Neutropenia in pediatric solid organ transplant
All authors contributed equally to the drafting and writing of this manuscript.
Abstract
Neutropenia is generally defined as an absolute neutrophil count in the circulation of less than 1500/mm3 and occurs in up to 25%–30% of pediatric solid organ transplant recipients (SOT) within the first year after transplantation. In the SOT population, neutropenia is most often a result of drug-induced bone marrow suppression but can also be secondary to viral infection, nutritional deficiencies, lymphoproliferative infiltrate, and inherited causes. Outcomes for patients with neutropenia vary by degree of neutropenia and type of solid organ transplant. Management of neutropenia should begin by addressing the underlying cause, including reducing or removing medications when appropriate, treating infections, and addressing nutrient deficiencies; however, consultation with an experienced pediatric hematologist and use of granulocyte colony-stimulating factor (G-CSF) may be helpful in some cases. Overall, data on clinical outcomes for G-CSF use remain limited, but observational studies may support its use in patients with infections or severe neutropenia.
Abbreviations
-
- ANC
-
- absolute neutrophil count
-
- aHR
-
- adjusted hazard ratio
-
- ATG
-
- antithymocyte globulin
-
- CBC
-
- complete blood count
-
- CMV
-
- cytomegalovirus
-
- EBV
-
- Epstein–Barr Virus
-
- G-CSF
-
- granulocyte-colony-stimulating Factor
-
- HR
-
- hazard ratio
-
- NGAL
-
- neutrophil gelatinase-associated lipocalin
-
- PTLD
-
- posttransplant lymphoproliferative disorder
-
- RR
-
- relative risk
-
- SOT
-
- solid organ transplant
1 THE NEUTROPHIL
Neutrophils, or polymorphonuclear leukocytes, are the most abundant white blood cells in circulation and play a key role in antimicrobial defense. Neutrophils develop from pluripotent stem cells in the bone marrow. During differentiation, the developing neutrophils undergo a number of intermediate stages, including acquisition of granules containing an array of toxins, specifically myeloperoxidase, defensins, protease 3, neutrophil gelatinase associated lipocalin (NGAL), lysozyme, and metalloproteases.1 Once fully mature, only 3%–5% of neutrophils are released from the bone marrow into circulation, where they survive anywhere from 5 to 135 h. The majority of neutrophils remain in the bone marrow, held in reserve in the “storage pool.”2 This means that readily quantifiable neutrophils in the peripheral blood represent only a fraction of the body's total reserve, and that the body can respond quickly to infections by mobilizing neutrophils from the bone marrow.
Neutropenia is generally defined as an absolute neutrophil count (ANC) in the circulation of less than 1500/mm3 (or 1500/μl or 1.5 × 109/L). Severity of neutropenia may be further classified as mild (1000/mm3 to 1499/mm3), moderate (500/mm3 to 1000/mm3), or severe (<500/mm3)3 or, alternatively by National Cancer Institute Common Terminology Criteria for Adverse events grade (Table 1).4 An absolute neutrophil count of <100/mm3 is defined as agranulocytosis. The clinical implications of neutropenia are related to the status of the bone marrow reserve pool. In situations where the bone marrow reserve is intact, there is no association between degree of neutropenia and risk of infection. The bone marrow in these patients can rapidly provide an adequate number of neutrophils in the setting of infection; however, if the bone marrow reserve is depleted, then grade or stage of neutropenia will correlate with infection risk.5
| Absolute neutrophil count | Grade | NCI stage | Risk of infection |
|---|---|---|---|
| >1500/mm3 | Normal | 1 | Baseline |
| 1000–1499/mm3 | Mild | 2 | No significant increased risk |
| 500–999/mm3 | Moderate | 3 | Some increased risk |
| <500/mm3 | Severe | 4 | Significant risk; typical infectious symptoms may be absent |
- Abbreviation: NCI, National Cancer Institute.
2 EPIDEMIOLOGY AND ETIOLOGY OF NEUTROPENIA AFTER SOLID ORGAN TRANSPLANTATION
Neutropenia is estimated to occur in approximately 25%–30% of solid organ transplant recipients in the first-year posttransplant. Table 2 shows the incidence of neutropenia within the first year following solid organ transplant as reported among both adult and pediatric solid organ transplant recipients by organ system.
2.1 Drug-induced neutropenia
Most epidemiologic studies after solid organ transplantation (SOT) attribute neutropenia to requirement for immunosuppressive medications. However, data on the incidence of neutropenia due to individual medications are largely limited to initial premarketing clinical trials; real-world reports are often complicated by polypharmacy. Here, we will review the incidence, etiology, and management of neutropenia associated with common and well-studied transplant-associated medications; however, neutropenia can also occur with a variety of medications beyond those discussed in detail here (Table 3).
| Common transplant medications associated with neutropenia |
|---|
| Immunosuppressives |
| Tacrolimus |
| Mycophenolate mofetil |
| Azathioprine |
| Antithymocyte globulin |
| Rituximab |
| Antihypertensives |
| Angiotensin-converting enzyme inhibitors |
| Propranolol |
| Furosemide |
| Anti-infectives |
| Trimethoprim-Sulfamethoxazole |
| Ganciclovir/Valganciclovir |
| Dapsone |
| Leflunomide |
| Semisynthetic penicillins |
| Vancomycin |
| Cephalosporins |
| Acyclovir |
| Oseltamivir |
Tacrolimus is the most commonly used posttransplant maintenance immunosuppression medication in the United States, prescribed to over 70% of intestine and pediatric liver and over 90% of pediatric heart, lung, and kidney recipients.6-10 It inhibits calcineurin by binding to FK506-binding protein, thereby blocking calcium-dependent events, including interleukin-2 gene transcription required for T-cell activation.11 Tacrolimus also inhibits glucuronidation of mycophenolic acid, leading to increased blood levels,12 which is the presumed mechanism by which it causes neutropenia. Zafrani et al.13 showed that the combination of tacrolimus and mycophenolate mofetil was an independent risk factor for neutropenia in kidney transplant recipients, and Alraddadi et al also showed that tacrolimus trough level was an independent predictor of neutropenia in liver transplant recipients.14 Cyclosporine does not have similar effects on mycophenolic acid levels, and a three-year study of 125 pediatric kidney transplant recipients reported a neutropenia incidence-rate of 0.064/patient-year with tacrolimus compared with 0.014/patient-year with cyclosporine.15 Multiple case reports of presumed tacrolimus-associated neutropenia report resolution with conversion of tacrolimus to cyclosporine16-18; however, in many of these cases, the neutropenia persisted after mycophenolate mofetil was initially discontinued, resolving only once tacrolimus was stopped. Therefore, it remains unclear if tacrolimus affects neutrophil counts by additional mechanisms.
Mycophenolate mofetil and mycophenolic acid are among the most commonly used maintenance immunosuppressive medications, prescribed to over 90% of pediatric heart, lung, and kidney transplant recipients, 35% of intestine recipients, and over 40% of pediatric liver recipients in the United States.6-10 Mycophenolic acid and its prodrug, mycophenolate mofetil, inhibit lymphocyte proliferation by blocking inosine monophosphate dehydrogenase, an enzyme involved in the synthesis of guanosine nucleotides. Mycophenolic acid has a stronger affinity for the type II isoform of the enzyme, which is found primarily in lymphocytes, than the type I isoform present in most other cells, thus contributing to its specificity as an immunosuppressive agent.19 The method by which mycophenolic acid causes neutropenia is less clear, but mouse models suggest that it may suppress T cells that produce interleukin-17, a cytokine that drives the production of granulocyte colony-stimulating factor, which is critical for neutrophil production.20
Mycophenolate mofetil has been associated with neutropenia in 2%–22%21, 22 of individuals, with higher rates reported with concomitant valganciclovir use. Ferrer-Machin et al reported that neutropenia occurred in 56% of 57 adult liver transplant recipients prescribed mycophenolate mofetil and valganciclovir, compared with 37% of the 87 recipients prescribed mycophenolate mofetil alone.23 Individuals with higher mycophenolic acid area under the curve (AUC) may be at higher risk of hematologic toxicities, but this has not been replicated in all studies. A target AUC0–12h of 30–60 mg-h/L has been recommended for pediatric kidney and adult liver transplant recipients, but data in heart and lung recipients are limited.24
Reduction in dose or discontinuation of mycophenolic acid can quickly improve the neutrophil count but is associated with an increased risk of acute rejection.25 Brar et al.26 reported that adult kidney transplant recipients whose mycophenolic acid was reduced or discontinued due to neutropenia doubled their risk of acute rejection, while Zafrani et al.13 reported an odds ratio for acute rejection of 1.11 per day off the medication. Due to the high incidence of neutropenia in SOT patients concomitantly receiving MMF and valganciclovir, and equally concerning acute rejection episodes when MMF is reduced or discontinued, alternative immunosuppressive medications have been considered. However, a Cochrane review did not find any difference in the incidence of hematologic side effects between mycophenolic acid and azathioprine.27
Antithymocyte globulin (ATG) is comprised of rabbit-derived polyclonal anti-T-cell antibodies and may be used as induction immunosuppression or treatment of rejection in transplantation. Targets for ATG include multiple molecules that are present on the neutrophil cell surface, including LFA-1, ICAM-1, CCR7, and CD16.28, 29 Data on the risk of neutropenia are limited. Büchler et al reported an ANC <1500/mm3 in 13% and an ANC <800/mm3 in 2.5% in renal transplant recipients within 14 days of initiation of therapy; all patients discontinued ATG with resolution of neutropenia. ATG was identified as an independent risk factor for neutropenia in a USRDA study of 6043 kidney transplant recipients with neutropenia.30, 31 Leukopenia is reported in 24.6%–33.5% of patients treated with ATG, but studies often do not differentiate if this is due to lymphopenia or neutropenia.32, 33 If a patient develops neutropenia while receiving a course of thymoglobulin, dose reduction is recommended.34
Rituximab is a humanized monoclonal antibody that targets CD20, which leads to binding and depletion of B cells. It may be used pretransplant, for ABO incompatible and highly sensitized patients, and posttransplant, for antibody-mediated rejection and CD20(+) posttransplant lymphoproliferative disease (PTLD). Late-onset neutropenia, occurring four or more weeks after rituximab infusion, has been reported in 4%–27.3% of patients.33, 35 The incidence may be higher in some solid organ recipients. Kabei et al.36 reported late-onset neutropenia in 48% of ABO incompatible heart transplant recipients at 2–12 months after rituximab treatment; 41.6% of those with late-onset neutropenia developed acute rejection. Similarly, Ahmadi et al reported neutropenia in four of six kidney transplant recipients at 35–93 days after treatment with rituximab. One of these patients died secondary to endocarditis.37 The reason for late-onset neutropenia and increased rejection risk is unknown. It has been hypothesized that depletion of immunoregulatory B cells leads to increased levels of B-cell-activating factor (BAFF) or other cytokines or competition between B-cell lymphopoiesis and granulopoiesis.36, 37 Granulocyte-colony-stimulating factor (G-CSF) has been reported as a possible treatment for rituximab-associated neutropenia.38
Trimethoprim-sulfamethoxazole is commonly used for prophylaxis against Pneumocystis jarovecii in immunocompromised patients. It inhibits thymidine synthesis by inhibiting folic acid synthesis and is associated with neutropenia in 34%–35% of children treated with trimethoprim-sulfamethoxazole for otitis media, often as early as 10 days of initiating treatment. Principi et al. showed that this incidence could be reduced to 17.5% with co-administration of folic acid.39, 40 Leukopenia, anemia, and thrombocytopenia have also been reported.33 If trimethoprim-sulfamethoxazole toxicity is suspected, the drug can be substituted with pentamidine, atovaquone, or dapsone, although dapsone is also associated with a rare and idiosyncratic but serious neutropenia risk.41, 42
Valganciclovir, a prodrug of ganciclovir, may be used for cytomegalovirus prophylaxis or treatment after solid organ transplant. Ganciclovir triphosphate accumulation in cells is associated with a decrease in neutrophil count within the first 3 months of treatment,43 where it is hypothesized to inhibit DNA polymerase.44 Kidney transplant recipients with a polymorphism in ABCC4, a multidrug efflux transporter, have been shown to be at risk of a decline in neutrophil count during valganciclovir treatment.44 Valganciclovir has been associated with neutropenia in 40%–77.3% of solid organ transplant recipients,45, 46 with several studies associating neutropenia with valganciclovir ‘overexposure’.47, 48 Incidence of neutropenia may be higher in pediatric than adult patients.49 Dose reduction in valganciclovir is not recommended due to the risk of development of resistant cytomegalovirus50; discontinuation of the drug with increased cytomegalovirus screening is an alternative for patients with significant toxicities.
2.2 Infection-induced neutropenia
Numerous common childhood infections can cause transient neutropenia, including respiratory syncytial virus, influenza, parvovirus, human herpes virus 6, measles, rubella, varicella, malaria, and shigella. Less common infectious causes include typhoid fever, brucellosis, tularemia, tuberculosis, kala-azar, and Rocky Mountain spotted fever. In most cases, the neutropenia is related to viral suppression of the bone marrow or viral-induced autoimmunity.51 The degree of neutropenia in these settings is typically mild-to-moderate and will resolve within days to weeks, depending on the pathogen. However, of particular concern to pediatric SOT patients is neutropenia related to Epstein–Barr virus and cytomegalovirus.
Epstein–Barr virus (EBV) is a member of the herpesvirus family that is acquired by 90% of children in developing countries prior to age 5 years.52 However, in the United States, 47%–60% of pediatric solid organ transplant recipients are EBV-naïve prior at the time of transplantation.6-9 Mild neutropenia occurs in 40% of immunocompetent children early in the course of infectious mononucleosis, and severe neutropenia has been reported.53 Data from transplant recipients are more limited, but Hyun et al.54 reported neutropenia in one of eight pediatric kidney transplant recipients with EBV DNAemia, while Hadou et al reported severe neutropenia in one of 18 pediatric kidney recipients with EBV DNAemia.55
Cytomegalovirus (CMV) is another human herpesvirus with a seroprevalence rate of 30%–97% worldwide.50 However, data from the United States show that 61%–71% of pediatric solid organ transplant recipients are CMV-naïve at the time of transplant.7-10 CMV does not typically cause neutropenia in immunocompetent individuals but is a component of the CMV syndrome that includes fever, malaise, atypical lymphocytosis, leukopenia, thrombocytopenia, and/or elevated hepatic transaminases as well as symptomatic end-organ disease.14, 50 High-risk CMV serostatus has been associated with risk of neutropenia in a cohort of 100 pediatric kidney and liver transplant recipients56 as well as large cohorts of adult liver, kidney, and heart recipients,30, 57, 58 although it can be difficult to separate the effects of CMV serostatus from those of prophylactic valganciclovir.
2.3 Autoimmune neutropenia
Antineutrophil antibodies can develop in the setting of infection, autoimmune disorders, ABO incompatible transplants, passenger lymphocyte syndrome, and immune system dysregulation related to immunosuppression. The antibodies target circulating neutrophils, usually the NA1 antigen, for destruction but generally do not affect bone marrow reserves. In a single-center cohort of 764 pediatric solid organ transplant recipients, Schoettler et al.59 reported autoimmune neutropenia in 0.3% of patients; all cases resolved after switching tacrolimus to either cyclosporine or sirolimus. In a cohort of 77 pediatric heart recipients with neutropenia, Rose-Felker et al.60 reported identifying antineutrophil antibodies in six of fourteen patients who were tested. Most children with autoimmune neutropenia will have spontaneous remission of disease in 7–24 months although serious infections occur in approximately 10%.61 Corticosteroids, intravenous immunoglobulin, and G-CSF have bene proposed as potential treatments for autoimmune neutropenia,51 but their usefulness is questionable given the low infection rate and normal bone marrow reserve.
Chronic benign neutropenia is thought to overlap autoimmune neutropenia in phenotype and typically presents in infants and children less than 3 years old with spontaneous resolution in two to three years; conversely, chronic idiopathic neutropenia tends to present in older children and does not remit. Both disorders are associated with normal bone marrow reserves and are not associated with increased infections.62
2.4 Neutropenia associated with nutritional deficiencies
Deficiencies in folate, vitamin B12, and copper may cause neutropenia due to decreased granulopoiesis and are often associated with macrocytosis and pancytopenia.63 While these nutritional deficiencies are uncommon in healthy children, they may be a consideration in chronically ill children with poor oral intake or malabsorption syndromes. Serum testing for folate and vitamin B12 levels may serve as an initial screen, while copper deficiency can be diagnosed by checking ceruloplasmin levels. Treatment is with supplementation of the deficient nutrient.
2.5 Other causes of neutropenia
There are a variety of reasons for a child to have neutropenia independent of SOT status. Benign familial neutropenia is a condition resulting in lower than “normal” absolute neutrophil counts and has been reported to be more common in African Americans (4.5%), South African blacks, West Indians, and Arab Jordanians.64, 65 It is hypothesized to be secondary to a defect in release of mature neutrophils from the bone marrow to the circulation; bone marrow neutrophil reserves are normal, and there is no increased infection risk.66, 67 In some cases, there may be an associated single nucleotide polymorphism in the Duffy antigen/receptor chemokine gene, a receptor for pro-inflammatory cytokines, that is protective against malaria.68-70 Examination of pretransplant blood counts should help make this diagnosis.
Hypersplenism can result in neutrophil trapping and neutropenia but does not affect bone marrow reserve. Infections are uncommon. G-CSF treatment is contraindicated in hypersplenism as it has been associated with splenic rupture.71 A variety of congenital disorders cause neutropenia including cyclic neutropenia, severe congenital neutropenia, Chediak-Higashi syndrome, and Shwachman-Diamond-Oski syndrome; the majority of these would be expected to be diagnosed early in life.72 Finally, bone marrow disorders, including leukemia, can present with neutropenia, but generally as part of a broader suppression of bone marrow cell lines.
3 OUTCOMES OF NEUTROPENIA IN SOLID ORGAN TRANSPLANT RECIPIENTS
Outcomes for patients with neutropenia vary by degree of neutropenia and type of solid organ transplant. Chow et al and Rose-Felker et al reported no increase in infection, acute rejection, or death among pediatric or adult heart transplant recipients with an ANC <1000/mm3.57, 60 Conversely, severe neutropenia among adult lung recipients was associated with a higher risk of mortality when compared with recipients with no neutropenia (aHR 2.97, p = .04) but with no difference in rejection or CMV infection. Gram-negative bacterial infection incidence was higher among those with moderate or severe neutropenia, but gram-positive infections were more common only among those with severe neutropenia.73
An analysis of 41 705 adult kidney transplant recipients in the US Renal Data System reported that neutropenia was associated with an increased risk of graft loss (aHR 1.59, p < .001), death (aHR 1.74, p < .001), and infectious causes of death (aHR 1.8, p < .001), and similar results have been reported in multiple smaller studies.26, 58, 74 Among pediatric kidney recipients, Jarasvaraparn et al.56 reported a higher risk of hospitalization (RR 2.7, p < .001) and infection (RR 1.8, p = .04) but not acute rejection, and Becker-Cohen et al.75 reported that those with an ANC <1500/mm3 maintained stable graft function during the neutropenic episodes.
Reported outcomes for neutropenic liver transplant recipients are similarly mixed. Alraddadi et al reported that an ANC <1000/mm3 was an independent predictor of mortality among adult liver recipients (HR 3.76, p < .001),14 but Perez et al reported no infections among liver recipients with an ANC <900/mm3.46 Among pediatric liver recipients, Jarasvaraparn et al.56 reported no increased risk of hospitalization or infection among those with neutropenia.
4 EVALUATION AND MANAGEMENT OF NEUTROPENIA
Initial evaluation of the neutropenic transplant recipient begins with an assessment of the severity of neutropenia and risk of infection. Patients with an ANC <500/mm3 are at highest infection risk and require counseling on strict hand washing, avoidance of sick contacts, and prompt medical evaluation of any fever. Patients should be screened for infectious symptoms, especially gingivitis, periodontitis, skin infections, and pneumonia symptoms.76 Evaluation of the cause of neutropenia includes review of the patient's medication list and testing for EBV and CMV DNAemia. Children should be assessed for signs or symptoms of other viral infections that can cause neutropenia. In patients with increased risk of nutritional issues (e.g., malabsorption or short-gut syndrome), vitamin B12, methylmalonic acid, homocysteine, copper, ceruloplasmin, and folate levels should be quantified to evaluate for micronutrient deficiencies that may be easily corrected.
Laboratory follow-up of neutropenia often occurs at least weekly in severe neutropenia and every 2–4 weeks in more moderate cases. If neutropenia is persistent without a clear underlying cause, further evaluation should include testing for antineutrophil antibodies and consideration to bone marrow examination as the most direct assessment of neutrophil reserve. Patients with neutropenia secondary to low bone marrow reserve will be at highest risk of infection, but those with normal marrow reserve, such as in chronic benign neutropenia and hypersplenism, will generally not be at increased risk.51 Hematology consultation should be initiated by the transplant clinician for patients with neutropenia in the setting of other cytopenias (anemia and/or thrombocytopenia), as this may be a symptom of a lymphoproliferative infiltrate or a broader bone marrow failure syndrome.
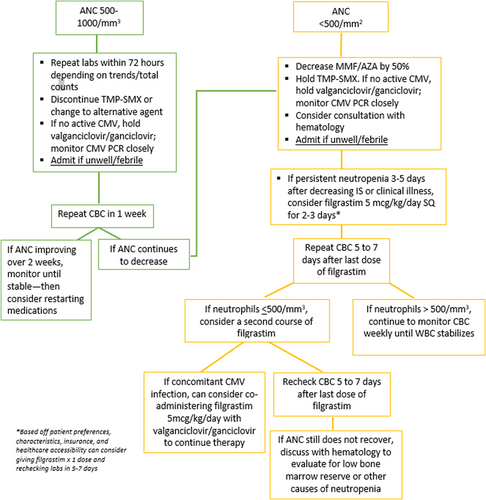
Management of neutropenia should begin by addressing the underlying cause, including reducing or removing bone marrow suppressing medications when appropriate, treating infections, and addressing nutrient deficiencies. A sample, single-center algorithm for the approach to management of neutropenia is shown in Figure 1. Providers should be cautious about reducing doses of antibiotics and antivirals due to the risk selecting for drug-resistant pathogens.50 Reduction in immunosuppressive medications carries an increased risk of allograft rejection. An alternative proposed therapy is G-CSF, a cytokine that stimulates production of neutrophil colonies and speeds their maturation and release into the circulation.77 It is most highly studied in children treated with chemotherapy, where meta-analytic data showed a modest reduction in the duration of hospitalization by 1.42 days (95% CI 0.62–2.22 days) for children receiving G-CSF compared to placebo.78 Reported side effects include fever, headache, skeletal pain, rash, nosebleed, diarrhea, anemia, and hair loss.34
Among adult solid organ transplant recipients, G-CSF use has been shown to increase white blood cell counts79-82 but its impact on clinical outcomes remains unclear. In a randomized control trial that treated liver transplant recipients with G-CSF for 21 days posttransplant, Winston et al found no beneficial impact for prevention of infection or death.83 A study using data from 41 705 US Medicare patients showed a decreased risk of death in unadjusted analysis, but this finding was no longer significant after adjustment in multivariable models.30 Among 228 lung transplant recipients, G-CSF use was associated with a higher risk of chronic allograft dysfunction in those with mild neutropenia but decreased risk of death in those with severe neutropenia (aHR 0.24).73
Turgeon et al reported that 8% of 50 kidney or liver recipients who were treated with G-CSF developed acute rejection within 2 months of G-CSF therapy.79 Similarly, Schneider et al.82 reported a trend toward increased rejection among 12 kidney recipients treated with G-CSF, and Nguyen et al reported an incidence rate of acute rejection of 0.067 episodes/month among heart transplant recipients treated with G-CSF compared with 0.011 episodes/month in the untreated group.77 Conversely, Hamel et al.81 found no rejection within one-month G-CSF treatment among 36 kidney transplant recipients. The mechanism of rejection following receipt of G-CSF is unclear and may conceivably be a result of immunosuppression reduction as a first-line response prior to use of G-CSF.
There are two cohort studies examining G-CSF use in pediatric kidney transplant recipients. Becker-Cohen et al.75 reported 46 neutropenic children, 16 of whom had an ANC <500/mm3. All patients with moderate-to-severe neutropenia also had discontinuation of oral valganciclovir and trimethoprim-sulfamethoxazole, a dose decrease in their antimetabolite immunosuppression, and discontinuation of the antimetabolite if ANC did not improve. Eight patients with severe neutropenia were treated with G-CSF at a dose of 5 mcg/kg/day for 1–4 doses. Patients were selected for treatment either due to infection (n = 5) or persistent severe neutropenia despite medication changes (n = 3). Neutrophil counts returned to the normal range within 3–10 days of treatment. In a multicenter cohort study, Engen et al reported 341 neutropenic patients, of whom 83 were treated with G-CSF at a median dose of 5 mcg/kg/dose for 2–7 doses. G-CSF was more commonly prescribed to patients with a lower ANC (median 310/mm3) compared with the untreated group (895/mm3). G-CSF treatment was associated with decreased incidence of hospitalization but no change in total duration of neutropenia, bacterial infections, rejection, or posttransplant lymphoproliferative disease in an adjusted analysis. Recurrence of neutropenia was more common in the G-CSF-treated group, likely related to the short (3.5 h) half-life of the medication.84 Overall, data on clinical outcomes for G-CSF use remain limited, but observational studies may support its use in patients with severe or recurrent infections associated with severe neutropenia.
5 CONCLUSION
Neutropenia is a common complication after SOT related to multiple, often interacting variables. While drug-induced neutropenia is common, a broad differential is necessary. Management of drug-induced neutropenia is a matter of trial and error, reducing various medication doses until ANC improves. Consultation with a pediatric hematologist is warranted, particularly where neutropenia is persistent and/or G-CSF use is being considered. G-CSF appears to be safe in the pediatric solid organ transplant population and may be helpful for those with severe neutropenia complicated by severe or recurrent infections. The clinical and allograft outcomes in this population are mixed and emphasize the need for more detailed longitudinal studies to assess the best management strategies associated with neutropenia after SOT.
CONFLICT OF INTEREST
Authors have no Conflict of Interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.