Paediatric deceased donor kidney transplant in Australia: A 30-year review—What have paediatric bonuses achieved and where to from here?
Funding information
This research was supporting in part by the Australian Organ and Tissue Authority through the funding of a National Review of Paediatric Transplant Recipients undertaken by the Transplantation Society of Australia and New Zealand (Work order 05.2018).
Abstract
Background
In this 30-year national review, we describe trends in DD transplantation for paediatric recipients, assess the impact of paediatric allocation bonuses and identify outstanding areas of need for this population.
Methods
A retrospective review of all DD kidney only transplants to paediatric recipients (<18 years old) in Australia between 1989 and 2018 was conducted using deidentified extracts from the ANZDATA.
Results
Of the 1011 kidney only transplants performed in paediatric recipients during the study period, 426 (42%) were from deceased donors. Paediatric candidates on the DD waiting list had consistently higher rates of transplantation and shorter time from dialysis initiation to transplantation compared with adult candidates (median 372 vs 832 days in 2018, for example). Donor characteristics remained more favourable for paediatric recipients, despite a decline in the overall quality of the donor pool. The mean number of HLA antigen mismatches for paediatric recipients of DD transplants increased each decade (2.86 [1989–1998], 3.85 [1999–2008], 4.01 [2009–2018]). Both patient and graft survival have improved for paediatric DD transplant recipients in the most recent era (5-year graft and patient survival 85% vs 65% and 99% vs 94%, respectively, for 2009–2018 vs 1999–2008).
Conclusions
The current DD kidney allocation system in Australia provides rapid access to high-quality organs for paediatric recipients, and early graft loss has decreased significantly in recent years; however, additional targeted interventions to address HLA matching may improve long-term outcomes in this population.
Abbreviations
-
- AKX
-
- Australian Paired Kidney Exchange
-
- ANZDATA
-
- Australia and New Zealand Dialysis and Transplant Registry
-
- ANZOD
-
- Australia and New Zealand Organ Donor Registry
-
- ANZSN
-
- Australian and New Zealand Society of Nephrology
-
- DCD
-
- donation after circulatory death
-
- DD
-
- deceased donor
-
- EPTS
-
- estimated post-transplant survival
-
- ESKD
-
- end-stage kidney disease
-
- HLA
-
- human leucocyte antigen
-
- KDPI
-
- kidney donor profile index
-
- KDRI
-
- kidney donor risk index
-
- OPTN
-
- Organ Procurement and Transplant Network's
-
- RRT
-
- renal replacement therapy
-
- TSANZ
-
- Transplantation Society of Australia and New Zealand
1 BACKGROUND
ESKD is a rare diagnosis in the paediatric population but has profound consequences for the child and their family.1 For most children with ESKD, kidney transplantation not only offers a survival benefit when compared with dialysis,2 but also provides improved opportunities for growth,3 development,4 education and social interaction,5 and a superior quality of life.6 Recognizing the unique needs and potential benefits of transplantation for this population, many transplant programmes worldwide have specific deceased donor organ allocation rules aimed at prioritizing access to transplantation for children with ESKD.7, 8
Widespread adoption of paediatric bonuses implies a broad international consensus on the value of positive discrimination in organ allocation of this population. A white paper produced by the OPTN/UNOS Paediatric Transplantation and Ethics Committees set out several philosophical justifications for paediatric priority including the prudential lifespan account, the principles of ‘fair innings’ and maximizing the minimum benefit to the least advantaged and utility considerations.9 Children also have unique growth and neurodevelopmental needs that can be optimized through earlier access to transplantation,3, 4 and studies into community preferences in the allocation or deceased donor organs have consistently shown support for prioritizing younger recipients.10-12
It is also important to consider potential unintended consequences of this prioritization in a system dependent on the availability of scarce organs.13, 14 Expedited access to deceased donor transplantation may negatively impact living donor transplant rates, affect donor selection or have detrimental effects for other populations on the waiting list. The impacts of the US Share35 programme also serve as an example of the potential for unintended consequences when implementing a priority allocation system. While this programme achieved its goal of improving access to deceased donor transplantation for paediatric candidates, the expedited access was associated with a decline in living donor transplant rates and increase in HLA mismatches for this population.15
Allocation of deceased donor kidneys in Australia is performed according to a national protocol developed by the TSANZ and implemented by the OrganMatch system that includes both national sharing and local allocation among the five transplanting regions.16 In brief, all kidneys are first allocated through a national programme that prioritizes highly sensitized patients and well-matched kidneys and addresses sharing imbalances between regions. Organs are then allocated within transplanting regions based on local algorithms that consider both HLA matching and recipient waiting time. If no suitable recipient is identified locally, the kidney is once again offered nationally to avoid organ wastage. Around 20% of kidneys are allocated through the national sharing programme and 80% through regional algorithms.
Over the past two decades, Australia has introduced a series of bonuses for paediatric transplant candidates in both the national and regional kidney allocation algorithms as outlined in Table 1. These bonuses range between 30 000 and 100 000 points. To put this in context, scores in the national allocation system range from 54 000 000 to over 60 000 000 and scores in the jurisdictional systems range from 0 to over 50 000 000.16 Therefore, the paediatric bonuses do not give absolute priority to children, but rather give them a priority over adults with similar HLA matching, sensitization and other bonuses, but who have a longer waiting time. These bonuses are not contingent on donor quality or HLA matching. It is also important to note that while the two largest transplant regions have formal bonus point systems for paediatric patients, the three smaller regions have informal local priorities for children (such as routine listing with ‘urgent’ status) that are not documented in the national guidelines but have been verbally communicated to the authors. Unlike some other transplanting jurisdictions, Australia does not allow pre-emptive listing on the deceased donor kidney transplant waiting list prior to the commencement of dialysis for either paediatric or adult candidates.
| Date of implementation | Jurisdiction | Description | Bonus points |
|---|---|---|---|
| 07/04/2000 | National | Age <18 years. First dialysis before 15th birthday. Duration of dialysis ≥1 year. | 30 000 |
| 04/08/2000 | NSW/ACT | Age <18 years. First dialysis before 15th birthday. Duration of dialysis ≥1 year. | 100 000 |
| 22/11/2011 | National | Age <18 years. Duration of dialysis ≥1 year | 30 000 |
| 10/12/2013 | VIC/TAS | Age <18 years. Duration of dialysis ≥1 year. | 100 000 |
| 14/02/2018 |
National NSW/ACT VIC/TAS |
Removal of requirement to have been on dialysis ≥1 year to be eligible for paediatric bonus. |
30 000 (National) 100 000 (NSW/ACT & VIC/TAS) |
- Abbreviations: ACT, Australian Capital Territory; NSW, New South Wales; Tas, Tasmania; VIC, Victoria.
We aimed to review trends in deceased donor renal transplantation for paediatric recipients in Australia over the last three decades to identify changes in transplant activity, patient and donor characteristics and graft survival. With incremental changes in the paediatric bonus over time, it is difficult to ascribe causation to any specific policy changes; however, we aimed to identify issues that may require further targeted policy interventions.
This work was commissioned by TSANZ's National Review of Paediatric Kidney Transplant Recipients.
2 METHODS
A deidentified data extract from the ANZDATA linked to data from the National Organ Matching Service (now OrganMatch) was used in this analysis. ANZDATA is a binational registry of all patients receiving RRT for the treatment of ESKD in Australia and New Zealand. The registry aims to include 100% of patients across both countries and is linked to the ANZOD to ensure complete capture of transplant activity. Participation in the registry is via a process of opt-out consent. Population data were sourced from the Australian Bureau of Statistics.17
2.1 Inclusion/exclusion criteria
All patients who commenced renal replacement therapy in Australia under the age of 18 years between 01/01/1989 and 31/12/2018 were included in the study. This period was chosen to give a long-term trend and to roughly coincide with the era of calcineurin use in kidney transplantation which were introduced into routine practice in the mid-1980s. For analysis relating to transplant activity, only patients aged under the age of 18 at the time of transplant were included. Patients waitlisted for multiorgan transplantation (n = 9) were excluded from the analysis. Patients receiving renal replacement therapy in New Zealand were not included. Selective comparisons are made with the non-paediatric population, aged 18 years and older.
2.2 Scope and definitions
We report on six areas including the following: incidence and prevalence of RRT, transplant activity, timeliness of access to deceased donor transplantation, donor characteristics, HLA matching, and patient and graft survival.
HLA mismatches were defined at the antigen level and considered at the HLA-A, HLA-B and HLA-DRB1 loci, giving a potential of up to six mismatches between donor and recipient. Although the number of HLA mismatches is a discrete variable, as well as the percentage in each discrete mismatch category, the mean number of mismatches is also presented to give a representation of overall trend in the population. KDPI was determined by calculating the KDRI based on the US OPTN donor only formula18 and scaling this from 1–100 based on a reference population of all utilized donors in Australia during the preceding 3 years.19 Donor race was assumed to be non-African American, and if hepatitis C status was missing, this was presumed to be negative. As a consequence of changes in data collection over time, some results are only reported for more recent years for which the relevant data field was available, this includes the following: some donor characteristics only reported from 1993, KDPI data only able to be calculated from 1996, allocation algorithm data only available from 2001 and waiting list data only reported from 2006.
2.3 Statistical methods
Due to the small number of deceased donor paediatric kidney transplants performed in Australia annually, this report is predominantly descriptive with information presented as summary statistics and in graphical form. Formal hypothesis testing has not been performed apart from in comparison of donor characteristics and the analysis of patient and graft survival.
Donor characteristics for adult and paediatric recipients were compared using Student's t test for normally distributed continuous variables, the Wilcoxon rank-sum test for non-normally distributed continuous variables and Pearson's chi-squared test for categorical variables.
For survival analysis, exposure was defined as decade of transplantation (1989–1998, 1999–2008, 2009–2018). The survival function was calculated using the Kaplan-Meier method. Differences were tested using the log-rank test. Graft survival was not censored for death, and survival statistics were unadjusted.
3 RESULTS
3.1 Incidence and prevalence of RRT
Over the three decades, a total of 1,157 patients under the age of 18 years commenced RRT in Australia. Figure 1 shows the change in annual incidence over time which increased by around 23% in the study period, from an average of 37 patients annually in the 1990s–2000s to an average of 45 patients annually in the last decade. Although there is considerable year on year variability, there was little difference in the trend in incident patients by age group.
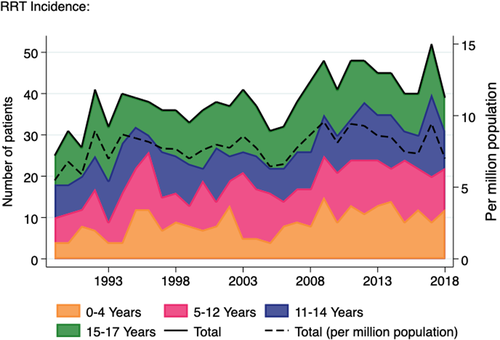
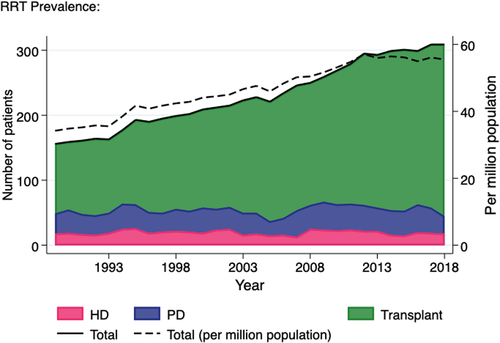
In contrast, the number of prevalent paediatric RRT patients almost doubled over the study period, from 156 in 1989 to 309 in 2017 (Figure 2). While the prevalent haemodialysis and peritoneal dialysis populations have remained static for many years (average 20 patients and 35 patients, respectively), the number of prevalent transplant patients has shown a 2.4-fold increase over the last 30 years, from 107 in 1989 to 264 in 2018. The prevalence per million population is also shown and rose at a similar rate to the change in absolute numbers.
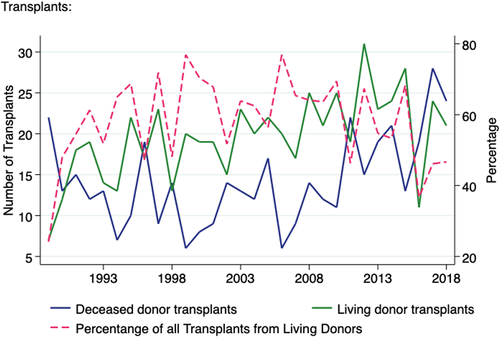
3.2 Transplant activity
Of the 19,210 kidney only transplants performed in Australia during this 30-year period, 1011 were transplanted into paediatric recipients, 585 (58%) from living donors and 426 (42%) from deceased donors. This accounts for 9.8% of all living donor kidney transplants and 3.2% of all deceased donor kidney only transplants performed in Australia during this period. There was an average of 20 deceased donor transplants to paediatric recipients annually that gradually increased over the last decade from a nadir of 6 in 2009 to a peak of 28 in 2017 (Figure 3). No clear trend was observed in the number of deceased donor kidney transplant by age group (Figure S1). Across the last two decades, there was a reduction in percentage of transplants from living donors for this population, from a peak of 77% 1999 to 47% in 2018 (Figure S2). This mirrored the trend seen in the adult transplant recipient population over the last decade, although the proportion remained significantly higher in the paediatric group (47% vs 21% in 2018). Of the living donor transplants performed in paediatric recipients, 12 (2%) were performed through the AKX programme and 16 (3%) were ABO blood group incompatible.
3.3 Timeliness of access to deceased donor transplantation
Paediatric patients on the deceased donor waiting list had a higher rate of transplantation compared with other age groups (averaging 103 transplants per 100 patient-years active on the deceased donor waiting list over the 13 years for which waitlist data were available) (Figure 4). All other age groups showed an increasing rate over time, such that in 2015/2016 the paediatric rate approximated that of patients aged over 60 years. However, in 2017/2018 there was a marked increase in the transplantation rate for paediatric patients reaching a peak of 214 transplants per 100 active years in 2018, more than double that of any other age groups.
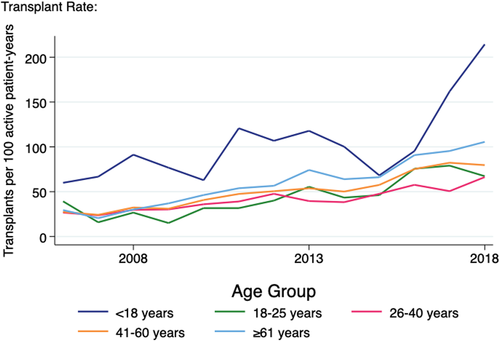
The median time since commencing RRT for paediatric recipients receiving a primary deceased donor renal transplant reached a peak of 2.46 years in 2000 which approximated the adult median at the time (Figure S3). Following the introduction of the initial paediatric bonus in 2000, this gradually declined and remained at an average of 1.25 years over the last decade of the study period with some fluctuation. In contrast, the adult median continued to increase to reach almost 4 years in 2005 and gradually reduced to 2.7 years in the most recent study year. When children under the age of 5 at the time of transplant were excluded to account for the period of dialysis required for many infants prior to achieving a transplantable weight, the average time spent on dialysis prior to deceased donor transplant for paediatric recipients in the last decade dropped slightly to 1.21 years. Children under 5 years of age spent an average of 1.64 years on dialysis prior to receiving a deceased donor transplant during the same period.
3.4 Deceased donor characteristics
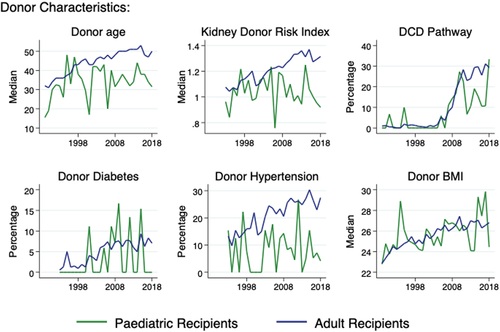
Donor characteristics for all recipients of deceased donor kidney transplants during the study period are shown in Table 2. Compared to the donors of adult recipients, donors of paediatric recipients were younger (median age 34 vs 46 years) and were less likely to have hypertension (6.8% vs 19.3%), diabetes (2.3% vs 4.8%) or to have donated via the DCD pathway (8.9% vs 13.7%). There was a higher percentage of donors to paediatric recipients donating after traumatic brain injury (32.9% vs 23.9%), and they were less likely to be overweight or obese.
| Donor characteristic | Paediatric recipients | Adult recipients | p-value |
|---|---|---|---|
| N | 426 | 12793 | |
| Donor age, median (IQR) | 34 (20, 46) | 46 (29, 57) | <.001 |
| KDRI, raw score, median (IQR) | 1.02 (0.86, 1.25) | 1.23 (0.98, 1.60) | <.001 |
| KDPI %, median (IQR) | 34.00 (17.50, 57.00) | 56.00 (30.00, 79.00) | <.001 |
| Donor cause of death category, n (%) | |||
| Intracranial haemorrhage | 140 (32.9%) | 5582 (43.6%) | <.001 |
| Traumatic brain injury | 140 (32.9%) | 3058 (23.9%) | |
| Cerebral infarct | 10 (2.3%) | 654 (5.1%) | |
| Cerebral hypoxia/ischaemia | 76 (17.8%) | 2134 (16.7%) | |
| Other neurological condition | 1 (0.2%) | 68 (0.5%) | |
| Non-neurological condition | 21 (4.9%) | 512 (4.0%) | |
| Missing | 38 (8.9%) | 785 (6.1%) | |
| Donation pathway, n (%) | |||
| DBD | 388 (91.1%) | 10996 (86.0%) | .004 |
| DCD | 38 (8.9%) | 1753 (13.7%) | |
| Missing | 0 (0.0%) | 44 (0.3%) | |
| Hypertension, n (%) | |||
| No | 327 (76.8%) | 8539 (66.7%) | <.001 |
| Yes | 29 (6.8%) | 2469 (19.3%) | |
| Missing | 70 (16.4%) | 1785 (14.0%) | |
| Diabetes, n (%) | |||
| No | 354 (83.1%) | 10608 (82.9%) | .022 |
| Yes | 10 (2.3%) | 619 (4.8%) | |
| Missing | 62 (14.6%) | 1566 (12.2%) | |
| Smoker, n (%) | |||
| No | 157 (36.9%) | 4580 (35.8%) | .36 |
| Yes | 200 (46.9%) | 6436 (50.3%) | |
| Missing | 69 (16.2%) | 1777 (13.9%) | |
| BMI (kg/m2), n (%) | |||
| Underweight (<18.5) | 20 (4.7%) | 350 (2.7%) | .003 |
| Normal (18.5–<25) | 202 (47.4%) | 5408 (42.3%) | |
| Overweight (25–<30) | 131 (30.8%) | 4360 (34.1%) | |
| Obese(≥30) | 59 (13.8%) | 2308 (18.0%) | |
| Missing | 14 (3.3%) | 367 (2.9%) | |
| Hepatitis C positive, n (%) | |||
| No | 364 (85.4%) | 11144 (87.1%) | .11 |
| Yes | 0 (0.0%) | 77 (0.6%) | |
| Missing | 62 (14.6%) | 1572 (12.3%) | |
| Creatinine—terminal, mean (SD) | 89.72 (69.69) | 93.51 (67.61) | .26 |
- BMI, body mass index; DBD, donation after brain death; IQR, interquartile range; SD, standard deviation.
While there was a gradual increase in donor age for adult recipients up until the last 3 years of the study period, the trend in donor age for paediatric recipients appeared to diverge from the late 1990s onwards (Figure 5). This divergence was mirrored in the trends in the composite KDRI over time, potentially amplified by differences in donor hypertension and donation pathway over time, which are two of the donor characteristics used to calculate KDRI.
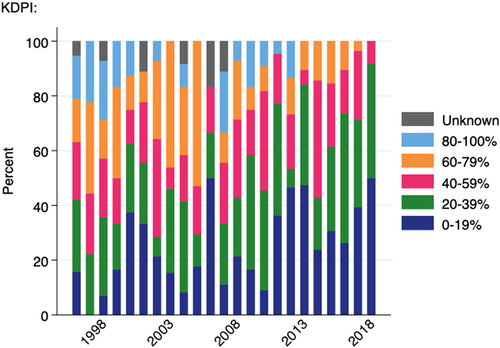
The KDPI is a scaling of the KDRI based on the population of deceased donors from whom at least one kidney was transplanted in the preceding 3 years. Figure 6 shows the KDPI quintile for paediatric deceased donor transplants over time. In the most recent year for which data are available (2018), over 90% of paediatric recipients received a kidney from a donor with KDPI <40% and no child in Australia had received a kidney from a donor with KDPI ≥80% since 2012. (Note that sufficient donor data required to determine this score is only available from 1996).
3.5 HLA matching in deceased donor transplants
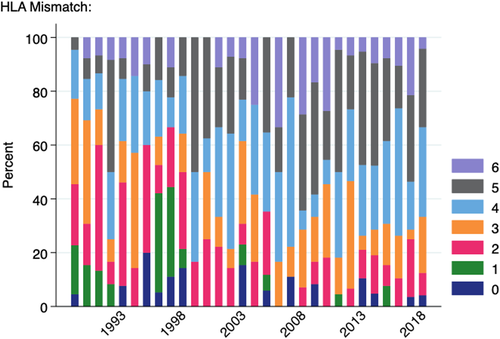
The average number of HLA antigen mismatches for paediatric patients receiving deceased donor grafts across the study period was 3.61. This increased each decade: 2.86 (1989–1998), 3.85 (1999–2008), 4.01 (2009–2018), mirroring a similar trend in the adult population (Figure S4). Over the last 2 decades of the study period, the mean number of HLA mismatches was higher for paediatric recipients of deceased donor transplants compared with adult recipients in all but 1 year, although the differences were relatively small (3.83 vs 3.70 mismatches in 2018, for example). Figure 7 shows the breakdown of HLA antigen mismatches for paediatric recipients of deceased donor transplants by year.
3.6 Patient and graft survival
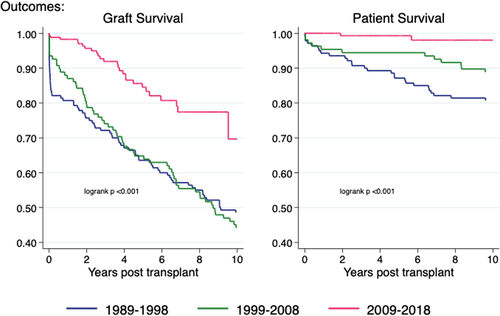
There was a significant improvement in both patient and graft survival in the most recent decade (2009–2018) for paediatric recipients of deceased donor kidney transplants (Figure 8). Five-year patient survival in the most recent era (2009–2018) was 99% [95%CI 95–100] compared with 94% [95% CI 88–97] in the decade prior (1999–2008). Similarly, there has been a dramatic improvement in 5-year graft survival over the same period from 65% [95% CI 55–73] to 85% [95% CI 77–90], mainly driven by reductions in early graft loss.
4 DISCUSSION
The optimal deceased donor kidney for a child with ESKD is one that can be accessed quickly to avoid the morbidity associated with dialysis, has the best possible long-term graft survival and reduces the risk of sensitization to preserve future transplant opportunities. Our analysis demonstrates that under Australia's current paediatric bonus system, children with ESKD received more timely access to higher quality kidneys from deceased donors compared with adult recipients; however, we highlight the need for better strategies to improve donor-recipient HLA matching for paediatric recipients.
Due to the incremental implementation of paediatric bonuses across national and regional allocation systems in Australia, and in light of informal priorities given to the population in some jurisdictions, it is challenging to determine the impacts of, or attribute causation for the observed trends to specific policy changes. However, since the introduction of Australia's first national priority for paediatric patients in 2000, this population have consistently maintained transplantation rates that are higher than the adult population, resulting in substantially less time spent on dialysis prior to deceased donor transplantation. The most recent two years saw a spike in transplantation rate for paediatric candidates, and this pre-dated a major policy change with the removal of the requirement for paediatric patients to spend 12 months on dialysis prior to becoming eligible for paediatric bonuses; however, this may have contributed to record high rates in 2018. Despite rapid access to transplantation, paediatric recipients are still spending on average over a year on dialysis prior to receiving a deceased donor graft. In the setting of a relatively small donor pool, this time may reflect a period of time waiting for a suitable organ offer. Since the late 2000s both the transplantation rate per waiting time and the time spent on dialysis prior to transplant have also shown ongoing improvements for the adult population, suggesting that in the setting of small absolute numbers, the expedited access to deceased donor transplants for paediatric recipients does not appear to have a discernible negative impact on transplantation rates for adult recipients.
In the context of increased access to deceased donor transplantation for both adult and paediatric recipients, a fall in the percentage of all transplants coming from living donors has emerged in both populations. For the paediatric population, this does not appear to be a result of reduced absolute numbers of living donor transplants. With historically high rates of living donor transplantation in this population, the decrease in the proportion in recent years may represent an exhaustion of suitable living donors and appropriate use of the deceased donation pathway for children who do not have access to an appropriate donor. Two large population studies in 2014 demonstrated small but significant increased long-term risks for living kidney donors20, 21 which may potentially have impacted clinical practice in the approach to accepting living donors, including for younger parents wishing to donate to children with ESKD.22 Living kidney donation can provide pre-emptive transplantation of well-matched, high-quality organs for paediatric recipients and should continue to be pursued as the primary transplant option where possible, with paired kidney exchange offering opportunities to further optimize transplant characteristics for this population.23
Although paediatric bonuses are not linked to donor age or organ quality in Australia, our results indicate that paediatric recipients are receiving higher quality kidneys in more recent years, despite changes in the overall donor pool. While the donor age has remained relatively static for this population in the context of increasing overall donor age, we demonstrate a trend towards lower KDRI over time. The differences in donor characteristics between paediatric and adult recipients suggest that clinicians are using the priority access to organs to be more discerning about the quality of organs accepted for these patients. KDPI is not used in the organ allocation algorithm in Australia; however, from March 2016 this score has been reported to clinicians with all organ offers.19 Despite no specific policy on the allocation of lower KDPI kidneys, in the most recent year over 90% of paediatric recipients received a kidney from a donor with KDPI <40%. This finding is similar to data from the United States where in 2018, 95.2% of paediatric recipients of deceased donor kidneys received an organ from a donor with KDPI <35%24 as a result of specific allocation rules prioritizing patients with low EPTS for low KDPI kidneys.
While it can be argued that the current system is achieving timely access to deceased donor transplantation and favourable donor characteristics for paediatric recipients, we have demonstrated that it is not resulting in superior donor-recipient HLA matching, compared with adult recipients. HLA mismatches are not only associated with poorer long-term graft survival but also increased sensitization and decreased future transplant opportunities following graft failure.25-27 These issues are of particular importance for paediatric recipients who, as a result of excellent long-term patient survival, require both extended primary graft survival and likely subsequent transplantation. A recent study by Ruck et al28 in the United States showed that children with 3–6 HLA mismatches had a 43% increased risk of graft failure compared to those with 0–2 mismatches. Despite the introduction of progressive paediatric bonuses in Australia, there has been a gradual increase in mean HLA mismatches for paediatric recipients of deceased donor kidney transplants over the past three decades. Similar trends have been seen in the United States where median HLA mismatch has increased from 4 in 1995–2004 to 5 in 2005–2014 for this population29 and a study reporting the impact of new KAS introduced in 2014 did not show an improvement in the percentage of transplants with ≤3 HLA mismatches for paediatric recipients.28 In stark contrast, a recent report on paediatric kidney transplantation in the UK where age-based allocation points are linked to HLA matching showed that in 2016 84% of children transplanted through the deceased donor programme received a well-matched graft (either 0 mismatches, or 0 -DR and 0/1 -B mismatches, this compared to only 27% in 1992).30 Although we show significant improvements in graft survival in the most recent decade, this is mainly driven by decreases in early graft loss and optimizing HLA matching may offer an opportunity to further decrease late graft loss.
In many ways, the failure to see improvement in HLA matching at the HLA-A, HLA-B and HLA-DR loci for paediatric recipients of deceased donor kidney transplants in Australia is to be expected as the current paediatric bonuses are not contingent on HLA matching, and therefore, in the setting of a relatively small donor pool, function to primarily give children priority for kidneys allocated solely on waiting time. The interaction between HLA matching and paediatric bonuses varies widely across international jurisdictions, in the United States the current Kidney Allocation System (2014) gives priority to paediatric candidates for kidneys with 0 HLA mismatches or from donors with a KDPI <35%31 and many European jurisdictions link paediatric bonuses to donor age or HLA matching.7 A novel approach has been taken in the UK, where paediatric bonuses per se have been removed from the allocation algorithm (except in the case of clinically urgent paediatric listings) and replaced with a combined HLA match and age score that is calculated for all patients.32 This functions to give paediatric candidates high priority only for well-matched transplants and does not have a specific age cut-off, but gradually reduces with increasing age, and may help to explain the improvement in HLA matching seen for paediatric recipients of deceased donor kidneys in the UK.
Our analysis of immunological matching in paediatric recipients of deceased donor kidney transplants is limited by the resolution of HLA typing data available from the ANZDATA registry. An increasing body of evidence suggests that considering HLA matching based on serological epitopes, rather than at the antigen level, may provide a more granular tool for assessing immunological risk in kidney transplantation33-35; however, the high-resolution molecular typing required to assess epitope matching was not available for this analysis. At least one paediatric transplant unit in Australia is using a novel HLA epitope-based approach to improving immunological matching where HLA antigens carrying a high number of epitope mismatches with the potential recipient are prospectively entered into the allocation system as unacceptable antigens, and therefore, organs from donors with unfavourable HLA profiles will not be allocated to these patients.36 Informal HLA epitope-based immunological assessment of deceased donor kidney offers in a number of other Australian paediatric transplanting centres has also been reported to the authors. With the widespread availability of HLA molecular typing, epitope-based organ allocation offers the potential for a more granular method of immunologic risk assessment in transplantation; however, more clinical outcome data and feasibility studies are required before this can be implemented at a system wide level.33, 37, 38
Deceased donation is an important pathway for children with ESKD to access kidney transplantation in Australia; however, the absolute numbers remain low and make up a small proportion of overall deceased donor transplant activity. Current paediatric bonuses have facilitated rapid access to deceased donor kidneys and enabled selection of high-quality organs for these recipients; however, immunological matching, considered at the HLA antigen level, has not improved with the introduction of these bonuses and could be optimized to improve long-term outcomes for this population. In the setting of a relatively small donor pool, increased priority linked to HLA matching coupled with novel approaches such as HLA epitope-based matching may assist in optimizing long-term graft survival and re-transplantation opportunities for children receiving deceased donor grafts.
ACKNOWLEDGEMENTS
The data reported here have been supplied by the Australia and New Zealand Dialysis and Transplant Registry. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the Australia and New Zealand Dialysis and Transplant Registry.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Matthew P Sypek was the primary author, participated in study design and performed data management, statistical analysis, manuscript drafting, editing, revision and submission. Chris E Davies assisted with statistical analysis and participated in review of the manuscript. Fiona Mackie, Joshua Y Kausman, Germaine Wong and Nicolas Larkins are members of the National Review of Paediatric Kidney Transplant Recipients in Australia working group and participated in study design, formulation of discussion and review of the manuscript. Amelia Le Page, Philip D Clayton and Peter Hughes participated in formulation of discussion and review of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the ANZDATA. Restrictions apply to the availability of these data, which were approved for use in the study. Data are available https://www.anzdata.org.au with the permission of the ANZDATA Registry.