Optimizing the approach to monitoring allograft inflammation using serial urinary CXCL10/creatinine testing in pediatric kidney transplant recipients
Abstract
Background
Urinary CXCL10/creatinine (uCXCL10/Cr) is proposed as an effective biomarker of subclinical rejection in pediatric kidney transplant recipients. This study objective was to model implementation in the clinical setting.
Methods
Banked urine samples at a single center were tested for uCXCL10/Cr to validate published thresholds for rejection diagnosis (>80% specificity). The positive predictive value (PPV) for rejection diagnosis for uCXCL10/Cr-indicated biopsy was modeled with first-positive versus two-test-positive approaches, with accounting for changes associated with urinary tract infection (UTI), BK and CMV viremia, and subsequent recovery.
Results
Seventy patients aged 10.5 ± 5.6 years at transplant (60% male) had n = 726 urine samples with n = 236 associated biopsies (no rejection = 167, borderline = 51, and Banff 1A = 18). A threshold of 12 ng/mmol was validated for Banff 1A versus no-rejection diagnosis (AUC = 0.74, 95% CI = 0.57–0.92). The first-positive test approach (n = 69) did not resolve a clinical diagnosis in 38 cases (55%), whereas the two-test approach resolved a clinical diagnosis in the majority as BK (n = 17/60, 28%), CMV (n = 4/60, 7%), UTI (n = 8/60, 13%), clinical rejection (n = 5/60, 8%), and transient elevation (n = 18, 30%). In those without a resolved clinical diagnosis, PPV from biopsy for subclinical rejection is 24% and 71% (p = .017), for first-test versus two-test models, respectively. After rejection treatment, uCXCL10/Cr level changes were all concordant with change in it-score. Sustained uCXCL10/Cr after CMV and BK viremia resolution was associated with later acute rejection.
Conclusions
Urinary CXCL10/Cr reliably identifies kidney allograft inflammation. These data support a two-test approach to reliably exclude other clinically identifiable sources of inflammation, for kidney biopsy indication to rule out subclinical rejection.
Abbreviations
-
- AUC
-
- area under the curve
-
- ALT
-
- alanine aminotransferase
-
- BCCH
-
- British Columbia Children's Hospital
-
- BK
-
- BK polyoma virus
-
- CMV
-
- cytomegalovirus
-
- CXCL10
-
- chemokine (C-X-C motif) ligand 10
-
- DAP
-
- Diagnostic Accreditation Program
-
- IFTA
-
- interstitial fibrosis and tubular atrophy
-
- IV
-
- intravenous
-
- IQR
-
- interquartile range
-
- PPV
-
- positive predictive value
-
- ROC
-
- receiver operating characteristic
-
- SR
-
- subclinical rejection
-
- uCXCL10/Cr
-
- urinary CXCL10/creatinine
-
- UTI
-
- urinary tract infection
1 INTRODUCTION
Acute rejection is associated with premature graft failure and impaired quality of life for pediatric kidney transplant recipients. Early acute cellular rejection is directly associated with later development of donor-specific antibodies and chronic inflammation in areas of interstitial fibrosis and tubular atrophy (i-IFTA),1, 2 which may progress to graft failure. Clinical functional monitoring will miss early or mild forms of rejection that are referred to as subclinical, but which are similarly associated with adverse allograft outcome.3-7 Kidney biopsy is the gold standard for diagnosis of acute rejection and is currently used for rejection surveillance. Improved graft outcome is associated with treatment of subclinical rejection, which occurs in 29%–44% of children within 3 months post-transplantation7-10 and 2.6%–50% of adult transplant recipients in the first year after transplant.3, 6, 11-21 However, the frequency with which biopsies may be performed for monitoring is limited by considerations of biopsy-related risks, cost, and impact on patient quality of life.22 The development of better tools to detect subclinical rejection is therefore essential.23
Urinary levels of the interferon-gamma-induced cytokine CXCL10 are associated with subclinical acute cellular rejection24-31 and may be reported as the ratio to urinary creatinine (uCXCL10/Cr),32, 33 with higher uCXCL10/Cr associated with greater severity of rejection.27 In previous work, our group established a diagnostic threshold of 12 ng/mmol that provided 90% specificity for the diagnosis of rejection.33 uCXCL10/Cr has been proposed as an ideal candidate for regular, non-invasive rejection surveillance in the clinic. As an indicator of acute inflammation, uCXCL10 is also associated with other sources of graft inflammation such as pyelonephritis/urinary tract infection (UTI) and polyoma BK virus nephropathy.27, 32, 34-39 The implementation as a clinical monitoring tool must therefore also account for other sources of inflammation when using uCXCL10/Cr as an indication for a diagnostic kidney biopsy to exclude rejection. The optimal application of uCXCL10/Cr into a diagnostic pathway has yet to be explored.
This study aims to explore the integration of serial uCXCL10/Cr testing into existing frameworks for transplant monitoring, including the number of tests required, when the tests should be performed, and how the results should be interpreted and acted upon in the context of rejection surveillance and post-infection monitoring. The results will help inform the best timing of kidney biopsy in patients where rejection is suspected, helping optimize testing, and improving patient care.
2 METHODS
2.1 Patient population
This is a cohort study of pediatric kidney transplant recipients from a single center to evaluate 2 different approaches to rejection surveillance and kidney biopsy indication, based on urinary CXCL10/Cr testing. Urine samples were collected in most patients under a local transplant research registry and biobanking protocol and then subsequently in a subset who had prospective serial uCXCL10/Cr testing to screen for rejection risk. The study protocol adheres to the Declaration of Helsinki and was approved by the research ethics board of the University of British Columbia (REB# H20-02105) with informed consent and assent where appropriate. Separate assent/consent was obtained for both the research registry and biobanks.
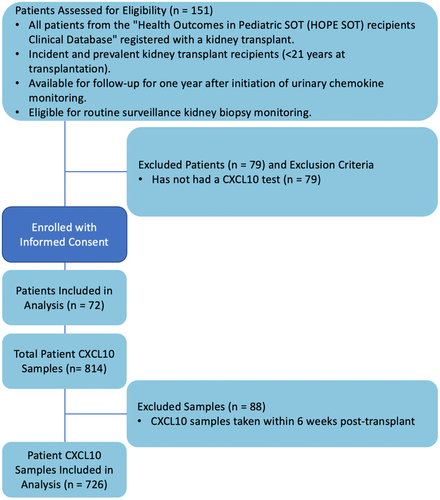
The transplant research registry was used to screen patients for inclusion in this study. Patients consented to both the registry and biobank were eligible if they were incident or prevalent pediatric kidney transplant recipients less than 21 years old at the time of transplant (Figure 1). Patients were excluded if they had not previously provided/biobanked urine samples that could be tested for CXCL10 or if they had primary cellular immunodeficiency syndromes that might impact uCXCL10/Cr monitoring.
Patients were treated according to the BCCH maintenance immunosuppression and rejection-treatment protocols. This included induction with intravenous methylprednisolone and either basiliximab or antithymocyte globulin and maintenance immunosuppression with a tacrolimus, mycophenolate, and prednisone. Acute rejection is typically treated with a 3-day course of IV methylprednisolone (300 mg/m2/dose), followed by an oral prednisone taper over 2 weeks.
2.2 Urine samples
Urine samples were collected in the prospective cohort for incident patients under biobank and serial testing protocols every 2 weeks post-transplant for 8 weeks, monthly for 4 months, and then every 3 months thereafter. For the prevalent cohort, 3 initial urine tests were obtained with at least 7 days between each test to establish a baseline and starting 3 months post-enrollment, and patients were tested every 3 months (range 2–4 months). Additional urine was obtained during the following situations: at the time of kidney biopsy, monthly for 3 months after treatment for acute rejection, and to follow up elevated uCXCL10/Cr levels. The retrospective (biobank) patients had samples collected at clinic visits, every 1–3 months.
2.3 Renal allograft biopsies
Kidney biopsies were conducted for indication (e.g., elevated creatinine) or for surveillance at 3, 6, 12, and 24 months post-transplant. Follow-up biopsies were routinely performed 6–8 weeks post-treatment for rejection. Kidney biopsies were graded according to the Banff 2013 criteria.40, 41 The Banff scores for acute inflammation (i) and tubulitis (t) were used to group as (1) no rejection (i < 1 or t < 1); (2) borderline grade rejection (i1t1–3; i2-3t1); and (3) Banff 1A or greater grade rejection (i ≥ 2 t ≥ 2).
2.4 Urine CXCL10 testing
Urinary CXCL10 levels were measured by electrochemiluminescence on a MESO QuickPlex SQ 120 analyzer, with V-PLEX CXCL10 plates using previously established protocols and a MSD CXCL10 standard curve.33 The percent coefficient of variation is <20% for intraassay and interassay variability, which provides excellent precision for an assay where the clinically important dynamic range exceeds a 10-fold difference between low and high levels of uCXCL10/Cr expression. The MSD assay for uCXCL10 has been established as a laboratory developed test for clinical use at the site. Testing and reporting validity are measured against the standards from Diagnostic Accreditation Program (DAP), a provincial laboratory accreditation body under the College of Physicians and Surgeons of British Columbia, Canada. Results are reported with normalization to urine creatinine (mmol/L) as the uCXCL10/Cr ratio (ng/mmol).
2.5 Validating reported thresholds for rejection diagnosis
We first sought to re-validate previously reported diagnostic thresholds for acute rejection in this independent cohort. Since previous reports have identified association between uCXCL10/Cr level and cases of BK, CMV viremia, and active UTI,27, 32, 34-39 these samples were withheld from the initial validation pertaining to rejection diagnosis and then evaluated separately for possible confounding. uCXCL10/Cr values from tests on the same day or up to 3 days pre-biopsy were associated with each biopsy.
After excluding the confounding samples, samples were organized by rejection group (no rejection, borderline rejection, and greater than Banff 1A rejection). The association of uCXCL10/Cr with each of the biopsy subgroups is reported as median and interquartile range (IQR) and visualized in box-whisker plots with reference to the previously reported diagnostic threshold.33 For comparisons between groups, repeated measures analysis of variance (ANOVA) was implemented as a regression model with a random subject effect to account for within-subject correlations. Paired t-tests to compare group means were also performed for each biopsy subgroup.
Receiver operating curve analysis was conducted to evaluate uCXCL10/Cr diagnostic validity, grouped based on the findings of the ANOVA analysis. The R library “cutpointr” was used to identify the optimal diagnostic threshold (Youden Index) in the dataset, and the area under curve (AUC) reported with 95% confidence interval (95% CI). The diagnostic threshold value of uCXCL10/Cr that represents 90% specificity was calculated to compare to the previously established thresholds.33
2.6 Simulating single versus serial testing to identify the cause of graft inflammation as indicated by uCXCL10/Cr
All available uCXCL10 tests were coded based on the value and timing of uCXCL10 results and then evaluated for the potential source of associated allograft inflammation. Samples taken within the first 6 weeks post-transplant were excluded from the analysis since early inflammation associated with ischemia-reperfusion injury is associated with elevated urinary uCXCL10/Cr levels.42
- Acute BK viremia
- CMV viremia
- Confirmed UTI
- Clinical acute rejection
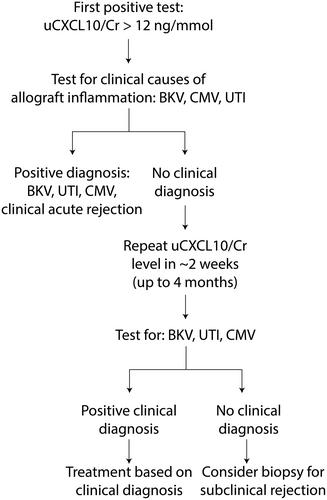
Viremia was defined as a measurable viral load above the minimum threshold for detection. CMV disease was defined as an elevated CMV viral load with manifestation of systemic CMV infection, such as elevated alanine aminotransferase (ALT) more than twofold, acute neutropenia, or other organ involvement. A confirmed UTI was defined as finding positive urine leukocytes and a single organism growth on urine culture or histologic confirmation of pyelonephritis. Clinical acute rejection included biopsies done for any indication other than elevated uCXCL10/Cr, such as elevated serum creatinine. The remainder were classified as “no clinical diagnosis.” Patients with acute rejection on surveillance biopsy within 4 months of the first elevated uCXCL10/Cr were identified and reported as the proportion with rejection relative to those with “no clinical diagnosis” who were biopsied and secondarily to the total with “no clinical diagnosis.” This represents the potential yield of biopsy indicated by otherwise unexplained uCXCL10/Cr elevation.
We next simulated the efficacy of serial uCXCL10/Cr testing to identify risk for subclinical acute rejection. Similar to how serum creatinine is used for monitoring, the purpose of a repeat test is to confirm a persistently positive result before proceeding to more invasive testing (i.e., kidney biopsy), after having excluded other causes. Patients with a second positive uCXCL10/Cr test within 4 months of the first-positive test were evaluated for persistently elevated uCXCL10/Cr. In the intervening time period until the repeat (second) test, additional clinical causes of explained elevated uCXCL10/Cr were accounted for (as above). In cases where uCXCL10/Cr was <10 ng/mmol on the second test, the case was treated as transient and resolved inflammation. The remaining cases with unexplained uCXCL10/Cr ≥12 ng/mmol after 2 tests were classified as a “persistently positive.” Patients found to have rejection on the day of the first-positive test were presumed to have persisted until diagnosis for the purpose of modeling serial sampling. Patients with persistently positive uCXCL10/Cr and acute rejection on biopsy within the 4-month period after the first-positive test were identified as subclinical rejection. Cases of clinically indicated biopsies around the time of the first uCXCL10/Cr test that demonstrate rejection were labeled as clinical acute rejection events. Only episodes classified as subclinical rejection were used to evaluate the proportion with rejection relative to those with “no clinical diagnosis” on a subsequent surveillance biopsy and secondarily to the total with “no clinical diagnosis.” This represents the potential yield (positive predictive value) of kidney biopsy indicated by unexplained persistent uCXCL10/Cr elevation, while indication or surveillance biopsies with rejection that occurred in the 4-month period after the initial positive uCXCL10/Cr test were labeled as subclinical acute rejection.
The temporal relationship between uCXCL10/Cr changes and biopsy-confirmed rejection was explored. For patients with a follow-up biopsy after rejection treatment, the persistence of rejection changes was reported in relation to the uCXCL10/Cr level at the time. Normalization was defined as uCXCL10/Cr levels that drop below 10 ng/mmol, and recurrence was defined as a relapse in uCXCL10/Cr level >12 ng/mmol.
Temporal trends were similarly evaluated for anticipation and resolution of uCXCL10/Cr associated with diagnosis and effective treatment of BK viremia, CMV, and UTI. Resolution of BK viremia and CMV infection was defined by the first test with a viral load persistently <1000 and 0 copies/mL, respectively. UTI resolution was defined as negative urine culture and the absence of recurrent pyuria following the antibiotic treatment. In cases that had a biopsy within 3 months after the infection had resolved, the finding of acute rejection was reported in relation to whether uCXCL10/Cr was persistently elevated after infection resolution.
3 RESULTS
3.1 Patient characteristics
152 kidney transplant patients were assessed for eligibility from the local research registry. Of these, 72 patients met criteria for inclusion (see Figure 1). One patient was excluded for declining to participate in the prospective trial (n = 1), and the remainder (n = 79) were not biobank participants and had no urine samples available. Participant characteristics are reported in Table 1, with a mean age of 10.5 ± 5.6 years at transplant and predominance for non-glomerular kidney disease (n = 42, 58%) and biologically male sex (n = 43, 60%).
| n | All patients (n = 72) |
|---|---|
| Total number of urine CXCL10 samples | 726 |
| Mean age at transplant (years) | 10.50 ± 5.56 |
| Mean age at CXCL10 test (years) | 12.8 ± 4.77 |
| Mean time to CXCL10 test from transplant (years) | 4.16 ± 3.57 |
| Mean follow-up time (years) from transplant to last CXCL10 | 14.70 ± 4.62 |
| Male sex | 43 (60%) |
| Race | |
| White | 31 (43%) |
| Asian: Southeast, Far East, India, Philippines | 22 (31%) |
| Other race | 17 (24%) |
| Etiology | |
| Glomerular disease | 30 (42%) |
| Non-glomerular disease | 42 (58%) |
| Median CXCL10 samples per patient | 10 (IQR 7.8, 15.2) |
| Biopsies linked to CXCL10 samples (0–3 days after test) | 236 |
| Median number of biopsies per patient | 3 (2, 5) |
| Biopsy reason | |
| Surveillance (%) | 124 (53%) |
| Follow-up (%) | 67 (28%) |
| Increased creatinine (%) | 50 (21%) |
| First transplant | 60 (83%) |
| Transplant characteristics | |
| Living-donor kidney | 31/72 (43%) |
| Mean HLA A mismatches | 1.26 ± 0.69 |
| Mean HLA B mismatches | 1.37 ± 0.65 |
| Mean HLA C mismatches | 1.24 ± 0.64 |
| Mean HLA DRB1 mismatches | 0.85 ± 0.62 |
| Mean HLA DQA mismatches | 0.52 ± 0.59 |
| Mean HLA DQB mismatches | 0.73 ± 0.63 |
| Antibody inductiona | |
| Basiliximab induction | 70/71 (99%) |
| Antithymocyte globulin induction | 3/71 (4.2%) |
- Note: Values are presented as mean ± SD, counts n (%), or median (IQR Q1, Q2).
- a Two patients were treated with both antithymocyte globulin and basiliximab.
There were 726 urine samples tested for uCXCL10, of which 236 were obtained at the time of a kidney biopsy (see Table 2). The average age at biopsy was 14.1 ± 8.2 years, and each patient had a median of 3 biopsies (IQR: 2, 5). Biopsies were performed at a mean 1.9 ± 2.2 years post-transplant. At the time of biopsy, 69 (29%) had acute rejection, of which 18 (26%) was Banff 1A severity or worse. None of the patients had a donor-specific antibody.
| n | All samples | No rejection | Borderline rejection | ≥Banff 1A rejection |
|---|---|---|---|---|
| Total number of samples | 236 | 167 | 51 | 18 |
| Mean years post-transplant | 1.9 ± 2.2 | 1.9 ± 2.3 | 2.0 ± 1.8 | 1.4 ± 1.9 |
| Median age at biopsy (years) | 14.1 (IQR 8.7, 16.9) | 13.9 (IQR 8.4, 16.6) | 14.8 (IQR 8.9, 19.0) | 14.1 (IQR 10.0, 15.4) |
| Urinary tract infection | 6 (2.5%) | 1 (0.6%) | 4 (7.8%) | 1 (5.6%) |
| BK viremia positive | 24 (10.2%) | 16 (9.6%) | 5 (9.8%) | 3 (16.7%) |
| CMV viremia; CMV disease | 10 (4.2%); 3 (1.3%) | 5 (3.0%); 0 (0%) | 2 (3.9%); 0 (0%) | 3 (16.7%); 3 (16.7%) |
| Urine samples excluding confounders | 198 (83.9%) | 145 (86.8%) | 40 (78.4%) | 13 (72.2%) |
| Median urinary CXCL10/Creatinine, excluding confounders | 4.1 (IQR 1.9, 9.1) | 3.9 (IQR 1.7, 8.5) | 3.7 (IQR 2.7, 7.7) | 16.2 (IQR 6.8, 27.7) |
- Note: Values are presented as mean ± SD, counts n (%), or median (IQR Q1, Q3). No rejection is defined as Banff scores of i0 (interstitial inflammation) t0 (tubulitis), i0 t ≥ 1, and i ≥ 1 t0. Borderline rejection is defined as Banff scores of i1 t1, i1 t2, i1 t3, i2 t1, and i3 t1. Banff 1A or greater rejection was defined as Banff scores of i ≥ 2 t ≥ 2.
The analysis of uCXCL10/Cr diagnostic threshold validity was completed after excluding samples with potential confounding from BK viremia (n = 24), CMV viremia (n = 10), and UTI (n = 6). The median uCXCL10/Cr with Banff ≥1A rejection was 16.2 (IQR: 6.8, 27.7) ng/mmol, which was significantly greater than for borderline (median = 3.7, IQR: 2.7, 7.7 ng/mmol; p < .01) and no-rejection samples (median = 3.9; IQR: 1.6, 8.5 ng/mmol; p < .01) (see Figure 2). The majority (54%) of samples with Banff ≥1A exceeded the previously reported diagnostic threshold of 12 ng/mmol.33 Findings were similar when only surveillance biopsies were evaluated.
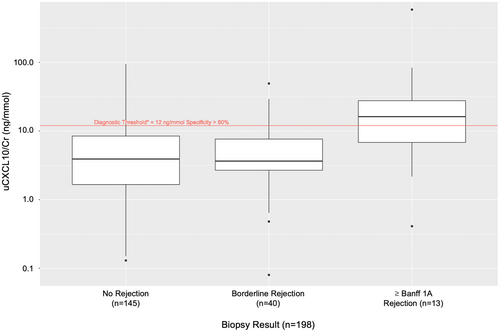
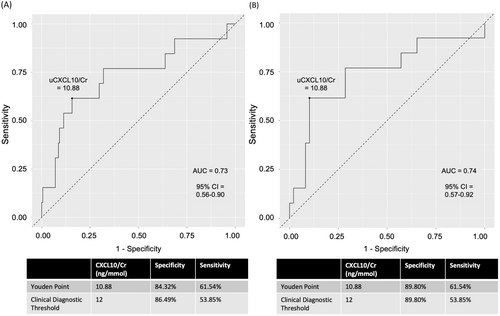
Figure 3A depicts the ROC curve for Banff ≥1A versus the no rejection and borderline groups combined (AUC = 0.73, 95% CI = 0.56–0.90). A sensitivity analysis (Figure 3B) that compared Banff ≥1A versus no rejection (excluding borderline) yielded a similar result (AUC = 0.74, 95% CI = 0.57–0.92). The Youden threshold of 10.9 ng/mmol (sensitivity = 62%, specificity = 84%) was similar to the previously reported threshold of 12 ng/mmol, although using the latter with this data yielded a sensitivity = 54% and specificity = 86%.
3.2 Implications of single versus serial uCXCL10/Cr testing
The breakdown of diagnostic outcomes (BKV, CMV, UTI, clinical acute rejections) and the positive predictive values of a biopsy based on the uCXCL10/Cr results are compared with the use of a one-step versus serial uCXCL10/Cr testing method in Table 3.
| BK viremia | CMV infection | UTI | Clinical acute rejectiona | Transient elevation | Unexplained positive | Subclinical acute rejection yield (PPV)b | |
|---|---|---|---|---|---|---|---|
| One-step (n = 69) | 14 (20%) | 5 (7%) | 8 (12%) | 4 (6%) | - | 38 (55%) | 24% (n = 7/29) |
| Two-step (n = 60) | 17 (28%) | 4 (7%) | 8 (13%) | 5 (8%) | 18 (30%) | 8 (13%) | 71% (n = 5/7) |
- Note: The two-step model only includes patients with a second uCXCL10/Cr test within 4 months of the first test.
- a On indication biopsy for elevated serum creatinine at the time of the first test positive with elevated uCXCL10.
- b Positive predictive value for subclinical acute rejection on uCXCL10/Cr-positive and includes only those with a surveillance biopsy anytime within 4 months of the first-positive test to identify subclinical rejection.
3.2.1 Single-step uCXCL10/Cr testing
Of the 179 high uCXCL10/Cr tests ≥12 ng/mmol, 69 events were considered a “first-positive” test (Table 3). There were 31 (n = 31/69, 45%) cases that had a clinical diagnosis confirmed at the time of the test: BK viremia (n = 14/69, 20%), CMV (n = 5/69, 7%), UTI (n = 8/69, 12%), and clinical acute rejection (n = 4/69, 6%). The remaining 38 (55%) did not have a confirmed clinical diagnosis associated with uCXCL10/Cr elevation. Of the 29 patients with a concurrent or subsequent surveillance biopsy, 7 had subclinical acute rejection (PPV = 24% among those biopsied). The median difference in uCXCL10/Cr increase and biopsy diagnosis was 0 days (n = 7, IQR: −4, 0). Acute rejection could not be excluded in the remaining 9 patients (13%) who were not biopsied.
3.2.2 Serial uCXCL10/Cr testing
Out of the 69 patients who had CXCL10 levels checked, 60 had repeat testing within 4 months (Table 3). The mean interval between tests was 2.3 ± 2.2 months. 34 cases (n = 34/60, 57%) were negative on the second test, of which 18 (n = 18/60, 30%) had no identified cause and were labeled as “transient elevation.” In the interval between the first and second tests, 5 additional patients received a clinical diagnosis associated with elevated uCXCL10/Cr as acute BK viremia (n = 4) and acute UTI (n = 2) and for a total of 33 infection-related cases in the 69 event cohort (n = 33/69; 48%). Nine cases (n = 8/26, 31%) remained unexplained by infection or clinical rejection after the second test. Seven of the unexplained cases had a surveillance biopsy, of which 5 (PPV = 71%) were identified with subclinical rejection. When comparing the subclinical acute rejection yield on biopsy, there is a 47% increase with serial testing compared with first-positive test approach (24% vs. 71%, p = .017).
Of the 3 samples (2 biopsied, 1 unbiopsied) with unexplained persistently elevated uCXCL10/Cr, potential sources of ongoing inflammation were identified in each case. For the 2 who were biopsied, 1 patient had slow post-transplant graft function recovery following ischemia-reperfusion injury, with samples taken on days 68 and 272 post-transplant. The second patient had ongoing sapovirus gastroenteritis through both time points. The unbiopsied patient had persistent obstructive uropathy and was noted to have associated chronic inflammation on a later kidney biopsy. These diagnoses were not included a priori in our diagnostic options, but represent potential additional sources for the uCXCL10/Cr elevation.
There were 18 transient cases, where the second uCXCL10/Cr test was negative and there was no identified cause (clinical rejection/infection) after the first test. Of these, 14 had a kidney biopsy within 4 months of the first-positive test and were informative to identify a potential source of inflammation. There were 2 cases (n = 2/14, 14%) with acute rejection identified on the day of the second test, when uCXCL10/Cr was negative. The first biopsy identified clinical rejection (for increased creatinine) at 75 days after the first-positive test (Banff score i1t1; uCXCL10/Cr = 3.2 ng/mmol). The second biopsy identified subclinical rejection at 85 days after the first-positive test (Banff score i1t2; uCXCL10/Cr = 4.8 ng/mmol). For the remainder of cases with transient elevation (n = 17), the mean uCXCL10/Cr was 26.7 ± 25.8 and 5.6 ± 2.8 ng/mmol (n = 17), at the first and second test points, respectively. The mean time to the second test was 65 ± 63 days (n = 17). Examination of uCXCL10/Cr levels in the context of variations in immunosuppression exposure or medication adherence was beyond the scope of this analysis.
3.3 Potential utility of serial monitoring with uCXCL10/Cr after treatment of acute rejection
The temporal change in uCXCL10/Cr was evaluated during and after treatment of the 11 cases of acute rejection (7 subclinical, 4 clinical acute rejection). There were insufficient cases with prior uCXCL10/Cr samples (n = 5) to reliably report on anticipation of rejection diagnosis. The mean uCXCL10/Cr at rejection diagnosis was 23.7 ± 23.6 ng/mmol, with a maximum level of 28 ± 22 ng/mmol during the episodes. All had a follow-up biopsy at 81 ± 42 days later to evaluate response to treatment, with an overall trend for decrease in response to treatment (mean uCXCL10/Cr = 11.8 ± 8.6 ng/mmol, p = .15) (Table 4; Figure 4). Five cases (45%) had persistent rejection on follow-up biopsy, of which 3 had worsened Banff scores compared to the first biopsy. Of those with increased rejection severity, the mean uCXCL10/Cr was 23.6 ± 6.4 ng/mmol, whereas those with improved histology had a mean uCXCL10/Cr of 7.4 ± 3.6 ng/mmol (p = .06 vs. index biopsy). In all cases of worsening rejection, the uCXCL10/Cr increased compared with the index biopsy, whereas in those that improved all had uCXCL10/Cr <12 ng/mmol. In the 5 cases where additional uCXCL10/Cr was available between the index and follow-up biopsy, the intervening level followed the general trend of increase/decrease as was identified at the follow-up biopsy.
| Case | Index biopsy Banff score (Indication) | uCXCL10/Cr level at index biopsy (ng/mmol) | Days to lowest CXCL10 between index biopsy + repeat biopsy | Lowest CXCL10 between index biopsy + repeat biopsy | Days from index biopsy to follow-up biopsy | Follow-up biopsy Banff score (Indication) | uCXCl10/Cr level on day of repeat biopsy (ng/mmol) |
|---|---|---|---|---|---|---|---|
| 1 | i3t3 (S) | 83.9 | N/A | N/A | 64 | i1t2 (S) | 10.5 |
| 2 | i1t2 (I) | 49.3 | 73 | 0.8 | 136 | i0t1 (I) | 10.9 |
| 3 | i1t1 (I) | 29.4 | N/A | N/A | 45 | i0t2 (S) | 6.7 |
| 4 | i2t2 (S) | 19.7 | N/A | N/A | 59 | i3t3 (S) | 26.8 |
| 5 | i2t3 (S) | 16.2 | N/A | N/A | 49 | i0t2 (S) | 11.0 |
| 6 | i2t2 (S) | 16.1 | N/A | N/A | 67 | i3t3 (S) | 27.7 |
| 7 | i1t2 (I) | 15.0 | 146 | 3.7 | 172 | i0t1 (S) | 2.0 |
| 8 | i1t2 (I) | 12.1 | N/A | N/A | 49 | i1t1 (S) | 4.5 |
| 9 | i1t1 (I) | 11.1 | N/A | N/A | 90 | i1t0 (S) | 9.32 |
| 10 | i1t2 (S) | 4.9 | 83 | 6.8 | 109 | i2t3 (S) | 16.2 |
| 11 | i1t1 (I) | 3.2 | 24 | 3.2 | 51 | i0t1 (I) | 3.9 |
| Median (IQR: Q1, Q3) | 16.1 (11.6, 24.6) (ng/mmol, n = 11) | 78 (61, 99) (days, n = 4) | 3.5 (2.6, 4.5) (ng/mmol, n = 4) | 64 (50, 100) (days, n = 4) | 10.5 (5.6, 13.6) (ng/mmol, n = 11) | ||
- Abbreviations: I, indication (increased creatinine); Indication, indication for biopsy; S, surveillance.
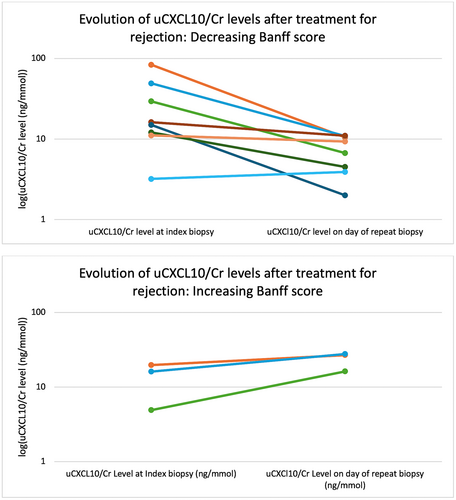
3.4 Temporal relationship between BK viremia and uCXCL10/Cr
We identified 17 patients in the cohort with BK viremia, which was associated with elevated uCXCL10/Cr in 15 cases (88%). Among those with uCXCL10/Cr elevation, the median uCXCL10/Cr on the first day of viremia was 38.2 ng/mmol (n = 14 available, IQR: 20.9, 65.4) and the maximum during the viremia period was 47.4 ng/mmol (n = 14, IQR: 24.0, 91.6). No patients received treatment with IV corticosteroids during the period of viremia, and the mean duration of viremia was 129 ± 112 days (n = 15). The median uCXCL10/Cr at resolution was 18.6 ng/mmol (n = 10; IQR: 7.8, 32.6; p = .088 vs. peak viremia, n = 9), with 6 (60%) exhibiting persistent uCXCL10/Cr elevation ≥12 ng/mmol. Only 2 patients (n = 2/15, 13%) exhibited anticipation with elevated uCXCL10/Cr (30.3, 644.8 ng/mmol) at 51 and 35 days prior to initial viremia detection.
Ten patients had a biopsy (Table 5; n = 4 indication, n = 6 surveillance) within the 4-month period following BK viremia resolution, of which 7 (70%) had acute rejection with uCXCL10/Cr measuring 22.1 ± 14.8 ng/mmol, compared with 6.1 ± 2.2 ng/mmol in those without rejection. The level of uCXCL10/Cr upon BK viremia resolution did not track consistently with subsequent diagnosis of rejection, although 3/4 cases of rejection (75%) exhibited sustained uCXCL10/Cr elevation. Rejection diagnosis was concordant with concurrent uCXCL10/Cr elevation in 7/9 cases (78%).
| Case | Maximum uCXCL10/Cr level during infection (ng/mmol) | Days to viremia resolution | uCXCL10/Cr level on day of viremia resolution (day 0) | Days from resolution to biopsy | Banff score of post-infection biopsy (Indication) | uCXCl10/Cr level on day of biopsy (ng/mmol) |
|---|---|---|---|---|---|---|
| 1 | 24 | 14 | 71.5 | 40 | i2t3 (S) | 20.2 |
| 2 | 61.3 | 114 | 7.8 | 108 | i2t2 (S) | 27.9 |
| 3 | 20.9 | 128 | N/A | 31 | i1t3 (I) | 9.7 |
| 4 | 644.8 | 64 | 46.2 | 0 | i1t2 (I) | 46.2a |
| 5 | 105.2 | 56 | 35.5 | 99 | i1t2 (I) | N/A |
| 6 | 16.8 | 53 | N/A | 77 | i1t1 (S) | 24.1 |
| 7 | 4.2 | 11 | N/A | 126 | i1t1 (S) | 4.4 |
| 8 | 60.1 | 95 | 4.2 | 10 | i0t2 (S) | 4.2 |
| 9 | 10 | 70 | 5.4 | 106 | i0t2 (I) | 5.7 |
| 10 | 91.6 | 245 | 24 | 84 | i0t0 (S) | 8.6 |
| Median (IQR: Q1, Q3) | 42.1 (17.8, 84.0) (ng/mmol, n = 10) | 67 (54, 109) (days, n = 10) | 24.0 (6.6, 40.9) (ng/mmol, n = 7) | 81 (33, 104) (days, n = 10) | 8.6 (5.1, 22.2) (ng/mmol, n = 9) |
- Note: Data are reported for 10 patients with kidney biopsy in the 3 months after BK viremia resolution, defined as BK PCR <1000 copies/mL.
- Abbreviations: Indication, indication for biopsy; S, surveillance; I, indication (increased creatinine).
- a The biopsy was done on the day of the first BK <1000 copies/mL.
3.5 Temporal trends between cytomegalovirus infection and uCXCL10/Cr
We identified 19 patients with CMV viremia, which was associated with elevated uCXCL10/Cr in 9 cases (47%). Among those with CXCL10 elevation, the mean uCXCL10/Cr on the first day of viremia was 26 ± 34 ng/mmol, which was also the maximum level during the viremia period. The median duration of viremia was 64 days (n = 12, IQR: 40, 122), and 83% (n = 10/12) were treated with valganciclovir to resolve viremia. The median uCXCL10/Cr at resolution in these cases was 8.7 ng/mol (n = 11, IQR: 3.0, 21). No patients had anticipation elevations in uCXCL10/Cr in the 2–8 weeks before CMV onset.
Ten patients underwent a kidney biopsy during the period of viremia, of which 3 (30%) also had significant elevation of ALT for a clinical diagnosis of CMV disease. uCXCL10/Cr was elevated (23.5 ± 12.2 ng/mmol) in patients with CMV disease and was associated with interstitial inflammation (all ≥i2t2) on biopsy, compared with uCXCL10/Cr mean 7.4 ± 4.6 ng/mmol in those with uncomplicated CMV viremia (Figure 5; p = .01). Only 2 cases with uncomplicated viremia (29%) had borderline grade inflammation <i2t2 (uCXCL10/Cr 7.4 and 11.1 ng/mmol, respectively), and the remainder had no acute inflammation. One of these cases was treated with IV methylprednisolone as borderline acute rejection stating on the day of viremia onset (before the result was known), whereas the other cases were not. Although CMV nephritis was suspected in the cases with inflammation and CMV disease, viral cytopathic effect was not identified and immunohistochemistry to identify CMV viral particles was not performed.
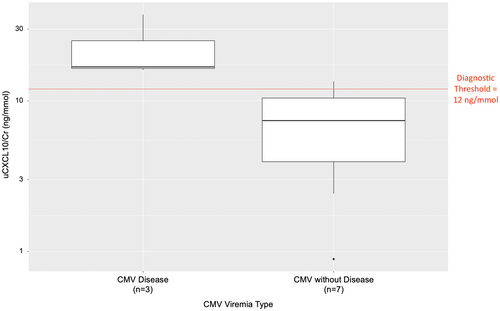
Nine patients had a surveillance biopsy within the 4-month period following viremia resolution (Table 6), of which 2 (22%) exhibited acute rejection. The case with Banff 1B grade rejection had elevated uCXCL10/Cr (i3t3; 27.7 ng/mmol), whereas the case with borderline grade did not (i2t1; uCXCL10/Cr = 3.6 ng/mmol). Of the 4 cases with uCXCL10/Cr ≥12 ng/mmol on the day of biopsy, only 1 of them was associated with the presence of post-infection rejection. In the 5 cases where uCXCL10/Cr was less than 12 on the day of the biopsy, 4 (80%) had no rejection. Therefore, the overall concordance between rejection histology and uCXCL10/Cr level was 55% in this setting.
| Case | Maximum uCXCL10/Cr level during infection (ng/mmol) | Days to viremia resolution | uCXCL10/Cr level on day of viremia resolution (day 0) | Days to lowest CXCL10 between end of infection and biopsy | Lowest CXCL10 between end of infection and biopsy | Days from resolution to biopsy | Banff score of post-infection biopsy (Indication) | uCXCl10/Cr level on day of biopsy (ng/mmol) |
|---|---|---|---|---|---|---|---|---|
| 1a | 16.07 | 8 | N/A | N/A | N/A | 61 | i3t3 | 27.7 |
| 2a | 7.40 | 757 | 3.61 | N/A | N/A | 0 | i2t1 | 3.6b |
| 3 | 13.67 | 113 | 1.84 | N/A | N/A | 0 | i0t2 | 1.8b |
| 4a | 30.39 | 43 | 47.44 | N/A | N/A | 0 | i0t1 | 47.4b |
| 5 | 4.99 | 27 | 11.07 | 7 | 5.5 | 29 | i0t1 | 13.5 |
| 6a | 5.35 | 260 | 2.85 | N/A | N/A | 0 | i0t0 | 2.9b |
| 7a | 19.97 | 147 | 41.49 | N/A | N/A | 54 | i0t0 | 19.7 |
| 8a | 3.40 | 30 | 1.57 | N/A | N/A | 75 | i0t0 | 1.0 |
| 9 | 28.48 | 49 | 24.00 | N/A | N/A | 97 | i0t0 | 8.6 |
| Median (IQR: Q1, Q3) | 13.7 (5.4, 20.0) (ng/mmol, n = 9) | 49 (30. 147) (days, n = 9) | 7.3 (2.6, 28.4) (ng/mmol, n = 8) | 29 (0. 61) (days, n = 9) | 13.5 (8.6, 19.7) (ng/mmol, n = 9) |
- Note: Data are reported for 10 patients with kidney biopsy in the 3 months after CMV viremia resolution, defined as CMV PCR of 0 copies/mL. All biopsies were surveillance biopsies.
- a ALT levels were elevated during CMV viremia.
- b The biopsy was done on the day of the first CMV < 0 copies/mL.
3.6 Temporal relationship between urinary tract infection and uCXCL10/Cr
We identified 7 patients in the cohort with UTI and elevated uCXCL10/Cr with a median uCXCL10/Cr of 34 ng/mmol (n = 7, IQR: 15, 54). All but 1 patient were treated with appropriate antibiotics, and the mean time to the first-negative culture was 50 days (n = 6, IQR: 27, 55). No patients (n = 8) with UTI exhibited anticipation with an elevated uCXCL10/Cr in the 2–8 weeks prior to onset of viremia.
Two patients underwent a subsequent kidney biopsy (1 surveillance, 1 indication) within the 3-month period post-UTI treatment (Table 7), of which 1 case exhibited borderline rejection (i1t1). Only 1 case had a uCXCL10/Cr level available on the day of biopsy, which was not elevated (8.4 ng/mmol) and the patient did not have rejection (i0t0).
| Case | Maximum uCXCL10/Cr level during infection (ng/mmol) | Days to lowest uCXCL10/Cr between treatment and biopsy | Lowest uCXCL10/Cr between treatment and biopsy | Days from treatment to biopsy | Banff score of post-treatment biopsy | uCXCl10/Cr level on day of biopsy (ng/mmol) |
|---|---|---|---|---|---|---|
| 1 | 33.6 | 84 | 7.9 | 98 | i0t0 (Surveillance) | 8.4 |
| 2 | 1.1 | N/A | N/A | 79 | i1t1 (Increased creatinine) | N/A |
- Note: Data are reported for 2 patients with kidney biopsy in the 3 months after UTI treatment, defined as the day of antibiotic prescription after positive urinalysis for leukocytes + positive urine culture.
4 DISCUSSION
This report modeled an approach to clinical surveillance utilizing serial monitoring of uCXCL10/Cr for rejection and the identification of other common causes of allograft inflammation. We were able to validate the previously reported diagnostic threshold of ≥12 ng/mmol, with a specificity of 80% for diagnosis of acute rejection.33 The superior approach to uCXCL10/Cr monitoring modeled serial testing, whereby initial elevation was followed by screening for alternate causes of inflammation and then repeat testing and biopsy in cases where it remains elevated. These findings further support the utility of uCXCL10/Cr monitoring after treatment of rejection to follow resolution and for monitoring of rejection following resolution of BK viremia, CMV viremia, and UTI (Figure 6).
As with previous reports, uCXCL10/Cr is most sensitive for rejection that is Banff 1A grade or greater.33, 34, 38, 43 For treatment of rejection, we confirm that uCXCL10/Cr is a sensitive indicator of histological response to treatment or lack thereof, which has also been reported previously.44-47 Whether elevated uCXCL10/Cr is prognostic of worse outcome in cases of borderline grade rejection could not be resolved in this analysis.
Urinary CXCL10 elevation is associated with acute rejection as well as inflammation associated with BK viremia, CMV, and UTIs.27, 32, 34-39 After the first instance of uCXCL10/Cr elevation, a majority of cases were attributable to non-alloimmune sources of allograft inflammation, some of which only became apparent between the first and second uCXCL10/Cr tests. Awaiting a second elevated level and excluding those cases where uCXCL10/Cr resolves is expected to substantially increase the yield of uCXCL10/Cr-indicated biopsy based on this modeling. This approach resembles the current use of functional monitoring with serum creatinine to indicate biopsy in the setting of persistent, unexplained elevation, but has superior sensitivity for diagnosis of subclinical rejection. This approach is also similar to pancreatic transplant rejection surveillance using granzyme B, perforin, and HLA-DR, where serial testing is also utilized.48 This serial strategy is expected to improve confidence in making clinical decisions based on the levels of the biomarkers.
There were numerous cases with transient uCXCL10/Cr elevation. Although it was not possible to identify a specific source of inflammation, it is possible that these were associated with self-limited episodes of systemic inflammation. However, there were also cases of rejection identified in a sub-cohort who were subsequently biopsied with resolved uCXCL10/Cr. It is possible that transient elevations may reflect the fluctuating intensity of alloimmune inflammation that may wax and wane with variations in medication adherence or self-limited infectious causes. This study design was unable to address whether the long-term outcome in cases of transient uCXCL10/Cr elevation differs from those who remain negative. However, the lesser sensitivity for diagnosis of borderline grade rejection suggests that a lack of uCXCL10/Cr elevation in the setting of graft dysfunction should not be sufficient to defer a biopsy to rule out rejection.
These data confirmed that BK viremia, CMV viremia, and UTI may be associated with uCXCL10/Cr level elevation and that typically levels fall with post-infection resolution. This has been observed previously, suggesting that the localization of the infections to the kidneys may contribute to the significantly elevated levels.27, 32, 34-39, 49, 50 Except for a minority of cases with BK viremia, there was little anticipation observed prior to infection diagnosis. This suggests a short latency period for infection before diagnosis is identified, with the exception of some BK viremia cases. In CMV viremia, this short latency frequently follows discontinuation of anti-viral prophylaxis in up to 57% of cases.51-53 UTIs can develop and worsen within hours of initial infection, which may contribute to the rapid increase and decline in uCXCL10/Cr observed in this study.54, 55 Conversely, BK viremia is transmitted through donor tissues and has been known to have a latency of 10 months with a slower progression to manifest infection.56-60 The nature of inflammatory signaling by uCXCL10/Cr elevation is most intense during BK viremia and UTI, which was substantially greater than those observed in rejection. This expands on previous reports of greater uCXCL10 elevation in UTI and BK viremia compared to CMV viremia, which is often below those of rejection.35, 39, 61, 62
For cases of CMV viremia, the intensity of inflammation (uCXCL10/Cr and histologic) was associated with other features of CMV disease, raising the possibility of CMV nephritis. Most cases were not treated with IV steroids and instead resolved with antiviral treatment. CMV nephritis has been reported post-kidney transplant but may be difficult to diagnose conclusively due to histologic features overlapping with acute rejection.63-65 The viral cytopathic effect may not always be seen and were not identified in these cases,65 and we did not perform PCR to identify whether CMV viral particles were present within the tissue. Unless there is progressive functional decline, our practice has generally been to defer treatment with steroids until the CMV viremia has resolved.
Elevated uCXCL10/Cr levels that persist or appear following infection resolution may identify an increased risk for acute rejection. This rejection risk has been previously reported following both CMV and BK infection49, 66, 67 and is likely associated with increased trafficking of immune cells to the allograft with parenchymal inflammation or as the result of decreasing immunosuppression medications during the active infection phase.68 There is disagreement on the extent to which allograft pyelonephritis contributes to subsequent rejection.69-71 This may be due in part to whether there is actual parenchymal involvement with UTI, since histologically documented pyelonephritis is associated with similar histological72 and molecular73 features of acute rejection. This report only followed cases of UTI with elevated uCXCL10, which may be an indicator of renal parenchymal versus lower UTI. Acute rejection may also be missed without surveillance biopsy, and indeed, most cases identified here were subclinical. One report identified 10% with acute rejection on surveillance biopsy 1 month after acute rejection.74 Since it is not common practice to perform biopsy surveillance after allograft infection, a similar strategy of serial monitoring with uCXCL10/Cr may also be effective for post-infection rejection surveillance.
Limitations of this report include lack of uniformity in the follow-up of patients involved. As data were compiled from both retrospective patients and patients undergoing ongoing monitoring, the schedule and interval between uCXCL10/Cr testing and biopsies differ significantly between participants. While the majority of patients with ≥Banff1A rejection had uCXCL10/Cr >12 ng/mmol, there are notably cases that fall below this diagnostic threshold. Further work is needed to understand whether this represents mechanistic differences in rejection pathophysiology in the type of inflammation that is present or differences in rejection severity. There were also incomplete data to track outcome after infection and rejection in cases where repeat uCXCL10/Cr did not coincide with follow-up. We were unable to characterize all UTI cases and only focused on those who had elevated uCXCL10/Cr paired with the infection. It has recently been reported that urinary detection of BV virus infection is associated with uCXCL10 in the absence of viremia,75 and inclusion of urinary PCR or decoy cell testing may provide more complete detection of clinical BKV infection when uCXCL10 is elevated. Strengths include the number of patients and the number of samples included, as well as the long duration of follow-up for each patient.
These data demonstrate the potential clinical utility of serial uCXCL10/Cr for non-invasive monitoring of subclinical allograft inflammation. Persistent uCXCL10/Cr elevation should prompt additional investigations for CMV, BKV, or UTI and in the absence of a clinically apparent cause should indicate a kidney biopsy to rule out subclinical rejection. Future implementation trials are needed to evaluate the clinical and cost benefit of uCXCL10/Cr monitoring.
ACKNOWLEDGMENTS
This project was funded by the BC Children's Hospital Foundation and the TRACE project. We are grateful to the young people and their families who agreed to participate in this study, as well as to research team members including Candice Weidman, Monica Ho, and the whole BCCH Multi-Organ Transplant Program for their help with this project. Thank you to the BCCHR Summer Studentship Program for funding this project.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.