Pathology of explanted pediatric hearts: An 11-year study. Population characteristics and implications for outcomes
Abstract
Background
As more pediatric patients become candidates for heart transplantation (HT), understanding pathological predictors of outcome and the accuracy of the pretransplantation evaluation are important to optimize utilization of scarce donor organs and improve outcomes. The authors aimed to investigate explanted heart specimens to identify pathologic predictors that may affect cardiac allograft survival after HT.
Methods
Explanted pediatric hearts obtained over an 11-year period were analyzed to understand the patient demographics, indications for transplant, and the clinical–pathological factors.
Results
In this study, 149 explanted hearts, 46% congenital heart defects (CHD), were studied. CHD patients were younger and mean pulmonary artery pressure and resistance were significantly lower than in cardiomyopathy patients. Twenty-one died or underwent retransplantation (14.1%). Survival was significantly higher in the cardiomyopathy group at all follow-up intervals. There were more deaths and the 1-, 5- and 7-year survival was lower in patients ≤10 years of age at HT. Early rejection was significantly higher in CHD patients exposed to homograft tissue, but not late rejection. Mortality/retransplantation rate was significantly higher and allograft survival lower in CHD hearts with excessive fibrosis of one or both ventricles. Anatomic diagnosis at pathologic examination differed from the clinical diagnosis in eight cases.
Conclusions
Survival was better for the cardiomyopathy group and patients >10 years at HT. Prior homograft use was associated with a higher prevalence of early rejection. Ventricular fibrosis (of explant) was a strong predictor of outcome in the CHD group. We presented several pathologic findings in explanted pediatric hearts.
Abbreviations
-
- ACR
-
- acute cellular rejection
-
- AMR
-
- antibody-mediated rejection
-
- CHD
-
- congenital heart disease
-
- CMP
-
- cardiomyopathy
-
- cPRA
-
- calculated panel reactive antibody
-
- DCM
-
- dilated cardiomyopathy
-
- HLA
-
- human leukocyte antigen
-
- HLHS
-
- hypoplastic left heart syndrome
-
- HT
-
- heart transplantation
-
- LV
-
- left ventricle
-
- PAP
-
- pulmonary artery pressure
-
- RV
-
- right ventricle
1 INTRODUCTION
Heart transplantation (HT) remains the best long-term option for most pediatric patients with end-stage heart failure. HT outcomes have improved in recent decades, due to better patient selection, improvement in surgical techniques and more effective immune suppression therapy,1 particularly for recipients with congenital heart defects (CHD).2-4 However, pediatric HT remains challenging because of higher mortality in children, limited availability of small donor hearts, and the complexity of underlying CHD.4-6 For example, patients at various stages of single ventricle palliation are reported to have a higher mortality than other CHD patients.7-12 Understanding pathologic predictors of HT outcomes that could potentially impact pre- and post- HT management may help improve outcomes.
We reviewed explanted hearts at our institution to better understand our end-stage heart failure population and the indications for HT. We sought pathologic predictors of outcomes and a comparison of clinical and pathologic findings.
2 MATERIALS AND METHODS
The Cardiac Registry database was queried from 2010 through 2021 to identify all explanted hearts, excluding multiorgan transplantation.
Clinical data were collected and outcomes recorded for patients wherein explanted heart specimens were available for review. Pretransplant data obtained from medical charts include demographics, cardiac diagnoses, clinical symptoms, hemodynamic data, genetic findings and syndromic status, cardiac surgical and catheter interventions (including pacemaker/implantable cardioverter-defibrillator placement), indications for heart transplantation, United Network for Organ Sharing (UNOS) status at the time of listing for transplantation, and calculated panel reactive antibody (CRP) levels at transplantation. Recorded outcomes include operative and late mortality, allograft survival, acute and late cellular and antibody-mediated rejection, reason for allograft failure/transplantation, cardiac allograft vasculopathy, anatomy and histology of explanted hearts, anatomy and histology of autopsy heart specimens, and histology of homograft associated with explant or autopsy hearts. For the purpose of this study, early rejection was diagnosed when clinical, cardiac imaging, and endomyocardial biopsy findings were consistent with rejection (acute cellular rejection [ACR] and/or antibody-mediated rejection [AMR]) and prompted additional immune suppression treatment within the first year posttransplantation, in accordance with previous reports.13-16 Late rejection was based on clinical, imaging, and biopsy findings regardless of additional immune suppression after 1-year posttransplantation.17 Calculated Panel Reactive Antibody (cPRA) (sensitization levels) for anti-human leukocyte antigen (HLA) Class I and Class II antibodies, and the total number of anti-HLA antibodies with various specificities were determined at HT as previously described.18 For those who underwent molecular testing, targeted cardiomyopathy gene next-generation sequencing panels were performed on cardiomyopathy patients, and a subset of CHD/syndromic patients underwent chromosomal microarray. Variants were classified using the American College of Medical Genetics and Genomics (ACMG) and the Association of Molecular Pathology (AMP) 2015 Guidelines.19
Cardiac anatomic diagnoses of explanted heart specimens were obtained from the Cardiac Registry reports and histopathological findings from Surgical Pathology reports. Residual homograft material was examined histologically for inflammatory and other cells. Myocardial fibrosis was quantified using representative photomicrographs of Masson trichrome-stained slides obtained using OLYMPUS BX51, DP72 microscope with camera and cellSens Standard 1.17 software (Olympus Corporation, Tokyo, Japan) (10X magnification) from the subepicardial, mid myocardial, and subendocardial regions of the right and left ventricular free walls using ImageJ software20 (Available at https://imagej.nih.gov/ij/). The total area of fibrous tissue was divided by the total myocardial area to yield % fibrosis. For functional single ventricle, fibrosis was assessed in both ventricles if there was distinct ventricular myocardial mass representing each side. Otherwise, only the dominant ventricle was evaluated. Of note, one heart specimen was excluded from assessment of fibrosis because the histology slides were not available for review.
Normally distributed data were presented as mean ± SD, otherwise as median and range. Age and BSA at HT, listing days for HT, pre-HT left ventricular (LV) ejection fraction, and days on ventricular assist device were presented as mean, median, and range in tables. Differences between groups were tested with the Welch's t-test for normally distributed continuous variables, the Mann–Whitney U test for nonnormally distributed continuous variables, and the Pearson's chi-squared test or the Fisher exact test for categorical variables. Kaplan–Meier curves for allograft survival were estimated overall and for various patient subgroups and compared using the log-rank test. A p value of <0.05 was considered statistically significant. All analyses were performed using IBM SPSS Statistics (Version 27.0. Armonk, NY: IBM Corp.).
The Boston Children's Hospital Institutional Review Board (IRB-P00040005) approved this retrospective study.
3 RESULTS
3.1 Demographics
This study included 149 explanted hearts (90 males, 60%), from 156 HT performed at our institution, with 7 (4%) explants unavailable for review. Sixty-nine explants had a CHD (46%) and 80 (including 4 retransplants) CMP (54%). The median age at HT was 9 years (mean 8.7, range 0–20 years). CHD patients were significantly younger and smaller at HT compared to CMP patients (Table 1). Indications for transplant are shown in Table 2.
| CHD | CMP | Total | p valuea | |
|---|---|---|---|---|
| Number of patients | 69 (46%) | 80 (54%) | 149 | |
| Sex | ||||
| Female (n) | 21 (30%) | 38 (48%) | 55 (40%) | .034 |
| Male (n) | 48 (69%) | 42 (52%) | 84 (60%) | .034 |
| At transplant | ||||
| Age (months) | ||||
| mean | 76 ± 73 | 129 ± 70 | 104 ± 76 | .001 |
| median (range) | 51 (2–249) | 158 (0–242) | 108 (0–249) | |
| BSA (m2) | ||||
| mean | 0.76 ± 0.49 | 1.21 ± 0.56 | 1.00 ± 0.57 | .001 |
| median (range) | 0.62 (0.18–1.98) | 1.26 (0.2–2.06) | 0.85 (0.18–2.06) | |
| UNOS statusb | ||||
| 1A | 57 (83%) | 54 (68%) | 111 (74%) | .035 |
| 1B | 5 (7%) | 12 (15%) | 17 (11%) | .296 |
| 2 | 5 (7%) | 13 (16%) | 18 (12%) | .096 |
| Listing-HTx (days) | ||||
| mean | 246 ± 359 | 168 ± 217 | 204 ± 292 | .075 |
| median (range) | 129 (4–2040) | 87 (2–1259) | 108 (2–2040) | |
| Pretransplant data | ||||
| LVEF (echo, %) | ||||
| mean | 34 ± 11 | 33 ± 18 | 33 ± 17 | .649 |
| median (range) | 32 (16–54) | 27 (9–72) | 28 (9–72) | |
| Mean PAP (mmHg) | 23 ± 9 (n = 63) | 28 ± 9 (n = 74) | 26 ± 9 | <.01 |
| PcwP (mmHg) | 16 ± 6 (n = 55) | 19 ± 7 (n = 73) | 17 ± 7 | .010 |
| PVR (Wood) | 2.1 (0.7–6.9) (n = 61) | 3.1 (0.9–9.7) (n = 65) | 3.0 (0.7–9.7) | .006 |
| TPG (mmHg) | 8.3 ± 5.6 (n = 62) | 9.3 ± 4.7 (n = 72) | 8.6 ± 5.2 | .236 |
| Intervention prior to HT | ||||
| Cardiac surgeries | 3.0 (0–12) | 1 (0–6) | 1 (0–12) | <.001 |
| Age at first surgery (months) | 0 (0–215) | 141.0 (0–220) | 1.5 (0–220) | <.001 |
| pacemaker/ICD | 1 (0–4) | 0 (0–3) | 0 (0–4) | <.001 |
| Catheter interventions | 1.0 (0–9) | 0 (0–2) | 1.0 (0–9) | .001 |
| Fontan | 20 (29%) | - | 20 | - |
| Last surgery to HTx or ECMO/VAD (mo) | 19 (0–237) | 22 (0–139) | 19 (0–237) | .556 |
| with VAD | 13 (18.8%) | 34 (42.5%) | 45 (31.5%) | .001 |
| Days on VAD | ||||
| mean | 108.0 ± 120.6 | 85.4 ± 114.0 | 91.5 ± 115.0 | .825 |
| median | 64 (10–371) | 56 (3–657) | 57 (3–657) | |
- Note: The statistically significant values, as defined in the materials and methods, appear in bold.
- Abbreviations: ECMO, extracorporeal membrane oxygenator; ICD, implantable cardioverter/defibrillator; LVEF, LV ejection fraction; PAP, pulmonary artery pressure; PcwP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; TPG, transpulmonary gradient; VAD, ventricular assist device.
- a CHD versus CMP.
- b UNOS status unknown in three patients.
| CHD (n = 69) | CMP (n = 80) | |
|---|---|---|
| Reason for HT | ||
| Systolic dysfunction | 41 (69.5%) | 50 (62.5%) |
| Diastolic dysfunction | 5 (7.2%) | 16 (20.0%) |
| Fontan failure | 9 (13.0%) | 0 |
| Arrhythmia | 0 | 7 (8.8%) |
| Elevated PVR | 2 (2.9%) | 3 (3.8%) |
| Right or left ventricular dependent coronary circulation ± ventricular dysfunction | 6 (8.7%) | 0 |
| No further surgical options | 5 (7.2%) | 0 |
| HOCM—no surgical option | 0 | 1 (1.3%) |
| Reason for re-HT | ||
| Cardiac allograft vasculopathy ± LV dysfunction | 1 (1.4%) | 3 (3.8%) |
- Abbreviations: HOCM, hypertrophic obstructive cardiomyopathy; PVR, pulmonary vascular resistance; other abbreviations as in previous tables.
3.1.1 Intervention prior to HT
Interventions prior to HT are shown in Table 1. CHD patients underwent significantly more operations, with the first operation at an earlier age, compared to CMP patients. The number of pacemaker or implantable cardioverter-defibrillator placements and catheter interventions was significantly greater in CHD patients, while CMP patients were significantly more likely to have ventricular assist device support.
3.1.2 Hemodynamics
Hemodynamic data are shown in Table 1. The echocardiographic LV ejection fraction did not differ significantly between CHD patients with a systemic LV and CMP patients. Right ventricular function was rarely quantified. Mean pulmonary artery pressure (PAP), pulmonary capillary wedge pressure, and pulmonary vascular resistance were significantly higher in CMP patients than in CHD patients.
3.1.3 Homograft exposure
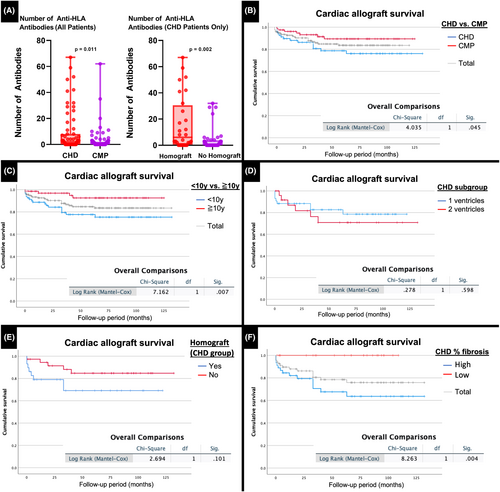
Homograft was used in 29/66 CHD hearts (3 hearts were excluded because of inavailability of operation reports) during repair or palliation (Table S1). The median duration of exposure was 41 months (mean 60, range 2–211 months). At transplant, the number of anti-HLA antibodies in CHD patients with homograft was significantly higher than in those without. Patients with CHD also had higher numbers of anti-HLA antibodies than those with CMP, mostly driven by those exposed to homograft (Figure 1A). Percent cPRA levels were also higher for both Class I and Class II antibodies at transplant for CHD patients with homograft compared to those without.
3.2 Breakdown of CHD/CMP diagnosis and genetic findings
3.2.1 CHD/CMP diagnosis
Table 3 shows the breakdown of CHD/CMP diagnosis. The most common CHDs were hypoplastic left heart syndrome (HLHS) (34.8%), congenitally physiologically corrected transposition of the great arteries {S, L, L} (8.7%) and critical aortic stenosis (7.2%). Forty-three of 69 CHD hearts (62%) had functional single ventricle at various stages of palliation, and the remaining 26 (38%) had 2 ventricles with or being staged toward biventricular repair. The most frequent CMP diagnosis was idiopathic dilated cardiomyopathy (DCM) (24%), followed by genetic DCM (20%) and secondary DCM (18%). Secondary DCM was due to some extrinsic cause other than a CHD or a genetic cardiomyopathy (e.g., myocarditis or chemotherapy).
| CHD | n = 69 | CMP | n = 80 |
|---|---|---|---|
| Functional single ventricle | 43 (62%) | Idiopathic DCM | 34 (42.5%) |
| HLHS | 24 (34.8%) | Secondary DCM | 14 (17.5%) |
| PA/IVS | 4 (5.8%) | RCM | 13 (16.2%) |
| MA/DORV | 3 (4.3%) | HCM | 6 (7.5%) |
| DI/DORV | 3 (4.3%) | ACM | 5 (6.3%) |
| Heterotaxy, CAVC/DORV | 3 (4.3%) | LV Non-compaction | 4 (5.0%) |
| DORV | 2 (2.9%) | Cardiac allograft failure | 4 (5.0%) |
| TA | 2 (2.9%) | ||
| DILV | 1 (1.4%) | ||
| Borderline LV | 1 (1.4%) | ||
| 2 ventricles | 26 (38%) | ||
| L-TGA | 6 (8.7%) | ||
| Critical AS | 5 (7.2%) | ||
| D-TGA | 4 (5.8%) | ||
| Borderline LV/CoA | 2 (2.9%) | ||
| Shone complex | 2 (2.9%) | ||
| Anomalous origin of LCA from right sinus | 1 (1.4%) | ||
| Posterior mitral leaflet cleft with severe MR | 1 (1.4%) | ||
| IAA/VSD | 1 (1.4%) | ||
| TOF | 1 (1.4%) | ||
| VSD | 1 (1.4%) | ||
| CoA/VSD with MR | 1 (1.4%) | ||
| CAVC | 1 (1.4%) |
- Note: Of note, one patient with CHD underwent retransplantation and this heart was included in the CMP group because the donor heart was structurally normal and had developed a posttransplant CMP.
- Abbreviations: ACM, arrhythmogenic cardiomyopathy; CAVC, common atrioventricular canal; CoA, aortic coarctation; DCM, dilated cardiomyopathy; DI, double inlet; DORV, double-outlet right ventricle; D-TGA, D-transposition of great arteries; HCM, hypertrophic cardiomyopathy; HLHS, hypoplastic left heart syndrome; IAA, interrupted aortic arch; LCA, left coronary artery; L-TGA, congenitally, physiologically corrected transposition of great arteries with two ventricles; LV, left ventricle; MA, mitral atresia; MR, mitral regurgitation; RCM, restrictive cardiomyopathy; TA, tricuspid atresia; TOF, tetralogy of Fallot; VSD, ventricular septal defect.
3.2.2 Syndromes and genetic abnormalities
Syndromes were identified in 7 CHD patients (4 heterotaxy syndromes, 1 PHACE [Posterior fossa anomalies, Hemangioma, Arterial anomalies, Cardiac anomalies, and Eye anomalies] syndrome,21 1 Sturge–Weber syndrome, and 1 Turner syndrome). Genetic testing was performed rarely in CHD patients and only 4 had abnormalities detected: 45, XO in Turner syndrome, 47, XYY in heterotaxy syndrome, 7p14.3 duplication in double-inlet right ventricle, and mosaic chromosome complement with 47, X, r(Y) x2 [9]/46, XY [11] in multiple left heart obstruction.
Genetic testing was performed more frequently in CMP patients and identified genetic variants in 39% (31/80). At least 51% of those (16/31) had >1 variant, of which 69% (11/16) had a combination of pathogenic/likely pathogenic variants and variants of uncertain significance. One patient with mitochondrial disorder by histology had negative genetic testing. Pathogenic and likely pathogenic variants identified for each main CMP phenotype are listed in Table 4.
| Case | Gene(s) | Details |
|---|---|---|
| Genetic DCM (n = 9/15) | ||
| #1 | TNNT | Heterozygous c.629_631delAGA (pLys210del), Exon 13 (P) |
| #2 (PRES syndrome) | TNNT | Heterozygous c.629_631delAGA (p.Lys210del), Exon 13 (P) |
| #3 | TNNT | Heterozygous c.629_631del (p.Lys210del), Exon13 (P) |
| #4 | TNNT | Heterozygous c.26752_26761del (p.Thr8918fs), Exon 105 (LP) |
| #5 | TTN | Heterozygous c.65683delG (p.Ala21895fs), Exon 275 (LP) |
| #6 | TTN | Heterozygous c.56614A>T (p.Arg18872x), Exon 257 (LP) |
| #7 | LMNA | Heterozygous 1621C>T (R541C), Exon 10, (Presumed Pathogenic) |
| #8 | TNNI3 | Heterozygous c.544G>A (p.Glu182Lys), Exon 7 (P) |
| #9 (Danon disease) | LAMP2 | X-linked dominant, details not available (P) |
| HCM (n = 3/6) | ||
| #10 | MYH7 | Heterozygous c.1357C>T (p.Arg453Cys), Exon 14 (P) |
| #11 (Noonan Syndrome) |
ALPK3 RRAS2 |
Heterozygous c.1018 C>T (p.Q340X) (P)Heterozygous c.35 A > G p.Q12R, De Novo (LP) |
| #12 | MYL3 | Homozygous, c.427G>A (p.Gu143Lys) (P) |
| RCM (n = 4/13) | ||
| #13 | DES | Heterozygous c.1360C>T (p.Arg454Trp), Exon 8 (P) |
| #14 | TNNI3 | Heterozygous c.575G>A (p.Arg192His), Exon 8 (P) |
| #15 | TNNI3 | Heterozygous c.575G>A (p.Arg192His), Exon 8 (P) |
| #16 | TNNI3 | Heterozygous c.509G>A (p.Arg170Gln) Exon 7 (P) |
| ACM (n = 5/5) | ||
| #17 | PKP2 | Heterozygous c.1034+1G>T, Intron 3 (LP) |
| #18 | PKP2 | Homozygous c.2509delA (p.Ser837fs), Exon 13 (P) |
| #19 | DSP | Heterozygous c.1873C>T (p.Gln625X), Exon 14, DSP, (P/LP) |
| #20 | DES | Heterozygous c.347A>G (p.Asn116Ser), Exon 1 (P/LP) |
| #21 | RBM20 | c.1913C>T (p.Pro638Leu) (P) |
- Abbreviations: ACM, arrhythmogenic cardiomyopathy; HCM, hypertrophic cardiomyopathy; RCM, restrictive cardiomyopathy.
3.3 Pathology
3.3.1 Primary diagnosis
The primary pathologic diagnosis differed from the clinical diagnosis in 8 explants (5%) (Table 5). Additionally, 10 valve abnormalities, 1 coronary anomaly, and 2 papillary muscle abnormalities were newly discovered in the explants. Multiple muscular ventricular septal defects reported clinically could not be confirmed pathologically in 1 explant.
| Differences in the main diagnosis (clinical vs. anatomic) (n = 8) | Identified anatomic abnormalities not detected clinically | Clinical findings not confirmed in the actual specimen | ||
|---|---|---|---|---|
| Valve abnormalities (n = 11) | Coronary anomalies (n = 1) | Others (n = 2) | ||
|
MA, DORV {S, X, D} to MA, DORV {S, L, D} DIRV/DORV {S, D, D} to DIRV/DORV {S, X, D} DIRV/DORV {S, X, D} to DIRV/DORV {S, D, D} TA, DORV {I, L, D} to TA, supero-inferior ventricles DORV {I, D, D} DORV to D-TGA (x 2) DILV to DIRV CAVC to VSD (membranous and mid-muscular) |
Bicuspid aortic valve (x 4) Bicuspid pulmonary valve Severe Ebstein anomaly in Pulmonary atresia/Intact ventricular septum Double orifice tricuspid valve Hypoplastic mitral valve Myxomatous pulmonary valve Dysplastic tricuspid valve Dysplastic mitral valve |
Left circumflex artery origin from right coronary artery | Unusual proximity of left ventricular papillary muscles (x 2) | Multiple muscular VSDs not identified in specimen (n = 1) |
- Abbreviations: CAVC, common atrioventricular canal; DILV, double inlet left ventricle; DIRV, double inlet right ventricle; TA, tricuspid atresia; VSD, ventricular septal defect.
Most diagnostic differences were regarding ventricular loop and ventriculo-arterial alignment. None would have affected HT outcome but could have influenced prior treatment. The valve abnormalities were minor and the coronary artery anomaly of no clinical significance.
3.3.2 Attached appliances
Explanted hearts had a variety of devices attached from prior interventions (Table S2).
3.3.3 Atria
The 4 hearts with restrictive CMP had evident atrial dilation (Figure S1). Atrial endocardial fibrosis was frequently observed in a variety of diagnoses.
3.3.4 Atrioventricular valves
About half of the hearts with systemic right ventricle (RV) and tricuspid valve regurgitation showed pathology clearly related to tricuspid valve dysfunction (Figure S2A,B). Both hearts with heterotaxy (asplenia) syndrome and severe common atrioventricular valve regurgitation had annular dilation and surgical valve plasty. Mitral valve dysfunction was less common, occurring in 2 hearts with left heart obstruction and in 1 with a posterior leaflet cleft and tethering (Figure S2C,D).
3.3.5 Ventricles
Myocyte hypertrophy and interstitial fibrosis were evident in many hearts, especially those with pressure and/or volume overload. Endocardial fibrosis was much more marked in the nonsystemic LV in single ventricle hearts or the systemic LV in 2-ventricle hearts (Figure 2A; Figure S3), likely due to the high prevalence of HLHS and borderline LV hearts. In contrast, endocardial fibrosis was mild or absent in the systemic LV in functional single ventricle hearts and in the RV regardless of subsystemic or subpulmonary position (Figure S3). Marked segmental LV dysplasia was noted in the heart from an infant who suffered a cardiac arrest within minutes of birth (Figure 2B).
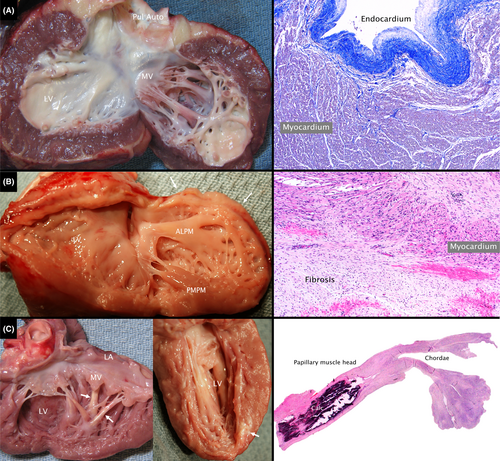
Extensive LV infarction with dystrophic calcification was present in 5 hearts including 2 with pulmonary atresia with intact ventricular septum and RV-dependent coronary circulation (Figure 2C), 1 following closure of multiple ventricular septal defects, 1 late after repair of tetralogy of Fallot, and 1 with anomalous aortic origin of the left coronary artery and out-of-hospital arrest.
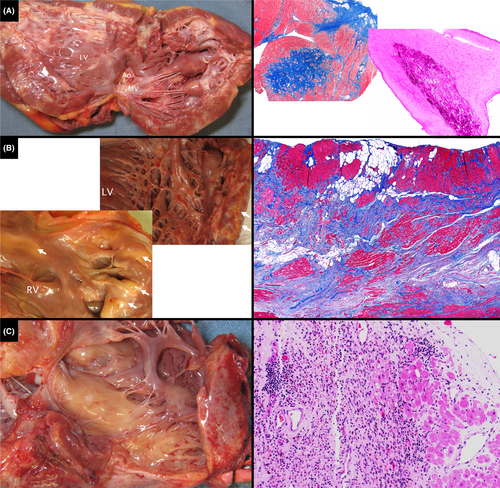
Explanted CMP hearts showed myocyte hypertrophy, interstitial fibrosis, replacement fibrosis and variable endocardial fibrosis. A Danon disease heart had cytoplasmic inclusions (Figure 3A). Arrythmogenic CMP hearts had multiple areas, often biventricular, of transmural fibro-fatty replacement of myocardium (Figure 3B). A heart with florid myocarditis showed extensive inflammatory cell infiltrate, myocyte necrosis, and replacement fibrosis (Figure 3C).
3.3.6 Coronary arteries
Congenital coronary artery anomalies were noted in 8 hearts: RV-dependent coronary circulation in 4 hearts with pulmonary atresia with intact ventricular septum (Figure S4A); LV-dependent coronary circulation in 2 hearts with HLHS; anomalous origin from the aorta in 2 hearts. Occlusion of 1 coronary artery was noted in 3 hearts with transposition {S, D, D} after arterial switch operation (Figure S4B). Coronary thrombosis or embolus was diagnosed by angiography prior to HT in 3 patients and was treated aggressively with thrombolysis and/or stenting. The explants demonstrated no residual thrombus.
Cardiac allograft vasculopathy was noted in the 4 explants from retransplantation. Coronary stents were in situ in 2, with minimal or no in-stent stenosis. Severe stenosis of the left anterior descending artery was noted in 1 (Figure S5, Left) and long-segment narrowing of the right coronary in another (Figure S5, Right).
3.4 Outcomes
During the study period, 21 patients died or underwent retransplantation (14%) and the allograft survival was 92% at 1 year, 84% at 5 years, and 83% at 7 years (Table 6, Figure 1B).
| Total (n = 149) | CMP (n = 80) | CHD (n = 69) | p valuea | |
|---|---|---|---|---|
| Death/re-Htx | ||||
| Total | 21 (14.1%) | 7 (8.8%) | 14 (20.3%) | .044 |
| Rejection | 13 (8.7%) | 4 (5.0%) | 9 (13.0%) | .083 |
| AMR | 6 (4.0%) | 0 | 6 (8.6%) | - |
| ACR | 2 (1.3%) | 1 (1.3%) | 1 (1.4%) | - |
| AMR + ACR | 1 (0.7%) | 1 (1.3%) | 0 | - |
| AMR + CAV | 1 (0.7%) | 0 | 1 (1.4%) | - |
| ACR + CAV | 2 (1.3%) | 2 (2.5%) | 0 | - |
| Clinical | 1 (0.7%) | 0 | 1 (1.4%) | - |
| Cardiac issue | 5 (3.4%) | 1 (1.3%) | 4 (5.8%) | .183 |
| Other | 3 (2.0%) | 2 (2.5%) | 1 (1.4%) | 1.0 |
| Lost to follow up | 2 (1.3%) | 1 (1.3%) | 1 (1.4%) | 1.0 |
| Cardiac event after transplantation | ||||
| iNO/Sildenafil | 42 (28.2%) | 18 (22.5%) | 24 (34.8%) | .097 |
| ECMO | 14 (9.4%) | 3 (3.8%) | 11 (15.9%) | .011 |
| Surgery | 1 (0.7%) | 1 (1.3%) | 0 | 1.0 |
| Cath intervention | 22 (14.8%) | 8 (10.0%) | 16 (23.2%) | .029 |
| Rejection | ||||
| None | 64 (43.0%) | 34 (42.5%) | 32 (46.4%) | .635 |
| Early (all) | 55 (36.9%) | 27 (33.8%) | 28 (40.6%) | .389 |
| AMR | 24 (16.1%) | 11 (13.8%) | 13 (18.8%) | - |
| ACR | 16 (10.7%) | 11 (13.8%) | 5 (7.2%) | - |
| Mixed | 15 (10%) | 5 (6.3%) | 10 (14.4%) | - |
| Lateb | 50 (39.1%) (n = 128) | 30 (42.3%) (n = 71) | 20 (35.1%) (n = 57) | .409 |
| AMR | 6 (4.0%) | 1 (1.3%) | 5 (7.2%) | - |
| ACR | 38 (25.5%) | 26 (32.5%) | 12 (20.3%) | - |
| Mixed | 5 (3.4%) | 3 (3.8%) | 2 (33.3%) | - |
| Clinical | 1 (0.7%) | 0 | 1 (1.4%) | - |
| Latest follow-up (echo) | ||||
| Follow-up (months) | 60.5 (0–125) | 63.0 (0–125) | 59.5 (0–122) | .637 |
| LVEF | 60.9 ± 4.6 | 60.9 ± 4.3 | 60.8 ± 4.9 | .830 |
| Valve dysfunction > mild | 27 (18.1%) | 15 (18.8%) | 14 (20.3%) | .773 |
| Transplant vasculopathy | 18 (12.1%) | 9 (11.3%) | 9 (13.0%) | .738 |
- Note: The statistically significant values, as defined in the materials and methods, appear in bold.
- Abbreviations: ACR, acute cellular rejection; AMR, antibody-mediated rejection; CAV, cardiac allograft vasculopathy; ECMO, extracorporeal membrane oxygenator; LVEF, LV ejection fraction.
- a CHD versus CMP.
- b For patients with at least 1-year follow-up.
3.4.1 CHD versus CMP
The overall outcomes for CHD and CMP groups were shown in Table 6, and survival was significantly higher in the CMP group at all follow-up intervals tested (Figure 1B).
3.4.2 Age at HT
Survival at all intervals was lower in patients ≤10 years of age at HT compared with patients >10 years of age, in part reflecting the greater proportion of patients with CHD in the younger cohort (Table S3; Figure 1C).
3.4.3 Cardiac allograft vasculopathy
Eighteen patients (13%), 9 CHD (13%), and 9 CMP (11%) developed cardiac allograft vasculopathy (Table 6). Two of the 18 have undergone coronary intervention and retransplantation (below).
3.4.4 Retransplantation
Four patients underwent a second HT during the study period. The pre-HT diagnosis was dilated CMP in 2, arrhythmogenic CMP in 1, and CHD (Shone complex) in 1. All patients had a history of rejection, 2 with early rejection (AMR, n = 2) and all alive patients with late rejection (AMR, n = 1; ACR, n = 1). All had cardiac allograft vasculopathy and 2 patients had undergone coronary intervention. One patient died due to bleeding and inability to separate from cardiopulmonary bypass at retransplantation. A second patient died suddenly 2 months after retransplantation. No evidence of rejection or coronary artery abnormality was seen at postmortem exam. The other 2 survived but have had recurrence of cardiac allograft vasculopathy.
3.5 Clinical and pathlogic correlation of increased risk of outcomes
3.5.1 1 versus 2 Ventricles
Deaths, allograft losses, and the 1-, 5-, and 7-year survival did not differ significantly between CHD patients with 1 and 2 ventricles (Table S4; Figure 1D) nor did use of extracorporeal membrane oxygenation or pulmonary vasodilators post-HT, prevalence of early or late rejection, or ventricular function on most recent echocardiogram.
3.5.2 Homograft exposure versus no exposure
Early rejection, including AMR (n = 9), ACR (n = 1), and mixed rejections (n = 6) for homograft exposure group and AMR (n = 3), ACR (n = 4), and mixed rejections (n = 3) for no homograft exposure group, was significantly more prevalent in patients exposed to homograft (55% vs 27%, p = .020) but not late rejection (32% vs 40%, p = .606), and there was a nonsignificant trend toward higher mortality (28% vs 14%, p = .101) (Figure 1E).
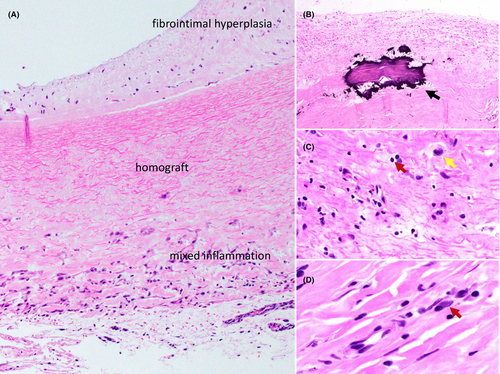
Six specimens had sufficient homograft attached for microscopic analysis. Sections were sparsely cellular (Figure 4) with myofibroblasts/stromal cells and inflammatory cells. Myofibroblasts were mostly located toward the luminal side, as a component of fibrointimal hyperplasia in 2 cases. Inflammatory cells were patchy, frequently toward the adventitial side, around areas of neovascularization. Histiocytes and lymphocytes were the most frequent inflammatory cells, with plasma cells present in half of cases. Calcifications were presented in all.
3.5.3 Venticular fibrosis
Myocardial fibrosis, predominantly interstitial with some replacement-type foci, measured as % fibrosis, was most marked in the subendocardial 1/3 of the wall in all hearts. The mortality/retransplantation rate was significantly higher and allograft survival lower in CHD hearts in which the % fibrosis for 1 or both ventricles was above the median (Table S5; Figure 1F). In fact, all deaths/retransplantation occurred in the former group and 64% were due to rejection, including AMR (n = 6), ACR (n = 1), AMR (n = 1) with cardiac allograft vasculopathy, and 1 clinical rejection (mostly early). The PAP but not resistance, was also significantly higher in this group. We did not find a significant relationship between ventricular fibrosis and allograft survival in CMP patients.
4 DISCUSSION
4.1 Recipient Characteristics
Compared with most recent International Society for Heart Lung Transplantation data,4 our patients were older (median age 9 vs 7 years) with a greater proportion of males (60% vs 54%). The proportion <1 year of age was lower compared with other North American centers (17% vs 30%), probably reflecting the local surgical philosophy favoring repair. The proportion with CHD was slightly higher than other North American centers (46% vs 40%), as was ventricular assist device support prior to HT (31% vs 25%). 62% of CHD patients had a functionally single ventricle, 40% after completion of Fontan, and another 20% had a borderline LV with a 2-ventricle repair. Dilated CMP accounted for 60% of CMP hearts.
4.2 Outcomes
The overall survival at 1-year was identical to International Society for Heart and Lung Transplantation data4 for North America (92%). The survival at 1 year was significantly better in those ≥10 years compared to those <10 years (97% vs 89%). Rossano and colleagues22 reported that the 1-year survival rate for infants transplanted under 1 year of age was significantly worse than older recipients based on International Society for Heart and Lung Transplantation data. However, the longest median survival (22 years) was seen in patients transplanted in infancy. Similarly, O'Connor and colleagues reported that late survival for patients who underwent HT in infancy was significantly better than for older patients23 and the authors speculate that perhaps greater immunologic tolerance in younger patients was the reason. The time course of our study (median follow-up 64 months) was too short to allow analysis of late survival.
Consistent with prior reports,3, 4, 6, 22, 24 the survival for CMP patients was significantly better than for CHD patients. The CHD patients were also significantly younger at transplant, confounding our ability to clearly distinguish between the effects of age and CHD on survival.
Homograft use was associated with significantly higher prevalence of early rejection, but not late rejection, and a nonsignificant trend toward reduced survival. The number of anti-HLA antibodies and the Class I and Class II percent cPRAs were higher in the CHD group. This was mainly due to elevated cPRA in the subgroup with homograft use. This material was only used in CHD patients and these were younger at HT, again confounding our ability to determine which factor(s) might be determining survival. We observed some inflammatory response in the attached homograft in 6 cardiac explants, consisting mostly of histiocytes (antigen-presenting cells) and lymphocytes. Plasma cells, mature antibody-producing cells, were present in half of the cases, consistent with the known association of homograft use and elevated cPRA.25 Although not current practice, this suggests a potential role for immunosuppressive pharmacotherapy in children receiving homografts as reported by Shaddy and colleagues.26 However, prevention of anti-HLA sensitization is likely the best approach through the use of decellularized homograft material,27, 28 use of prosthetic alternatives, or immune response modulation.26
Most prior reports indicate that patients with single ventricle CHD at various stages of palliation are at increased risk for death following HT.7-12 Of our single ventricle patients, 44% had undergone completion of Fontan with the remainder at earlier stages of palliation. Similar to Crossland and colleagues,24 we found no difference in survival between CHD patients with 1 ventricle and 2 ventricles. Most of the 2-ventricle hearts had borderline LV size and significant diastolic dysfunction resulting in significantly higher PAP and pulmonary vascular resistance compared with the single ventricle group. This finding could have contributed to the lack of difference in outcomes for the 2 groups.
Genetic testing was performed in few patients with CHD, likely reflecting historical barriers to access and the relatively low yield of actionable findings. This is likely to change with the rapid discovery of more disease-causing variants and the availability and decreasing cost of new technology such as next-generation sequencing. In contrast, genetic testing was performed in the majority of CMP patients and yielded known or potential disease-causing variants in 39%. The relatively high yield of genetic testing in patients undergoing HT is consistent with findings at other centers.29
The extent of ventricular fibrosis in the CHD explanted hearts was highly predictive of allograft survival. All deaths and allograft loss were in the CHD group where the % fibrosis for 1 or both ventricles was above the median for the CHD group. The distribution of CHD types was similar in the 2 groups. PAP, but not calculated pulmonary vascular resistance, was higher in the high-fibrosis group. In addition, homograft was used in more patients in the high-fibrosis group (47% vs 30%). This group also tended to be younger and had undergone more catheter interventions after HT. The cause of death or retransplantation was rejection (mostly early) in 64% of cases.
It is unclear how fibrosis in the explant could influence survival after HT. There was some selection for higher risk patients in the high-fibrosis group (elevated PAP and homograft use), but this seems insufficient to explain the marked difference in outcome between the groups. Accumulating evidence indicates that myocardial fibrosis could be a marker for enhanced inflammation and fibrosis pathway activity in the recipient,30-32 which to a certain degree, could be related to an altered immune status. The unexplored consequences of partial or total thymectomy during open-heart surgery in infants is increasingly becoming a subject of interest as recent investigations gear toward understanding the various mechanisms of immune alterations in patients with CHD,31 and how these could possibly impact the heart and other organs in the long term. Anti-heart autoantibodies, thought to form as a result of prior myocardial injury, potentially play an essential role in early T-cell mediated rejection.31, 33-35 Outside of hearts with abnormal coronary/ostium anatomy, the coronary vessels in the other explanted native hearts were unremarkable, with no significant macroscopic and microscopic luminal narrowing, thereby hinting an alternative etiology of fibrosis aside from ischemic events. It is possible that an enhanced inflammatory state could carry over into the post-HT period leading to a higher likelihood of rejection, the main cause of failure of HT in the high-fibrosis group. Pathophysiologic pathways that link altered immunity, pro-inflammatory state, rejection, and fibrosis together warrant further study.
4.3 Study limitations
We acknowledge that this study has limitations. It was retrospective in nature. Additionally, not all explanted CHD specimens in which previous repairs utilized homografts had the homografts still attached to the specimen and available for sampling.
5 CONCLUSIONS
Nearly half of our patients had CHD, mostly HLHS after palliation and other forms of borderline left heart. Most CMP hearts had idiopathic DCM and in 39% a potential genetic cause was identified. Survival was significantly better for those with CMP and for patients >10 years at HT. Use of homograft for palliation of CHD was associated with a higher prevalence of early rejection, but not late, and a trend toward worse survival. We were able to demonstrate antigen-presenting cells and antibody-producing cells in residual homograft material in 6 explants, which is causally linked to the development of preformed anti-HLA antibodies. However, we did not observe a difference in survival between patients with 1 and 2 ventricles. Ventricular fibrosis in the explant was a strong predictor of outcome in the CHD group, but not the CMP group. This finding could be due in part to selection bias, but ventricular fibrosis could be a marker for patients at increased risk for early rejection. We presented some of the variety of pathologic findings in cardiac explants in a pediatric population.
AUTHOR CONTRIBUTIONS
TY, SPS, CKC: Conceived and designed the analysis, collected the data, performed the analysis, drafted the manuscript. RJH, CM: Contributed data, contributed in drafting the manuscript. JEM, FFT, EDB, KPD: Reviewed the data and manuscript.
ACKNOWLEDGMENTS
The authors do not have any acknowledgment.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.