Current practice of antithrombotic prophylaxis in pediatric kidney transplantation—Results of an international survey on behalf of the European Society for Paediatric Nephrology
Abstract
Background
Renal graft thrombosis (RGT) is one of the main causes for early graft loss in pediatric kidney transplantation (KTx). Despite the lack of evidence-based recommendations, antithrombotic prophylaxis (aP) is used to prevent RGT.
Methods
An online survey supported by the European Society for Pediatric Nephrology was developed to investigate the current practice of aP in pediatric KTx recipients <18 years.
Results
A total of 80 pediatric KTx centers from 37 countries participated in the survey. Antithrombotic prophylaxis was performed in 96% of the pediatric renal transplant centers (all/selected patients: 54%/42%). The main overall used drugs were as follows: low-molecular-weight heparin (89%), unfractionated heparin (UFH) (69%), and acetylsalicylic acid (ASS) (55%). Ten different aP management strategies were identified as follows: 51% used a single drug and 48% combined two drugs sequentially. The corresponding centers started aP predominantly within 24 hours after pediatric KTx; 51% preferred UFH for starting aP. In centers switching to a second drug (51%), this change was performed after 10 ± 6 days; of these 57% preferred ASS for maintenance aP. Reported median aP duration was 51 days (range 1-360).
Conclusions
Despite the use of aP in almost all responding pediatric KTx centers, there is no uniform management strategy. Notwithstanding, UFH seems to be the preferred drug for the early post-operative period of pediatric KTx, and ASS for maintenance prophylaxis following pediatric KTx. Prospective studies are needed to further evaluate the benefits and risks of aP, preferably resulting in guidelines for the management in pediatric KTx.
Abbreviations
-
- aP
-
- antithrombotic prophylaxis
-
- ASS
-
- acetylsalicylic acid
-
- ESPN
-
- European Society for Pediatric Nephrology
-
- KTx
-
- kidney transplantation
-
- LMWH
-
- low-molecular-weight heparin
-
- RGT
-
- renal graft thrombosis
-
- UFH
-
- unfractionated heparin
1 INTRODUCTION
Renal transplantation is the treatment of choice for children and adolescents with end-stage renal disease.1 Although transplant survival rates have markedly improved over the last decades, renal graft thrombosis (RGT) remains a significant cause of early graft loss in pediatric kidney transplantation (KTx).2 Renal graft thrombosis occurs mostly within the first week following KTx.3 The reported incidence of RGT varies between 2.3% and 5.1%, mainly due to differences in study populations and associated risk factors.2, 4 The available evidence does not allow a quantitative differentiation of RGT into arterial or venous thromboses.5, 6 The risk for developing a thrombotic episode due to modifiable and non-modifiable risk factors seems to be higher in pediatric than in adult renal transplantation.7-9 Hence, antithrombotic prophylaxis (aP) with anticoagulants and antiplatelets is widely used to prevent RGT in pediatric KTx.10-13 To date, an evidence-based management strategy is still lacking.10, 11, 14, 15 Thus, neither recommendations nor consensus guidelines on the use of aP are available, and the limited data have to be extrapolated from the adult population.16 For that reason, an online survey was designed to investigate current practice of aP management strategies in children and adolescents <18 years undergoing KTx.
2 MATERIALS AND METHODS
2.1 Study design
The electronic, questionnaire-based survey (SurveyMonkey Inc, www.surveymonkey.com) was developed in accordance with given recommendations on behalf of the “Transplantation Working Group” of the European Society for Pediatric Nephrology (ESPN).17
The questionnaire was structured into four sections with 38 items (open and multiple-choice questions): (a) demographic information about the responding center; (b) general characteristics about the transplant center; (c) detailed questions addressing screening for thrombophilia during pretransplant evaluation; and (d) comprehensive information about aP including type of anticoagulants and antiplatelets, timing, dosage, mode of application, monitoring, and selection criteria of aP (Supporting Information S1).
The questionnaire was tested in advance by three physicians for the following aspects: clarity, utility, and redundancy. Changes were conducted following the suggestions. The adapted survey was finally tested by another five physicians.
An invitation to complete the survey and a reminder were sent to all ESPN members (n = 518 [state 12/2019]) with provided information about the objective of the study, the investigators involved and the link to the survey website (Supporting Informations S2 and S3).
The research project was not approved by an ethics committee, because the study neither involved patients directly nor was any specific patient data required.
2.2 Study duration and study population
The survey was carried out between July 10, 2019 and January 16, 2020.
Overall, 108 responses were retrieved. After elimination of double (n = 17) and triple data entries (n = 3), 85 responding pediatric nephrology centers were identified. Responders who did not perform renal transplantation in the pediatric population <18 years (n = 4) or could not be assigned to a particular institution (n = 1) were excluded from the analysis. Finally, 80 pediatric KTx centers were included for data evaluation.
2.3 Statistical analyses
The responses were collected in an electronic database and checked before the final analysis. Double and triple responses from one center were combined into a single answer. The overall completion rate of the questions within the entire survey was 84% (67/80) excluding two mandatory questions (Q24 and Q30) both completed by <50% of the responders. The statistical analyses were conducted and reported based on the number of total answers for each question. Details of data completion including missing and valid data for all items are provided in the Supporting Information S4. If data were missing or ambiguous, responders were contacted via email for further information.
Data were analyzed using the statistical package SPSS for Windows, release 21 (IBM Corp.). Differences between subgroups were calculated using the Mann-Whitney U test for continuous variables, and P < .05 was considered significant. Data were checked for normal distribution according to the Kolmogorov-Smirnov test. Continuous variables following a normal distribution were expressed as mean and standard deviation, and non-normally distributed as median and range. Categorical variables were expressed as frequencies and percentages.
3 RESULTS
3.1 General information about the participating centers
3.1.1 Demographic and institutional characteristics
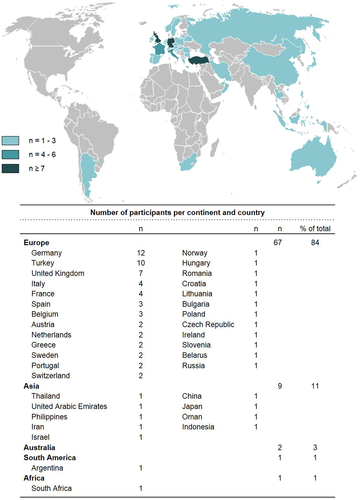
The 80 participating centers originated from 37 countries (84% [67/80] from Europe) (Figure 1).
A standardized institutional protocol for pediatric KTx was available in 96% (77/80). The number of pediatric KTx per year was reported as follows: <5:24% (19/80); 5-10:49% (39/80); 11-20:20% (16/80), and >20:8% (6/80). In 71% (57/80) of all centers, the transplant procedure was carried out by a surgeon specialized in pediatric transplantation. Ninety-nine percent (79/80) targeted a minimum required body weight of the recipient as follows: 3 to <7 kg: 1% (1/79); 7-10 kg: 32% (25/79); and >10 kg: 67% (53/79). Grafts from donors with a body weight <5 kg were accepted by 25% (20/80) of the survey responders.
3.2 Preparation for pediatric kidney transplantation
3.2.1 Screening for thrombophilia
Screening for thrombophilia was performed in 94% (75/80) of all transplant centers with 55% (44/80) in all transplant candidates and 39% (31/80) in a selected population only. Positive family history and previous thromboembolic complications (both 97% [30/31]) were the main reasons for selective screening (Table 1). An overview including all thrombophilia screening parameters is displayed in Table 2.
| Reasons | n | % of total |
|---|---|---|
| Positive family history for thromboembolic events | 30 | 97 |
| Previous thromboembolic complications | 30 | 97 |
| Re-kidney transplantation | 8 | 26 |
| Deceased donor | 2 | 7 |
| AB0-incompatible kidney transplantation | 2 | 7 |
| Living donor kidney transplantation | 1 | 3 |
| Age | 1 | 3 |
| 0 to < 6 years | 1 | 3 |
| 6 to <12 years | 0 | 0 |
| 12-18 years | 0 | 0 |
| Other reasons, specified by respondersa | 5 | 16 |
- Abbreviations: n, number.
- a Other reasons: congenital nephrotic syndrome; concomitant oral contraception; clinical suspicion of thrombophilia; underlying condition such as systemic lupus erythematosus; age <4 years of age.
| Parameters |
n |
% of total |
|---|---|---|
| Quick/international normalized ratio, partial thromboplastin time, fibrinogen, thrombin time | 74 | 100 |
| Platelet count | 73 | 99 |
| Protein C | 63 | 85 |
| Protein S | 62 | 84 |
| Antithrombin | 61 | 82 |
| Factor V Leiden mutation | 58 | 78 |
| Antiphospholipid antibodies | 51 | 69 |
| Lupus anticoagulant | 49 | 66 |
| Factor VIII | 47 | 64 |
| Homocysteine level | 45 | 61 |
| Prothrombin mutation | 40 | 54 |
| Methylenetetrahydrofolate reductase polymorphism | 38 | 51 |
| Lipoprotein (a) | 25 | 34 |
| Other parameters, specified by respondersa | 5 | 7 |
- Abbreviations: n, number.
- a Other screening parameters: activated protein C resistance; in case of factor V Leiden or prothrombin mutation: plasminogen activator inhibitor-1 polymorphism; in case of increased homocysteine level: genetic testing for mutations in the methylenetetrahydrofolate reductase gene; in special conditions (not specified): closing time/ thrombocyte aggregation (measured with adenosine triphosphate or epinephrine); in special conditions (not specified): platelet factor assay.
3.3 Antithrombotic management
3.3.1 General aspects of antithrombotic prophylaxis
A protocol for aP was available in 81% (60/74) of all participating centers. A stratified risk assessment for aP was reported by 70% (52/74). Ninety-six percent (76/79) of the corresponding centers performed aP. In 54% (43/79) of all centers, aP was used in all renal transplant patients. In the remaining centers (42% [33/79]), aP was limited to a selected recipient population with specific reasons (Table 3; reasons only specified by 31 responders).
| Parameters | n | % of total |
|---|---|---|
| Previous thromboembolic complications of the patients | 27 | 87 |
| Positive thrombophilia screening | 27 | 87 |
| Positive family history for thromboembolic events | 19 | 61 |
| Small donor graft to small recipient | 17 | 55 |
| Arterial or venous anomalies | 17 | 55 |
| Large donor graft to small recipient | 14 | 45 |
| Small donor graft to large recipient | 13 | 42 |
| Dependent on the surgical placement of the transplant | 11 | 35 |
| Age | 10 | 32 |
| 0 to < 6 years | 9 | 29 |
| 6 to <12 years | 1 | 3 |
| 12-18 years | 1 | 3 |
| Surgical re-intervention | 9 | 29 |
| Re-kidney transplantation | 4 | 13 |
| Deceased donor kidney transplantation | 4 | 13 |
| Living donor kidney transplantation | 3 | 10 |
| Other conditions, specified by respondersa | 11 | 35 |
- Abbreviations: n, number.
- a Other reasons for selective antithrombotic prophylaxis: adolescent patients immobilized >2-3 days after kidney transplantation; recipients with a body weight <30 kg; recipients with polyuria; congenital thrombophilia; prothrombotic immunological disease; history of thrombosis of at least 2 vessels; recipients receiving already prophylactic or therapeutic anticoagulant therapy; intraoperative complications with high risk of thrombosis (n = 2); kidney transplant surgeon- and/or multi-professional decisions (n = 2).
3.3.2 Anticoagulants and antiplatelets
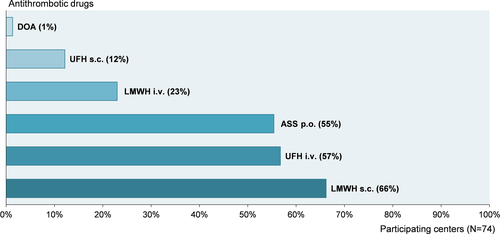
Low-molecular-weight heparin (LMWH) (89% [66/74]) and unfractionated heparin (UFH) (69% [51/74]) were the main drugs used for aP, followed by oral acetylsalicylic acid (ASS) (55% [41/74]). A summary of the used antithrombotic drugs is displayed in Figure 2.
3.3.3 Antithrombotic prophylaxis strategies
A total of 10 different center-specific standard strategies for aP were identified (Figure 3A). A single drug was used in 48% (33/69) of all responding centers, and a change to another antithrombotic drug for maintenance prophylaxis was made in 51% (35/69) (one center reported individualized aP for every patient). Alternative non-standardized aP management strategies for individual patients were reported by 41% (28/68). In 25% (16/64) of the participating centers, simultaneous use of drugs for aP was considered under specific circumstances (Supporting Information S5).
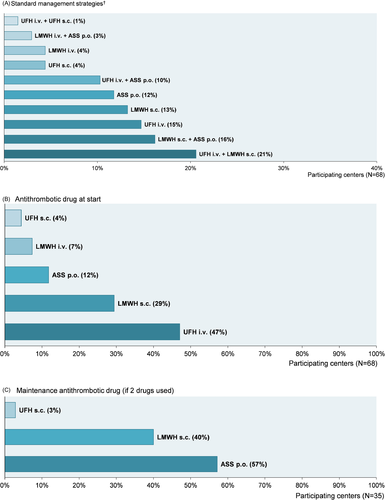
UFH intravenously or subcutaneously was administered at pediatric KTx in 51% (35/68) of the transplant centers as initial prophylaxis (Figure 3 B). For centers changing to another maintenance antithrombotic drug (51% [35/68]), the favored drug was oral ASS (57% [20/35]) (Figure 3C).
3.3.4 Timing of aP
Antithrombotic prophylaxis was started preoperatively in 11% (8/74), intra-operatively in 22% (16/74), within 12 hours in 50% (37/74), between 12 and 24 hours in 14% (10/74), and more than 24 hours after pediatric KTx in 4% (3/74) of the corresponding centers.
The time-point for changing the drug to maintenance prophylaxis in centers using two drugs sequentially was on average 10 ± 6 days following pediatric KTx (29/35; 6 centers did not specify the time-point). Antithrombotic prophylaxis was discontinued after a median of 51 days (range 1-360) after pediatric KTx (48/69; 21 centers did not report) (Figure 4). Reported criteria for an individual point of time to maintenance antithrombotic regimen are shown in Supporting Information S6 and for discontinuation in Supporting Information S7. ASS-based aP strategies were conducted significantly longer than UFH- or LMWH-based strategies (127 ± 111 days vs 52 ± 78 days, respectively; P < .001). Detailed information for aP duration is provided in Table 4.
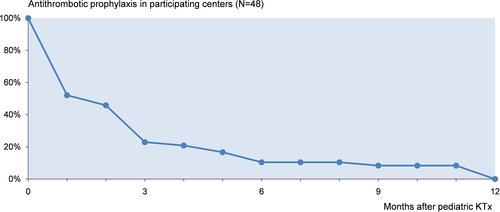
| Strategy | Duration | Centers | Missinga | ||
|---|---|---|---|---|---|
| Mean ± SD, d | Median, d | R, d | n | n | |
| UFH iv | 25 ± 30 | 18 | 1-90 | 7 | 3 |
| UFH iv + UFH s.c. | 9 | — | — | 1 | 0 |
| UFH iv + LMWH s.c. | 88 ± 111 | 28 | 12-360 | 9 | 5 |
| UFH iv + ASS p.o. | 237 ± 135 | 270 | 60-360 | 5 | 2 |
| UFH s.c. | 16 ± 20 | 16 | 2-30 | 2 | 1 |
| LMWH iv + ASS p.o. | 75 ± 21 | 75 | 60-90 | 2 | 0 |
| LMWH s.c. | 60 ± 76 | 17 | 4-180 | 9 | 4 |
| LMWH s.c. + ASS p.o. | 107 ± 99 | 87 | 30-360 | 10 | 1 |
| ASS p.o. | 79 ± 68 | 90 | 5-180 | 5 | 3 |
- Abbreviations: ASS, acetylsalicylic acid; d, days; iv, intravenously; LMWH, low-molecular-weight heparin; n, number; p.o., per os; R, range; s.c., subcutaneously; SD, standard deviation; UFH, unfractionated heparin.
- a Data regarding the duration of antithrombotic prophylaxis were provided by only 48 out of 68 pediatric kidney transplantation centers; therefore, for each strategy the number of missing data is reported.
3.3.5 Dosing and therapeutic drug monitoring
Available data of drug dosing for aP were limited (Supporting Information S8). Drug monitoring was done in 54% (36/67). The reported monitoring parameters are shown in Supporting Information S9.
4 DISCUSSION
This survey clearly demonstrates that the vast majority of the participating pediatric renal transplant centers favor aP in pediatric KTx even though studies show conflicting results toward the beneficial effects for prevention of RGT.11, 15, 18
While a significant number of renal transplant centers perform aP in all renal transplant patients, other transplant centers restrict aP to selected patients with a suggested distinct higher risk for developing RGT.4, 9, 13, 15 Interestingly, criteria for assignment of renal transplant candidates to the high-risk group for RGT differ among the particular renal transplant centers. The main criteria for aP in selected patients, however, were previous thromboembolic complications, positive thrombophilia screening results, and positive family history for thromboembolic events. Of note, despite the high proportion of surgeons specialized in pediatric KTx and encouraging trends for non-inferior outcome of small pediatric donors and recipients, body weight of donor and recipient still seem to play a pivotal role in decision-making for aP besides renal vascular anomalies, presumably due to the associated surgical difficulties and risks.9, 19 Only a minority of reporting centers do not use aP at all which might be supported by the findings of a few studies.9, 11, 20
Most pediatric renal transplant patients develop RGT within a few hours post-transplant.3, 21 Hence, it is not remarkable that almost all renal transplant centers initiate aP in the peri- or early post-operative phase following surgical intervention. The timing of the management strategy for aP reflects study protocols of previous published studies.10, 22 In contrast to the comparatively uniform starting time-point of aP at pediatric KTx, the overall duration of aP varies significantly among the participating centers. Renal graft thrombosis most frequently occurs within the first month after KTx, with a peak in the first week post-transplant.3, 21 Therefore, it is even the more astonishing that a few transplant centers prolong the aP to more than three months which does not correspond to other studies.5, 10, 11 Nevertheless, the prolonged use of aP is astonishing as both early and late thromboses are more often related to numerous other factors such as hyperacute rejection by preformed antibodies, surgical experience regarding vessel anastomoses, small vessels in either the donor graft or the recipient, which are not associated with coagulation disorders and therefore cannot be modified by anticoagulants and antiplatelets.23 Within this context, a limited use of aP for only a few days or weeks following pediatric KTx could be discussed. In centers practicing a management strategy with a change to another maintenance antithrombotic drug, most centers switchover within one to two weeks following pediatric KTx.9, 24 We assume that the selection of this time period correlates positively with the clinical improvement of the renal transplant patient, the associated renal transplant function, and lastly with the discharge from the hospital.
The considerable high multitude of antithrombotic management strategies represents uncertainty to the best practice which might arise from the limited evidence from few predominantly retrospective studies with small sample sizes.10-12, 15 In addition, there is only moderate to low quality evidence that anticoagulants are superior in prevention of venous thromboses and antiplatelets in arterial thromboses.25, 26 Notwithstanding, the favored antithrombotic drug is heparin in accordance with published studies in pediatric and adult KTx.10, 11, 21 The preference for the anticoagulant heparin compared to the antiplatelet ASS may be traced back to short-term pharmacokinetic effects, the availability of an antidote, and therefore the lower attributed risk of clinical significant bleeding complications.11, 12 There is also a time-dependent preference toward the initial use of intravenous UFH compared to the subcutaneous application which might be explained by the already existing (central) venous lines and the assigned lower associated costs of UFH.9, 27 Notwithstanding, LMWH subcutaneously is the most often used overall antithrombotic drug, followed by UFH intravenously and oral ASS.10-12, 21
It remains uncertain if anticoagulation with heparin or antiplatelet management with ASS is superior in prevention of RGT.5, 9, 18 There are a number of studies which suggest that the use of ASS decreases significantly the risk of RGT compared to heparin; however, the quality and reliability of the data needs to be determined.5, 18 For this reason, it is not surprising that oral ASS is the drug of choice for maintenance prophylaxis in these transplant centers which use two drugs sequentially for aP.22 However, the convenient mode of oral application of ASS may play a more determining role in the decision-making process than the suggested higher effect of an antiplatelet management strategy compared to heparin. Of note, even though the alteration from heparin to oral ASS adds up from the clinical point of view, there is only one study showing a beneficial effect.28 The evidence for combination of different drugs for aP is scarce and inconclusive.15 Therefore, it is not a surprise that the simultaneous use of two drugs for aP was reported only as an alternative option for specific individual patients.
Interestingly, the information about dosing of the single drugs for aP was not only very limited due to an insufficient response rate but also highly heterogeneous which precludes a conclusive analysis. This finding is somewhat unclear as there are existing dosing recommendations and available blood parameters to monitor the effects of aP.29, 30 In addition, drug monitoring for aP was part of the management strategy in only slightly more than half of the transplant centers which is an inscrutable finding because thereby a critical evaluation of a dose-response correlation is not possible.31 With respect to a critical evaluation of the risk-benefit balance of aP, drug monitoring making it all the more important as there are only few studies showing the efficacy of aP in preventing RGT outweighing risk of bleeding complications.14, 24, 32 Moreover, it may be especially of interest for preventing harms for patients with LMWH and compromised renal function after pediatric KTx.29 Expectedly, the most often used monitoring parameter for aP are partial thromboplastin time and anti-Xa-activity.33
Though thrombophilia screening in children prior to pediatric KTx is a matter of debate due to conflicting results and limited data, most transplant centers perform thrombophilia screening per protocol as standard of care in renal transplant patients.9, 34 The parameters of routine investigation for thrombophilia differ hardly among the different transplant centers corresponding to common recommendations.34, 35 Within this context, screening for known genetic causes of thrombophilia including factor V Leiden mutation, prothrombin mutation, or methylenetetrahydrofolate reductase polymorphism was performed by 54 to 78% of the transplant centers prior to pediatric KTx.35 Interestingly, while screening for thrombotic risk factors is done in all renal transplant candidates in more than half of the transplant centers, about one third of all transplant centers restrict thrombophilia screening to a selected population with an estimated higher hazard of developing RGT.34 Albeit the risk factors attributed for increasing the likelihood of RGT are diverse, positive family history, previous events of thromboembolic episodes, and re-transplantations appear to be the main determining factors in the pediatric transplant centers similar to data from adult renal transplantation.36 Surprisingly, the selected thrombophilia screening does not necessarily result in a tailored aP for the patients with positive thrombophilia markers. About one third of centers perform aP in all renal transplant patients independent from the results of the thrombophilia screening.34
The survey has several limitations: First, the precise number of registered pediatric transplant centers within the ESPN remains unknown, and therefore, the findings of the survey might be significantly biased by an indeterminate non-response rate. Nevertheless, compared to previous published ESPN-based surveys focusing on aspects of pediatric KTx, the number of responses was rather above-average.37, 38 Second, though a homogenous distribution of the responding pediatric KTx centers all-over Europe endorses the representative value for the investigation of the current practice of aP, only a minority of pediatric KTx centers outside Europe participated in the survey. Consequently, the findings might not reflect the management strategies in other underrepresented transplant centers, particularly in Northern America. Otherwise, the reliability of the reported data is corroborated by an available standard protocol for renal transplantation including the management of aP in almost all participating centers. Therefore, the generalizability of the findings to a wider base of renal transplant centers might be assumed.
In conclusion, despite the use of aP in almost all responding pediatric KTx centers, there is no uniform management strategy for aP related to indications, drug choice, mode of application, and dosing and monitoring. Notwithstanding, UFH seems to be the drug of choice as initial prophylaxis, and ASS for maintenance of aP.
The heterogeneous results of this survey indicate that a harmonization and standardization of aP management strategies in pediatric renal transplant recipients is urgently needed. Prior to the development of consensus recommendations, for instance by selecting the Delphi survey method, more data on aP in pediatric KTx should be generated. Within this context, a complementary systematic review could be helpful to collate all the available current information and to elucidate important criteria for further studies. These studies, preferably prospective randomized controlled trials, are essential to further evaluate the benefits and risks of aP in pediatric KTx.
5 COLLABORATORS (IN ALPHABETICAL ORDER)
Brigitte Adams (Department of Pediatric Nephrology, Hôpital des Enfants Reine-Fabiola, Université Libre de Bruxelles, Brussels, Belgium); Ángel Alonso-Melgar (Pediatric Nephrology, Hospital Universitario La Paz, Madrid, Spain); Atif Awan (Pediatric Nephrology and Transplantation, Children's University Hospital, Dublin, Ireland); Sergey Baiko (Department of Pediatrics, Belarusian State Medical University, Minsk, Belarus); Elisa Benetti (Pediatric Nephrology, Dialysis and Transplantation Unit, Department of Women's and Children's Health, University Hospital of Padua, Padua, Italy); Rajendra Bhimma (University of KwaZulu-Natal, Durban, South Africa); Martin Bitzan (Al Jalila Children's Hospital, Dubai, United Arabic Emirates); Anna Kristina Bjerre (Division of Pediatric and Adolescent Medicine, Oslo University Hospital HF, Rikshospitalet, Oslo, Norway); Caroline Booth (Evelina London Children's Hospital, London, United Kingdom); Antonia Bouts (Department of Pediatric Nephrology, Emma Children's Hospital, AMC, Amsterdam, The Netherlands); Per Brandstrom (Queen Silvia Children's Hospital, Gothenburg, Sweden); Anja Büscher (Pediatric Nephrology, University Children`s Hospital Essen, Essen, Germany); Burcu Bulum (Department of Pediatric Nephrology, Acıbadem Mehmet Ali Aydınlar University School of Medicine, Istanbul, Turkey); Roberta Camilla (Regina Margherita Children's Hospital, Turino, Italy); Benedetta Chiodini (Department of Pediatric Nephrology, Hôpital des Enfants Reine-Fabiola, Université Libre de Bruxelles, Brussels, Belgium); Martin Christian (Department of Pediatric Nephrology, Nottingham University Hospitals NHS Trust, Nottingham, United Kingdom); Marlies Cornelissen (Radboud University Medical Centre, Nijmegen, The Netherlands); Patrícia Costa-Reis (Pediatrics Department, Hospital de Santa Maria, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal); Francisco de la Cerda-Ojeda (Virgen del Rocio Children's Hospital, Seville, Spain); Luca Dello Strologo (Bambino Gesu Children's Hospital, Rome, Italy); Nida Temizkan Dinçel (Department of Pediatric Nephrology, Dr Behcet Uz Children's Hospital, University of Health Sciences, Ege University, Izmir, Turkey); Katalin Dittrich (Pediatric Nephrology, University Children`s Hospital Leipzig, Leipzig, Germany); Ismail Dursun (Division of Nephrology, Department of Pediatrics, Erciyes University Faculty of Medicine, Kayseri, Turkey); Laura Espinosa-Román (Pediatric Nephrology, Hospital Universitario La Paz, Madrid, Spain); Markus Feldkötter (Department of Pediatric Nephrology, University Children`s Hospital Bonn, Bonn, Germany; Pediatric Nephrology Department; University Children`s Hospital Zurich, Zurich, Switzerland); Jorge Ferraris (Hospital Italiano de Buenos Aires, Buenos Aires, Argentina); Kibriya Fidan (Department of Pediatric Nephrology, Gazi University Medical School, Gazi, Turkey); Marc Fila (Pediatric Nephrology—CHU Arnaud de Vielleneuve, Montpellier University Hospital, France); Michaela Gessner (Department of General Pediatrics and Hematology/Oncology, University Children`s Hospital, University Hospital Tübingen, Tübingen, Germany); Ryszard Grenda (The Children's Memorial Health Institute, Warsaw, Poland); Jérôme Harambat (Pediatric Nephrology Unit, Bordeaux University Hospital, Bordeaux, France); Wesley Hayes (Department of Pediatric Nephrology, Great Ormond Street Hospital for Children NHS Foundation Trust, London, United Kingdom); Maria Herthelius (Pediatric Nephrology, The Children's and Women's Health Theme, Karolinska University Hospital, Stockholm, Sweden); Nakysa Hooman (Aliasghar Children Hospital, Aliasghar Clinical Research Development Center, Iran University of Medical Sciences, Tehran, Iran); Lilian Johnstone (Monash Children's Hospital, Melbourne, Australia); Michael Kaabak (Organ transplant division in National Medical Research Center for Children's Health, Boris Petrovsky National Research Center of Surgery, Moscow, Russia); Beltinge Demircioğlu Kılıç (Department of Pediatric Nephrology, Faculty of Medicine, Gaziantep University, Gaziantep, Turkey); Günter Klaus (Pediatric Nephrology, University of Marburg, Marburg, Germany); Noel Knops (University Hospital Leuven, Leuven, Belgium); Jens Koenig (University Children`s Hospital Münster, Germany); Matjaz Kopac, (Children's Hospital Ljubljana, University Clinical Centre Ljubljana, Ljubljana, Slovenia); Eda Didem Kurt-Şükür (Department of Pediatric Nephrology, Ankara Dr Sami Ulus Maternity and Children Hospital, Ankara, Turkey); Angela Lamb (Pediatric Renal Unit, Royal Hospital for Children Glasgow, Glasgow, United Kingdom); Guido F. Laube (Pediatric Nephrology Department; University Children`s Hospital Zurich, Zurich, Switzerland); Mercedes Lopez (Vall d´Hebrón Hospital, Barcelona, Spain); Ma. Angeles Marbella (National Kidney and Transplant Institute, Quezon City, Philippines); Jurate Masalskiene (Hospital of Lithuanian University of Health Sciences, Kaunas, Lithuania); Marta Melgosa (Pediatric Nephrology, Hospital Universitario La Paz, Madrid, Spain); Omega Mellyana (Faculty of Medicine Diponegoro University, Semarang, Indonesia); Kenichiro Miura (Department of Pediatric Nephrology, Tokyo Women's Medical University, Tokyo, Japan); Henry Morgan (Pediatric Nephrology, Alder Hey Children's NHS Foundation Trust, Liverpool, United Kingdom); Conceição Mota (Centro Hospitalar do Porto, Porto, Portugal); Dominik Müller (Department of Pediatric Gastroenterology, Nephrology and Metabolic Diseases, Charité University Medicine Berlin, Berlin, Germany); Luisa Murer (Pediatric Nephrology, Dialysis and Transplantation Unit, Department of Women's and Children's Health, University Hospital of Padua, Padua, Italy); Bogna Niwińska-Faryna (Pediatric Nephrology, The Children's and Women's Health Theme, Karolinska University Hospital, Stockholm, Sweden); Robert Novo (University Hospital Lille, Lille, France); Lars Pape (Department of Pediatric Kidney, Liver and Metabolic Diseases, Hannover Medical School, Hannover, Germany); Licia Peruzzi (Regina Margherita Children's Hospital, Turino, Italy); Martin Pohl (Pediatric Nephrology Department, University Hospital Freiburg, Freiburg, Germany); Nikoleta Printza (Pediatric Nephrology Unit, First Pediatric Department, Hippokration General Hospital, Aristotle University, Thessaloniki, Greece); Agnieszka Prytula (Ghent University Hospital, Ghent, Belgium); Andreaa Rachisan (University of Medicine and Pharmacy Cluj-Napoca, Cluj-Napoca, Romania); George S. Reusz (First Department of Pediatrics, Semmelweis University; Budapest, Hungary); Ben Reynolds (Pediatric Renal Unit, Royal Hospital for Children Glasgow, Glasgow, United Kingdom); Pornpimol Rianthavorn (Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand); Dimitar Roussinov (Pediatric University Hospital, Medical University, Sofia, Bulgaria); Rina Rus (Children's Hospital Ljubljana, University Clinical Centre Ljubljana, Ljubljana, Slovenia); Raphael-Sebastian Schild (Pediatric Nephrology Department, University Hospital Hamburg Eppendorf, Hamburg, Germany); Anne-Laure Sellier-Leclerc (Pediatric Nephrology Department, Hôpital Femme Mère Enfant, Hospices Civils de Lyon, Bron, France); Fatma Lale Sever (Pediatric Nephrology Department, Cerrahpasa School of Medicine, Istanbul University-Cerrahpasa, Istanbul, Turkey); Mohan Shenoy (Pediatric Nephrology, Royal Manchester Children's Hospital, Manchester, United Kingdom); Jasna Slaviček (Department for Pediatric Nephrology, Dialysis and Transplantation, Department of Pediatrics, University Hospital Centre Zagreb, University of Zagreb Medical School, Zagreb, Croatia); Stella Stabouli (Pediatric Nephrology Unit, First Pediatric Department, Hippokration General Hospital, Aristotle University, Thessaloniki, Greece); Julie Tenenbaum (Pediatric Nephrology—CHU Arnaud de Vielleneuve, Montpellier University Hospital, France); Sara Testa (Pediatric Nephrology, Dialysis and Transplantation Unit, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy); Rezan Topaloğlu (Division of Pediatric Nephrology, Hacettepe University Faculty of Medicine, Ankara, Turkey); Peter Trnka (Children's Health Queensland, Brisbane, Australia); Sibylle Tschumi (Division of Pediatric Nephrology, Children's Hospital, University of Bern, Bern, Switzerland); Yincent Tse (Department of Pediatric Nephrology, Great North Children's Hospital, Newcastle upon Tyne, United Kingdom); Siegfried Waldegger (Department of Pediatrics I, Medical University of Innsbruck, Innsbruck, Austria); Lutz T. Weber (Pediatric Nephrology, University Children´s Hospital of Cologne, Cologne, Germany); Nurdan Yıldız (Division of Pediatric Nephrology, Marmara University School of Medicine, Istanbul, Turkey); Eugene Yu-hin Chan (Pediatric Nephrology Centre, Hong Kong Children's Hospital, Hong Kong, China); Selçuk Yüksel (Division of Pediatric Nephrology, Department of Pediatrics, Pamukkale University Faculty of Medicine, Denizli, Turkey); Jakub Zieg (Department of Pediatrics, Second Faculty of Medicine, Charles University Prague, University Hospital Motol, Prague, Czech Republic); further responses were retrieved from the following institutions (corresponding physicians unknown): Children's Hospital P. and A. Kyriakou, University of Athens School of Medicine, Greece; Royal Hospital, Muscat, Oman; Shaare Zedek Medical Center, Jerusalem, Israel; and Medical University of Vienna, Austria.
ACKNOWLEDGMENTS
The authors would like to thank all responders who answered the survey. Furthermore, the authors thank the previous und current presidents of the ESPN, Elena Levtchenko, and Rezan Topaloğlu, for their full endorsement of the study as well as their precious assistance in distributing the survey.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHORS’ CONTRIBUTIONS
MW, KB, BT: Involved in research idea and study design; KB and MW: Involved in data acquisition; KB, MW, and MZ: Analyzed/interpreted the data; KB: Performed statistical analysis; KB, MW, MZ, SB, SN, and BT: Wrote the manuscript. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies with human participants or animal performed by any of the authors.