Timing of reconstruction of the lower urinary tract in pediatric kidney transplant recipients: A CERTAIN multicenter analysis of current practice
Burkhard Tönshoff and Joanne Nyarangi-Dix contributed equally to this manuscript.
Abstract
Background
Preexistent LUTD are considered a hostile environment, which might negatively impact KTx survival. In such cases, surgical reconstruction of the bladder is required. However, there is still disagreement on the optimal timing of the reconstruction procedure.
Methods
This is a multicenter analysis of data from the CERTAIN Registry. Included were 62 children aged 8.18 ± 4.90 years, with LUTD. Study endpoints were the duration of initial posttransplant hospitalization, febrile UTIs, and a composite failure endpoint comprising decline of eGFR, graft loss, or death up to 5 years posttransplant. Outcome was compared to matched controls without bladder dysfunction.
Results
Forty-one patients (66.1%) underwent pretransplant and 14 patients (22.6%) posttransplant reconstruction. Bladder augmentation was performed more frequently in the pretransplant (61%) than in the posttransplant group (21%, p = .013). Outcome in the pre- and posttransplant groups and in the subgroups of patients on pretransplant PD with major bladder surgery either pre- (n = 14) or posttransplant (n = 7) was comparable. Outcomes of the main study cohort and the matched control cohort (n = 119) were comparable during the first 4 years posttransplant; at year 5, there were more events of transplant dysfunction in the study cohort with LUTD than in controls (p = .03).
Conclusions
This multicenter analysis of the current practice of LUTD reconstruction in pediatric KTx recipients shows that pre- or posttransplant surgical reconstruction of the lower urinary tract is associated with a comparable 5-year outcome.
Abbreviations
-
- CERTAIN
-
- Cooperative European Pediatric Renal Transplant Initiative
-
- eGFR
-
- estimated glomerular filtration rate
-
- HD
-
- hemodialysis
-
- HLA
-
- human leukocyte antigen
-
- IQR
-
- interquartile range
-
- KTx
-
- kidney transplantation
-
- LUTD
-
- disorders of the lower urinary tract
-
- PD
-
- peritoneal dialysis
-
- n.a.
-
- not applicable
-
- SD
-
- standard deviation
-
- SE
-
- standard error
-
- UTI
-
- urinary tract infection
-
- VUR
-
- vesicoureteral reflux
1 INTRODUCTION
About one-fifth of children suffering from chronic kidney disease stage 5 has associated anatomical or functional abnormalitiess of the lower urinary tract.1, 2 These disorders often complicate clinical management.3 Lower urinary tract disorders play an important role in the deterioration of native kidney function, but they can also affect posttransplant long-term outcomes and kidney graft survival.4, 5 Thorough assessment of the lower urinary tract is therefore an essential part of the routine pretransplant work-up.6 In most cases, conservative treatment modalities are sufficient.7 In severe cases, however, surgical interventions such as bladder augmentation or the placement of a continent appendicostomy are required.7, 8 Due to limited data, optimal timing for surgical reconstruction of the lower urinary tract is still a matter of debate, and evidence-based guidelines are lacking. Therefore, different opinion-based approaches shape the current practice pattern. The main doctrines are reconstruction of the urinary tract before or after KTx.9-15 Though technically feasible, KTx and simultaneous lower urinary tract reconstruction are rarely performed in children.16, 17
The majority of published data on this topic are currently derived from single-center studies with rather small patient cohorts.10, 18, 19 However, a statistically meaningful analysis allowing for a solid recommendation can only be reached by multicenter studies. We therefore performed this multicenter retrospective registry analysis of the current practice pattern and outcome of pre- or posttransplant reconstruction of the lower urinary tract in pediatric KTx recipients with lower urinary tract dysfunction.
2 MATERIALS AND METHODS
This is a multicenter, multinational retrospective registry analysis of the current practice pattern and outcome of children after KTx and surgical reconstruction of the lower urinary tract by bladder augmentation, bladder substitution, or placement of a continent appendicostomy. The study is based on data derived from the CERTAIN Registry (www.certain-registry.eu).
2.1 Data source
The web-based CERTAIN Registry collects clinical and laboratory data of pediatric KTx recipients in a detailed and comprehensive manner. This allows a thorough patient characterization in general and also of specific patient subcohorts.20 Specific and additional data collection for this analysis was performed according to a defined protocol (see Supporting Information). Written informed consent was obtained from all parents/guardians to participate in the registry, with assent from patients when appropriate for their age. The CERTAIN Registry has been approved by the respective ethics committee of each contributing center and is kept in full accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The study was designed, analyzed, and reported according to the STROBE guidelines (https://www.strobe-statement.org).
2.2 Patient attrition model
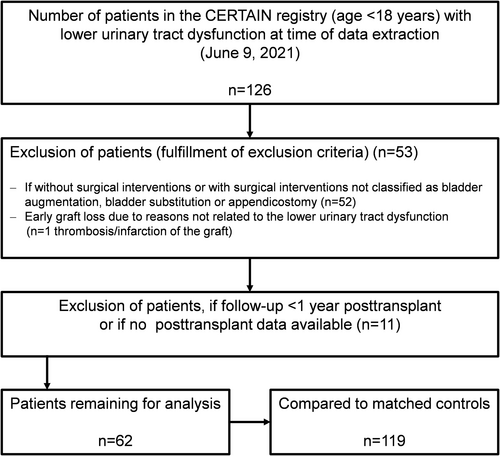
Data collection from CERTAIN for this study was based on the following inclusion criteria: patient age <18 years at the time of transplantation, and the presence of lower urinary tract dysfunction defined as: bladder disorder with either (i) urethral valves, (ii) urethral stricture, atresia or dysplasia, (iii) bladder exstrophy, (iv) bladder hypoplasia, (v) detrusor sphincter dyscoordination, and/or (vi) neurogenic bladder disorder associated with myelomeningocele, traumatic spinal cord injury, hydrocephalus, caudal regression, spinal and cerebral tumors; Figure 1). Exclusion criteria were patients without surgical interventions classified as (i) bladder augmentation, (ii) bladder substitution, (iii) appendicostomy, (iv) early graft loss posttransplant due to reasons not related to the urinary tract dysfunction and/or bladder surgery, and (v) patient follow-up for less than 1 year posttransplant (Figure 1).
2.3 Definition of subgroups and study endpoints
We describe patient and transplant characteristics, primary kidney diseases, and initial immunosuppressive medication. Children whose urinary tract was reconstructed prior to transplant were compared to those reconstructed posttransplant. A small subgroup of children had the urinary tract reconstruction performed during KTx. We defined a subgroup “major operations” as the performance of bladder augmentation or bladder substitution surgery with or without preceding or subsequent appendicostomy; we defined the subgroup “minor operations” as the placement of an appendicostomy only.
The following endpoints were analyzed for up to 5 years posttransplant: (i) duration of initial hospitalization after transplant surgery, (ii) rate of first febrile UTI occurrence, and the time to first febrile UTI occurrence, (iii) cumulative duration of hospitalization due to febrile UTI; (iv) eGFR at day 30, and years 1, 3, and 5 posttransplant; (v) graft loss, (vi) patient death, (vii) detection of VUR, and (viii) occurrence of a composite failure endpoint comprising eGFR decline below 30 ml/min/1.73 m2 or below 50% of baseline eGFR (100%) as measured at day 30 posttransplant, graft loss or death, whichever event came first. If an event of eGFR decline below 50% of baseline was due to a decline of eGFR below 30 ml/min/1.73 m2, this event was classified as a decline below 50% of baseline. eGFR values above 120 ml/min/1.73 m2 at day 30 posttransplant (in 11 patients) were set to 120 ml/min/1.73 m2. The cumulative duration of hospitalization due to febrile UTIs was calculated in the subgroup of patients with at least one febrile UTI. We assessed and described the reasons for kidney allograft loss and patient death. We performed a subgroup analysis of patients on pretransplant PD with major bladder surgery. We analyzed specific urodynamic investigation results prior to bladder surgery. Normal bladder compliance was defined as a value >30 ml/cm H2O. The urinary bladder was categorized as unstable when autonomic detrusor contractions occurred in the storage phase; a high-pressure bladder was defined as an increase in pressure of >40 cm H2O in the storage phase. eGFR was calculated according to the revised Schwartz formula: eGFR = (ml/min per 1.73 m2) = 0.413 × [height (cm)/serum creatinine (mg/dl)].21
We further compared patients on pretransplant PD with patients on pretransplant HD (Figure 1). The occurrence rate of first febrile UTI in this subgroup could be calculated only for the first 3 years posttransplant because no patient with urinary tract reconstruction posttransplant had a follow-up longer than 3 years.
We identified control patients without bladder dysfunction from the CERTAIN Registry and matched these to the study cohort (Figure 1). The matching criteria were: recipient age at KTx stratified into the three groups 0–5.9, 6–11.9, and 12–17.9 years, donor age (≤15.9 years or >16 and ≤50 years), initial immunosuppressive medication (tacrolimus or ciclosporin), cold ischemia time (<12, 12–24, >24 h), first or second KTx, living or deceased donor, and era of transplantation (date of transplantation ±5 years). Each patient (n = 62) was matched with two controls (n = 119) in the majority of cases. Only for one patient, there was no matched control patient, and for three patients, there was only one matched control patient. If there were more than two matching controls available, we chose two matched control patients at random and blinded.
2.4 Statistical analyses
The program R (version 4.1.0) was used for data analysis. Patient and transplant characteristics, the distribution of primary kidney diseases, and the initial immunosuppressive medication are presented in a descriptive manner. Categorical parameters are presented as the number and percentage of patients, while results for continuous variables are expressed as mean ± SD or as median and first and third quartile, as appropriate. Event rates of time-to-event endpoints were calculated using the Kaplan–Meier estimator, SDs are based on the complementary log–log transformation of the survival function. Continuous variables were compared using either the Welch's t-test or Mann–Whitney U-test, as appropriate. Categorical variables were compared using the Boschloo's test for 2 × 2 contingency tables and the Fisher's exact test for higher-dimensional contingency tables. For time-to-event variables, the log-rank test was used. The main study cohort and the matched control cohort were compared using a Cox frailty model, where a random intercept was specified for each group of matched patients. Specific urodynamic investigation results prior to bladder surgery are shown in a categorical manner: high bladder pressure (yes, no), bladder compliance (increased, normal, reduced), and urodynamic instability (yes, no). A p value <.05 is considered as statistically significant. Since this is an exploratory analysis, we did not adjust for multiplicity.
3 RESULTS
3.1 Patients, transplant characteristics, and types of bladder surgery
For this specific analysis, 126 patients were documented. The main study cohort comprised 62 patients aged <18 years with the diagnosis of LUTD and major or minor bladder surgery (Figure 1). Of these, 41 (66%) underwent pretransplant reconstruction of the lower urinary tract (pretransplant group) compared to 14 patients (23%) with posttransplant reconstruction (posttransplant group). In seven patients, KTx and lower urinary tract reconstruction were performed simultaneously (during-transplant group). Table 1 shows clinical characteristics and the distribution of primary kidney diseases, type of bladder surgery, and the initial immunosuppressive regimen in the main study cohort and the three subgroups.
| Characteristics | Entire cohort n = 62 | Bladder surgery during KTx n = 7 | Bladder surgery pretransplant n = 41 | Bladder surgery posttransplant n = 14 | p valuee |
|---|---|---|---|---|---|
| Age at KTx (years), mean ± SD | 8.18 ± 4.90 | 5.81 ± 4.25 | 9.05 ± 4.80 | 6.82 ± 5.11 | .168 |
| Male sex, n (%) | 48 (77) | 6 (86) | 30 (74) | 12 (86) | .401 |
| Caucasian, n (%) | 58 (94) | 6 (86) | 39 (95) | 13 (93) | .978 |
| Second RTx, n (%)a | 6 (10) | 0 (0) | 5 (12) | 1 (7) | .807 |
| Living RTx, n (%) | 18 (29) | 1 (14) | 15 (37) | 2 (14) | .129 |
| Prior HD, n (%) | 19 (31) | 2 (29) | 16 (39) | 1 (7) | .025f |
| Prior PD, n (%) | 30 (48) | 3 (43) | 16 (39) | 11 (79) | .025g |
| HLA-A/B mismatches, median (IQR) | 2 (1–3) | 3 (2–4) | 2 (1–3) | 2.5 (1–3) | .856 |
| Cold ischemia time (hours), mean ± SD | 11.1 ± 7.96 | 12.7 ± 3.77 | 10.8 ± 8.49 | 11.3 ± 8.2 | .841 |
| eGFR (ml/min/1.73 m2)b, mean ± SD | 82.7 ± 29.5 | 89.6 ± 27.6 | 81.8 ± 29.4 | 82.0 ± 32.4 | .983 |
| Primary kidney disease, n (%) | .071 | ||||
| Posterior urethral valves and kidney hypo-/dysplasia, n (%) | 44 (71) | 6 (86) | 28 (68) | 10 (71) | – |
| Obstructive uropathy/nephropathy, n (%) | 6 (10) | 0 (0) | 6 (15) | 0 (0) | – |
| Cystic kidney disease, n (%) | 6 (10) | 1 (14) | 3 (7) | 2 (14) | – |
| Complex malformations, n (%) | 4 (6) | 0 (0) | 4 10) | 0 (0) | – |
| Nephrotic syndrome, n (%) | 1 (2) | 0 (0) | 0 (0) | 1 (7) | – |
| Unknown, n (%) | 1 (2) | 0 (0) | 0 (0) | 1 (7) | – |
| Type of bladder operation | |||||
| Major surgery, n (%) | 43 (69) | 5 (71) | 31 (76) | 7 (50) | .089h |
| Bladder augmentation, n (%) | 28 (45) | 0 (0) | 25 (61) | 3 (21) | .013 |
| Bladder substitution, n (%) | 15 (24) | 5 (71) | 6 (15) | 4 (29) | .298 |
| Minor surgery n (%) | 19 (31) | 2 (29) | 10 (24) | 7 (50) | n.a. |
| Initial immunosuppressive regimen, n (%)c,d | |||||
| Tacrolimus, n (%) | 48 (77) | 6 (86) | 33 (80) | 9 (64) | .249 |
| Ciclosporin, n (%) | 14 (23) | 1 (14) | 8 (20) | 5 (36) | .249 |
| Mycophenolic acid, n (%) | 51 (82) | 5 (71) | 36 (88) | 10 (71) | .195 |
| Azathioprine, n (%) | 7 (11) | 2 (29) | 4 (10) | 1 (7) | 1 |
| Everolimus, n (%) | 1 (2) | 0 (0) | 1 (2) | 0 (0) | 1 |
| Glucocorticoids, n (%) | 59 (95) | 7 (100) | 38 (93) | 14 (100) | .608 |
| IL-2 receptor antagonist, n (%) | 2 (3) | 0 (0) | 1 (2) | 1 (7) | .674 |
- aFor patients with more than one KTx, only the most recent one was evaluated; bDefined as eGFR at day 30 posttransplant; cAt day 30 posttransplant; dNo patient received sirolimus, rituximab, or thymoglobulin; ep value is calculated for pre- versus posttransplant; f,gp value is calculated for the distribution of HD, PD, and preemptive KTx; hp value is calculated for the comparison: major surgery versus minor surgery in the pretransplant group vs. the posttransplant group.
Posterior urethral valves and kidney hypo−/dysplasia were the most common primary kidney diseases (68%), followed by obstructive uropathies and/or nephropathies (10%). Major bladder surgery was performed in 76% of patients in the pretransplant group and in 50% in the posttransplant group (Table 1). In total, there were 28 bladder augmentations and 15 bladder substitutions (13 ileal conduits) performed. Bladder augmentation was performed more frequently in the pretransplant group (61%) than in the posttransplant group (21%, p = .013). In total, there were 15 bladder substitutions performed (13 ileal conduits). The initial immunosuppressive medication regimen was comparable between the groups (Table 1). Table S1 shows the urodynamic study results obtained before bladder surgery in the group of patients with posterior urethral valves and kidney hypo−/dysplasia. The respective frequencies of reduced bladder compliance and high-pressure bladder were comparable among the pre-, post-, and during-transplant groups.
3.2 Primary endpoint analysis
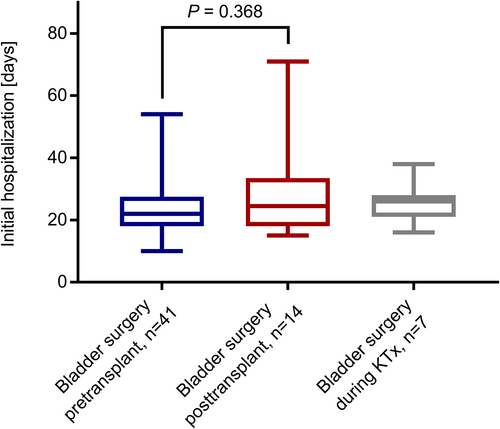
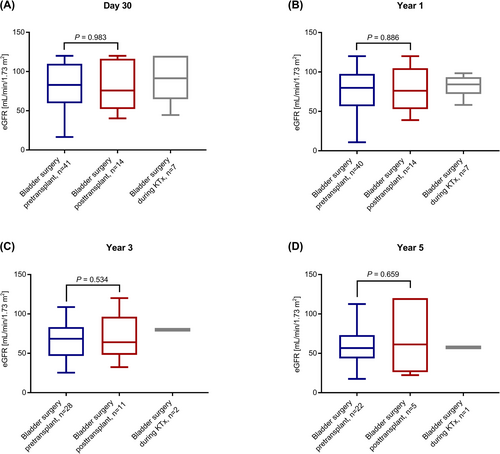
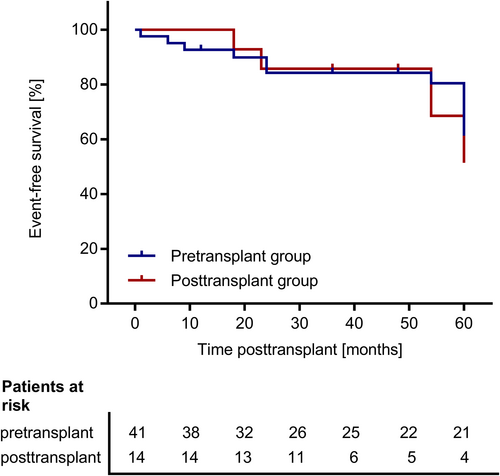
We found no significant differences between the pre- and posttransplant groups regarding the duration of initial hospitalization after transplant surgery (Figure 2) and regarding eGFR at day 30, year 1, 3, and 5 posttransplant (Figure 3). There was one event of graft loss in the pretransplant group (due to urological complications at year 4 posttransplant) and one event of graft loss in the posttransplant group (due to graft infection at year 2 posttransplant) (Table 2). There were no deaths in the study cohort. The composite failure endpoint comprising eGFR decline, graft loss, or death did not differ between the pre- and posttransplant group (p = .77; Figure 4). This analysis was based on 17 events in the main study cohort (n = 62): There were 4 events of eGFR decline below 30 ml/min/1.73 m2, seven events of eGFR decline below 50% of baseline eGFR, and one event of graft loss in the pretransplant group and two events of eGFR decline below 30 ml/min/1.73 m2, one event of eGFR decline below 50% of baseline eGFR and one event of graft loss in the posttransplant group. There was one event of eGFR decline below 50% of baseline eGFR in the during-transplant group. Table 2 shows the proportion of patients with at least one febrile UTI during the follow-up of up to 5 years posttransplant. The rate of first febrile UTI occurrence, the time to first febrile UTI occurrence posttransplant and the cumulative duration of hospitalization due to febrile UTIs did not differ significantly between the pre- and the posttransplant group (Table 2; Figure 5). Also, the time to the first detection of VUR within 5 years posttransplant did not differ between the pre- and posttransplant groups (Figure S1).
| Endpoint | Entire cohort n = 62 | Bladder surgery during KTx n = 7 | Bladder surgery pretransplant n = 41 | Bladder surgery posttransplant n = 14 | p valuea |
|---|---|---|---|---|---|
| Rate of allograft loss occurrence within 5 years posttransplant, % (SE) [patients] | 5.1 (3.7) | 0.0 (0.0) | 4.2 (4.1) [1] | 7.1 (6.9) [1] | n.a. |
| Death, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | n.a. |
| Rate of febrile UTI occurrence within 5 years posttransplant, % (SE) | 50.2 (7.29) | 50.0 (20.4) | 44.1 (8.6) | 66.7 (16.3) | .345b |
| Cumulative duration of hospitalization (days) due to febrile UTIs (n = 27), median (IQR) | 10 (4–16) | 6 (4–10) | 13 (7–16) | 7 (2.5–26.5) | .581c |
- Note. Statistical tests: aP value is calculated for pre- versus posttransplant. blog-rank test, cU-test.
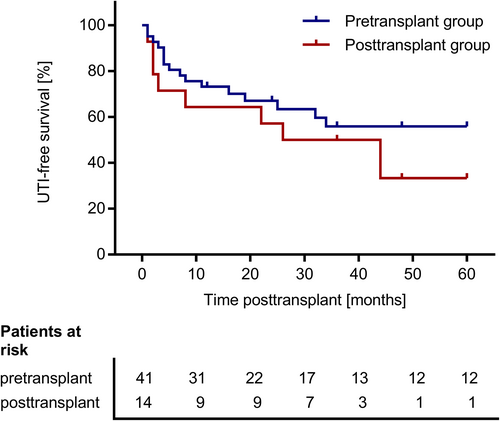
3.3 Subgroup analysis of PD patients with major bladder surgery pre- or posttransplant
In the entire study cohort of 62 patients, there were 23 PD patients with major bladder surgery (37%). The respective patient and transplant characteristics are shown in Table S2. We found no significant outcome differences in PD patients with pre- or posttransplant bladder surgery regarding all investigated analysis endpoints as described above and shown in Table 3. There was no allograft loss in this subgroup (Table 3).
| Endpoints | Bladder surgery pretransplant n = 14 | Bladder surgery posttransplant n = 7 | p value |
|---|---|---|---|
| Duration of hospitalization (days) posttransplant, median (IQR) | 23 (18–27) | 24 (18–37) | .653a |
| Rate of febrile UTI occurrence within 3 years posttransplant, % (SE) | 44.9 (13.9) | 57.1 (18.7) | .449b |
| Duration of hospitalization (days) due to febrile UTIs (n = 11), median (IQR) | 13 (10–31) | 4 (3–25) | .175c |
| Allograft loss within 5 years posttransplant, n (%) | 0 (0) | 0 (0) | n.a. |
| Death, n (%) | 0 (0) | 0 (0) | n.a. |
| Event-free survival without composite efficacy failure endpoint occurrence 5 years posttransplant, % (SE) | 81,2 (12) | 42.9 (22) | .17d |
| eGFR (ml/min/1.73 m2) at day 30 posttransplant mean ± SD | 89.1 ± 31.8 | 81.1 ± 33.8 | .612 |
| eGFR (ml/min/1.73 m2) at year 1 posttransplant mean ± SD | 76.7 ± 27.7 | 65.1 ± 22.6 | .318 |
| eGFR (ml/min/1.73 m2) at year 3 posttransplant mean ± SD | 71.4 ± 28.7 | 64.6 ± 29.3 | .661 |
| eGFR (ml/min/1.73 m2) at year 5 posttransplant mean ± SD | 63.1 ± 31.9 | 58.4 ± 44.4 | .856 |
| Occurrence rate of first VUR detection, % (SE) | 58.1 (15.0) | 14.3 (13.2) | .169e |
- Note: The group of patients with lower urinary tract repair during KTx comprises only two patients and is therefore not shown here. The composite efficacy failure endpoint comprises eGFR decline below 30 ml/min/1.73 m2 or below 50% of baseline eGFR (100%) as measured at day 30 posttransplant, graft loss, or death, whichever event came first; aDefined as eGFR at day 30 posttransplant; a,cU-test; b,d,eLog-rank test.
- Events in the composite efficacy failure analysis: Pretransplant bladder surgery: one event of eGFR decline below 30 ml/min/1.73 m2 and one event of eGFR decline below 50% of baseline eGFR (100%) as measured at day 30 posttransplant. Posttransplant bladder surgery: two events of eGFR decline below 30 ml/min/1.73 m2 and one event of eGFR decline below 50% of baseline eGFR (100%) as measured at day 30 posttransplant.
3.4 Subgroup analysis of pretransplant bladder surgery in PD or HD patients
Table S3 shows patient and transplant characteristics in the pretransplant group stratified according to dialysis mode. Sixteen of 19 (84.2%) patients on HD underwent pretransplant bladder surgery compared with 16 of 30 (53.3%) patients on PD (p = .025; Table 1). As expected, patients on PD were younger than patients on HD (p = .0015). The event-free survival (without the occurrence of the composite failure endpoint) was better in patients on PD than in patients on HD (p = .04, log-rank test). After 5 years posttransplant, event-free rates were 84% in PD patients compared with 43% in HD patients (Table 4). There were more events of eGFR decline in the HD group (three events of eGFR decline below 30 ml/min/1.73 m2, three events of eGFR decline below 50% of baseline eGFR, and one event of graft loss) than in the PD group (one event of eGFR decline below 30 ml/min/1.73 m2 and one event of eGFR decline below 50% of baseline eGFR). Mean eGFR until 5 years posttransplant did not differ significantly between PD and HD patients; however, eGFR at day 30 posttransplant tended to be higher in PD patients, presumably due to their younger age (Table 4).
| Endpoints | PD patients n = 16 | HD patients n = 16 | p value |
|---|---|---|---|
| Duration of hospitalization (days) posttransplant, median (IQR) | 22 (17.5–26) | 22 (18.5–30.5) | .678a |
| Rate of febrile UTI occurrence within 5 years posttransplant, % (SE) | 38.9 (12.6) | 47.8 (15.0) | .987b |
| Duration of hospitalization (days) due to febrile UTIs (n = 12), median (IQR) | 13 (10–31) | 11.5 (6–16) | .422c |
| Rate of allograft loss occurrence within 5 years posttransplant, % (SE) [patients] | 0.0 (0.0) [0] | 14.3 (13.2) [1] | n.a. |
| Death, n (%) | 0 (0) | 0 (0) | n.a. |
| Event-free survival without composite efficacy failure endpoint occurrence, % (SE) | 84.4 (10) | 43.8 (15) | .04d |
| eGFR (ml/min/1.73 m2) at day 30 posttransplant mean ± SD | 88.6 ± 31.4 | 67.5 ± 28.1 | .054 |
| eGFR (ml/min/1.73 m2) at year 1 posttransplant mean ± SD | 77.9 ± 25.9 | 62.9 ± 33.1 | .175 |
| eGFR (ml/min/1.73 m2) at year 3 posttransplant mean ± SD | 72.0 ± 26.1 | 56.7 ± 27.9 | .22 |
| eGFR (ml/min/1.73 m2) at year 5 posttransplant mean ± SD | 63.8 ± 28.6 | 60.5 ± 28.2 | .824 |
| Occurrence rate of first VUR detection, % (SE) [patients] | 66.1 (13.4) [8] | 100 (n.a.) [3] | .363e |
- Note: The group of patients with lower urinary tract repair during KTx comprises only two patients and is therefore not shown here. The composite efficacy failure endpoint comprises eGFR decline below 30 ml/min/1.73 m2 or below 50% of baseline eGFR (100%) as measured at day 30 posttransplant, graft loss, or death, whichever event came first. at-test, cU-test; b,d,elog-rank test.
3.5 Comparison between the main study cohort and the matched control cohort
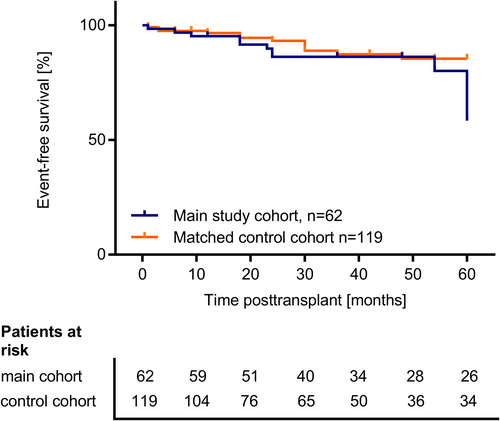
Table S4 shows the patient and transplant characteristics of the main study cohort (n = 62) in comparison to the matched control cohort (n = 119). Patients and controls were well comparable regarding most characteristics besides sex and primary kidney disease. The outcome as assessed by the occurrence of the composite failure endpoint in the main study cohort and the matched control cohort was comparable during the first 4 years posttransplant (Figure 6); only at year 5, there were more events of transplant dysfunction in the study cohort (six events of eGFR decline below 30 ml/min/1.73 m2; nine events of eGFR decline below 50% of baseline eGFR and 2 events of graft loss) than in the control cohort (one event of eGFR decline below 30 ml/min/1.73 m2; seven events of eGFR decline below 50% of baseline; two events of graft loss; two events of death; log-rank test p = .029). The mean eGFR course over 5 years posttransplant did not differ significantly between the study cohort (n = 62) and the matched control cohort (n = 119; Table S4).
4 DISCUSSION
This is the first multicenter analysis and also the largest study to date of the current practice pattern regarding lower urinary tract reconstruction in pediatric KTx recipients focusing on the timing of bladder surgery. Our data show that regarding all analysis endpoint outcome did not differ significantly in children with lower urinary tract reconstruction pre- versus posttransplant.
As specific clinical practice guidelines are lacking in this field, there are different opinion-based approaches regarding the optimal timing of bladder reconstruction.22-26 One major argument against pretransplant bladder augmentation emphasizes the bacterial load in augmented bladders, which might endanger the graft at the time of transplantation especially under initial intensive perioperative immunosuppressive therapy.27 However, postoperative infections and delayed healing processes might also complicate bladder reconstruction performed after KTx when the patient's immune system is suppressed.18 At the same time, the opportunity for, for example, pretransplant optimization of clean intermittent self-catheterization may also reduce posttransplant complications.
There are few single-center studies that compare outcomes of children with KTx and bladder dysfunction with regard to the timepoint of lower urinary tract reconstruction: Taghizadeh et al. observed in their single-center study of 16 patients with bladder augmentation no differences in the duration of initial hospitalization and UTI occurrence within 3 months posttransplant; however, they reported more graft losses (4 of 8 vs. 1 of 8) in patients with posttransplant bladder reconstruction, while patients with pretransplant bladder augmentation had more complications related to Mitrofanoff channels in (3 of 8 vs. 1 of 8).18 Basiri et al. observed in their single-center study on 44 pediatric patients with bladder augmentation no differences in UTI occurrence and a similar rate of graft failure between children with augmentation pre- or posttransplant.27 Our results also show no differences regarding the duration of initial hospitalization after transplant surgery, the rate of febrile UTI, and the cumulative hospitalization duration due to febrile UTI. We also observed, in accordance with previous works, that graft survival within 5 years posttransplant did not differ between children with pre- versus posttransplant lower urinary tract reconstruction. It is reassuring that event-free survival and graft function of pediatric KTx recipients with bladder dysfunction who required surgical reconstruction and those without bladder dysfunction were comparable in the first 4 years posttransplant. Thereafter, the event-free survival in patients with bladder dysfunction was slightly lower than in controls, indicating that a hostile bladder environment may affect long-term outcomes.
Our study investigates for the first time specifically the subgroup of children on pretransplant PD who had a surgical bladder reconstruction before or after KTx. These children are at higher risk because an open bladder surgery via the abdominal cavity imposes the hazard of an imperative permanent switch of dialysis mode to HD due to PD failure.28 However, in our analysis no significant differences between pre- or posttransplant reconstruction of the lower urinary tract were found in all investigated study endpoints. Furthermore, most investigated study endpoints were also comparable between PD and hemodialysis patients with pretransplant bladder surgery.
Timely identification and interdisciplinary management of children with a hostile bladder environment are necessary in order to avert complications that may culminate in an untimely loss of kidney transplant function. There are several arguments in favor of pretransplant reconstruction of the lower urinary tract: (i) it may offer more favorable conditions for the graft, because it allows for the timely increment of bladder capacity and realization of a low-pressure reservoir, for example after augmentation cystoplasty; (ii) it permits physiologic reduction in mucous production and bacteriuria with time; (iii) it accommodates a learning curve of clean intermittent catheterization for optimal bladder emptying well before KTx; (iv) our data suggest that transperitoneal surgery can be safely performed without an increased risk of the permanent switch of dialysis mode from PD to HD. On the other hand, favorable results have been published on pediatric kidney transplant recipients with small capacity defunctionalized urinary bladders receiving adult-sized kidney without prior bladder augmentation, thus avoiding an unnecessary surgery.29 More research is needed to precisely define the criteria for which dysfunctional bladders require augmentation and which bladders are able to accommodate the kidney graft's urine production by rapidly increasing its capacity posttransplant.
Our study has several limitations. Firstly, only few urodynamic data in patients prior to surgical bladder reconstruction were available. Nevertheless, there were apparently few differences in the severity of the underlying bladder dysfunction between pediatric patients with posterior urethral valves and kidney hypo−/dysplasia and bladder reconstruction pre- or posttransplant. Secondly, this is a registry analysis that depends on the completeness of data entry into the registry, which cannot be verified in individual cases. Third, further information on specific subtypes of bladder surgery besides augmentation -and substitution and further information on center-specific pretransplant clinical assessment strategies regarding the characterization of bladder dysfunction could not be reported as they were not provided by the study-specific CERTAIN registry dataset.
In conclusion, this multicenter study on the current practice pattern of operative reconstruction of the lower urinary tract in pediatric KTx shows that more bladder reconstructions are performed pretransplant. However, was the bladder surgery performed posttransplant for specific reasons, the outcome of these children was comparable to those with pretransplant surgical bladder reconstruction. This statement holds also true for the subgroup of children on pretransplant PD. The urologic surgical care of these complex patients can therefore be tailored to suit individual, patient-specific factors.
4.1 AUTHOR'S CONTRIBUTION
Each author fulfills all criteria of the ICMJE definition of authorship. Each author is able to identify, which co-authors are responsible for specific other parts of the work.
5 ACKNOWLEDGMENTS
The authors gratefully acknowledge the funding of the CERTAIN Registry by a grant from the Dietmar Hopp Stiftung, the European Society for Pediatric Nephrology (ESPN), the German Society for Pediatric Nephrology (GPN), and by grants from the pharmaceutical companies Astellas and Novartis. The authors' thanks are due to our study nurse Annette Mechler for her continuous excellent contribution to the CERTAIN Registry.
6 CONFLICT OF INTEREST
The authors declare that they have nothing to disclose and that there is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the submitted files and in the supplementary material of this article.