Varicella vaccine meningoencephalitis in a child receiving autologous bone marrow transplantation
Abstract
Background
Varicella vaccine, a live-attenuated Oka-strain of varicella zoster virus (VZV), is a recommended childhood vaccine by many countries. As with wild varicella strain, after primary infection, the live-attenuated virus can establish latency in sensory ganglia and reactivate causing vaccine-strain illnesses: herpes zoster (HZ), visceral or peripheral and central nervous system dissemination. We report a case of early reactivation of live-attenuated virus—HZ and meningoencephalitis—in an immunocompromised child.
Methods
This is a retrospective descriptive report of a case, in a tertiary pediatric hospital, CHU Sainte-Justine (Montréal, Canada).
Results
An 18 month-year old girl diagnosed with a primitive neuro-ectodermal tumor (PNET) received the day prior to diagnosis, a first varicella vaccine (MMRV). She received chemotherapy 20 days post MMRV vaccine and autologous bone marrow transplantation 3 months post vaccination. She was considered not eligible, to acyclovir prophylaxis prior transplantation (positive for VZV IgG and negative for herpes simplex virus IgG by ELISA). At day 1 post transplantation, she developed dermatomal HZ and meningoencephalitis. Oka-strain varicella was isolated, she was treated with acyclovir and foscarnet. Neurologic status improved in 5 days. Control of VZV viral load in cerebrospinal fluid showed a slow decrease to from 5.24 log 10 copies/mL to 2.14 log 10 copies/mL in 6 weeks. No relapse was observed. She recovered without neurological sequelae.
Conclusions
Our experience highlights the importance of conducting a thorough medical history regarding vaccination and serological status of newly immunocompromised patients. Intensive chemotherapy succeeding live vaccine administration <4 weeks could have influenced early and severe viral reactivation. Early initiation of prophylactic antiviral treatment is questioned in such circumstances.
Abbreviations
-
- auto-HSCT
-
- autologous hematopoietic stem cell transplantations
-
- CNS
-
- central nervous system
-
- CSF
-
- cerebrospinal fluid
-
- HZ
-
- herpes zoster
-
- MMRV
-
- measles–mumps–rubella–varicella
-
- PCR
-
- polymerase chain reaction
-
- VZV
-
- varicella zoster virus
1 INTRODUCTION
Varicella vaccine is recommended in the Canadian immunization program since April 2000. The vaccine comprises a live-attenuated Oka-strain of varicella zoster virus (VZV) and should be administered at least 4 weeks before immunosuppressive therapy is started because of the potential for vaccine-related diseases.1 As with wild VZV strain, after primary infection, Oka-strain can establish latency in dorsal root sensory ganglia and reactivate causing vaccine-strain illnesses: herpes zoster (HZ), visceral or peripheral and central nervous system dissemination. However, risk and severity of developing HZ are lower in vaccinated children as compared to children who have had wild varicella.2
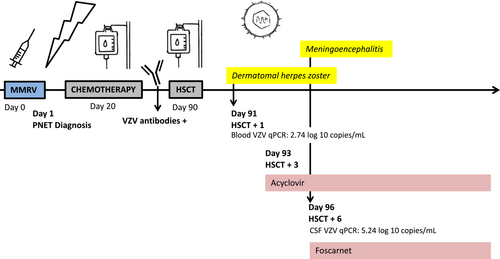
We report a case of early reactivation of live-attenuated virus, HZ and meningoencephalitis, in an immunocompromised child who received chemotherapy 20 days post MMRV vaccine and autologous bone marrow transplantation 3 months post vaccination (Figure 1).
2 CASE REPORT
An 18 month-year old girl was diagnosed with a primitive neuroectodermal tumor. She was up to date with the regular immunization program of the province of Québec, Canada. She received the day prior to diagnosis, a first dose of live-attenuated MMRV vaccine in the right deltoid. She had no previous history of varicella exposure (vaccine or disease).
Her treatment included three cycles of chemotherapy followed by three courses of high-dose chemotherapy, supported by three autologous stem cell transplantations (auto-HSCT). Chemotherapy started 20 days after MMRV vaccine and the first HSCT was at 3 months post vaccine. Prior to transplant, the patient was HSV-seronegative and VZV-seropositive. Prophylaxis, with acyclovir, is initiated at the start of the conditioning therapy in HSV-seropositive patients to prevent viral reactivation which can lead to life-threatening conditions (viremia, organ disease, and meningoencephalitis).3 Indeed, reactivation rate among this population (approximately 80%) occurs early—during the first 4 weeks—post transplant.4 Patients with isolated VZV-positive serological profile start acyclovir at day 21 post HSCT only. Therefore, the patient was not candidate to antiviral prophylaxis at the onset of her HSCT.
At day 1 after first auto-HSCT, she presented several papulovesicular lesions on her right hand. On day 3, number and size of vesicles increased and became painful. The lesions had a dermatomal distribution, C6-C7, covering the base of the right index, the dorsal face of the right hand and the right forearm. No extra dermatomal lesions, meningismus, or focal neurologic deficit were present. Polymerase chain reaction (PCR) of lesion scrapings was positive for VZV and negative for HSV. Intravenous acyclovir (1500 mg/m2/day) was started on day 3. In whole blood, VZV qPCR (Altona diagnostics, Hamburg, Germany) detected 550 copies/mL (2.74 log 10 copies/mL). Three days after starting acyclovir, the lesions started to crust and VZV qPCR in blood was negative. On day 6, new symptoms of meningoencephalitis occurred: fever, vomiting, irritability with inconsolable crying, and episodes of alteration of mental status. No new skin lesion was observed. Cerebral computed tomography did not reveal any sign of increased intracranial pressure or new cerebral lesion. Lumbar puncture showed an absence of cell in cerebrospinal fluid (CSF) (peripheral leucocytes 0.07 G/L), proteinorachia was 0.39 g/L, and glycorrhachia 7.02 mmol/L (glycemia 4.4 mmoL/L). Bacterial and viral cultures of CSF remained negative. VZV qPCR in CSF was highly positive: 173 780 copies/mL (5.24 log 10 copies/mL). Viral sequencing data performed at the National Microbiological Laboratory (Winnipeg, Canada) isolated an Oka varicella vaccine strain. Intravenous foscarnet was added in combination with acyclovir in the hypothesis of a viral resistance (Figure 1). Neurologic status improved in 5 days. No other confounding cause explained her neurological status.
Control of VZV viral load in CSF showed a slow decrease to 138 copies/mL (2.14 log 10 copies/mL) at 6 weeks. VZV qPCR remained negative in blood. She received foscarnet during the first two autologous bone marrow transplantations (42 days) and acyclovir as secondary prophylactic treatment during the third transplantation. No relapse was observed. She recovered from meningoencephalitis caused by the Oka varicella vaccine strain without neurological sequelae.
3 DISCUSSION
We present a case of early HZ and meningoencephalitis caused by reactivation of Oka-strain varicella with a proven HZ rash, neurological symptoms, high levels of VZV qPCR in the CSF, response to antiviral treatment, and absence of other cause. Varicella is usually a benign childhood illness; however, serious complications can occur including bacterial sepsis, pneumonia, encephalitis, and meningitis among others. Central nervous system (CNS) complications of primary VZV infection occur in 0.5–1.5 per 1000 pediatric cases.5 CNS manifestations as a consequence of VZV reactivation are infrequently described in the pediatric literature. However, with facilitated access to VZV PCR in CSF, more cases are being diagnosed and VZV neurological complications are increasingly reported. VZV, along with enterovirus and HSV, is now considered among the three first causes of viral meningitis in adults.6
Prior immunization programs, in developed countries, ~5 out of 1000 people with varicella were hospitalized and 2–3 per 100 000 patients died.7, 8 Immunization programs worldwide with the varicella-attenuated Oka-strain virus led to 85% decrease in chickenpox-related hospitalizations. MMRV vaccination effectiveness, against varicella, after two doses is 95%.9 The safety profile is excellent, varicella-like rash occurs in 2%–2.8% in healthy children, within 6 weeks postvaccination.9 The strain is attenuated for replication in skin, but less in other target tissues. Like the wild strain, it retains neurotropic properties and can establish lifelong latency in the dorsal root ganglia and reactivate to cause HZ or further neurological complications. MMRV should be administered at least 4 weeks before immunosuppressive therapy is started because of the potential for vaccine-related diseases.1 One can argue that prophylaxis should have been offered to the patient at the start of the first cycle of chemotherapy because of the short delay between the vaccine and the immunosuppressive therapy, 20 days.
The most serious complication in healthy and immunocompromised children exposed to the virus, remains viral reactivation and CNS infections. Studies in children with leukemia who received the live-attenuated varicella vaccine documented a significantly lower incidence of HZ than in children who had the natural disease.2
A study conducted in the USA,10 where vaccination has been implemented since 1995 (single-dose regimen until 2006, then double-dose regimen at 12 months and 4–6 years), reported a total of 12 children who developed varicella vaccine meningitis as a complication of vaccine-related HZ over the last 25 years. Eight occurred in once-immunized patients, with no pattern to the time interval between vaccination and the onset of symptoms (22 months–11 years in immunocompetent patients): two were diagnosed with cancer shortly after. Four occurred in twice-immunized adolescents, 2–10 years after second immunization: one had cancer. Majority of children had HZ prior to developing meningitis. Dermatomal distribution was unusual, zoster rashes were predominant in cervical dermatomes. Immunization site for most cases was not reported. Noteworthy, our patient was injected with vaccine on the ipsilateral side of the HZ. All were treated with intravenous acyclovir and had favorable outcome.
High-dose intravenous acyclovir is the recommended therapy to treat VZV meningo/encephalitis. However, some authors recommend a combination treatment with foscarnet, which has excellent CNS penetration and is active against thymidine kinase-deficient VZV strains (acyclovir resistant).11, 12 A unique case report describes the development of resistance to acyclovir during chronic infection with the Oka vaccine strain of VZV, in an immunosuppressed child. A mutation in the viral thymidine kinase gene was detected.13
Evidence for immunocompetent children experiencing CNS complications of wild-strain varicella reactivation is emerging. A recent study5 summarizes case reports of pediatric meningitis/encephalitis, without zosteriform rash among unvaccinated adolescents. Eleven cases of VZV meningitis/encephalitis in immunocompetent children with a prior history of childhood varicella are reported. Adolescents predominate, there was no pattern to the time interval between primo-infection and reactivation.
Viral reactivation appears to be largely controlled by T-cell-mediated immunity, immunosurveillance and early virion clearance during reactivation events, thus preventing viral spread. In our case, the short delay between vaccination and deep immunosuppression, 20 days, is very likely to have resulted in impaired varicella immunity and triggered this early reactivation. Antibody titers prior to auto-HSCT were within a protective range. However, in accordance with Hardy et al's observations, humoral immunity to varicella zoster virus is relatively unimportant in controlling the reactivation of latent infection.
This rare case or HZ and CNS reactivation is unusual both through the early viral reactivation (3 months) post MMRV vaccination and the timing with the HSCT (median 60 days post HSCT14). This case stresses the importance of conducting a thorough medical history, MMVR should be administered >4 weeks prior immunosuppression per guidance.1 In the event of the necessity of introducing immunosuppressive agents <4 weeks post MMRV, antiviral prophylaxis should be discussed. Furthermore, viral reactivation in patients receiving high-dose chemotherapy should be closely tracked through clinical examination and regular laboratory screening.
4 CONCLUSION
We describe a rare case of meningoencephalitis caused by the Oka-strain varicella vaccine administered 20 days prior chemotherapy and 3 months before HSCT for PNET. Our experience highlights the importance of conducting a thorough medical history analysis regarding vaccination status, including the delay prior immunosuppression. In a context of high immunosuppression, antibodies are not necessarily an accurate marker of robust immunity and early initiation of prophylactic antiviral treatment must be questioned. This report stresses the importance of having a close clinical and biological monitoring of immunocompromised patients who develop a varicelliform of zosteriform rash: dermatological manifestation and central neurological complications can appear separately.
FUNDING INFORMATION
This work was not supported by any funding.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest or financial relationship with the organization that set up the research. There is no funding source.
ETHICS STATEMENT
The patients' legal guardians have provided written consent for publication of the case report.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.