8.7%) | 37/47 (78.7%) |
| Reduced dose/no MMF in age groups, n (%) | ||
| 0–5 (12) | 0/11a (0%) | 10/11 (90.9%) |
| 6–11 (15) | 2/15 (13.3%) | 13/15 (86.7%) |
| ≥ 12 (22) | 2/20a (10.0%) | 14/21 (66.7%) |
| p-value | 0.67 | 0.27 |
| Prednisolone, n (%) | 10/49 (20.4%) | 5/47 (10.6%) |
| Dose/BSA (mg/m2), median (IQR; range) | 55.9 (23.3; 17–60) | 2.5 (2.7; 1.6–11.0) |
| Other, n (%) | ||
| Azathioprin | 0 | 1/47 (2.1%) |
| Number of immunosuppressive drugs, n (%) | ||
| One drug therapy | 0/49 (0%) | 9/47 (19.2%) |
| Two drug therapy | 39/49 (79.6%) | 35/47 (74.5%) |
| Three drug therapy | 10/49 (20.4%) | 3/47 (6.4%) |
| Reduced IS (one IS, OR two IS AND MMF < 600 mg/m2/day) | 3/47a (6.4%) | 34/47 (72.3%) |
| 0–5 (12) | 0/12 (0%) | 9/11 (81.8%) |
| 6–11 (15) | 1/15 (6.7%) | 13/15 (86.7%) |
| ≥ 12 (22) | 2/20a (10.0%) | 12/21 (57.14%) |
| p-value | 0.78 | 0.13 |
- Abbreviations: BSA, body surface area; IS, Immunosuppressive medication; MMF, mycophenolate mofetil.
- a Three BSA missing at year 0.
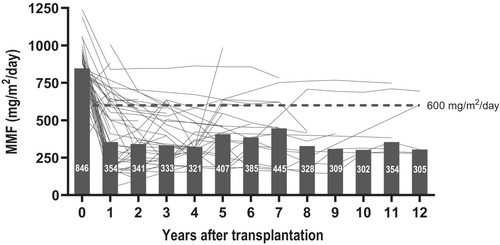
Similarly, all recipients received MMF from transplantation, with 79% still receiving it after the first year, but at year one, 73% received a dosage below the desired of 600 mg/m2/day (Table 2; Figure 2). MMF dosage was targeted at 900 mg/m2/day at transplantation, and a median dosage of 846 mg/m2/day (IQR 124 mg/m2/day) was obtained. At year one, the median dosage in the cohort was only 354 mg/m2/day (IQR 419 mg/m2/day) (< 0.001).
Ten recipients were treated with prednisolone from transplantation, with eight tapering to hold during the first year (Table 2). In three recipients, prednisolone was initiated during the first year—one as part of anti-rejection therapy and two to replace tacrolimus due to chronic Epstein–Barr viremia.
After 1 year, 72% of the cohort received either mono- or dual therapy with an MMF dosage below 600 g/m2/day. The reduction was numerically more pronounced in recipients from 1 to 11 years (Table 2). MMF was successfully increased to the standard dose in some, but most continued a reduced dose (Figure 2).
3.3 Side Effects Prompting Reduction in Immunosuppressive Medication
In one-third of the cohort, gastrointestinal side effects led to a reduction in MMF (Table 3). Leukopenia was observed in 79%, causing a reduction of MMF in the majority (77%). Ten recipients had EBV infection, resulting in a reduction of immunosuppressive medication in nine, while 14 recipients had CMV infection, causing a reduction in half. BK polyoma viral replication was present in nine recipients, and seven were reduced in MMF.
| Type of side effects | First year | Year 2–13 |
|---|---|---|
| Diarrhea, nausea, n (%) | 18/47 (38.3%) | 3/47 (6.4%) |
| 0–5 (11) | 5/11 (45.5%) | |
| 6–11 (15) | 5/15 (33.3%) | |
| ≥ 12 (21) | 8/21 (38.1%) | |
| p-value | 0.82 | |
| Leukopenia, n (%) | 37/47 (78.7%) | 9/47 (19.1%) |
| Reduced IS due to leukopenia | 36/47 (76.6%) | |
| 0–5 (11) | 8/11 (72.7%) | |
| 6–11 (15) | 12/15 (80.0%) | |
| ≥ 12 (21) | 16/21 (76.2%) | |
| p-value | 0.91 | |
| Epstein–Barr virus, n (%) | 10/47 (21.3%) | 6/47 (12.8%) |
| Reduced IS due to EBV | 9/47 (19.2%) | |
| 0–5 (11) | 5/11 (45.5%) | |
| 6–11 (15) | 3/15 (20.0%) | |
| ≥ 12 (21) | 1/21 (4.8%) | |
| p-value | 0.016* | |
| Cytomegalovirus, n (%) | 14/47 (29.8%) | 1/47 (2.1%) |
| Reduced IS due to CMV | 7/47 (14.9%) | |
| 0–5 (11) | 2/11 (18.2%) | |
| 6–11 (15) | 2/15 (13.3%) | |
| ≥ 12 (21) | 3/21 (14.3%) | |
| p-value | 1.00 | |
| BK polyomavirus, n (%) | 9/47 (19.1%) | 7/47 (14.9%) |
| Reduced IS due to BK | 7/47 (14.9%) | |
| 0–5 (11) | 2/11 (18.2%) | |
| 6–11 (15) | 2/15 (13.3%) | |
| ≥ 12 (21) | 3/21 (14.3%) | |
| p-value | 1.00 |
- Abbreviations: CMV, cytomegalovirus; EBV, Epstein–Barr virus; IS, immunosuppressive medication. *p-value indicates < 0.05.
Recipients with gastrointestinal side effects in the first year did not undergo further medication changes or reductions, but three additional recipients developed gastrointestinal side effects, leading to a reduction or change in MMF (Table 3). Recurrent or persistent leukopenia and EBV infection remained problematic beyond the first year in eight and six recipients, respectively. Only one recipient was changed in medication due to CMV after the first year, and it was an incident case. BK had an equal distribution of new-onset and persistent replication after the first year (Table 3).
Reduction of MMF due to side effects, leukopenia, CMV, or BK was similarly distributed between age groups. MMF was reduced significantly more due to EBV in children < 6 years compared to children over 11 years (Table 3).
3.4 Infections and Hospitalizations Over Time
During the first year after KT, the median number of hospitalizations due to all causes was one (IQR 3), and infections were the main reason for hospitalization, with 60% being hospitalized at least once with infection. After the first year, the median number of hospitalizations due to infections decreased to zero (Table S1). Recipients under the age of six were significantly more likely to be hospitalized than older age groups (p = 0.002), especially with infections (p = 0.003) (Figure 3a,b).
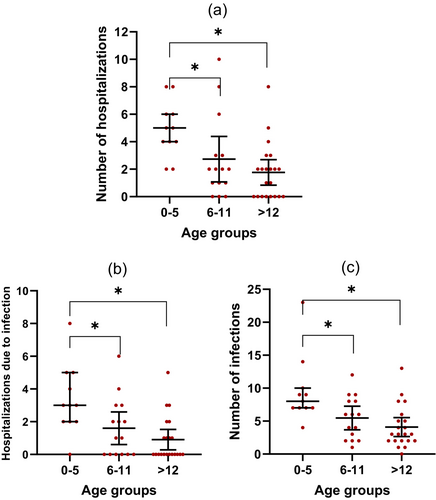
A median of five infections (IQR 6) requiring either hospitalization or outpatient care was observed during the first year. Recipients below 6 years old had significantly more infections than older age groups (p < 0.001) (Figure 3c). No association was found between baseline immunosuppressive medication and the number of infections when comparing standard immunosuppression (basiliximab, tacrolimus, and MMF) with other regimens (p = 0.19). Bacterial infections, particularly urinary tract infections (UTI), were the most frequent, with 72% experiencing at least one UTI during the first year. After the first year, the number of infections declined to a median of between zero and two per year (Table S1).
Recipients were routinely screened for CMV and EBV DNA replication. During the first year, 32% had CMV infections and 23% had EBV infections. CMV infections were typically asymptomatic or mild, while EBV infections often caused malaise and hospitalizations. One patient developed PTLD during the first year, with a total of two cases during the follow-up period, both associated with EBViremia. After the first year, only two recipients had incident CMV infections and one had an incident EBV infection. Eighteen percent had BK viruria or viremia, but the virus did not cause biopsy-verified BK nephropathy. CMV and BK had an equal distribution among ages; only EBV was more frequent in the youngest recipients.
3.5 Alloimmunological Outcomes
3.5.1 HLA Sensitization
During the follow-up period, 11.4% of the cohort developed dnDSA, and if including recipients with DSA but without pretransplant solid phase assay, a maximum of 17.4% developed dnDSA (Table 4). The time to the first detection of dnDSA was 3.91 years (IQR 2.28), and the 5-year dnDSA-free survival was 87.4% [95% CI 69.5; 95.1] (Figure S2).
| HLA antibodies, n (%) | |
|---|---|
| All | 28/47 (59.6%) |
| HLA Cl I | 7/28 (25.0%) |
| HLA Cl II | 9/28 (32.1%) |
| HLA Cl I & HLA Cl II | 11/28 (39.3%) |
| Unknown | 1/28 (3.6%) |
| Non-DSA | 14/47 (29.8%) |
| DSA | 14/47 (29.8%) |
| Pretransplant | 6/14 |
| Posttransplant | 8/14 |
| De novo donor-specific antibodies, n (%) | |
|---|---|
| DnDSA during follow-up | 5/44 (11.4%) |
| HLA Cl I | 0/5 |
| HLA Cl II | 3/5 (60%) |
| HLA Cl I & HLA Cl II | 2/5 (40%) |
| Time to dnDSA (years), median (IQR; range) | 3.9 (2.3; 2.2–9.2) |
| MFI, first detected dnDSA, median (IQR; range) | 1700 (6100; 1400–14 600) |
| DnDSA in age groups | |
| 0–5 (12) | 2/12 (16.7%) |
| 6–11 (13) | 0/13 (0%) |
| ≥ 12 (19) | 3/19 (15.8%) |
| p-value | 0.36 |
- Abbreviations: dnDSA, de novo donor-specific antibodies; DSA, donor-specific antibodies; HLA, human leukocyte antigen; MFI, mean fluorescence intensity.
All five recipients with dnDSA were categorized as low-immunological risk at the time of transplantation and received maintenance therapy with tacrolimus and MMF. We found no differences in baseline characteristics between recipients with and without dnDSA (Table 1). When statistically examining tacrolimus and MMF levels over time in the recipients with dnDSA compared to the rest, we did not find significantly different levels (Table S3). Neither did we observe a difference between the groups when examining whether their overall immunosuppression was reduced at 1 year (monotherapy or dual therapy with MMF < 600 g/m2/day) (Table S3). However, the medical histories of the recipients with dnDSA revealed periods of reduced immunosuppression, particularly notable for reductions in tacrolimus. One recipient was strongly suspected of nonadherence; two recipients received reduced or no CNI due to PTLD; one recipient had reduced MMF and tacrolimus due to multiple infections; and the last recipient had reduced MMF but maintained normal tacrolimus levels.
HLA antibodies were detected in 59.6% of the recipients. Most of the antibodies were weak (MFI < 3000), the majority (39%) were a combination of HLA class I and II antibodies, and the non-DSA HLA antibodies were predominantly class I. In comparison, none of the dnDSA were exclusively class I (Table 4).
3.5.2 Rejections
The cumulative incidence of rejection was 4.1% [95% CI −1.5; 5.5] after 1 year, 10.4% [95% CI 1.6; 18.7] after 5 years, and 14.6% [95% CI 3.0; 25.9] after 10 years when death and graft loss were considered competing risks (Figure 4).
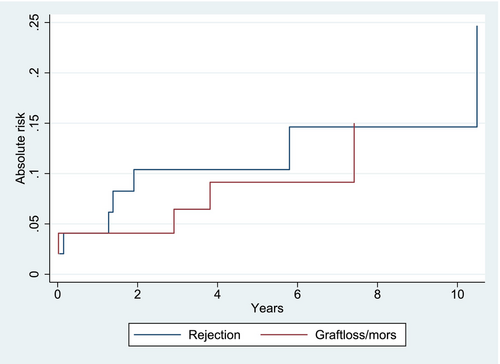
During the follow-up period, seven recipients had eight rejections. Two rejections occurred within 3 months of transplantation, three occurred between one and 2 years after transplantation, and two occurred more than 5 years after transplantation. The median age of the recipients at the time of rejection was 15.5 years (IQR 12.3).
In 75% of the recipients with rejection, the medical records reported a reduction in immunosuppression before the rejection. One-third were not taking their medication as prescribed, one-third had reduced medication due to infections, and one-third had dysregulated B-tacrolimus due to gastrointestinal side effects (early rejections). Two recipients with rejection lost their grafts later, but none of the graft losses were directly related to the rejection episodes.
All the rejections were T-cell-mediated rejections. Five were classified as Banff 1A, one as Banff Ib, one as Banff IIa, and one as Banff IIb.
One recipient with rejection had dnDSA, and 86.3% were sensitized, which was numerically higher than the rest of the population. However, the hazard ratio of rejection was not significantly higher in recipients who were sensitized or who had dnDSA (4.0 [95% CI 0.5; 34.9] and 1.97 [95% CI 0.2; 17.8], respectively). Neither could we statistically show an association between reduced immunosuppression at year one and rejections (only considering rejections occurring after the first year [Table S4]). The baseline characteristics of the recipients with rejection did not differ from the rest of the cohort, except for better HLA compatibilities in the recipients with rejection (data not shown).
3.6 Patient Survival and Graft Function
One recipient died from infection. Five-year patient survival was 100%, while 10-year was 93.8 [95% CI 63; 99].
During the follow-up period, six recipients lost their grafts. The median time from transplantation to graft loss was 3 years (IQR 3.8). Two recipients lost their graft immediately after transplantation: one due to graft thrombosis and the other due to the recurrence of undiagnosed primary hyperoxaluria. The remaining four lost their grafts due to: chronic Parvo B19 infection (after 3 years), multiple UTIs and suspected nonadherence (after 3.5 years), recurrence of membranoproliferative glomerulonephritis (after 3 years), and recurrence of C3 glomerulopathy (dense deposit disease) (after 6 years) (Figure 5).
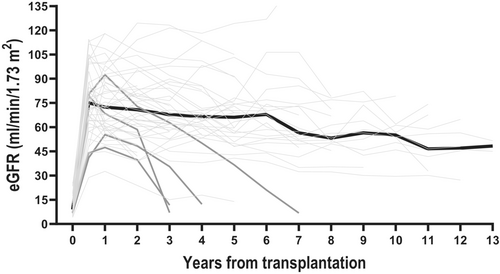
Death-censored graft survival rates were 95.9% [95% CI 84.7; 99] at year 1, 88.2% [95% CI 73.7; 95] at year 5%, and 84.0% [95% CI 66.7; 92.8] at year 10 (Figure 4; Figure S5). There was no significant difference in graft survival between recipients receiving kidneys from deceased or living donors (p-value = 0.43), in graft survival based on age (p-value = 0.46), gender (p-value = 0.58), HLA-DR mismatches (p = value 0.56), rejection status (p-value = 0.26), or immunological risk pretransplant (p-value = 0.86). However, there was a tendency toward decreased graft survival in recipients with dnDSA (p-value = 0.06).
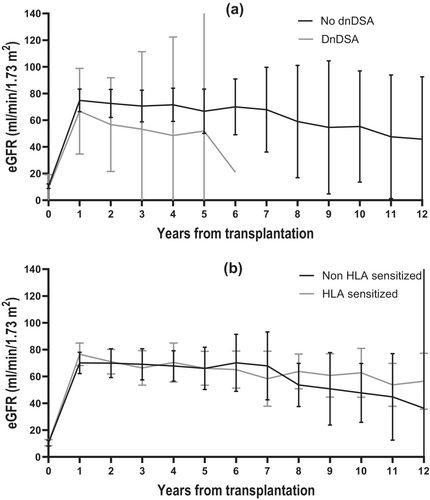
Evaluating the graft function, the mean eGFR increased to 75.5 mL/min/1.73 m2 [95% CI 69.3; 81.5] 6 months after transplantation, after that decreasing with an average of 2.1 mL/min/1.73 m2 73 m2 [95% CI 1.3; 2.9] each year (Figure 5). To investigate the impact of dnDSA and HLA sensitization on kidney function, a linear mixed-effect model was applied, and the mean eGFR was 21.0 mL/min/1.73 m2 [95% CI 5.1; 36.8] higher in recipients without dnDSA compared to recipients with dnDSA (p = 0.009) (Figure 6a). There was no difference in kidney function between HLA-sensitized and nonsensitized recipients (Figure 6b).
4 Discussion
This retrospective single-center study examined 49 pediatric kidney transplant recipients, of which 80% were completely corticosteroid-free. By the end of the first year, 72% of the patients received either one immunosuppressive drug or two drugs and MMF below the targeted dose of 600 mg/m2/day. The median dosage of MMF was 354 mg/m2/day, nearly half of the intended dose at year one, and it remained reduced over the following years. However, tacrolimus trough levels were maintained at around 5 ng/mL throughout follow-up. Despite the minimized immunosuppression, rejection rates, dnDSA development, and graft losses were low compared to previously published data. Infection rates were high in the first year but decreased subsequently, likely due to reduced immunosuppression. Side effects like infections, leukopenia, and diarrhea primarily caused the reduction in immunosuppression, but these complications also markedly decreased after the first year. Recipients under six experienced significantly higher infection rates, whereas other side effects were evenly distributed among the age groups.
The deleterious effect of dnDSA was indicated by findings of a lower mean eGFR in recipients with dnDSA. We could not identify any statistically significant risk factors for developing dnDSA. However, a review of the recipients' medical histories revealed a marked reduction in CNI in most.
In our study, MMF primarily caused side effects, a well-known trait of MMF. The rates of side effects were comparable to other studies [10, 31, 32] and declined markedly after the first year. We observed equally distributed side effects among age groups, similar to the study of Bunchmann et al. [10]. In contrast, other studies have found more side effects among the youngest recipients [17, 32, 33]. On the other hand, we showed that infections generally affected children under six significantly more than the oldest (Figure 3), which Hogan et al. also observed in their study [34]. In our cohort, the most abundant number of infections and side effects were observed during the first year, decreasing during follow-up after reductions in immunosuppression. The MMF dosage of 354 mg/m2/day at year one was lower than reported in two studies, reporting 718 mg/m2/day in a tacrolimus-based population and 991–1084 mg/m2/day in CsA-based populations [10, 17]. The recommended target dose of 900–1200 mg/m2/day has been established from a target MPA Area under the curve (AUC of 30–60 mg h/L), extrapolated from MPA AUC in the adult populations [11, 12], but supported by evidence of decreased rejections also in pediatric recipients [3, 9, 31]. Studies of MMF toxicity have not established an upper threshold, and the efficacy of MPA TDM is debatable [11, 12]. We have not used MPA TDM-guided therapy in our center as routine for more reasons. First, measurements for MPA AUC are time-consuming for the recipients and their families. Second, the evidence supporting its clear benefits is controversial [12]. However, we acknowledge that in a corticosteroid-free population, MPA TDM is recommended due to the decreased immunosuppression and the increased MPA exposure without concomitant corticosteroids. The absence of MPA TDM in this study poses a significant limitation, precluding definitive evidence regarding whether our cohort received a lower dosage than recommended. However, our cohort's targeted dosage of 600 mg/m2/day was marginally lower than the recommended [8, 28], and studies have shown a tendency toward underexposure in fixed dosage regimes [35]. On the other hand, the absence of corticosteroids could potentially increase the MPA AUC. Furthermore, the dose-normalized exposure of MPA has been shown to increase in the first months after transplantation [11].
In our cohort, the death-censored 5-year graft survival was 88.2%, comparable to previously published data from Europe, Australia, and the United States [36, 37]. In contrast, our 1-year rejection rate of 4.1% was lower than the rejection rates reported in other studies, which ranged from 10.5% to over 25% [10, 38-40]. Six of the eight rejections occurred after the first year, primarily in adolescents, and were associated with either nonadherence or reduced immunosuppression due to infection.
During the follow-up period, 11.4% of the recipients developed dnDSA, while 59.6% were HLA sensitized. Compared to similar studies, the incidence of dnDSA was low, reporting dnDSA rates ranging from 10% to 73%. However, in most pediatric kidney transplant recipients, the incidence of dnDSA is about 30%–40% using similar MFI cut-offs [21, 41-46]. We speculated whether the HLA compatibility was higher in our cohort, but when reported, the HLA-A, -B, -Cw, -DRB1, and-DQB1 incompatibility varied from 3.9 to 7.4 [42, 43, 46], similar to our findings. A follow-up period of 5 years, with a median time of almost 4 years to the first detection of dnDSA, also seems adequate for assessing dnDSA as an outcome measure. In most studies, the median time to detection of dnDSA is from 2 to 4 years [42, 46-49], similar to our observation of about 4 years.
DnDSA is associated with inferior graft survival and rejection [19-21], while the effect of HLA sensitization is debatable. Cioni et al. found in a study of 144 nonsensitized pediatric kidney transplant recipients that 56% developed de novo HLA antibodies, which were not associated with inferior graft outcomes [50]. In a study of 82 nonsensitized pediatric kidney transplant recipients, Ginevri et al. found that 45% developed de novo HLA antibodies, but only the DSA were associated with graft failure [49]. In contrast, Susal et al. found that 42% of 83 adult recipients developed HLA antibodies, which was associated with graft loss [51]. We found a tendency (NS) toward increased graft failure in recipients with dnDSA, but this tendency was not observed with HLA sensitization. However, we did see reduced eGFR in the recipients with dnDSA compared to recipients without dnDSA (p = 0.009), while HLA sensitization did not affect eGFR (Figure 6). Nevertheless, all persistent HLA antibodies complicate retransplantation [22, 52]. To investigate the clinical relevance of HLA sensitization and its potential impact on retransplantation, our definition of HLA sensitization included both pre and posttransplant DSA combined with non-DSA HLA antibodies posttransplant. This broad definition might have contributed to the high sensitization rate. However, on closer examination, the non-DSA HLA antibodies were weaker and predominantly HLA class I. Consequently, the non-DSA HLA antibodies appear less clinically relevant and may not pose a significant challenge in retransplantation.
To our knowledge, no studies have investigated the relationship between the level of MMF and dnDSA in pediatric KT. Two studies have examined the association of dnDSA and corticosteroids, one in adult KT [53] and one in pediatric [54]. Both studies failed to demonstrate a difference in dnDSA between corticosteroid-based and corticosteroid-free/-withdrawal protocols, but the follow-up period of only 24 months in the pediatric study may have been insufficient for evaluating dnDSA formation [54]. In our transplant center, we have performed corticosteroid-free transplantations for more than three decades to avoid the side effects of corticosteroids and enhance the growth and metabolic health of the recipients [25]. With 80% of the cohort being corticosteroid-free (increasing to 89% at year one), our findings do not support excessive dnDSA formation in corticosteroid-free and MMF-reduced recipients.
Tacrolimus, on the other hand, does seem to inhibit the formation of dnDSA. Urzykowska et al. evaluated the effect of triple therapy (tacrolimus/MMF/corticosteroids) on dnDSA and found that only tacrolimus level was associated with dnDSA [55]. The median tacrolimus trough level was 7.9 ng/mL in the dnDSA-negative recipients and 7.1 ng/mL in the dnDSA positive. In a study of extended-release tacrolimus in adult KT on a corticosteroid-free protocol, Gatault et al. concluded that tacrolimus trough level above 7 ng/mL reduced the risk of alloimmunological events [56]. Hiramitsu et al. reported a trough level below 4.9 ng/mL to be associated with dnDSA [57]. In addition, maintaining a stable trough level with minimal variation seems vital in preventing dnDSA [44, 47]. We did not study individual variations in tacrolimus trough levels, but the median level remained close to the desired target over time.
Apart from those mentioned above, our study has certain limitations. One major limitation is that the study was observational and retrospective. Moreover, our cohort was heterogeneous, with diverse pretransplant immunological risk profiles, reflecting the real-life situation. This heterogeneity makes it more challenging to extrapolate our findings to specific subgroups. Pretransplant HLA antibody screening was not routinely performed until 2011, and recipients without were also included. The absence of data regarding pretransplant HLA antibodies affects the interpretation of de novo HLA sensitization, and this complicated the inference of whether the HLA sensitization was clinically relevant. Furthermore, the outcomes of rejection and dnDSA were sparse, limiting the statistical robustness and the ability to adjust for confounders. Furthermore, rejections might be underestimated as surveillance biopsies are not standard care in our center.
4.1 Conclusion and Perspective
Balancing immunosuppressive medication to prevent alloimmunity while optimizing the quality of life and minimizing side effects is challenging in pediatric kidney transplant recipients.
In this study, immunosuppressive medication was significantly reduced throughout the first year due to side effects and frequent infections, leading to nearly halving the intended dosage of MMF while maintaining a well-regulated level of tacrolimus. Subsequently, infections and side effects notably decreased, with immunosuppression remaining low. Remarkably, despite the reduced immunosuppression, no increased alloimmunity was observed.
Our findings suggest that reducing MMF while maintaining an adequate trough tacrolimus level in a population of mainly corticosteroid-free pediatric recipients does not lead to unacceptable alloimmunity. Moreover, it may mitigate the risk of infections. Particularly noteworthy is the potential benefit for children under six, where reducing MMF dosage might prevent infections without elevating the risk of acute rejections and HLA sensitization.
A prospective study aimed at reducing the MMF dosage early after transplantation is essential to determine the optimal dosage for pediatric kidney transplant recipients. Such a study would require the use of MPA-TDM and the collaboration of multiple centers.
Author Contributions
Ann-Maria Gramkow: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing – original draft, writing – review and editing, visualization, project administration, funding acquisition. Emilie T. Gramkow: investigation, writing – review and editing. Per Wittenhagen: writing – review and editing. Johanne H. Baatrup and Pernille Koefoed-Nielsen: resources, writing – review and editing. Helle C. Thiesson: conceptualization, methodology, validation, resources, writing – review and editing, supervision, funding acquisition.
Acknowledgments
We acknowledge the biomedical laboratory scientists, nurses, and doctors at the Department of Nephrology, OUH, The Research Unit, OUH, Department of Immunology, AUH, and the Department of Pediatrics, OUH.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.