Hepatic artery flow, inspired oxygen, and hemoglobin determine liver tissue saturation measured with visible diffuse reflectance spectroscopy (vis-DRS) in an in vivo swine model
Abstract
Background
Prompt diagnosis of vascular compromise following pediatric liver transplantation and restoration of oxygen delivery to the liver improves organ survival. vis-DRS allows for real-time measurement of liver tissue saturation.
Methods
The current study used vis-DRS to determine changes in liver saturation during clinically relevant conditions of reduced oxygen delivery. In an in vivo swine model (n = 15), we determined liver tissue saturation (StO2) during stepwise reduction in hepatic artery flow, different inspiratory oxygen fraction (FiO2), and increasing hemodilution. A custom vis-DRS probe was placed directly on the organ.
Results
Liver tissue saturation decreased significantly with a decrease in hepatic artery flow. A reduction in hepatic artery flow to 25% of baseline reduced the StO2 by 15.3 ± 1.4% at FiO2 0.3 (mean ± SE, p < .0013), and by 8.3 ± 1.9% at FiO2 1.0 (p = .0013). After hemodilution to 7–8 g/dl, StO2 was reduced by 31.8% ± 2.7%, p < .001 (FiO2 0.3) and 26.6 ± 2.7%, p < .001 (FiO2: 1.0) respectively. Portal venous saturation during low hepatic artery flow was consistently higher at FiO2 1.0. The gradient between portal venous saturation and liver tissue saturation was consistently greater at lower hemoglobin levels (7.0 ± 1.6% per g/dl hemoglobin, p < .001).
Conclusions
Vis-DRS showed prompt changes in liver tissue saturation with decreases in hepatic artery blood flow. At hepatic artery flows below 50% of baseline, liver saturation depended on FiO2 and hemoglobin concentration suggesting that during hepatic artery occlusion, packed red blood cell transfusion and increased FiO2 may be useful measures to reduce hypoxic damage until surgical revascularization.
Abbreviations
-
- AAALAC
-
- Association for Assessment and Accreditation of Laboratory Animal Care
-
- ETCO2
-
- end-tidal CO2
-
- FiO2
-
- fraction of inspired oxygen
-
- Hb
-
- hemoglobin
-
- IACUC
-
- Institutional Animal Care and Use Committees
-
- IM
-
- intramuscular
-
- IV
-
- intravenous
-
- LED
-
- light-emitting diode
-
- LPR
-
- lactate/pyruvate ratio
-
- LR
-
- Ringer's lactate solution
-
- MCW
-
- Medical College of Wisconsin
-
- NIRS
-
- near-infrared spectroscopy
-
- SE
-
- standard error
-
- StO2
-
- tissue hemoglobin oxygen saturation
-
- Vis-DRS
-
- visible diffuse reflectance spectroscopy
1 INTRODUCTION
Early hepatic artery thrombosis is a serious complication after orthotopic liver transplantation that may lead to graft failure and need urgent management or re-transplantation. The patency of the anastomosis and the flow in the hepatic artery are examined directly with frequent ultrasonographic surveillance or indirectly with blood sampling and trending of biochemical markers that reveal hepatic tissue damage. Although the ultrasound is a very reliable bedside test, it provides a snapshot and may reveal the problem hours after it has occurred since it is not a continuous monitor. Similarly, abnormal blood tests indicate cellular damage that has already occurred and that is not necessarily due to acute ischemia but can be, for example, acute rejection.
Direct measurement of StO2 allows conclusions regarding the oxygen delivery to and extraction from the tissue. Oxygen delivery depends not only on vascular patency, cardiac output, and organ perfusion pressure, but also on hemoglobin concentration and oxygen saturation. Currently, the most widely used method in clinical practice for StO2 is NIRS,1, 2 but clinical studies showed wide variability at baseline values and inconsistency in identifying changes in oxygen delivery.3 We have recently validated vis-DRS as a method for measuring StO2 in the highly pigmented hepatic tissue.4 In this study, we used vis-DRS in an acute, in vivo swine model to determine changes in hepatic StO2 during typical clinical scenarios that may be encountered intraoperatively or in the immediate posttransplant phase. While the vis-DRS surface probe used in this study only allowed intraoperative measurements, we are developing a needle probe that can be inserted into the liver tissue with the goal to continuously measure StO2 in the postoperative period, even with a closed abdomen. For the current study, we hypothesized that an increase in FiO2 and hemoglobin concentrations can improve liver tissue saturation during hepatic artery occlusion.
2 MATERIALS AND METHODS
2.1 vis-DRS instrument
The setup consisted of a self-calibrating fiber optic probe and a portable vis-DRS system equipped with a laptop computer for data acquisition as described before.5 In brief, our spectroscopy system included a white-LED as the light source and two visible spectrometers (Avantes BV, The Netherlands) for spectrum detection.4 The fiber optic probe was composed of nine 200/220-µm multimode optical fibers with six for DRS illumination, the seventh one for DRS detection, and the remaining two for self-calibration illumination and detection, respectively. At the distal end, the six DRS illumination fibers are arranged as a ring around the DRS detection fiber. The real-time diffuse reflectance and self-calibration spectra (430–630 nm) were collected by a custom LabVIEW program. For calibration, the tissue spectrum is divided by the self-calibration spectrum collected concurrently to account for real-time instrument drifts and fiber bending loss. A 3D printed dark stopper was added to the distal end of the probe to maintain a stable tissue contact while blocking background noise from the room light.
The device was calibrated with tissue-mimicking liquid phantoms using human Hemoglobin powder (H0267, Sigma-Aldrich Co. LLC) as the absorber and 1-µm polystyrene microspheres (07310-15, Polysciences Inc) as the scatterer. The phantoms were obtained through 16 successive titrations of the Hb from 46.36-112.01 µM and fixing the number of scatterers. The absorption coefficients (µa) of the phantoms were independently determined by measuring the µa of the hemoglobin stock solution using a spectrophotometer (Lambda35, PerkinElmer) and the Beer-Lambert law. The reduced scattering coefficients (µs') were calculated using Mie theory with known particle refractive index, size, and density. Diffuse reflectance and self-calibration spectra in the wavelength range of 435–630 nm for each of the 16 phantoms were collected using the fiberoptic probe and spectrometer. Self-calibration was performed by dividing the phantom spectrum by the calibration spectrum (point-by-point). A Monte Carlo inverse model was used to extract the phantom µa and µs' as well as hemoglobin concentration from the calibrated phantom spectrum, following the procedures described by Yu et al.6
2.2 Surgical preparation
The use of animals in this study was approved by the IACUC at the MCW and Zablocki Veterans Affairs Medical Center. The animal use programs at both institutions are fully accredited by the AAALAC International. Experiments were carried out on adult (42–52 kg) female pigs. The animals were fasted for 12 h and premedicated/sedated with an IM injection of Tiletamine 3–5 mg/kg, Zolazepam 3–5 mg/kg, and xylazine 2.2 mg/kg. When the animal was sedated enough it was taken into the intubation suite. Atropine IM (50 mcg/kg) was given, and an auricular IV catheter was placed prior to intubation. Ocular reflexes were tested for depth of anesthesia, and additional propofol (1–2 mg/kg) was administered to achieve appropriate intubating conditions. A single dose of enrofloxacin (5 mg/kg) IM was administered as antibiotic prophylaxis on account of the long, invasive surgical preparation. Animals were transferred intubated and ventilated under continuous pulse oximetry to the surgical suite where they were continuously ventilated using an anesthesia machine (Ohmeda CD, GE, Datex Ohmeda). General anesthesia was maintained with 1.5–3% isoflurane. Throughout the experiments, care was taken to increase anesthetic depth for any signs of “light anesthesia”, for example, an increase in blood pressure or heart rate. Animals were maintained at 38.5 ± 0.5°C with a warming blanket. Inspiratory oxygen fraction, expiratory carbon dioxide concentration, and expiratory isoflurane concentration were continuously displayed with an infrared analyzer (POET II, Criticare Systems). An esophageal probe was placed for non-invasive cardiac output measurements in the initial experiments in which cardiac output values showed good correlation with blood pressure and pulse pressure amplitude (ECOM, ECOM Medical Inc).
Animals were placed in the supine position. The right groin was infiltrated with 1% lidocaine, and a femoral venous and arterial catheter were placed via cut down for monitoring, drug administration, and blood sampling. All incision sites were infiltrated with 1% lidocaine, and a midline laparotomy was performed with extension to the bilateral subcostal areas and sternum. The stomach was retracted, and two stay sutures were placed for better exposure. The biliary duct was divided and ligated, and a gallbladder drain was placed and kept to gravity to prevent biliary stasis. The liver hilum was dissected to identify the portal vein and hepatic artery. The portal vein was dissected free, and vessel loops were placed around it. The hepatic artery was dissected proximally to the celiac trunk. The gastroduodenal arteries were carefully identified and ligated. The liver was partially mobilized to gain access to the hepatic veins. Depending on the protocol, a 22G angiocath with an extension tubing was inserted into the portal vein or one of the hepatic veins for repeated blood sampling.
A 5-mm vascular occluder was placed around the common hepatic artery. A flowmeter (Transonic flow-probe, precision S-series 6 mm) was placed on either the common hepatic artery or the left hepatic artery depending on the anatomy/length of the common hepatic artery. Heparin 100Units/kg IV was administered prior to any vascular occlusion. The administration was repeated every 2 h. Supported by a holder, the vis-DRS probe rested against the liver surface, which allowed free motion during the respiratory cycle without changing the pressure against the tissue. Hepatic artery flow, ETCO2, systemic blood pressure, and heart rate were continuously recorded using a digital acquisition system and stored on a computerized chart recorder (Powerlab/16SP; ADInstruments). Expiratory isoflurane concentration, systemic oxygenation (pulse oximeter), and temperature were manually recorded in the same record in 20-min intervals.
2.3 Experimental protocols
2.3.1 Incremental hepatic artery occlusion
We reduced hepatic artery flow with a vascular occluder in three increments of 25% of baseline flow to a minimum of 25% flow. At each flow rate, flow was maintained steady for ~2 min before sampling of 2–3 sets of vis-DRS values at the steady state (20 measurements per set, ca. 6 s per measurement, i.e., 2–3 min sampling time per set). As shown below, there was little delay between flow reduction and drop in tissue saturation (2.3.2). Initial testing showed no change in saturations over the 2–3 data sets; the results thus show the pooled values for all data sets per flow rate. In our previous study, the coefficient of variation for similar data sets was 0.1–1%.4 Flow was then increased in 25% increments to baseline. Protocols were performed at FiO2 values 0.3 and 1.0. Blood gas samples were obtained at baseline and at 25% hepatic artery flow.
2.3.2 Continuous vis-DRS measurements
To determine the time lag between the change in hepatic artery flow and the change in tissue saturation, we decreased hepatic artery flow from baseline to a minimum of 5–10% of baseline over 4–5 min and immediately increased flow back to baseline over 5 min while measuring tissue saturation continuously. The duration of the protocol was limited to ~10 min because datasets are limited to maximally 200 vis-DRS values with each value requiring ~6 s of sampling time.
2.3.3 Hemodilution without vascular occlusion
To determine the effect of hemoglobin concentration on liver tissue saturation during unobstructed hepatic artery flow, we placed a 22G angiocath in the portal vein or a hepatic vein with an extension tubing to facilitate blood sampling. 250–350 ml aliquots of blood were gradually removed through the femoral venous line and stored in a citrated bag and replaced with 500–700 ml lactated ringer's solution. Care was taken to maintain a hemodynamic steady state. We targeted decreases in hemoglobin of 2–3 g/dl per step. At each hemoglobin level, we obtained 2–3 sets of StO2 values at FiO2 0.21, 0.3, and 1.0. Samples were obtained for blood gas analysis during each vis-DRS measurement.
2.3.4 Hemodilution with vascular occlusion
To determine whether the hemoglobin concentration affected liver tissue saturation when hepatic artery flow was reduced, we reduced hemoglobin concentration with normovolemic hemodilution as described above to hemoglobin of 7–8 g/dl. We measured tissue saturations during incremental hepatic artery occlusion (similar to the procedures described in 2.3.1.) at the high and low hemoglobin concentrations.
In two experiments, we completely occluded the hepatic artery after hemodilution and then re-transfused the stored blood, which resulted in an increase in hemoglobin value by 1–1.5 g/dl. This protocol was performed once per animal in ~5 min. Tissue saturation values were recorded continuously at baseline, during hepatic artery occlusion, and during re-transfusion.
2.4 Euthanasia
At the conclusion of the experiment, animals were euthanized with 1 meq/kg potassium chloride IV under general anesthesia. Liver tissue saturation was recorded continuously for 10 min from the beginning of euthanasia to complete cardiovascular collapse as confirmed by loss of blood pressure and end-tidal CO2.
2.5 Statistical analysis
The vis-DRS values for tissue scattering and absorption were derived from the measured diffuse reflectance spectra using the Monte Carlo inverse model. Tissue hemoglobin concentrations and StO2 were calculated from the extracted tissue absorption spectra using the Beer-Lambert law. Post-hoc data reduction, data plotting, and statistical analysis of the pooled data were performed using SigmaPlot 11 (Systat Software) and R 3.5.0 (R foundation for statistical Computing). Values for stepwise occlusion were averaged for each data set. For continuous measurements, we measured the time difference between reaching minimal flow (95% decrease) and minimal liver tissue saturation. The individual statistical tests for each sub-study are described in the Results section. Statistical significance was assumed for p < .05. Corrections for multiple comparisons were made within each analysis. Values are expressed as mean ± SE or median/25-75% range as appropriate.
3 RESULTS
3.1 Incremental hepatic artery occlusion
A total of seven animals were used for this protocol. A mixed-effects model with random pig and run-within-pig effects and two-way fixed effects of time-point, FiO2, and hemodilution, and their interaction was fitted to the data. The conclusions are presented in two ways: pair-wise comparisons of time-points within each FiO2 level and among FiO2 levels within each time-point, as well as the interaction comparisons of the drop from baseline between the FiO2 levels.
We performed 22 sets of protocol 2.3.1. at FiO2 0.3. As shown in Figure 1a,b, liver tissue saturation was decreased from 90.6 ± 1.5% to 84.7 ± 2.3% (−5.8 ± 1.4%, p = .0022) when the hepatic artery flow was reduced from 100% to 50%. An additional reduction in hepatic artery flow from 50% to 25% resulted in a further decrease by 9.4 ± 1.4% (p < .001) to 75.3 ± 3.1%. The coefficient of variation for each data point (20 measurements per data point) was consistently between 0.1 and 1%. Tissue saturation values during reperfusion closely matched the values during the initial flow reduction.
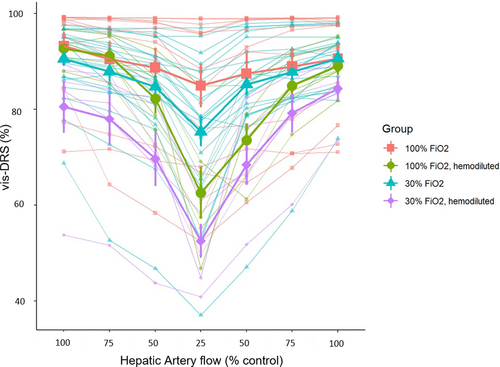
Twelve experimental protocols were performed in the same animals at FiO2 1.0. Liver tissue saturation dropped from 93.3 ± 2.8% to 88.7 ± 4.1% when flow was reduced from 100% to 50% (p < .001) and to 84.9±4.5% when flow was further reduced to 25%. The reduction of the hepatic flow from baseline to 25% resulted in a reduction in liver tissue saturation by 8.3 ± 1.9%, p = .0013.
Liver tissue saturation was consistently lower at FiO2 0.3 compared to FiO2 1.0., however the difference was not statistically significant at 100% flow (Δ (vis-DRSFiO2 1.0–vis-DRSFiO2 0.3) 4.7 ± 2.7%, p = .9859), 75% flow (Δ (vis-DRSFiO2 1.0–vis-DRSFiO2 0.3): 4.6 ± 2.7%, p = .9897), or 50% flow (Δ (vis-DRSFiO2 1.0–vis-DRSFiO2 0.3): 6.1 ± 2.7%, p = .8081). At 25% hepatic artery flow, vis-DRSFiO2 1.0 was significantly greater than vis-DRSFiO2 0.3 by 11.6 ± 2.7% (p = .0022). The hemoglobin was repeatedly measured and maintained in the range of 10–12 g/dl throughout the experiments.
Arterial pO2 measured via blood gas analysis exceeded 100 mmHg for both FiO2 0.3 and 1.0, that is, the blood delivered to the liver through the hepatic artery was fully saturated. The observed difference in the vis-DRS values between FiO2 1.0 and FiO2 0.3 could not be explained by a difference in the oxygen delivery through the hepatic artery but pointed to a difference in oxygen delivery from the portal venous system. We hypothesized that the observed difference in liver tissue saturation was due to higher oxygen content in the portal vein, resulting from increased oxygen delivery to the splanchnic circulation. In order to examine how much the oxygen-carrying capacity of the blood affected the oxygen delivery to the liver during hepatic artery occlusion, we repeated the hepatic artery occlusion protocol (2.3.1.) before and after isovolumic hemodilution to hemoglobin of 7–8.5 g/dl. Two animals were used for a total of 13 additional sets of the experimental protocol (FiO2 0.3, n = 7 and FiO2 1.0, n = 6).
Following hemodilution, at FiO2 0.3 (blue lines in Figure 1), a reduction in hepatic artery flow from baseline to 50% decreased the liver vis-DRS tissue saturation from 80.5 ± 5.5% to 69.7 ± 5.7%, that is, by10.9 ± 2.7% (p = .0049). An additional reduction in hepatic artery flow from 50% to 25% resulted in a further decrease by 17.1 ± 2.7% to 52.5 ± 3.6% (p < .001).
After hemodilution, at FiO2 1.0 a reduction in hepatic artery flow from baseline to 50% decreased the liver tissue saturation from 92.7 ± 1.2% to 82.2 ± 3.1% (10.5 ± 2.7%, p = .0094). An additional reduction in hepatic artery flow from 50% to 25% resulted in a further decrease by 19.7 ± 2.7% to 62.6 ± 5.4% (p < .001). The coefficient of variation for these measurements remained in the range of 0.1–1% and tissue saturation values during reperfusion closely matched the values during the initial flow reduction. (Figure 1).
After hemodilution, the difference in tissue saturation between FiO2 0.3 and FiO2 1.0. was not statistically significant at all flow levels (at 100% flow: Δ (vis-DRSFiO2 1.0–vis-DRSFiO2 0.3) = 12.2 ± 3.2%, p = .1785; at 75% flow Δ (vis-DRSFiO2 1.0–vis-DRSFiO2 0.3) = 13.2 ± 4%, p = .0832; at 50% flow Δ (vis-DRSFiO2 1.0–vis-DRSFiO2 0.3) = 12.6 ± 4%, p = .1356; and at 25% flow Δ (vis-DRSFiO2 1.0–vis-DRSFiO2 0.3) = 10.0 ± 4.0%, p = .5648).
At FiO2 0.3 the decrease in tissue saturation with hemodilution was not statistically significant at 100% flow (Δ (vis-DRSFiO2 0.3–vis-DRSFiO2 0.3 hemodiluted) = 10.5 ± 4.4%, p = .6612) and at 75% flow (Δ (vis-DRSFiO2 0.3–vis-DRS FiO2 0.3 hemodiluted) = 10.4 ± 4.6%, p = .6841). At 50% flow hemodilution significantly decreased liver tissue saturation (Δ (vis-DRSFiO2 0.3–vis-DRS FiO2 0.3 hemodiluted) = 15.5 ± 4.4%, p = .0361) as well as at 25% hepatic artery flow (Δ (vis-DRSFiO2 0.3–vis-DRSFiO2 0.3 hemodiluted) = 23.3 ± 4.6%, p < .001).
At FiO2 1.0 the decrease in tissue saturation with hemodilution was not statistically significant at 100% flow (Δ (vis-DRSFiO2 1.0–vis-DRSFiO2 1.0 hemodiluted) = 3.1 ± 4.6%, p = 1.00), or at 75% flow (Δ (vis-DRSFiO2 1.0–vis-DRSFiO2 1.0 hemodiluted) = 1.8 ± 4.6%, p = 1.00), or at 50% flow (Δ (vis-DRSFiO2 1.0–vis-DRSFiO2 1.0 hemodiluted) = 9 ± 4.6%, p = .9357). Tissue saturation was significantly decreased at 25% hepatic artery flow (Δ (vis-DRSFiO2 1.0– vis-DRS FiO2 1.0 hemodiluted) = 24.9 ± 4.6%, p < .001).
In short, these results showed that liver tissue saturation decreased significantly with a decrease in hepatic artery flow. Tissue saturation was different between FiO2 0.3 and 1.0 when hepatic artery flow was reduced to 25%. Hemodilution caused an additional reduction in liver tissue saturation when flow was reduced to 50% and 25% for FiO2 0.3, and when flow was reduced to 25% for FiO2 1.0.
3.2 Hemodilution without vascular occlusion
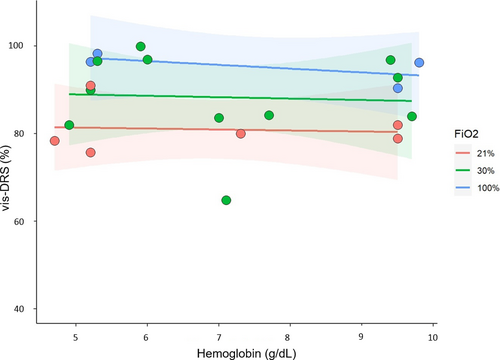
Three animals underwent hemodilution without vascular occlusion (Protocol 2.3.3). A linear mixed-effects regression model with fixed hemoglobin and FiO2 effects, and a random pig-specific intercept was fitted. Blood pressure and organ perfusion were maintained constant throughout the experiment. The range of hemoglobin values between 4.5 and 11 g/dl represents the range that may be encountered in liver transplant patients where anemia is routinely sought for improved rheology. Figure 2 illustrates that liver tissue saturation remained constant with hemodilution for each FiO2 level, but was higher at FiO2 1.0 compared to FiO2 0.21 (11.2 ± 3.6%, p = .0072, n = 21).
3.3 Hemodilution during 25% hepatic artery flow
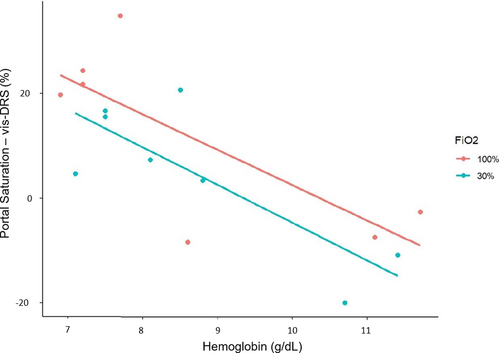
In two animals, portal venous saturations were measured during the “Hemodilution with vascular occlusion” protocol (2.3.4). When hepatic artery flow was reduced to 25%, portal venous saturation was higher at FiO2 1.0 compared to FiO2 0.3 (Table 1). Liver tissue saturations (vis-DRS) were lower at lower hemoglobin concentrations (Table 1). A linear regression analysis correlating the difference between portal venous saturation and liver tissue saturation with hemoglobin, adjusted for FiO2 was performed without adjusting for animal effect. Figure 3 illustrates that the difference increased with decreasing hemoglobin concentrations (7 ± 1.6% per g/dl hemoglobin, p < .001). This may suggest an increase in oxygen extraction in the liver when oxygen delivery is reduced due to low hemoglobin and hepatic artery flow. The effect of FiO2 was not significant (p = .24).
| Hb (g/dl) | SPortalVO2% | vis-DRS % | Δ (SPortalVO2% - vis-DRS) % |
|---|---|---|---|
| FiO2: 0.3 | |||
| 11.5 | 65 | 80.1 | −15.1 |
| 11.4 | 67.7 | 77.2 | −9.5 |
| 8.5 | 61.5 | 39.0 | 22.5 |
| 8.8 | 61.8 | 51.5 | 10.3 |
| 7 | 76.8 | 56.5 | 20.3 |
| 7.5 | 61.5 | 42.9 | 18.6 |
| 7.5 | 65.1 | 47.6 | 17.5 |
| 7.1 | 65.9 | 59.2 | 6.7 |
| 8.1 | 67.9 | 58.7 | 9.2 |
| FiO2: 1.0 | |||
| 11.7 | 73.4 | 74.6 | −1.2 |
| 11.1 | 76.8 | 83.2 | −6.4 |
| 8.6 | 74.5 | 83.8 | −9.3 |
| 7.2 | 84 | 60.4 | 23.7 |
| 7.2 | 79.6 | 53.3 | 26.4 |
| 6.9 | 77.2 | 55.5 | 21.7 |
| 7.7 | 81.6 | 44.8 | 36.8 |
Note
- Concomitant hemoglobin levels and FiO2 are indicated. The difference between SPortalVO2 and vis-DRS describes the oxygen extraction in the liver tissue.
3.4 Time delay between changes in oxygen delivery and vis-DRS values
To determine the delay between changes in organ perfusion and liver tissue saturation we performed continuous measurements where hepatic artery flow was slowly decreased to 5–10% of baseline (Protocol 2.3.2, n = 10). The average time delay between the nadir in hepatic artery flow and vis-DRS values was 17 ± 5 s (Figure 4a). Vis-DRS values recovered promptly with increasing hepatic artery flow (Figure 4a). Values were similar for comparable flow rates before and after the nadir, which agreed with our observations in 3.1. “Incremental hepatic artery occlusion” (Figure 1).
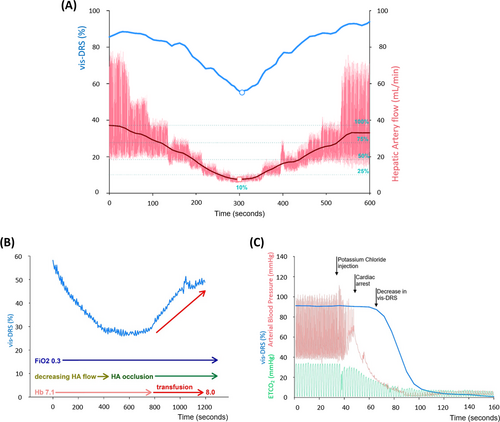
In two animals whose hemoglobin concentration had been significantly reduced by hemodilution, vis-DRS values decreased with complete hepatic artery occlusion but increased promptly with autologous transfusion of stored blood (Figure 4b, Protocol 2.3.4). Finally, after intravenous administration of potassium chloride for euthanasia, vis-DRS values dropped rapidly, starting approximately 30 s after loss of pulsatility (cardiac arrest) (Figure 4c, Protocol 2.4., [n = 2]). Vis-DRS values decreased to near-zero in <2 min, indicating the complete lack of oxygenated hemoglobin soon after cessation of circulation.
4 DISCUSSION
Our study used vis-DRS to identify changes in liver tissue oxygen saturation with changes in hepatic artery flow, FiO2, and hemoglobin. We found that liver tissue saturation changed promptly with oxygen delivery. At hepatic artery flows ≤25% of baseline, liver tissue saturation depended on FiO2 and hemoglobin concentration. Our results highlight the importance of portal venous oxygen content for oxygen delivery to the liver when hepatic artery flow is compromised. This suggests that should hepatic artery occlusion occur in the early post-transplant period, an increase in hemoglobin and FiO2 may be beneficial to reduce ischemic damage to the liver until hepatic artery flow can be surgically restored.
Postoperative patient management after liver transplantation routinely includes permissive anemia to improve the rheology of blood, combined with anticoagulation and antiplatelet therapy.7-9 FiO2 is usually maintained at the minimum level to achieve normoxia in order to protect the lungs from oxygen toxicity.10-13
In our model, during unimpeded hepatic artery flow liver tissue saturation did not change over a wide range of hemoglobin (4.5–11 g/dl) (Figure 2), suggesting that permissive anemia alone is well tolerated. In contrast, Figure 1 shows that when hepatic artery flow was reduced to 25%, lower hemoglobin values were associated with lower liver tissue saturations. At this flow, an increase in FiO2 to 1.0 raised liver saturations at high and low hemoglobin concentrations.
The results highlight the importance of portal venous saturation for hepatic oxygen delivery. While the magnitude of oxygen delivery through the hepatic artery depends mostly on flow and hemoglobin concentration, and hemoglobin is usually fully oxygenated, oxygen delivery through the portal vein also depends on oxygen delivery to and oxygen extraction in the splanchnic circulation. An increase in inspired oxygen fraction was shown to increase portal venous blood flow and oxygen saturation in in vivo rats.14 On the other hand, oxygen extraction increases with decreasing oxygen delivery, for example, a reduction in hemoglobin. We must consider that in our experiments, oxygen extraction in the splanchnic circulation was relatively low because animals were fasted and under general anesthesia. The risk for hypoxic injury to the liver with hepatic artery occlusion is likely much greater in patients who are awake and receive enteral feeds. Future goals are to investigate how changes in global cardiac output, organ perfusion pressure, and individual drugs affect oxygen delivery to the liver and splanchnic circulation.
4.1 Methodologic considerations
We have recently validated the ability of vis-DRS technology to measure liver tissue saturation and reached a goodness-of-fit (R2) of .76, compared to hepatic venous blood gas analysis.4 The current data highlight the short delay between changes in oxygen delivery and tissue saturation (Figure 4). The time delay is at least in part due to measurement time of the vis-DRS instrument, which requires 3–6 s per value, depending on the number of iterations set for the Monte Carlo model. After cessation of circulation (euthanasia), vis-DRS values decreased to near zero, accurately reflecting the lack of oxygenated hemoglobin (Figure 4b). One shortcoming of the method may be the small penetration depth of 1–2 mm below the probe,15 which in the current study limited investigation to the liver surface and may not have fully represented oxygen delivery to the entire organ. On the other hand, liver tissue directly below the surface is homogenous, and results are not likely to be confounded by larger blood vessels. We are developing a vis-DRS needle probe that can be inserted deeper into the liver tissue and will allow more permanent measurements as well as a comparison between StO2 at different depth.
This feature is different from NIRS, which has been used for more than a decade as a bedside and intraoperative monitor of tissue oxygenation, and which has a penetration depth of 1–4 cm, depending on probe size and tissue.16 In an in vivo piglet model, Skowno et al found a decrease in surface liver NIRS saturations (neonatal Foresight NIRS probe, source-detector separation 25 mm) of ~15% during 50% reduction in hepatic artery flow, and a decrease during complete occlusion of ~30% for animals 15–20 kg and ~20% for animals 5–7 kg.3 Total hepatic ischemia decreased NIRS values by 80–90%.3 No blood gas values were obtained for validation, but the decrease in tissue saturation was roughly comparable to our observations (Figure 1). Since the authors reported only changes in saturation, not absolute values, a closer comparison of the techniques was not possible. Interestingly, despite the greater penetration depth transcutaneous NIRS values (Foresight NIRS probe, source-detector separation 10 & 40 mm and 15 & 55 mm) did not correlate with the values obtained on the liver surface.3 This discrepancy was also observed in other studies: In a porcine endotoxemic shock model, transcutaneous NIRS (NIRO-300, Hamamatsu Photonics) correlated well with systemic perfusion parameters, but there was no clear correlation with the liver surface NIRS.17 In patients undergoing cardiac catheterization, transcutaneous hepatic NIRS values (INVOS 5100C, Somanetics) correlated well with the cardiac output (R = .808; p = .0047) but not with the directly measured hepatic venous saturation (R = −.035; p = .9238). 18 Even in young children with a short distance between skin and palpable liver surface, the correlation between transcutaneous NIRS and directly measured hepatic venous saturation was poor.19 Considering that transcutaneous NIRS values in these studies reflected the oxygen saturation of the tissues overlying the liver rather than of the liver itself, the penetration depth of near-infrared light into the highly pigmented liver tissue remains unclear.
The direct measurement of tissue oxygenation through spectrometry may be more specific for oxygen delivery than determination of the LPR as a marker for anaerobic metabolism with intrahepatic microdialysis. While Haugaa et al. determined that a lactate level >3 mM and LPR >20 were highly predictive for post-transplant hepatic artery thrombosis,20 these cut-off values were not sufficient to diagnose or rule out hepatic artery thrombosis in an observational follow-up study, possibly due to dependence of these values on the blood glucose level.21 The predictive value of vis-DRS measurements in the clinical arena where other determinants of oxygen delivery are more variable than in our experimental setting remains to be determined.
5 CONCLUSION
In conclusion, vis-DRS showed prompt changes in liver tissue saturation with decreases in hepatic artery blood flow. At hepatic artery flows below 50% of baseline, liver saturation depended on FiO2 and hemoglobin concentration, highlighting the importance of portal venous saturation for oxygen delivery to the liver. We propose that during hepatic artery occlusion in the post-transplant period, packed red blood cell transfusion and increasing FiO2 may be useful, temporizing measures to reduce hypoxic liver damage until hepatic artery flow can be reestablished.
ACKNOWLEDGEMENTS
This research was supported by new-investigator funding, Department of Anesthesiology, Medical College of Wisconsin, to Dr. Voulgarelis, as well as Marquette University Startup grants to Dr. Yu.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: Voulgarelis, Stucke. Experimental preparation: Voulgarelis, Stucke, Chatzizacharias, Allen. Data acquisition: Fathi, Yu, Voulgarelis, Stucke, Palkovic. Data analysis: Voulgarelis, Stucke, Palkovic, Fathi, Yu. Manuscript preparation: Voulgarelis, Stucke, Palkovic, Yu. Critical revision: Voulgarelis, Stucke, Yu, Chatzizacharias, Palkovic, Fathi, Allen.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.