Combining living and deceased donation for pediatric first isolated liver transplantation: A win-win even in countries with high deceased donor donation rates
Abstract
Background
Split and living donor liver transplantations are both key surgical strategies for development of pediatric liver transplant programs. Often, however, teams tend to prioritize only one preferentially.
Methods
In the context of a very active national split liver graft allocation program (Italy), retrospective study of 226 consecutive pediatric first isolated liver transplants performed by a single team using organs from both deceased and living donors. Clinical characterisitics and outcome were compared.
Results
In the context of a steadily slowly decreasing split graft offer, living donation activity steadily increased. Deceased and living donation accounted for 52.6% and 47.4% of transplantations, respectively. Both strategies were equally used for transplanting patients up to 30 kg of weight, while deceased donors were predominantly used for older recipients. Technical variants represented 86% of all transplants, with 183 conisting of left lateral segment grafts (76 split liver grafts and 107 left grafts from living donors). Outcome of both surgical strategies was similar, with excellent outcomes at early, mid-, and long-term.
Conclusions
Splitting livers of deceased donors and using living donation were complementary and non-competitive strategies for developping pediatric liver transplant activity. Implementing both activities in parallell allowed to maintain stable the number of annual transplant in Italy and allowed to reach superior outcomes. This analysis provides evidence that living donation plays a role in Italy despite an existing very active “mandatory-split” national policy.
Abbreviations
-
- BAP
-
- biliary atresia patients
-
- DcD
-
- deceased donor
-
- DcD-LT
-
- deceased donor liver transplantation
-
- ELTR
-
- European Liver Transplant Registry
-
- KMAS
-
- Kaplan–Meier actuarial survival
-
- LD
-
- living donor
-
- LD-LT
-
- living donor liver transplantation
-
- LT
-
- liver transplantation
-
- LLS
-
- left-lateral-segment
-
- PELD
-
- pediatric end-stage liver disease
-
- SD
-
- standard deviation
-
- SLG
-
- split liver graft
1 INTRODUCTION
With the introduction of living donor (LD) liver transplantation (LD-LT) into the pediatric liver transplant armamentarium in the early nineties, the clinical application thereof naturally extended very quickly in the countries where deceased donation numbers were low or extremely low.1, 2 LD-LT rapidly gained an important role as a complementary strategy in some expert centers, especially those offering liver transplantation (LT) to foreign children who did not qualify to be be allocated organs in a the local allocation program. LD-LT was progressively integrated in the routine practice of these centers, and offered to any candidate. In Europe, this phenomenon increased with time, in terms of number of LT and the number of centers using LD-LT, partly as a result of the scarcity of other types of liver grafts. The current situation is well illustrated by the reports of the European Liver Transplant Registry (ELTR), with pediatric LD-LT representing 39.3% of all pediatric LT in the last decade.
This analysis details the 15-years of experience of pediatric LT by a single team, and the results of a combined deceased donors (DcD)–and LD transplant program in Italy–a country where successful DcD donation and split liver graft (SLG) programs have been run very successfully for decades. The progressive and successful introduction of LD-LT, is described with a focus on the advantages that LD-LT brought to the program. Benefits for the patients are highlighted, as well as residual weaknesses.
2 MATERIALS AND METHODS
2.1 Inclusion criteria
All consecutive patients who underwent transplantation by the author team, between November 1, 2008 and December 31, 2022, were enrolled if they fulfilled the following criteria: first liver transplantation, indication for isolated liver transplant, and <18 years of age at time of LT.
2.2 Study design
This is an observational retrospective study of all selected children. All data were collected prospectively in the patient's electronic medical and operational records during the study period, and these data were retrieved retrospectively for the purposes of this analysis. The study was conducted in accordance with the local ethics committee guidelines and conforms to the recognized standards. Informed consent from families for the use of their child's data had been obtained prospectively, and at the time, patients were assessed as candidates for transplantation and at further admission for transplantation or follow-up. Patient's privacy rights were guaranteed by anonymization for data analysis. In the case of living donation, informed consent was also obtained from donors.
The objective of the study was to analyze: (1) the incidence of living or deceased donor use with time and in accordance with national (Italian) allocation rules; (2) the type of liver grafts and their use according to age and weight of the recipient; (3) the death rate on the waiting list during the study period; and (4) the long-term outcomes in terms of graft and patient survival.
Over the 15-year period of time of the study, 13 children (5.7%) were lost to follow-up after LT. Seven patients were lost after transition to an adult center – with a mean initially followed-up of 6.8 ± 3.1 years; a further six cases were lost after they returned to their country of origin, within the first year after LT. The former group was included in the long-term survival analysis, with censoring at the time of the transition, while the six children lost during the first year after LT were excluded from the survival analysis.
As the cohort of patients was inhomogenous by definition for (1) primary diseases and (2) graft types, a sub-analysis was then performed after selecting (1) only left liver grafts (either from LD or DcDs donors) in order to get an homogenous group from a surgical technique point of view, and (2) all and only biliary atresia patients (BAP) to get an homogenous group in term of clinical condition and disease recurrence risk.
2.3 Enrolment of patients in the living donor program
In Italy, by law, living donor LT is authorized only if it is proposed as an alternative to- and in the context of- conventional organ allocation; this means that all families are given both the information and the access to both procedures. In practice, all candidates are registered on the national list for DcD transplantation at the end of the assessment, but then decide the timing of LD- transplantation – which may be very shortly afterwards, depending of their preferences. The Italian law, however, prohibit explicitly proposing LD in the case of emergency LT (as a full priority status is offered in the case of an emergency).
By law also, the process of selecting LD is scrutinized under the law, and checked by a judge at the end. It includes (1) the obligation of a third party involvement in the process of selection, (2) a final check by a judge to confirm that the whole procedure has been run in accordance with the law and that the donor is free of any pressure for donation, and (3) the authorization by the National Transplant Center before proceeding to procurement. In addition, to avoid circumstantial pressure on the potential donor candidates, the law in Italy also prohibits (1) LD for emergencies (UNOS status 1), and (2) Samaritan donation.
In the program, LD activity started in 2011; since then, all the families of children assessed for LT were given information about LD-LT and were given the opportunity to opt in. Donors were selected on the condition of a normal health status and the absence of specific risks; any candidate with a risk profile was not considered.
2.4 Transplant techniques and perioperative management
Liver grafts were procured from either deceased or living donors. For the analysis, graft types were categorized as “full-size liver”, “reduced-liver”, “right SLG”, and “left-lateral-segment (LLS; DcD-SLG or living-donor) graft”. Well-described conventional transplant techniques were used, including piggy-back caval reconstruction, portal vein longitudinal venoplasty in the case of portal vein hypoplasia, aiming at direct arterial anastomosis without graft interposition, and using either the trans-umbilical or trans-hilar liver division techniques for procuring LLS grafts.3-5 As per protocol, all patients had Doppler ultrasonography at regular intervals (daily during the first week) and received anticoagulant and/or antiaggregant therapy aimed at reducing the incidence of vascular complications during the first 3 months, as described in a previous analysis.6
All patients received immunosuppression induction by anti-Interleukin 2 monoclonal antibodies and two doses of steroids on day 0 and day 1, followed by maintenance immunosuppression therapy with tacrolimus. In the long term, the aim was to maintain monotherapy with tacrolimus; the latter was converted to a combination of tacrolimus and mycofenolate mofetil, or mycofenolate mofetil and steroids according to individual patient's clinical needs. Switch from tacrolimus to ciclosporine A or mTor-inhibitors was considered in cases with specific indications (toxicity, lymphoproliferative disease).
2.5 Statistical analysis
Continuous variables are expressed as mean and standard deviations, or as median and range where appropriate. They were compared with the T-Test, Wilcoxon rank sum test or the Mann–Whitney test, and the ANOVA test, together with Levene's Test, for assessing the homogeneity of variance between the groups. The categorical variables were compared by using Fisher's exact test or the Chi-square test when appropriate. Kaplan–Meier analyses were used in order to estimate the graft and the patient's long-term survival. p < .05 was considered statistically significant.
3 RESULTS
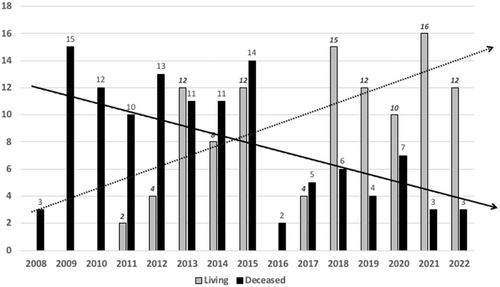
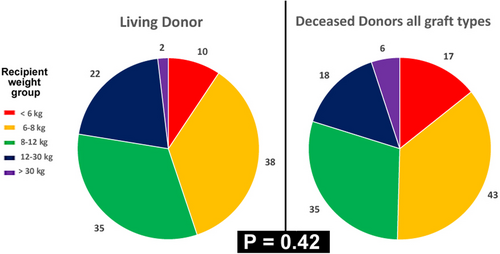
Between November 1, 2008, and December 31, 2022, 226 consecutive first pediatric liver transplantations were performed. The demographics and the characteristics of the patients and the grafts are detailed in Table 1. LD-LT was distributed inhomogeneously in time during the study period (Figure 1), with a progressive increase of the annual ratio LD-LT/DcD-LT with time, from 0% in first 3 years of activity to 84% of LT in 2021. Interestingly, the use of grafts from either DcD or LD donors was evenly spread among recipient weight groups (Figure 2). As to be expected, there were several significant differences between LD- and DcD- LT groups (Table 1); less-expected differences were that LD-LT was associated with significantly shorter operative time, intensive care and hospital stay despite a significantly lower age and smaller weight of recipients, and significantly higher number of patients needing portal vein reconstruction. Interestingly, all these findings were confirmed when analyzing the highly selected and very homogenous group of children – the BAP-only subgroup (Table 2).
| Whole series (N = 226) | Living donor transplants (N = 107) | Deceased donor transplants (N = 119) | p | |
|---|---|---|---|---|
| Demographics | ||||
| Recipient age (months) | 43.0 ± 51.5 | 23.2 ± 25.6 | 60.8 ± 61.6 | <.001 |
| Recipient weight (kg) | 15.4 ± 13.9 | 10.3 ± 5.6 | 19.9 ± 17.2 | <.001 |
| Exceptions to PELD/MELD | 66 (29%) | 11 (10%) | 55 (46%) | <.0001 |
| Status 3 PELD/MELD | 160 (71%) | 96 (90%) | 64 (54%) | |
| PELD score of status 3 patients | 17.3 ± 11.9 | 15.8 ± 12.2 | 19.6 ± 11.2 | .047 |
| Indications | ||||
| Biliary atresia | 138 (61%) | 82 (77%) | 56 (47.0%) | <.0001 |
| Metabolic disease | 23 (10%) | 8 (7.5%) | 15 (12.6%) | |
| Cholestasis | 22 (9.7%) | 8 (7.5%) | 14 (11.8%) | |
| Liver tumors | 20 (8.8%) | 1 (0.9%) | 19 (16.0%) | |
| Alagille | 7 (3.1%) | 3 (%) | 4 (3.4%) | |
| Fulminant failure | 7 (3.1%) | 1 (0.9%) | 6 (5.0%) | |
| Cirrhosis | 4 (1.8%) | 2 (1.9%) | 2 (1.7%) | |
| Other | 3 (1.3%) | – | 3 (2.5%) | |
| Abernethy | 2 (0.9%) | 2 (1.9%) | – | |
| Recipient age | ||||
| <6 months | 15 (6.6%) | 8 (7.5%) | 7 (5.9%) | <.0001 |
| 6–12 months | 76 (33.6%) | 51 (47.7%) | 25 (21.0%) | |
| 1–2 years | 40 (17.7%) | 20 (18.6%) | 20 (16.8%) | |
| 2–5 years | 40 (17.7%) | 18 (16.8%) | 22 (18.5%) | |
| 5–12 years | 40 (17.7%) | 10 (9.3%) | 30 (25.2%) | |
| 12–18 years | 15 (6.6%) | – | 15 (12.6%) | |
| Recipient weight | ||||
| <6 kg | 18 (8.0%) | 10 (9.3%) | 8 (6.8%) | <.001 |
| 6–8 kg | 59 (26.1%) | 38 (35.5%) | 21 (17.6%) | |
| 8–12 kg | 62 (27.4%) | 35 (32.7%) | 27 (22.7%) | |
| 12–30 kg | 64 (28.3%) | 22 (20.5%) | 42 (35.3%) | |
| >30 kg | 23 (10.2%) | 2 (1.9%) | 21 (17.6%) | |
| Waiting time | 94 ± 174 | 62 ± 132 | 124 ± 200 | <.01 |
| Graft type | ||||
| Whole liver | 31 (14%) | – | 31 (26%) | |
| Reduced liver | 8 (3.6%) | – | 8 (6.7%) | |
| Right split | 4 (1.8%) | – | 4 (3.3%) | |
| LLS | 166 (73%) | 95 (89%) | 71 (60%) | .5 |
| Extended LLS | 9 (4.0%) | 7 (6%) | 2 (1.7%) | |
| Hyper-reduced LLS | 8 (3.6%) | 5 (5%) | 3 (2.3%) | |
| Donor | ||||
| Weight | 63.8 ± 19.0 | 67.8 ± 12.7 | 60.1 ± 22.7 | <.01 |
| Age | 29.5 ± 13.4 | 33.4 ± 6.8 | 26.0 ± 16.6 | <.001 |
| DR/RW ratio | 6.4 ± 3.8 | 7.9 ± 3.4 | 5.0 ± 3.6 | <.001 |
| Technique | ||||
| Piggy-back | 216 (96%) | 104 (97%) | 112 (94%) | .26 |
| Porto-plasty | 73 (32%) | 50 (47%) | 23 (19%) | <.0001 |
| End-to end artery | 223 (99%) | 107 (100%) | 116 (97%) | .1 |
| Bilio-jejunostony | 196 (87%) | 103 (96%) | 93 (78%) | <.0001 |
| Timing | ||||
| Cold ischemic time (min) | 373 ± 174 | 221 ± 75 | 510 ± 112 | <.001 |
| Operative time (min) | 537 ± 125 | 489 ± 92 | 580 ± 135 | <.001 |
| Hospital stay (days) | 26.5 ± 17.6 | 23.1 ± 12.0 | 29.7 ± 21.1 | <.01 |
| Intensive care stay ≤36 h | 120 (53%) | 53 (49.5%) | 67 (56.3%) | <.001 |
| Intensive care stay ≤7 days | 188 (83%) | 98 (92.1%) | 90 (75.6%) | |
| Intensive care stay 7–30 days | 33 (15%) | 9 (8.4%) | 24 (20.2%) | |
| Complications | ||||
| Vena cava (stenosis) | 2 (0.9%) | 1 (0.9%) | 1 (0.8%) | .9 |
| Portal vein stenosis/thrombosis | 10/9 (8.4%) | 7/7 (13.1%) | 3/2 (4.2%) | <.05 |
| Artery stenosis/thrombosis | 3/1 (1.8%) | 1/1 (1.9%) | 2/0 (1.7%) | .9 |
| Splenic arterial steal | 6 (2.7%) | 1 (0.9%) | 5 (4.2%) | .1 |
| Bile duct stenosis/leak | 17 /8 (11.1%) | 7/2 (8.4%) | 10/6 (13.4%) | .2 |
- Note: Values are expressed as numbers and (% of the group), or as mean ± SD. Significant if p < .05.
- Abbreviations: DR/RW, donor/recipient weight; LLS, left lateral segment; MELD, model for end stage liver disease; PELD, pediatric end-stage liver disease.
| Biliary atresia patients |
N N = 138 |
Living donor N = 82 (59%) |
Deceased donor N = 56 (41%) |
p |
|---|---|---|---|---|
| Demographics | ||||
| Recipient age (Months) | 17.4 ± 18.3 | 16.1 ± 18.5 | 19.2 ± 18.0 | .33 |
| Recipient weight (kg) | 9.41 ± 4.41 | 9.15 ± 4.48 | 9.79 ± 4.32 | .4 |
| Exceptions to PELD/MELD | 11 8%) | 3 (3.7%) | 8 (14%) | .05 |
| Status 3 PELD/MELD N | 127 (92%) | 79 (96%) | 48 (86%) | |
| PELD Score of status 3 patients | 19.3 ± 10.7 | 18.3 ± 11.39 | 20.9 ± 9.59 | .18 |
| Recipient age | ||||
| <6 months | 10 (7.2%) | 7 (8.5%) | 3 (5.4%) | .34 |
| 6–12 months | 71 (51%) | 47 (57%) | 24 (43%) | |
| 1–2 years | 34 (25%) | 17 (21%) | 17 (30%) | |
| 2–5 years | 18 (13%) | 9 (11%) | 9 (16%) | |
| 5–12 years | 5 (3.6%) | 2 (2.4%) | 3 (5.4%) | |
| 12–18 years | – | – | – | – |
| Recipient weight | ||||
| < 6 kg | 13 (9.4%) | 9 (11%) | 4 (7.1%) | .35 |
| 6–8 kg | 55 (40%) | 35 (43%) | 20 (36%) | |
| 8–12 kg | 47 (34%) | 27 (33%) | 20 (36%) | |
| 12–30 kg | 21 (15%) | 9 (11%) | 12 (21%) | |
| >30 kg | 2 (1.4%) | 2 (2.4%) | – | |
| Waiting time | 112 ± 206 | 66.8 ± 142 | 179 ± 262 | <.01 |
| Graft type | ||||
| Whole liver | 5 (3.6%) | – | 5 (8.9%) | .6 |
| Reduced liver | 2 (1.4%) | – | 2 (3.6%) | |
| Right split | 1 (0.7%) | – | 1 (1.8% | |
| LLS | 118 (86%) | 73 (89%) | 45 (80%) | |
| Extended LLS | 3 (2.2%) | 2 (2.4%) | 1 (1.8%) | |
| Hyper-reduced LLS | 9 (6.5%) | 7 (8.5%) | 2 (3.6%) | |
| Donor | ||||
| Weight | 66.5 ± 17.9 | 67.8 ± 13.3 | 64.5 ± 23.0 | .32 |
| Age | 30.9 ± 11.8 | 33.3 ± 6.51 | 27.4 ± 16.3 | .012 |
| DR/RW ratio | 7.94 ± 3.12 | 8.43 ± 2.97 | 7.23 ± 3.22 | .028 |
| Technique | ||||
| Piggy-back | 133 (96%) | 79 (96%) | 54 (96%) | 1 |
| Porto-plasty | 74 (54%) | 50 (61%) | 24 (43%) | .036 |
| End-to end artery | 138 (100%) | 82 (100%) | 56 (100%) | – |
| Bilio-jejunostony | 138 (100%) | 82 (100%) | 56 (100%) | – |
| Timing | ||||
| Cold ischemic time (min) | 343 ± 169 | 228 ± 77.0 | 512 ± 115 | <.001 |
| Operative time (min) | 532 ± 113 | 499 ± 98.4 | 581 ± 116 | <.001 |
| Hospital stay (days) | 27.0 ± 18.3 | 23.4 ± 12.6 | 32.5 ± 23.6 | .011 |
| Intensive care stay <36 h N | 40 (29%) | 33 (40%) | 7 (13%) | |
| Intensive care stay <7 days | 74 (54%) | 40 (49%) | 34 (63%) | <.001 |
| Intensive care stay 7–30 days | 21 (15%) | 8 (9.8%) | 13 (24%) | |
| Intensive care stay >1 month or dead | 1 (0.74%) | 1 (1.2%) | 0 (0%) | |
| Complications | ||||
| Vena cava (stenosis) | 2 (1.4%) | 1 (1.2%) | 1 (1.8%) | .8 |
| Portal vein stenosis/thrombosis | 6/5 (8.0%) | 4/3 (8.5%) | 2/2 (7.1%) | .8 |
| Artery stenosis/thrombosis | 3/1 (2.9%) | 1/1 (2.4%) | 2/0 (3.6%) | .7 |
| Splenic arterial steal | 4 (2.9%) | 1 (1.2%) | 3 (5.4%) | .1 |
| Bile duct stenosis/leak | 8/4 (8.7%) | 4/2 (7.3%) | 4/2 (10.7%) | .5 |
- Note: Values are expressed as numbers and (% of the group), or as Mean ± SD. P significant if <.05.
- Abbreviations: DR/RW, donor/recipient weight; LLS, left lateral segment; MELD, model for end stage liver disease; PELD, pediatric end-stage liver disease.
Patients weighing < 8 kg were 77/226 in this series (34.1%); 11 children had a priority status while the median pediatric end stage liver disease (PELD) score of the 66 left was a score of 24 (SD: 8.5). Indications for LT in this group of patients were as follows: metabolic disease (N = 1), acute liver failure (N = 5), and cholestasis (N = 71 (BAP in 68/71)). In the whole series, BAP was the commonest indication (N = 138 (61%); Table 1), and the outcome was the best with a single death and no need for retransplantation (99.3% graft and patient actual survival) with a median follow-up of 7.3 years (SD: ±3.7); the latter group had a median age at LT of 10.2 months (SD: ±14.7). For these 77 LT recipients(<8 kg recipient), grafts were procured from DcD donors in 29 cases (two whole livers, five reduced livers and 22 left SLG) and from related LD in 48 cases (all LLS grafts). Out of these 77 patients, none has been retransplanted to date and 75/77 (97.4%) are currently alive and well; death in two infants was caused by multiple organ failure due to refractory multiresistant bacterial sepsis in one case, and recurrence of neonatal fulminant failure of unknown cause in the other.
During the study period, eight patients who had been listed for isolated first liver transplantation died on the waiting list, and therefore are not included in the analyzed cohort. At the time of their death, four children were listed as emergencies for a fulminant-type liver failure (N = 4: one toxic, one metabolic, and two post-chemotherapy), and three others had acute-on-chronic decompensation of a cholestatic disease (one BA, two familial cholestasis); PELD score of these seven children was >29. Of note, three candidates died during urgent workup for LD-LT transplantation, and two others deaths were directly related to the COVID pandemic.
Last but not least, two patients who were registered on the national waiting list as emergency (status 1 – because of acute deterioration of end-stage liver disease) at a time when they were assessed for LD-LT – were then converted (after 5 days of waiting for a DcD graft) into an emergency LD-LT after a judge authorized this as an exception to the Italian law.
Overall, out of the 234 children considered for LT, the intent-to-transplant rate was thus 158/160 for status 3 patients (99%), 7/11 for fulminant failure (64%), 19/19 for oncologic patients (100%), and 43/45 for those with other priority status (96%). Taking into account these non-transplanted candidates and the outcome of LT in this series, the overall intent-to-treat survival is 157/160 for status 3 patients (98%), 5/11 for fulminant failure (46%), 16/19 for oncologic patients (84%), and 42/45 for those with other priority status (93%).
3.1 Specific technical aspects and post-transplant surgical complications
Technical aspects and post-transplant surgical complications are detailed in Tables 1 and 2.
Living donor LT was performed in 10/18 infants weighing <6 kg of weight (56%), for 38/59 small children weighing 6–8 kg (64%), for 35/62 children weighing 8–12 kg (56%), for 21/64 children weighing 12–30 kg (33%), and for 2/23 children >30 kg (9%; Figure 2). All the 107 grafts were procured from the donor left liver, using the transhilar technique in 84 cases (78.5%) and the transumbilical division in 23 donors (21.5%); of these grafts, 7 were “extended” to include most of segment IV and another 8 were “reduced” LLS (resection of 10–60% of the LLS mass).
Causes of graft loss were as follows: one patient had primary non-function at first LT and died during attempt at retransplant; one had recurrence of primary sclerosis cholangitis and later underwent successful retransplantation in a different hospital; six more grafts were lost by the death of the recipient for multiple organ failure secondary to multiresistant bacterial refractory sepsis (N = 1) and recurrence of disease in five other cases (tumor (N = 3), macrophagic activation syndrome (N = 1), neonatal subfulminant liver failure (unknown cause; N = 1)). In this series, no graft was lost secondary to vascular or biliary problem.
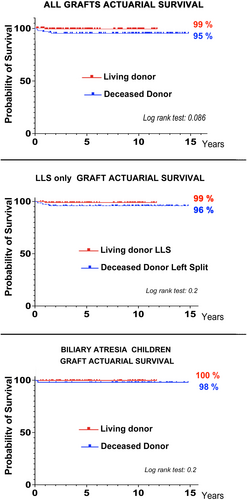
At the time of the writing, a 8 months minimum follow-up information was available for 223/226 transplants, and more than 40 months follow-up was available for 212 of the 219 patients who survived after LT (seven children were lost for follow-up during the first year after LT as they returned to a foreign country). Overall, 212/219 patients (96.8%), are alive and well at the time of writing, with a mean Follow-up of 5.3 ± 4.3 years for LD-LT and of 8.9 ± 4.2 years for DcD-donor-LT (p < .001). Overall, 211 of 219 grafts with a complete follow-up (96.4%) are well and functioning; a single patient was re-transplanted in this series (for recurrence of disease). Kaplan–Meier actuarial survival (KMAS) showed an overall 95% DcD-graft 10-year survival, and did not show a significant difference in outcome between LD- and DcD- LT (p < .05; Figure 2). In order to eliminate bias due to the heterogeneity of the series, KMAS was also analyzed for (1) only left liver grafts (Figure 3B), and (2) only BAP (Figure 3C); in both selected subgroups, the high survival rates for either LD- or DcD- grafts and patients were confirmed. Regardless of the type of donor, BAP reaches a 99% long-term survival, confirming that an excellent outcome can be also be reached in this pediatric-specific transplant disease.
4 DISCUSSION
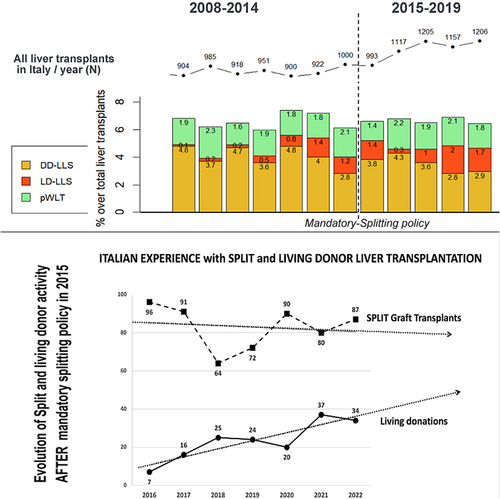
Slowly but surely, Italy is moving forwards to parallel Europe where the pediatric LT activity is currently based on LD-LT for one third of the liver transplants, overall (Figure 3). Italy has been slower to develop LD-LT because it had developed both a strong donation network with high donation rates (approaching 25 organ donors per million population in 20227 and a very active DcD SLG program). This combination ensured a very efficient system of allocation of DcD SLG to children, and for many years, there was little incentive to develop LD-LT. However, a steadily changing DcD profile (age, clinical characteristics and co-morbidities) contributed to a progressive decline in SLG offering, to the point that the National Transplant Organization decided in 2015 to convert the system from “split-incentive” to “mandatory-split” (REFER). Although the latter initiative did increase DcD SLG activity in 2016, the trend has been again later for decreasing SLG7 and increasing LD-LT; the latter currently plays an important role in recently maintaining the annual pediatric transplant activity at its previous levels (Figure 3). In parallel to increase LD activity, Italy recently reported the lowest pediatric waiting list mortality rate; 1.5% in 20218 compared to 2.5% in 2016.9
The early inclusion of LD-LT as an option for LT and the rapid expansion of the LD activity (Figure 4) is a strong hypothesis for the success of this program. Combined with a very active national split program that benefited the children who had no opportunity for LD-LT, it resulted in high intention-to-transplant rates and very successful transplantation outcomes. Families who opted for LD-LT often did understand that a rapid transplant would benefit the child and avoiding clinical deterioration while waiting… while LD allowed rapid access to transplant and proposing transplant while they were in a satisfactory clinical condition. It is very likely that this has been the major reason for the rapidly increasing numbers, and so many families opting for LD in recent years. At the same time, families who did not desire LD-LT (and those who were not eligible) benefited from this “cultural change”, in that they had a quicker access to DcD grafts from the national split program, as the competition for a liver graft was decreasing on the list as they were less candidates: a Win-Win.
LD-LT has been a great success in this cohort overall, but in particular for proposing LT in time to BAP: out of 139 BAP listed for LT, 138/139 (99.3%) were transplanted and 137/138 (99.3%) after alive and well after LT – none has been retransplanted. The BAP sub-analysis (Table 2) gives a unique opportunity for comparing LD-LT and SLG techniques in a very homogenous group of patients – as LT were performed with LLS grafts in 94.3% of these patients (N = 130 LLS grafts: 82 LD and 48 DcD donors). This sub-analysis provides evidence that (1) LD and DcD groups were similar in many aspects (demographic or technical characterisitcs), (2) significantly longer waiting time was associated with significantly higher use of exception status in the DcD group, (3) LD was associated with a significantly shorter stay in Intensive care and in hospital, and (4) outcomes were similar with LLS from either donor type. The last finding and the good outcome of DcD SLG (Figure 2) call for comment, as the most recent analysis of SLG outcome in Italy brought much lower figures for left SLG survival even in most recent years (83% and 79.1% graft survival at 1 and 3 years, respectively).10 It is conceivable that a LD program, by being highly demanding from a technical and surgical point of view, provides in turn an expertise that also helps to reach the best results with DcD-SLG LT. This is possibly an indirect, but important, benefit of LD activity for a pediatric transplant center.
In-situ splitting in Italy has been a successful story and an example for the world – showing that splitting can be implemented on a large scale and offer a large number of liver grafts to children without limiting the graft offer to adult recipients.9, 11, 12 There are however some drawbacks. The preferred surgical approach is an in-situ trans-umbilical division3-5; the latter however limits strictly the graft mass to that of segments II and III, and also does not deal well with anatomical arterial variations within the umbilical scissure. This limitation is most likely responsible for the observation by Angelico et al. that left SLG used in Italy for children >25 kg were associated with a higher risk and hazard ratio.9 Interestingly, the latter risk was not confirmed in this series, in which both trans-umbilical and trans-hilar approaches were used (either in LD or DcD donors), aiming at dealing at best with the donor anatomy on one side, and the recipient liver mass needs on the other side.3-5 The trans-umbilical technique was used in only 29% of liver divisions, while all other divisions were conducted with a trans-hilar technique: in 4% of divisions, the whole of segment IV was procured to the left graft (Extended LLS graft: Tables 1 and 2). It can be speculated that the latter surgical strategy has contributed to the better outcome of LLS grafts (a 96% long-term survival figure in this series), and compares well with most international series.13-17 It eventually compares very well with the 79.1% 3-years graft survival recently published outcomes for left SLG in Italy.10
Coincidentally, the growth of the LD program in Italy during the last decade happened as the national split program was losing strength (Figure 4). This double but inverse trend has allowed the total number of pediatric LTs to remain stable in the last decade (Figure 4). A possible direct benefit of a combination of LD and DcD programs was the recent observation that the waiting list mortality decreased to numbers that had not been observed earlier in Italy. As previously mentioned, in their paper reporting on the successful introduction in 2015 of the “mandatory-split policy, Angelico et al. suggested that this resulted in a low mortality rate on the pediatric waiting list (2.5% in 2016). More recently the mortality rate has however further dropped to 1.5% (2021).8 Interestingly, since 2016, the trend was a decreasing split- and increasing LD-activity, there are good reasons to hypothetize that LD was instrumental not only in maintaining the overall number of pediatric LT but also in lowering the waiting list death rate to one of its lowest numbers in decades. This consideration is even more crucial given that LD activity in Italy is limited to very few centers. Interestingly, the dynamics observed in Italy (mandatory split policy and progression of LD activity with subsequent decreased waiting list mortality) eventually mirrors the observations by Esmati et al.18; though the waiting-list mortality decreased from 6.7% to 2.3 in Eurotransplant area, after prioritization of donor organ allocation to BAP <2 years of age, Esmati et al. concluded that development of LD-LT activity played in fact the dominant role in the decrease as the use of LD-LT for BAP increased from 55% to 74% (p = .001) during the studied period.18 This is precisely the situation observed in Italy in last decade as well.
LD and DcD split programs must be complementary and not competitive, and their combination may be the way forward for pediatric LT. The latter includes learning form each other to develop better strategies: for example, a very homogenous group in this analysis (BAP), demonstrated an ICU stay, operative time and hospital stay all shorter after LD, suggesting that improving the timing of LT and the clinical preparation of the recipient has a direct effect on ealry morbidity and outcome. Although the latter strategy has been called for many times in litterature, this analysis confrims that transplanting candidates earlier and in better condition would not only allow better outcome but likely would be extremely efficient economically speaking.
It remains however critical that the development of LD-LT does not disencentivize transplant teams to split DcD livers. Ethically speaking, it is necessary in developing LD, for there to be parallel efforts in pursuing to maintain and develop DcD splitting in order to decrease pre-transplant mortality. Mazariegos et al. wrote recently that “the goal is zero of waiting list mortality in pediatric liver transplantation”.19 Eight children died while awaiting for LT in this series; though not a high number over a 15 years period, this must be addressed. One child died from direct complications of Covid, but seven others died from terminal liver insufficiency (three acute-on-chronic decompensation and four fulminant failures); two of the children who had acute-on-chronic decompensation were on the edge of benefiting of LD-LT. This is a call for an earlier referal of children with chronic disease–early enough to give time for assessment, pre-transplant management, listing and elective transplant. Moreover, acute-on-chronic decompensation should become a problem of the past and pre-emptive LT should become the rule.20, 21 The second call is about managing emergency cases as typically fulminant failures: although 7/8 children who died before LT in this series were on the highest emergency status, they could not benefit from a transplant in time. In Italy, while the mean waiting time for elective pediatric transplant was 3 months for status 3 children in 2020,22 it was 4.4 days for those in status 1 in 2021.23 It has been speculated that the difficult donor to recipient size matching was the cause of the long waiting time for status 1 children. Interestingly, during the last 2 years of this transplant program, two children who were assessed for LD-LT presented with acute decompensation before the LD process could be finalized; because of their clinical deterioration, they were offered status 1, and this, by the Italian law, halted the LD process. However, as a graft was not found after few days, the LD process was finalized by obtaining the authorization from a judge. These two children were successfully transplanted from LD, as an emergency procedure, 6 days after they had been listed as status 1. Although the use of LD for emergency indications is not an issue in many countries,24, 25 the story of these two children opens a major question in Italy (and in other countries with similar legislations). The fact that the mean waiting time for children in status 1 was it was 4.4 days in 202123 brings a question: should the Italian law be revisited to allow possible consideration of LD-LT in specific emergency conditions?
“With great power comes great responsibility”: developing LD-LT for the benefit of children brings an ethical and moral duty of delivering the best for both the donors and the recipients. Not only should the surgery be safe for the donor but it should also ensure the highest rate of success for the recipient – the only way to respect the donor gift. It has been extensively shown that early graft loss mostly relates to technical complications (primary non-function, vascular thrombosis, abdominal infections, biliary or digestive leaks) and many early deaths are surrogate for surgical complications (including liver dysfunctions, sepsis and collateral organ dysfunctions).26-28 In this cohort, with only one immediate graft loss (and patient death), the early graft loss was extremely low compared to most series; in fact, graft loss was mostly that caused by patient death in the context of disease recurrence in 5/ 7patients. Interestingly, there was no graft loss due to vascular issues, which is likely related to the careful, constant, dedicated program of prevention of vascular complications, as previously reported6, 29; of note, similar results are reported by many expert pediatric centers in the world, and there is growing evidence that LD-LT can play an important role in not only offering LT in time to young candidates but also contributes significantly optimizing outcomes after LT.26, 27, 30, 31 Last but not least, a very interesting observation is that graft survival curves are extremely flat after the first year after LT, suggesting that optimal grafts, adequate techniques, and attention given to all the peri-operative aspects of LT, may be the key for the very best long-term outcome (Figure 3).
5 CONCLUSION
The cohort is large and the results of the analysis allow for robust conclusions. Firstly, it suggests that the combination of very active programs of both DcD-SLG transplantation and LD-LT can offer transplantation at the right time to the majority of candidates for LT. Secondly it shows that with low rate of major technical transplant complications (Tables 1 and 2), excellent early outcomes can resull in 95% graft survival figures, not only at 5 years after LT but possibly at 10 years after LT and above (Figure 3).
Referring children in time is increasingly a critical aspect for offering successful LT to all candidates and avoiding acute clinical deterioration of children while awaiting LT. This series highlights that even with the best allocation programs, there is still a need to improve the timing of allocation of liver graft for emergency cases; there may be room to revisit a role for LD in these cases.24, 25
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.